ABSTRACT
In the intestine water has to be reabsorbed from the chymus across the intestinal epithelium. The osmolarity within the lumen is subjected to high variations meaning that water transport often has to take place against osmotic gradients. It has been hypothesized that LI-cadherin is important in this process by keeping the intercellular cleft narrow facilitating the buildup of an osmotic gradient allowing water reabsorption. LI-cadherin is exceptional among the cadherin superfamily with respect to its localization along the lateral plasma membrane of epithelial cells being excluded from adherens junction. Furthermore it has 7 but not 5 extracellular cadherin repeats (EC1-EC7) and a small cytosolic domain. In this study we identified the peptide VAALD as an inhibitor of LI-cadherin trans-interaction by modeling the structure of LI-cadherin and comparison with the known adhesive interfaces of E-cadherin. This inhibitory peptide was used to measure LI-cadherin dependency of water transport through a monolayer of epithelial CACO2 cells under various osmotic conditions. If LI-cadherin trans-interaction was inhibited by use of the peptide, water transport from the luminal to the basolateral side was impaired and even reversed in the case of hypertonic conditions whereas no effect could be observed at isotonic conditions. These data are in line with a recently published model predicting LI-cadherin to keep the width of the lateral intercellular cleft small. In this narrow cleft a high osmolarity can be achieved due to ion pumps yielding a standing osmotic gradient allowing water absorption from the gut even if the faeces is highly hypertonic.
KEYWORDS: cadherin-17, CDH17, enterocytes, intercellular cleft, LI-cadherin, osmoregulation, water reabsorption
Introduction
The inner and outer surfaces of the body of eumetazoic animals are covered by epithelia.1-3 Thus, the epithelium represents the primary barrier for controlled transport of water or dissolved molecules into or out of the body.4 The intestinal mucosa contains a simple epithelium that fulfils the selective uptake of nutrients and ions, the prevention of pathogen invasion and the reabsorption of water. In the present work we will focus on the last point, the reabsorption of water. It has to be emphasized that controlled transport in this context means that on one side unwanted loss of water, ions or nutrients must be avoided1,3,5-9 while necessary nutrients have to be absorbed, i.e. transported over the cellular barrier.4,8,10-12 There exist two main transport pathways: transcellular and paracellular.12 In the first case, the substances have to be taken up by the epithelial cells either directly by transporters or by pinocytosis. A modification of the substances within the enterocytes may then take place, followed by the secretion of the substances on the basolateral or luminal side, respectively. However, a lot of substances are transported at least partially paracellular through the intercellular cleft (IC) at the lateral boarders of the cells.5 The importance of the IC as a coupling compartment for transport is well established.5,6,13 The IC has to be sealed tight enough to avoid uncontrolled transport while on the other hand, ions and water molecules shall be transported through the IC in a controlled fashion. The shape of the IC as well as it's tightness and transport properties between the compartments are determined by the so called junctional complex linking the enterocytes together. The junctional complex consists of tight junctions,9,11,14,15 adherens junctions16-20 and desmosomes.17,21 In addition, in intestinal epithelium one adhesion molecule, i.e. LI (Liver-intestine)-cadherin, is uniformly distributed along the whole lateral membrane22,23 and it has been suggested that LI-cadherin might be important for regulation of the width of the IC.24-27
LI- cadherin, also termed cadherin-17 is expressed in intestinal epithelial cells of human and other mammals.27-31 It was the first identified member of the family of so called 7D-cadherins. The second member is KSP, which is expressed in kidney ducts exclusively.32 In contrast to classical cadherins, 7D-cadherins have 7 instead of 5 extracellular cadherin repeats. Human LI-cadherin is a protein of 832 amino acids (120 kD) and shares 20–30% overall homology with classical cadherins.22,30 Another striking difference between LI-cadherin and classical cadherins is the very short (20 amino acids) cytoplasmic domain of LI-cadherin showing no similarity to the highly conserved cytoplasmic region of classical cadherins or to the cytoplasmic portion of any other cadherin subfamily. Due to its structural similarity with E-cadherin, it is assumed that LI-cadherin may have originated from a 5-repeat cadherin by a duplication of the first two aminoterminal repeats.22,30,33
The exact biologic function of LI-cadherin is currently not known. Besides the idea that it might be important during embryonic development and organogenesis,23 it has been speculated due to the expression of 7D-cadherins exclusively in epithelia which are involved in water resorption under various osmotic conditions that LI-cadherin might be involved in regulating the width of the lateral intercellular cleft and thereby influencing the water transport.22,26,34 It is worth noticing that LI-cadherin is not found in the adherens junction but is almost uniformly distributed along the lateral plasma membrane, but can also be found basal and apical.24
In this study we show that inhibition of LI-cadherin function impaired water transport across an epithelial monolayer at hypertonic but not isotonic conditions. These data are in line with a theoretical model predicting that changes of the width of the IC between neighboring epithelial cells by LI-cadherin could regulate the direction and efficiency of water transport through a simple epithelium.
