Abstract
Objective
To identify differences in health factors, neuromuscular attributes and performance-based mobility among community-dwelling older adults with symptomatic lumbar spinal stenosis (SLSS); and to determine which neuromuscular attributes are associated with performance-based measures of mobility. We hypothesized that: (1) community-dwelling older adults with SLSS would have poorer health, greater neuromuscular impairment and worse mobility; and (2) greater impairment in trunk extensor muscle endurance, leg strength, leg speed and knee flexion range of motion (ROM) would be associated with performance-based mobility.
Design
Cross-sectional; secondary data analysis of a cohort study
Setting
outpatient rehabilitation center
Participants
Community-dwelling adults ≥ 65 years with self-reported mobility limitations and SLSS
Interventions
N/A
Main Outcome Measures
Short Physical Performance Battery Score, Habitual Gait Speed, and Chair Stand Test
Results
SLSS was classified using self-reported symptoms of neurogenic claudication and imaging. Among 430 community-dwelling older adults 54(13%) met criteria for SLSS. Compared to participants without SLSS, those with SLSS had more comorbidities, higher BMI, greater pain and less balance confidence. Participants with SLSS had greater impairment in trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion range of motion (ROM), knee extension ROM, and ankle ROM compared to participants without SLSS. Five neuromuscular attributes were associated with performance-based mobility among participants with SLSS: trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion ROM, and knee extension ROM asymmetry.
Conclusions
Community-dwelling older adults with self-reported mobility limitations with SLSS exhibit poorer health characteristics, greater neuromuscular impairment and worse mobility when compared to those without SLSS. Poorer trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion ROM, and knee extension ROM asymmetry were associated with performance-based mobility among participants with SLSS.
Keywords: aged, spinal stenosis, impairment, mobility limitation
Symptomatic lumbar spinal stenosis (SLSS) is a degenerative spinal condition prevalent among 10–14% of community-dwelling older adults.1–3 SLSS is a major contributor to mobility limitations and is recognized as the primary reason for spinal surgery among patients > 65 years.4 SLSS is defined as the combination of imaging-detected lumbar spinal stenosis (LSS) and self-reported neurogenic claudication; pain, numbness or weakness in the lower extremities that worsens with spinal extension and improves with spinal flexion.5 Neurogenic claudication, the hallmark clinical symptom of SLSS is a major cause for the manifestation of mobility limitations among older adults.6–8
Despite the major impact of SLSS on mobility, major gaps remain in the evidence guiding effective non-surgical rehabilitative care for older adults with SLSS.1, 9 A clear understanding of the impairments that contribute to limitations in walking and mobility-related tasks is essential to guide the development of rehabilitative care plans that target those impairments. This point is conceptualized within the International Classification of Functioning, Disability and Health, which defines rehabilitation as treatment that reduces impairments and improves activities and participation of patients receiving care.10 Although, mobility limitations and the associated neuromuscular attributes have been described in the literature among older adults in general, they have not been well-described among older adults with SLSS.6, 7, 11, 12
The Boston Rehabilitative Study of the Elderly (Boston RISE) is a prospective cohort study of community-dwelling older adults that evaluated a large scope of neuromuscular attributes linked to mobility skills among older adults.13 Boston RISE evaluated performance-based mobility as well as the presence of neurogenic claudication making it an ideal platform to study impairments that are linked to mobility among patients with SLSS.
The purposes of this study were: (1) to identify differences in health factors, neuromuscular attributes and measures of performance-based mobility among community-dwelling older adults with SLSS compared to those without SLSS; and (2) to determine which neuromuscular attributes were associated with performance-based measures of mobility among participants with SLSS. It was hypothesized that community-dwelling older adults with SLSS would have poorer health, greater impairment among neuromuscular attributes and worse mobility. In addition, greater impairment in trunk extensor muscle endurance, leg strength, leg speed and knee flexion range of motion (ROM) would be associated with performance-based mobility.
Methods
Boston RISE conducted assessments across 4 years of follow-up and evaluated neuromuscular attributes as well as self-reported and performance-based measures of mobility.13 It included 430 community-dwelling older adults ≥ 65 years who were at risk for mobility decline, as defined by self-reported difficulty or the inability to walk one-half mile and/or climb one flight of stairs without assistance. Boston RISE also contains questions that assess components of neurogenic claudication, the hallmark symptom characterizing SLSS.5 Boston RISE and this secondary analysis received approval from the Spaulding Rehabilitation Network IRB.