Materials and methods
Protein modeling
For each identified template, the template's quality has been predicted from features of the target-template alignment. The templates with the highest quality have then been selected for model building. Template search with BLAST and HHBlits has been performed against the SWISS-MODEL template library (SMTL, last update: 2016–04–13, last included PDB release: 2016–04–08). The target sequence was searched with BLAST35 against the primary amino acid sequence contained in the SMTL. A total of 69 templates were found. An initial HHblits profile has been built using the procedure outlined recently,36 followed by 1 iteration of HHblits against NR20. The obtained profile has then be searched against all profiles of the SMTL. A total of 144 templates were found, most of which were cadherins, especially E-cadherin molecules of different species exhibited highest quality ranks.
Models are built based on the target-template alignment using Promod-II. Coordinates which are conserved between the target and the template are copied from the template to the model. Insertions and deletions are remodelled using a fragment library. Side chains are then rebuilt. Finally, the geometry of the resulting model is regularised by using a force field. In case loop modeling with ProMod-II37 does not give satisfactory results, an alternative model is built with MODELLER.38,39 The global and per-residue model quality has been assessed using the QMEAN scoring function.40
Recombinant LI-cadherin-Fc fusion protein
As a tool for checking the effect of the selected LI-cadherin inhibitory peptide, a chimeric protein consisting of the full extracellular domain of human LI-cadherin fused to the Fc-portion of human IgG1 was created. Such chimeric cadherin-dimers were found to be functional in previous studies.41-48
For that purpose total RNA was isolated from confluent Caco-2 cells using the ReliaPrep™ RNA Cell Miniprep System (Promega, Mannheim, Germany) according to the manufacturer's protocol. RNA was then subjected to reverse transcription with the ReadyScript™ cDNA Synthesis Mix (Sigma, Taufkirchen, Germany) and stored at −80°C. The extracellular domain of LI-cadherin (bp 127 – bp 2475, NM_001144663.1) was amplified from the cDNA using Phusion U Hot Start polymerase (Thermo Scientific) and 5′-tatctaactagtATGATACTTCAGGCCCATCTTCACTC-3′ and 5′-cgtcgtctcgagGGGTATCCCAGTCTGGTGACCTG-3′ (introduced SpeI and XhoI restriction sites underlined) as forward and reverse primers, respectively. Subsequently, the purified PCR-product was cloned into the pT757R/T vector (InsTAclone PCR cloning kit, Thermo Fisher Scientific) and transformed into E.coli XL1 Blue. For fusion to the Fc-portion of IgG1 and expression in eukaryotic cells a modified pEGFP-N3 vector containing the extracellular domain of N-cadherin (which was replaced by the LI-cadherin EZ domain in the following) fused to human IgG1 and a 6x histidine-tag followed by a stop codon preventing GFP transcription was used.49 After insertion of an additional restriction site (SpeI) into this N-cad-EZ-Ig-pEGFP-N3 vector the plasmid was digested with SpeI and XhoI leading to excision of the N-cadherin EZ-domain, and this linearized plasmid was gel-purified (Wizard SV Gel and PCR Clean-Up System, Promega). The pT757R/T vector containing the verified sequence of the LI-cadherin-EZ domain was also digested with SpeI and XhoI, the insert gel-purified and ligated into the Ig-pEGFP-N3 plasmid by T4 DNA ligase (New England Biolabs, Frankfurt, Germany). The correct sequence of the LI-cadherin-EZ domain fused to Fc was controlled by sequencing and is shown in the supplementary material.
Chinese hamster ovarian (CHO) cells were transfected with LI-cad-Fc-pEGFP-N3 using Roti-Fect plus transfection reagent (Carl Roth, Karlsruhe, Germany) following the manufacturer's protocol. After selection of cells by application of 1.4 mg/ml geneticin, cells were seeded on culture flasks (150 cm2) and cultured with α modified DMEM supplemented with 10% FCS. About 200 ml cell culture supernatant was collected and after addition of protease inhibitors (aprotinin, pepstatin, leustatin; 1 μg/ml each, Sigma, Taufkirchen, Germany) the supernatant was centrifuged for 25 min at 10000 × g and 4 °C. Purification of LI-Cadherin-Fc was done by affinity chromatography using protein A-sepharose column (Amersham Pharmacia Biotech, Freiburg, Germany). The protein was eluted by citrate buffer (25 mM, pH 2.4), immediately dialyzed against Hanks' Balanced Salt Solution (HBSS, Applichem, Darmstadt, Germany) for 16 h at 4°C and stored in aliquots at −80°C. Protein purity was checked by Coomassie Blue staining of 7.5% SDS–PAGE and by western blot analysis.
Affinity shift chromatography
The method of affinity shift chromatography for the determination of cadherin-cadherin trans-interaction was performed as described recently.28,43 Briefly, 100 mg of CNBr-activated sepharose (Fluka) was allowed to swell for 45 min at 4° C in 1 mM HCl (10 ml). The swollen sepharose (1 ml) was transferred to a column (diameter 5 mm) and washed with 100 ml of 1 mM HCl, followed by 3 ml of distilled water (H2O). The column was equilibrated with 1 ml of coupling buffer (100 mM NaHCO3, 500 mM NaCl, pH 8.4) and then loaded with 2 ml of coupling buffer containing a mixture of 0.45 mg/ml LI-cadherin-Fc and 0.4 mg/ml bovine serum albumin (BSA) and allowed to react for 2 h at RT under slow overhead rotation. Afterwards the column was washed once with 3 ml coupling buffer followed by a wash with 300 ml blocking buffer (200 mM glycine, pH 8.0) and then subjected to incubation for 3 h at RT in blocking buffer. After 3 washes with 3 ml acetate buffer (100 mM acetate, 500 mM NaCl, pH 4.5), the column was washed and equilibrated with either 10 ml of HBSS containing 2 mM Ca2+ or PBS.
For determination of mobility shift, typically 20 µl (0.45 mg/ml) of LI-cadherin-Fc in HBSS (without or with 2 mM EGTA and without or with inhibitory peptide) was loaded onto the column and allowed to flow through with a velocity of approximately 0.14 ml/min. Fractions of 120 µl (3 droplets) were collected and subjected to dot blot analysis and determination of protein amount (see below).
Protein concentration determination and dot blot analysis
Protein concentrations were measured using the BCA protein assay kit (Perbio Science, Germany).
For dot blot analysis, 20 µl of the fractions from chromatography were adsorbed to Hybond nitrocellulose membranes (Amersham, Braunschweig, Germany) using a home built dot-blot apparatus. After washing the membrane 3 times in TBS-T (15 mM Tris/HCl, pH 7.5, 120 mM NaCl, 0.05% Tween 20) for 10 min, the membranes were incubated for 60 min with horseradish peroxidase-conjugated polyclonal goat-anti-human antibodies (Fc-specific, Dako, Germany) diluted 1:2000 in blocking solution. After another wash with TBS-T, antibody complexes were visualized using the ECL detection system (Amersham Pharmacia Biotech, Germany) and Kodak BioMax films (Kodak, Germany). Data analysis for mobility shift chromatography was performed as described in detail recently.43
Peptide use and labeling
The VAALD-peptide was purchased from Genaxxon (ULM, Germany) and freshly dissolved for each experiment and used at a final concentration of 1 mg/ml. The solution was neutralised by adding 1 M NaOH solution dropwise until a pH-value of 7.4 was reached.