Inclusion criteria for Boston RISE were as follows: age ≥ 65 years, ability to speak and understand English, receiving primary care services from Brigham and Women’s Hospital or Massachusetts General Hospital at the time of the study, difficulty or inability to walk one-half mile and/or climb one flight of stairs, no planned major surgery, and no planned move from the area for two years. Exclusion criteria were as follows: significant visual impairment, uncontrolled hypertension, amputation of a lower extremity, use of supplemental oxygen, myocardial infarction or major surgery in the previous 6 months, Mini-Mental State Examination (MMSE) score < 18,14 and Short Physical Performance Battery score < four.15
Participants
Only participants who completed a lumbar spinal stenosis (LSS) questionnaire at one of three annual visits (baseline, year 1, or year 2) as well as measures of neuromuscular attributes and performance-based mobility were included in this analysis. The LSS questionnaire describes the presentation of neurogenic claudication: pain, numbness or weakness in either leg that is aggravated with walking and decreases or ceases with flexion postures. The questions have a high diagnostic accuracy for detecting the clinical syndrome of lumbar spinal stenosis.5
SLSS was diagnosed using self-reported symptoms of neurogenic claudication combined with spinal imaging demonstrating imaging-detected LSS. Spinal imaging was not part of the study methods for Boston RISE, however, imaging from two separate sources was used in order to define the presence of imaging-detected LSS. As the first source, electronic medical records for participants within Boston RISE with self-reported neurogenic claudication were accessed to extract either computed tomography (CT) or magnetic resonance imaging (MRI) reports that identified imaging-detected LSS. Imaging reports were interpreted by the primary author (CTS). Based on previously validated criteria, imaging-detected LSS was defined as the presence of at least moderate to severe central canal stenosis, or severe neuroforaminal stenosis.5 Lateral recess stenosis was not recorded for this analysis due to poor reliability in previous studies and a lack of consensus on optimal grading.16 An inter-rater reliability study for extraction of imaging-detected LSS based on our definition was conducted in support of the methods (Kappa statistic = 0.87; 95% agreement).
The second source of evidence for imaging-detected spinal stenosis came from a separate ancillary study of Boston RISE with different scientific aims. Within that study lumbar spine CT scans were conducted among 50 participants. Scans were read by an experienced spine physiatrist trained in the assessment of lumbar spine imaging for research purposes (PS). CT scans that identified the presence of moderate to severe central canal stenosis or severe neuroforaminal stenosis were included in this study.17
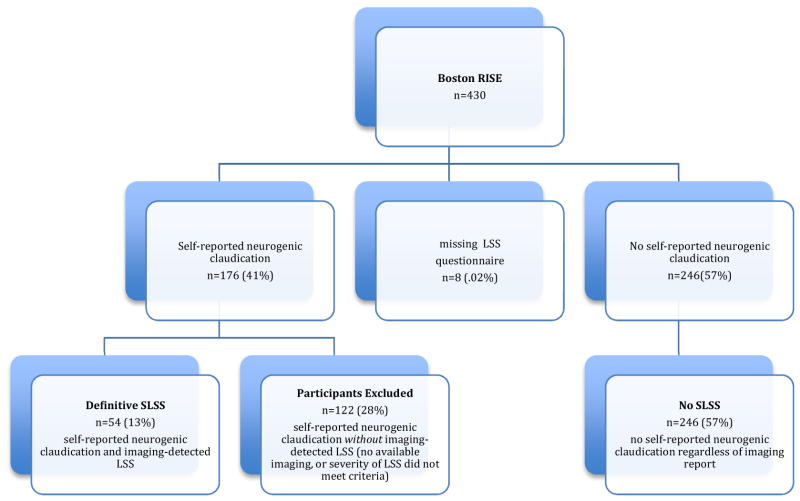
Using responses from the lumbar spinal stenosis questionnaire and information extracted from the electronic medical record and/or CT scans two separate categories were defined (Figure 1). Participants with self-reported neurogenic claudication symptoms from at least one annual visit and imaging-detected LSS were classified as Definitive Stenosis. Participants reporting no neurogenic claudication at all three time points were classified as No SLSS. Participants with self-reported neurogenic claudication symptoms but without imaging reports available, or a severity of LSS that did not meet study criteria were excluded from this study.
Figure 1.
Flowchart Indicating Inclusion
Demographics and Health Characteristics
Baseline demographics of age, gender, and education were evaluated along with selected health factors. The number of comorbidities was ascertained from a validated self-reported questionnaire developed by Sangha and colleagues.18 Participants were questioned about the presence of selected chronic diseases. The final score was the total number of chronic diseases reported by the patient (0–13).18 BMI was calculated as weight (kilograms)/height2 (meter2).19 Global pain was evaluated using the Brief Pain Inventory Pain Severity Subscale. This scale includes four questions that are each scored from 0–10 where 0 indicates no pain and 10 indicates the highest level of pain. The final score is comprised of an average of the four ratings: pain at its worst in the last week, at its least in the last week, on average, and at its current status.20 Sensory loss was recorded if a participant was unable to feel less than 3 out of 5 touches for both, the 4.17 and 5.07 monofilaments over the dorsum of one or both great toes.21 Self-efficacy was measured using the Activities Balance Confidence Scale (0–100) with lower scores indicating less balance confidence. Cognitive status was measured using the MMSE (0–30) with lower scores indicating worse cognitive performance.14 The Patient Health Questionnaire was evaluated to measure depression (0–24) with higher scores indicating a greater level of depressive symptoms.