To determine if the peptide alters the free Ca2+-concentration, peptide solution or only solute (control) was added to a final concentration of 5 mg/ml to culture medium and the free Ca2+ -concentration was determined photometrically using the Ca2+-sensitive chromophore Eriochrome Black-T as described recently.47 Briefly, 500 mM Eriochrome Black-T were dissolved in 100 mM Tris (pH 11) and 100 µl of the medium was added to 500 µl of the chromophore solution. The Ca2+-concentration was determined photometrically by measuring the transmission at 600 nm.
For fluorescence labeling the Atto 488 protein labeling kit (No. 92313, Fluka, Buchs, Switzerland) was used. Besides the fact that label and peptide were incubated at a molecular ratio of 1:1, the labeling was performed according to the manufacturers recommendations.
Generally CACO2-cells were grown in DMEM-Medium (Gibco, Vienna, Austria) which was supplemented with 10% FCS. For peptide labeling experiments CACO2-cells were grown on glass coverslips and kept confluent for at least 2 weeks for them to differentiate. The fluorescenctly labeled peptide VAALD was applied at a concentration of 10µg/ml for 1h in HBSS. Afterwards cells were fixed at RT with 2% formaldehyde in HBSS (pH 7.4, Life Technologies, Karlsruhe, Germany) for 10 min. For immunofluorescence staining cells were additionally permeabilised by Triton X-100 (Sigma) to a final concentration of 0.1%. Then cells were pre-incubated with 10% normal goat serum (NGS, Jackson ImmunoResearch Laboratories, West Grove, USA) and 1% bovine serum albumin (BSA, Sigma, Germany) for 30 min at 4°C. Monolayers were incubated for 1 h at RT with a 1:400 dilution of polyclonal rabbit-anti-LI-cadherin (antikoerper-online, Aachen, Germany). After washing with PBS (3 × 5 min) cells were incubated with 1:300 diluted Alexa Fluor 488 goat-anti rabbit IgG (Dianova, Hamburg, Germany) for 1 h at RT. Finally cells were washed in PBS (3 × 15 min), mounted in 60% glycerol in PBS containing 1.5% n-propyl gallate (Sigma) as antifading compound.
Transwell water flux and transepithelial resistance (TER) measurements
As peptides will typically be metabolised rather fast, we had to find a measurement technique that determines water flux within 1h to avoid peptide degradation during the time of measurement. CACO2-cells were grown on transwell-filters Thin-Certs-TM for 24-well plates (Greiner Bio, Frickenhausen, Germany). The inserts (corresponding to the luminal side) were filled with 300µl DMEM-medium (Gibco) and the companion (corresponding to the basal side of the epithelium) with 1 ml medium. The cells were grown for at least 2 weeks to allow the cells to differentiate. The pore-size of the filter was below 0.4 µm. This was necessary to prevent outgrowth of the cells. Due to this rather dense mesh of the filter the fluid and solute exchange from the filter to the companion chamber is rather limited. So one will get a good initial transport of solutes and water through the cell layer until the medium in the filter has an osmolarity that hinders further water transport through the cells. So we established a new method of discontinuous measurement to avoid this problem. Every 5 minutes the inserts were taken out and the medium from the filter was soaked off with a tissue in a reproducible way by placing the basal side of the filter twice for 1 s onto a dry flat tissue (Kleenex, Kimberly-Clark, USA) to reduce the osmolarity within the filter itseld back to control conditions. Then the insert was weighed by a microscale (VWR, Vienna, Austria) and placed back in the companion. The mass loss of the insert was a useful value for reproducible determination of the water transport out of the insert. The experiments had to be done cyclically to be sure that the cell layers were not modified during the experiments. This means that the mass transport was initially determined under isotonic conditions without inhibitory peptides or other modifications for 30′. Then the flux was determined under the actual conditions of interest for 30′ and finally the control flux measurement was repeated. Between the measurements the cells were allowed to rest for 15′. If the initial and the final control measurement deviated strongly, the experiment was removed from further analysis. The osmolarity of the medium in the insert was varied by addition of NaCl and/or sucrose while the medium in the lower compartment (companion) was kept constant. For LI-cadherin inhibition experiments the peptide VAALD was added to a final concentration of 1 mg/ml.