Neuromuscular Attributes
The following 10 neuromuscular attributes were analyzed: trunk extensor muscle endurance; leg strength; leg strength asymmetry; leg speed; maximum knee flexion range of motion and asymmetry; maximum knee extension ROM and asymmetry; ankle ROM; and kyphosis.13 Trunk extensor muscle endurance was measured using a specialized plinth that stabilized the participant’s pelvis and lower extremities in a position 45 degrees from a horizontal plane. Trunk extensor muscle endurance was recorded as the time in seconds that a participant could maintain a neutral trunk position with arms crossed over his/her chest (0–150 seconds).22 Leg strength and leg speed were measured using a pneumatic leg press as a composite lower extremity assessment evaluating leg strength and power generation. The 1 repetition maximum was conducted for both legs. The higher value of either leg (right or left) was recorded as the 1 repetition maximum and normalized based on body weight (Newtons/kilogram). Leg speed (m/s) was derived as the speed of movement of the leg as measured on the pneumatic leg press during the measurement of a participant’s maximum leg power (speed = power/force).23 Leg strength asymmetry was measured as the ratio of the higher value of a participant’s right or left leg strength divided by the lower value of the opposing side.
Lower extremity range of motion was measured using a goniometer and based on standardized protocols.24 Maximum knee flexion and extension ROM were recorded with the participant positioned in supine and measured in degrees. Knee flexion and extension ROM asymmetry were recorded as the absolute difference between the right and left ROM measures. Impaired ankle range of motion (yes/no) was recorded as the inability to dorsiflex greater than or equal to 90 degrees, or < 20 degrees of total ankle motion on either lower extremity. Kyphosis was measured using a flexicurve ruler placed over the participant’s thoracic spine. The curvature of the ruler was traced onto a paper and a measure of height/length × 100 was recorded for the amount of thoracic kyphosis.25
Measures of Mobility
Performance-based mobility limitations were measured using the Short Physical Performance Battery Score15, habitual gait speed (HGS)26 and the chair stand test.15 The Short Physical Performance Battery Score is comprised of 3 tests: standing balance, usual paced walking speed, and a 5-repitition chair stand. Each of the 3 tests is scored from 0–4 with the total score being a sum of the 3 tests (0–12), and a higher score indicating better performance. HGS (m/s) was derived from the usual walking speed subcomponent of the Short Physical Performance Battery Score, which measures the time it takes to walk 4 meters. The chair stand test measures the time it takes for participants to rise from a chair 5 times with their arms folded over their chest. These performance-based measures of mobility were selected as they represent a range of mobility performance including walking and basic mobility tasks and are reliable and valid measures among older adults.15, 26–28
Statistical Analysis
Measures of neuromuscular attributes and performance-based mobility were analyzed from the annual visit that occurred closest to the imaging report identifying imaging-detected LSS. Imaging that occurred greater than 5 years prior to or 3 years following the presence of neurogenic claudication symptoms was excluded as changes in anatomic LSS are minimal at follow-up durations of ≤ 3 years.29 For participants in the No SLSS group assessment data was analyzed from the earliest annual visit when no neurogenic claudication was reported. Weighted imputation, an approach advocated for addressing missing data among studies of older adults was used for the analysis of missing neuromuscular attributes.30
Demographics, health factors, measures of neuromuscular attributes and performance-based mobility were analyzed for normality and compared for participants with Definitive SLSS vs No SLSS using independent samples t-tests for continuous variables and Chi Square tests for categorical variables.
Three separate multivariable linear regression models evaluating the relationship between neuromuscular attributes and measures of mobility were conducted using an iterative process. First, the neuromuscular attributes were evaluated for collinearity using Spearman or Pearson correlation coefficients. For any attribute that had a correlation coefficient > 0.40 the neuromuscular attribute that had the stronger association with the outcome was considered for inclusion in the proceeding analysis. Next, bivariate associations for each neuromuscular attribute and mobility outcome were evaluated. Neuromuscular attributes that had an association with the outcome of p<0.1 were selected for inclusion into the initial regression models. For each model neuromuscular attributes that resulted in a statistically significant association (p<0.05) with the outcome were included in the final model. Fit statistics were evaluated to ensure that the statistical assumptions for each statistical model were met appropriately.
Adjustment variables were selected based on their association with the outcomes. Adjustment variables that were statistically significant and/or changed the beta estimate greater than 20% were considered for the final model. Electronic medical record data was collected using REDCap.31 SAS 9.4 was used for all analyses.
Sensitivity analyses were conducted by combining participants with self-reported neurogenic claudication and imaging-detected LSS of lesser severity (i.e., central canal stenosis less than moderate to severe and/or neuroforaminal stenosis less than severe) with participants with Definitive SLSS. Demographics, health factors, measures of neuromuscular attributes and performance-based mobility were compared for the sensitivity group vs the No SLSS group. Also, the association of neuromuscular attributes with the three separate measures of performance-based mobility was conducted among the sensitivity group using the same statistical methods as the primary analysis.
Results
Among the 430 participants from Boston RISE, 176 (41%) reported and 246 (57%) did not report the presence of neurogenic claudication symptoms. Among those who reported the presence of neurogenic claudication 54(13%) met criteria for Definitive SLSS, 246 (57%) were classified as No SLSS, and 122 (28%) did not meet criteria for imaging-detected LSS (did not have spinal imaging measures available within the medical record to ascertain imaging-detected LSS, or the severity of LSS did not meet study criteria) and were excluded (Figure 1).
Participants with SLSS experienced a greater number of comorbidities, a higher BMI, greater global pain, and less balance self-efficacy compared to participants without SLSS (Table 1). In addition, the number of participants with post high school education was significantly less among patients with SLSS compared to those without SLSS.
Table 1.
Mean Differences for Demographics, Health Factors, Neuromuscular Attributes and Measures of Mobility among Participants with and without SLSS
| SLSS* (n=54) | n | No SLSS* | n | p value | 95% CI | |
|---|---|---|---|---|---|---|
| Demographics/Health Factors | ||||||
|
| ||||||
| Age (years) | 76.9(6.6) | 54 | 77.1(7.2) | 246 | 0.86 | (−1.9, 2.3) |
|
| ||||||
| Gender (% female) | 36(67%) | 54 | 164(67%) | 246 | 1.00 | ---- |
|
| ||||||
| Education | ||||||
| ≤ high school | 28(53%) | 53 | 92(37%) | 246 | 0.04 | ---- |
| ≥ college | 25(47%) | 154(63%) | ||||
|
| ||||||
| Comorbidities (0–13) | 4.9 (2.1) | 54 | 3.6(1.7) | 246 | <0.001 | (−1.9, −0.7) |
|
| ||||||
| BMI | 30.9(5.7) | 52 | 28.7(5.7) | 245 | 0.01 | (−3.9, −0.5) |
|
| ||||||
| Brief Pain Inventory (0–10) | 4.0(1.6) | 53 | 2.1(1.8) | 246 | <0.001 | (−2.5, −1.4) |
|
| ||||||
| Sensory Loss (% impaired) | 20(40%) | 51 | 69(28%) | 243 | 0.13 | ---- |
|
| ||||||
| Self efficacy ABC Scale (0–100) | 68.1(20.1) | 53 | 78.1(15.8) | 246 | <0.01 | (4.1,15.9) |
|
| ||||||
| MMSE (0–30) | 27.4(2.3) | 53 | 27.4(2.4) | 246 | 0.94 | (−0.7,0.7) |
|
| ||||||
| Depression (0–24) | 1.9(4.8) | 54 | 1.4(3.2) | 246 | 0.41 | (−1.9, 0.8) |
|
| ||||||
| Neuromuscular Attributes | ||||||
|
| ||||||
| Trunk Extensor Muscle Endurance (seconds) | 69.1(55.9) | 54 | 101.9(56.8) | 246 | <0.001 | (15.2, 50.4) |
|
| ||||||
| Leg Strength (Newtons/kg) | 8.4(2.5) | 54 | 9.6(2.7) | 246 | <0.01 | (0.4,2.0) |
|
| ||||||
| Leg Strength Asymmetry | 1.3(0.6) | 54 | 1.2(0.2) | 246 | 0.04 | (−0.2,−0.002) |
|
| ||||||
| Leg Speed (meters/second) | 0.97(0.31) | 54 | 1.0(0.23) | 246 | 0.50 | (−0.06, 0.1) |
|
| ||||||
| Knee Flexion ROM (degrees) | 120.1(18.8) | 54 | 126.2(13.1) | 246 | 0.01 | (1.84, 10.4) |
|
| ||||||
| Knee Flexion ROM Difference (degrees) † | 7.3(10.5) | 54 | 5.3(6.2) | 246 | 0.19 | (−5.0, 1.0) |
|
| ||||||
| Knee Extension ROM (degrees) † | 6.4 (5.5) | 54 | 9.6(6.1) | 246 | <0.001 | (1.4, 5.0) |