In parallel to the flux for n = 3 measurements the transepithelial resistance (TER) was determined in parallel using a TER-meter (Millicell-ERS, Millipore, Darmstadt, Germany) according to the manufacturers recommendations.
Transmission electron microscopy (TEM)
Cells were grown on transwell filters and exposed to different osmotic conditions in the presence and absence of the inhibitory peptide as described above. Then cells were fixed in 2.5% glutaraldehyde / 2% formaldehyde in PBS at 4°C for 48 h and then post-fixed for 1 h in 1% osmium tetroxide (Fluka, Switzerland) in PBS, washed 3 times for 15 min in ddH2O and stained for 1 h with 2% uranyl acetate solution in ethanol (Merck, Germany) in a dark environment. Afterwards, the samples were gradually dehydrated in ethanol (at 70, 80, 90, 96 and 100%, for 15 min each). Then filters were removed from the plastic and transferred to glass petri dishes. They were washed twice for 30 min in propylene oxide (SERVA, Germany), then stored for 16 h in a mixture of propylene oxide and epoxy resin (Epon; SERVA, Germany). After washing in Epon twice for 2 h, the samples were embedded and polymerised for 48 h at 57°C. Ultra thin sections were cut with a Reichert OmU3 ultramicrotome (Reichert AG, Austria) and placed on 200 mesh (200 division bars on 25.4 mm) nickel grids (Plano GmbH, Germany). To increase the contrast of the tissue compartments, the ultra thin sections were incubated with 2% uranylacetate for 20 min followed by incubation with 0.2% lead citrate in a CO2 -free atmosphere for 7 min. After each incubation, the sections were washed 4–5 times with water and air-dried for 15 min. Then samples were analyzed using a Philips REM 500 which was equipped with a home built STEM-holder.
Results
Peptide design for inhibition of LI-cadherin trans-interaction
For inhibition of LI-cadherin function we searched for a peptide which fits into the probable binding interface of LI-cadherin and, thus, can be expected to inhibit LI-cadherin trans-interaction as previously done for VE-cadherin49 or N-cadherin.50,51 Since the crystal structure of LI-cadherin has not been yet identified we compared the protein sequence of LI-cadherin with other cadherins with known structures. For that purpose the SWISS-MODEL template library (SMTL version 2016–04–13, PDB release 2016–04–08) was searched with BLAST35 and HHBlits36 for evolutionary related structures. Based on these templates, from which most were E-cadherin structures from different species, a 3D-model of the EC1 domain of LI-cadherin was built (see Fig. 1). Similar to methods published earlier,49 by comparing the structures of cadherins with resolved structure in which the interaction sites are known and our model of LI-cadherin, the interaction sites were predicted and the loop between β-sheets β6 and β7 from the alanin at position 69 onwards was identified to be most promising. In Fig. 1A the aspartic acid of the peptide sequence is marked in the protein structure for orientation. We decided to start within the β-sheet to conserve the structure and we tried to keep the peptide as short as possible to make access to the LI-cadherin protein simple. Thus, the peptide sequence COOH-VAALD-NH2 was selected as a promising candidate for an inhibitory peptide. Presumably the loop interacts with the pocket in the vicinity of the β4-sheet (Fig. 1B), similar to the trans-interaction of VE-cadherin.49
Figure 1.