|
| ||||||
| Knee Extension ROM Difference (degrees) | 2.9(2.4) | 54 | 3.3(3.0) | 246 | 0.33 | (−0.4, 1.3) |
|
| ||||||
| Ankle ROM (% impaired) | 26(49%) | 54 | 67(27%) | 246 | <0.01 | ---- |
|
| ||||||
| Kyphosis‡ | 10.3(3.4) | 54 | 10.6(3.1) | 246 | 0.64 | (−0.7, 1.2) |
|
| ||||||
| Mobility Measures | ||||||
|
| ||||||
| Short Physical Performance Battery Score (0–12) | 8.2(2.5) | 54 | 9.0(2.2) | 246 | 0.03 | (0.1, 1.4) |
|
| ||||||
| Habitual Gait Speed (meters/second) | 0.82(0.25) | 54 | 0.93(0.21) | 246 | <0.001 | (0.1, 0.2) |
|
| ||||||
| Chair Stand Test (seconds) | 16.1(7.0) | 54 | 15.5(6.9) | 246 | 0.58 | (−2.6, 1.5) |
Mean(standard deviation) or frequency(percentage),
Mean(SD) calculated from non-imputed data
ABC Scale=Activities-specific Balance Confidence Scale
kyphosis measure=height/length of flexicurve measure 100
On average participants with SLSS experienced greater impairment in trunk extensor muscle endurance (p<.001), leg strength (p<.01), leg strength asymmetry (p<.04), knee flexion ROM (p<.01), knee extension ROM (p<.001) and ankle ROM (p<.01). In addition, participants with SLSS scored lower on the Short Physical Performance Battery Score (p=.03) and slower on habitual gait speed performance (p<.001) (Table 1).
Among the neuromuscular attributes, knee flexion ROM and knee flexion ROM asymmetry demonstrated collinearity (r=0.41). Knee flexion ROM had a greater association with the mobility outcomes and therefore was used for this analysis. Using multivariable regression models trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion ROM and knee extension asymmetry were associated with cross-sectional performance-based measures of mobility (Table 2).
Table 2.
Multivariable Linear Regression Models Evaluating the Association of Neuromuscular Attributes with Performance-based Measures of Mobility among Participants with SLSS
| Outcome | Variables | N | Beta Estimate | SE | 95% CI | p value |
|---|---|---|---|---|---|---|
| R2=0.24 | ||||||
| SPPB Total | trunk extensor muscle endurance | 54 | 0.02 | 0.005 | (0.01,0.03) | <0.01 |
| leg strength asymmetry | −1.16 | 0.49 | (−2.13,−0.19) | 0.02 | ||
| leg speed | 2.01 | 1.03 | (−0.06, 4.09) | 0.057 | ||
| R2=0.35 | ||||||
| HGS | trunk extensor muscle endurance | 54 | 0.001 | 0.0005 | (0.0003,0.003) | 0.02 |
| knee flexion ROM | 0.004 | 0.002 | (0.001,0.007) | 0.01 | ||
| knee extension asymmetry | 0.04 | 0.01 | (0.02,0.07) | <0.01 | ||
| leg speed | 0.17 | 0.10 | (−0.03, 0.38) | 0.09 | ||
| R2=0.19 | ||||||
| Chair stand | leg strength | 54 | −1.53 | 0.43 | (−2.40,−0.66) | <0.01 |
95% CI=95% confidence interval, R2=adjusted R2, multivariable linear regression models were adjusted for age and gender.
When comparing the sensitivity group (N=79) with the No SLSS group, differences in education level and leg strength asymmetry identified in the primary analysis were no longer significant. Also, participants in the sensitivity group had significantly greater impairment in knee extension ROM asymmetry than participants in the No SLSS group. The neuromuscular impairments associated with performance-based mobility in the primary analysis remained associated in the sensitivity analysis, however, leg speed, additionally predicted Short Physical Performance Battery Score and habitual gait speed performance within the sensitivity group (Table 2).
Discussion
The main findings of this study are: (1) that Boston RISE participants with SLSS manifested poorer health factors, greater impairment in neuromuscular attributes, and worse performance-based mobility when compared to participants without SLSS; and (2) among participants with SLSS, trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion ROM and knee extension ROM asymmetry were associated with the performance of basic mobility skills.