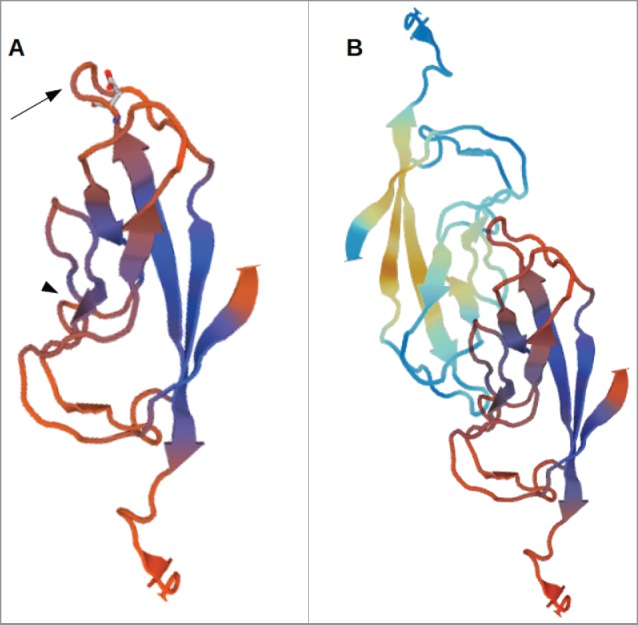
3D-model of the EC1-domain of LI-cadherin. (A) A model of the EC1-domain of LI-cadherin. The model is based on an energy minimisation of the primary sequence of LI-cadherin EC1 initiated by a template based on X-ray crystallography of E-cadherin EC1. Based on comparison with other cadherins (VE-cadherin, N-cadherin and E-cadherin) the loop between the β-sheets β6 and β7 (indicated by the arrow) was identified to be one binding site for LI-cadherin trans-interaction and an inhibitory peptide of the sequence VAALD was identified there. The aspartic acid of this peptide in the loop between β6 and β7 is indicated. (B) A model of the trans-interaction of 2 LI-cadherins. The orientation of the lower EC1 is identical to the single EC1 shown in (A). The loop between β6 and β7 interacts with corresponding amino acids in the vicinity of β4. This binding region close to β4 is indicated in (A) by the arrow-head.
The selected peptide VAALD binds to LI-cadherin and prevents its trans-interaction
First, we determined if the selected peptide can in fact inhibit LI-cadherin interaction by a mobility shift assay.43 For that purpose a recombinant LI-cadherin-Fc chimeric protein was covalently coupled to an agarose column and soluble LI-cadherin-Fc was applied on this column at constant flow with and without the peptide VAALD and as a control without Ca2+. Fig. 2 shows dot blots of fractions collected from a column loaded with LI-cadherin-Fc at the different experimental conditions. At control conditions (without peptide, with Ca2+) LI-cadherin-Fc was found mainly in fraction 5 whereas at Ca2+-free conditions (addition of EGTA) it was detected mainly in fraction 4. This typical delay of mobility of LI-cadherin-Fc in the presence of calcium is due to the trans-interaction of soluble LI-cadherin-Fc to the immobilised LI-cadherin-Fc of the column which is inhibited in the absence of Ca2+. Addition of 1 mg/ml peptide VAALD to LI-cadherin-Fc resulted in a similar elution profile of LI-cadherin-Fc as at Ca2+-free conditions demonstrating inhibition of LI-cadherin trans-interaction by the peptide.
Figure 2.
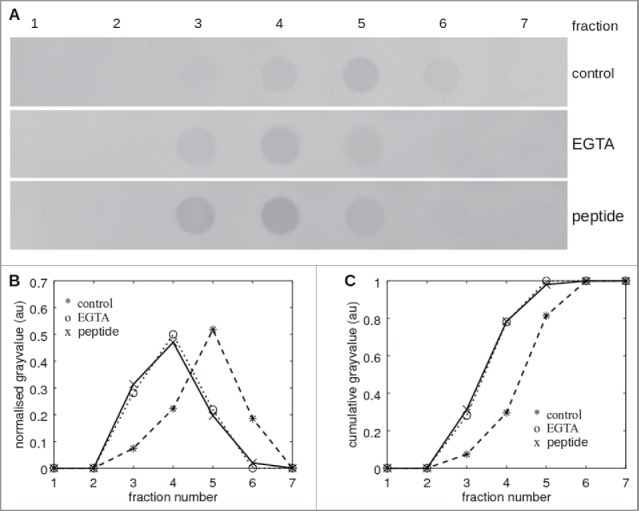
Affinity shift chromatography of LI-cadherin in the presence and absence of VAALD. (A) Dot-blot of the fractions from the eluation of a LI-cadherin-Fc column. While there is a significant delay under control conditions of the eluation of free LI-cadherin-Fc, in the presence of VAALD or in the absence of Ca2+, the LI-cadherin is eluated directly without specific interaction. A quantification is shown in (B) and (C).
Next we checked, whether or not the peptide VAALD is able to bind to endogenous LI-cadherin of cultured epithelial cells meaning that it is able to penetrate into the intercellular cleft to have access to LI-cadherin within the lateral plasma membrane. We applied fluorescently labeled VAALD to a confluent differentiated monolayer of CACO-2 cells. Fig. 3 B shows a clear staining pattern along the cell boarders which is similar to the staining pattern of LI-cadherin after immunolabelling (Fig. 3A). This indicates that the peptide VAALD binds to natively expressed LI-cadherin.
Figure 3.
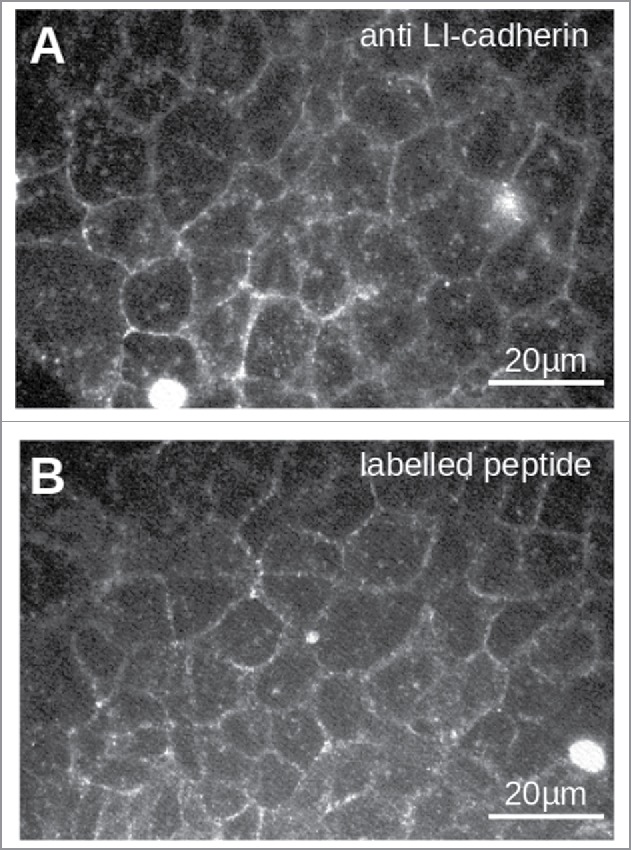
Staining of LI-cadherin in CACO2-cells with LI-cadherin specific antibodies (A) or with the fluorescently labeled peptide VAALD (B). Clearly the LI-cadherin is mainly found at cell-cell-boarders. Clearly there is a localization of the peptide and the antibody along the cell boarders. It has to be emphasized that the cells for the peptide staining were not permeabilised to see whether or not the peptide can enter the lateral intercellular cleft for our transport measurements.
Transepithelial water transport is affected by the inhibitory peptide VAALD
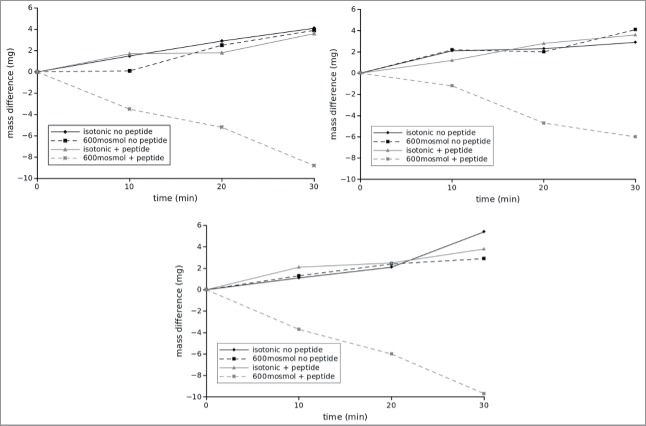
To address the question if LI-cadherin is important for water transport through intestinal epithelium against osmotic gradients we measured the water flux across confluent monolayers of CACO2-cells at different osmolarities in the presence and absence of the inhibitory peptide VAALD. CACO2-cells were grown on transwell filters and the water flux from the insert (luminal side) to the companion (basolateral compartment) was determined by the mass difference of the insert over time. By convention we define that a flux from the insert to the companion is positive which corresponds to a flux from the gut lumen to the interstitium of the body. We measured water flux at 3 different osmolarities of the medium within the insert, i.e., isotonic (∼300 mosmol/l), 600 mosmol/l and 1200 mosmol/l in the presence and the absence of the peptide VAALD. The osmolarity in the companion was not changed. Figure 4 shows a representative example of a time course of water flux at isotonic and hypertonic (600 mosmol/l) conditions. In this example one can see that under isotonic conditions 4 µl of water flowed from the insert to the companion (according to 4 mg mass loss of the insert) within 30 minutes. This did not change if the LI-cadherin inhibiting peptide VAALD was added. At hypertonic conditions in the absence of the inhibitory peptide the amount of water transport was similar compared with control. However, in the presence of the inhibitory peptide, the direction of the net water transport was inverted and about 9µl of water were transported within 30 minutes into the insert. The results of n = 6 independent measurements for each condition are summarised in Fig. 5. Again, while there was no change in water transport due to the inhibitory peptide in the case of isotonic conditions, the peptide hinders the transport out of the insert in the case of hypertonic conditions significantly (marked by asterisks) at both conditions investigated, i.e., 600 mosomol/l and 1200 mosmol/l. It has to be mentioned, that we adjusted the osmolarity using a mixture of NaCl and sucrose, so additional transportable sodium and chloride ions were available for ATPases. If only sucrose was used to adjust hypertonic conditions, the water flux was even in the absence of the peptide reversed earlier. It reached almost 0 at 600mosmol and was about −15 µl/h at 1200 mosmol/l and the effect in the presence of the peptide was even more pronounced (data not shown).