This study is among the first to evaluate a broad range of neuromuscular attributes that are linked to mobility and amenable to rehabilitative care among older adults with SLSS. When compared to participants without SLSS, trunk extensor muscle endurance, leg strength, leg strength asymmetry, and leg ROM were more impaired for participants with SLSS. Additionally, participants with SLSS had significantly worse performance in measures of mobility compared to those without SLSS. Furthermore, the magnitude of the observed differences for baseline measures of performance-based mobility of the Short Physical Performance Battery Score and HGS between the groups surpassed minimal clinically important differences (MCIDs) previously identified in the literature (Short Physical Performance Battery Score MCID=1.0 unit, HGS= 0.1 m/s).32–34 The statistical and clinically meaningful differences provide a better understanding of the level of impairment among neuromuscular attributes and degree of limitation in mobility among community-dwelling older adults with SLSS.
Previous cross-sectional analysis of the Boston RISE cohort identified five categories of neuromuscular attributes that were associated with patient-reported mobility status: leg strength; leg speed of movement; leg ROM; leg strength asymmetry; and trunk extensor muscle endurance. This study among Boston RISE participants with SLSS was consistent except that leg speed was not associated with any of the mobility measures within this analysis. Interestingly, leg speed was not significantly different between those with and without SLSS. This finding may imply that participants with SLSS do not prioritize use of this neuromuscular attribute when performing basic mobility tasks. Also, it may be that due to a small sample size of participants with SLSS sufficient statistical power was not achieved to detect a meaningful relationship for this variable.
A novel finding of this study is the relationship between trunk extensor muscle endurance and performance-based mobility. Targeting trunk extensor muscle endurance has been studied among younger adults, for the purposes of enhancing athletic performance and preventing low back pain.35, 36 The current study suggests that trunk extensor muscle endurance is an important neuromuscular attribute among older patients with SLSS relevant to poor mobility performance. This is also supported by another Boston RISE analysis among participants with low back pain that highlighted the importance of trunk extensor muscle endurance.37 While the other neuromuscular attributes linked to mobility are commonly prioritized within existing SLSS rehabilitative care, findings from this study suggest that future approaches should address trunk extensor muscle endurance.22, 23, 38 The association between trunk extensor muscle endurance and mobility skills may provide insights into existing rehabilitative approaches and in turn functional outcomes may be improved.
Among participants with self-reported neurogenic claudication 54(31%) met our criteria for having SLSS, however 25(14 %) had a severity of imaging-detected stenosis that did not meet our criteria. This study employed strict study criteria for the Definitive SLSS group, excluding participants with a lesser severity of imaging-detected LSS, which may have led to some misclassification. We explored this through sensitivity analyses combining participants with a lesser severity of imaging-detected LSS with those with moderate to severe LSS (sensitivity group and Definitive SLSS N=79). The group that had a less severe presentation of imaging-detect LSS did not result in material differences with regard to mean comparisons for neuromuscular impairment measures or performance of mobility tasks. However, leg speed was a significant predictor of Short Physical Performance Battery Score and habitual gait speed performance, despite estimates for leg speed being equivalent within the regression models for the primary analyses and sensitivity analyses. Although this may suggest a limitation in power, the findings highlight that leg speed is an important neuromuscular impairment relevant to mobility tasks among patients with SLSS regardless of the severity of imaging-detected LSS, which has not been previously studied.
Study Limitations
There are other potential limitations to this study. Boston RISE was not a population based study and therefore findings from this study can only be generalized to community-dwelling older adults with SLSS and in accordance with the criteria used to define imaging-detected SLSS. Although the prevalence of SLSS within the Boston RISE cohort (13%) is similar to that of a population based study the small sample size may have limited observing other clinically relevant associations.39 Also, the specific criteria for diagnosing the severity of imaging-detected spinal stenosis may have varied among those reading the images. However, all physicians belong to the Partner’s HealthCare System and used consistent documentation systems.
Conclusions
Community-dwelling older adults with SLSS exhibit important differences in health characteristics and measures of neuromuscular attributes and performance-based mobility when compared to those without SLSS. Worse impairment in four neuromuscular attributes, trunk extensor muscle endurance, leg strength, leg ROM, and asymmetry of strength and ROM, were associated with performance-based mobility among participants with SLSS. These findings can help inform the development of interventions targeting basic mobility skills among older patients with SLSS.
Highlights.
Community-dwelling older adults with symptomatic lumbar spinal stenosis (SLSS) have more comorbidities, higher BMI, greater pain and less balance confidence compared to participants without SLSS.
Community-dwelling older adults with SLSS have more impaired trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion range of motion (ROM), knee extension ROM, and ankle ROM compared to participants without SLSS.
Five neuromuscular attributes are associated with performance-based mobility among community-dwelling older adults with SLSS: trunk extensor muscle endurance, leg strength, leg strength asymmetry, knee flexion ROM, and knee extension ROM asymmetry.
Acknowledgments
Source of Funding
Dr. Schmidt reports grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K24HD070966), grants from National Institute on Aging (R01 AG032052), during the conduct of the study.
Dr. Jonathan Bean
Dr. Bean reports grants from NIH, during the conduct of the study; grants from NIH, grants from RX Foundation, outside the submitted work.
Boston Rehabilitative Impairment Study of the Elderly is supported by the National Institute on Aging (R01 AG032052), by the National Center for Research Resources in a grant to the Harvard Clinical and Translational Science Center (UL1 RR025758), and by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (1K24HD070966).
Dr. Ward reports grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K24HD070966), grants from National Institute on Aging (R01 AG032052), during the conduct of the study.
Dr. Suri reports personal fees from Spaulding Rehabilitation Hospital, during the conduct of the study.
Dr. Anderson reports grants from National Institute on Aging, grants from Beth Israel Deaconess Medical Center, during the conduct of the study; grants from American Society for Bone and Mineral Research, outside the submitted work
Dr. Dennis Anderson: provided CT Scan data from his previous ancillary study.
Departmental Research Grant Award from the Department of Orthopedics, Beth Israel, Deaconess Medical Center
National Institute on Aging grant T32-AG023480
Abbreviations
- BMI
Body Mass Index
- Boston RISE
Boston Rehabilitative Study of the Elderly
- CT
computed tomography
- HGS
Habitual Gait Speed
- LSS
lumbar spinal stenosis
- MCIDs
Minimal clinically important differences
- MMSE
Mini-mental State Examination Score
- MRI
Magnetic Resonance Imaging
- ROM
range of motion
- SLSS
symptomatic lumbar spinal stenosis
Footnotes
Presentation
An abstract/poster of portions of this work will be presented at the APTA Combined Sections Meeting, San Antonio Texas, 2017.
Another manuscript, “Which Neuromuscular Attributes are Associated with Change in Mobility Among Older Primary Care Patients with Symptomatic Lumbar Spinal Stenosis?” has been submitted to the Physical Therapy Journal. Contents of that manuscript are similar to this submission to Archives including the sample used and the methods section. Otherwise, the objectives of the manuscript and outcome variables were different.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catherine T. Schmidt, Center for Interprofessional Studies and Innovation, MGH Institute of Health Professions, 36 1st Avenue, Boston, MA 02129-4557, Phone: 401-863-1281.
Rachel E. Ward, New England Geriatric Research Education and Clinical Center, GRECC, VA Boston Healthcare System, Boston, MA, 150 South Huntington St., Boston, MA 02130. Department of Physical Medicine and Rehabilitation, Harvard Medical School, Phone: 857-364-2786, Fax: 617-952-6965.
Pradeep Suri, Staff Physician, Division of Rehabilitation Care Services, VA Puget Sound Health Care System, Seattle, WA. Investigator, Seattle Epidemiologic Research and Information Center (ERIC), VA Puget Sound Health Care System. Associate Professor, University of Washington, Department of Rehabilitation Medicine, Seattle, WA, Phone: 206-277-1812, Fax: 206-716-5977.
Dan K. Kiely, Data Analyst, Spaulding Rehabilitation Hospital, Cambridge, MA, Phone: 617-952-6951, Fax: 617-952-6965.
Ni Pensheng, Health and Disability Research Institute, Boston University, Boston, MA, 617-638-1989.
Dennis E. Anderson, Center for Advanced Orthopaedic Studies, Beth Israel Deaconess Medical Center, R N 115, 330 Brookline Avenue, RN115, Boston, MA 02215. Department of Orthopedic Surgery, Harvard Medical School, Phone: 617-667-5380, Fax: 617-667-7175.
Jonathan F. Bean, New England Geriatric Research Education and Clinical Center, GRECC, VA Boston Healthcare System, Boston, MA, 150 South Huntington St., Boston, MA 02130. Spaulding Rehabilitation Hospital, Boston, MA. Department of PM&R, Harvard Medical School, Boston, MA, Phone: 857-364-2786, Fax: 617-952-6965.
References
- 1.Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev. 2013;8:Cd010712. doi: 10.1002/14651858.CD010712. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358(8):818–825. doi: 10.1056/NEJMcp0708097. [DOI] [PubMed] [Google Scholar]
- 3.Ishimoto Y, Yoshimura N, Muraki S, et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthritis Cartilage. 2012;20(10):1103–1108. doi: 10.1016/j.joca.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Ciol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44(3):285–290. doi: 10.1111/j.1532-5415.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 5.Suri P, Rainville J, Kalichman L, Katz JN. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA. 2010;304(23):2628–2636. doi: 10.1001/jama.2010.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iversen MD, Katz JN. Examination findings and self-reported walking capacity in patients with lumbar spinal stenosis. Phys Ther. 2001;81(7):1296–1306. [PubMed] [Google Scholar]
- 7.Tomkins-Lane CC, Holz SC, Yamakawa KS, et al. Predictors of walking performance and walking capacity in people with lumbar spinal stenosis, low back pain, and asymptomatic controls. Arch Phys Med Rehabil. 2012;93(4):647–653. doi: 10.1016/j.apmr.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz JM, Delitto A, Welch WC, Erhard RE. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil. 1998;79(6):700–708. doi: 10.1016/s0003-9993(98)90048-x. [DOI] [PubMed] [Google Scholar]
- 9.Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update) Spine J. 2013;13(7):734–743. doi: 10.1016/j.spinee.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Organization WH. International Classification of Functioning, Disability and Health (ICF) Geneva: World Health Organization; 2001. [Google Scholar]
- 11.Iversen MD, Kale MK, Sullivan JT., Jr Pilot case control study of postural sway and balance performance in aging adults with degenerative lumbar spinal stenosis. J Geriatr Phys Ther. 2009;32(1):15–21. doi: 10.1519/00139143-200932010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Stucki G, Liang MH, Lipson SJ, Fossel AH, Katz JN. Contribution of neuromuscular impairment to physical functional status in patients with lumbar spinal stenosis. J Rheumatol. 1994;21(7):1338–1343. [PubMed] [Google Scholar]
- 13.Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Arch Phys Med Rehabil. 2013;94(2):347–355. doi: 10.1016/j.apmr.2012.08.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RN, Gallo JJ. Dimensions of the Mini-Mental State Examination among community dwelling older adults. Psychol Med. 2000;30(3):605–618. doi: 10.1017/s0033291799001853. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Drew R, Bhandari M, Kulkarni AV, Louw D, Reddy K, Dunlop B. Reliability in grading the severity of lumbar spinal stenosis. J Spinal Disord. 2000;13(3):253–258. doi: 10.1097/00002517-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DE, Bean JF, Holt NE, Keel JC, Bouxsein ML. Computed tomography-based muscle attenuation and electrical impedance myography as indicators of trunk muscle strength independent of muscle size in older adults. Am J Phys Med Rehabil. 2014;93(7):553–561. doi: 10.1097/PHM.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 19.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16(12):1509–1515. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS. Measurement of pain by subjective report. In: Chapman C, Loeser JD, editors. Advances in pain research and therapy. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 21.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract. 2001;54(2):115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 22.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. Pm r. 2009;1(10):916–924. doi: 10.1016/j.pmrj.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bean JF, Latham NK, Holt N, et al. Which neuromuscular attributes are most associated with mobility among older primary care patients? Arch Phys Med Rehabil. 2013;94(12):2381–2388. doi: 10.1016/j.apmr.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins MA, Riddle DL, Lamb RL, Personius WJ. Reliability of goniometric measurements and visual estimates of knee range of motion obtained in a clinical setting. Phys Ther. 1991;71(2):90–96. doi: 10.1093/ptj/71.2.90. discussion 96–97. [DOI] [PubMed] [Google Scholar]
- 25.Milne JS, Williamson J. A longitudinal study of kyphosis in older people. Age Ageing. 1983;12(3):225–233. doi: 10.1093/ageing/12.3.225. [DOI] [PubMed] [Google Scholar]
- 26.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 29.Jarvik JG, Hollingworth WP, Heagerty PJP, et al. Three-Year Incidence of Low Back Pain in an Initially Asymptomatic Cohort: Clinical and Imaging Risk Factors. Spine. 2005;30(13):1541–1548. doi: 10.1097/01.brs.0000167536.60002.87. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter JR, Kenward MG, White IR. Sensitivity analysis after multiple imputation under missing at random: a weighting approach. Stat Methods Med Res. 2007;16(3):259–275. doi: 10.1177/0962280206075303. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 33.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract. 2014;20(4):295–300. doi: 10.1111/jep.12158. [DOI] [PubMed] [Google Scholar]
- 35.Chok B, Lee R, Latimer J, Tan SB. Endurance training of the trunk extensor muscles in people with subacute low back pain. Phys Ther. 1999;79(11):1032–1042. [PubMed] [Google Scholar]
- 36.Dejanovic A, Harvey EP, McGill SM. Changes in torso muscle endurance profiles in children aged 7 to 14 years: reference values. Arch Phys Med Rehabil. 2012;93(12):2295–2301. doi: 10.1016/j.apmr.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Makris UE, Paul TM, Holt NE, et al. The Relationship Among Neuromuscular Impairments, Chronic Back Pain, and Mobility in Older Adults. Pm r. 2016;8(8):738–747. doi: 10.1016/j.pmrj.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67(1):66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishimoto Y, Yoshimura N, Muraki S, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthritis Cartilage. 2013;21(6):783–788. doi: 10.1016/j.joca.2013.02.656. [DOI] [PubMed] [Google Scholar]