Figure 4.
Typical flux measurements under different osmotic conditions in the presence and absence of the inhibitory peptide. The flux was determined indirectly by measuring the mass difference of the insert over time. While normally the cells can cope with a rather high osmotic gradient for water into the lumen, the inhibitory peptide impairs this ability. Under isotonic conditions the peptide has no visible effect. The panels correspond to 3 independent measurements on different trans well filters.
Figure 5.

Analysis of flux measurements under different osmotic conditions in the presence and absence of the inhibitory peptide. Here values to the right correspond to flux out of the gut into the interstitium while columns to the left indicate flux into the lumen. All columns correspond to at least 6 independent measurements, i.e., 6 different inserts. Asterisks indicate statistical significant differences (2 sided unpaired t-test, p < 0.01).
To determine if the peptide could possibly indirectly affect LI-cadherin trans-interaction by changing the free Ca2+-concentration of the medium, we determined the free Ca2+-concentration of culture medium in the presence and absence of 5 mg/l peptide. This was done by using the Ca2+-sensitive chromophore Eriochrome Black-T. We found absolutely no effect of the peptide on the calcium levels.
Thus, direct inhibition of LI-cadherin function by the peptide VAALD hampered (and even inverted) water transport from the luminal to the basolateral side at hypertonic but not at isotonic conditions.
The lateral intercellular cleft but not the junctional complex is affected by the inhibitory peptide
To determine whether the inhibitory peptide induces morphological changes of the CACO2-cells exposed to different osmotic conditions, transmission electron microscopy (TEM) was performed. Representative images are shown in Fig. 6. In the presence of VAALD under hypertonic conditions (600 mosmol/l) the cells still adhere to the transwell filter but the intercellular cleft was widened (Fig. 6A). The effect increases from apical to basal. However, the structure of the junctional complex appeared not to be impaired (Fig. 6B). No effect could be seen in the absence of the inhibitory peptide. To see whether or not the junctional complex, especially the tight junctions were affected by the peptide, flux measurements were performed in parallel to the determination of the transepithelial resistance (TER). The results are summarised in Fig. 7 for n = 3 independent measurements. While the peptide influenced the water transport under hypertonic conditions, the TER did not change significantly. Also other experiments using different tracer molecules showed that only the water transport, but no other transport was altered by the VAALD-peptide (not shown).
Figure 6.
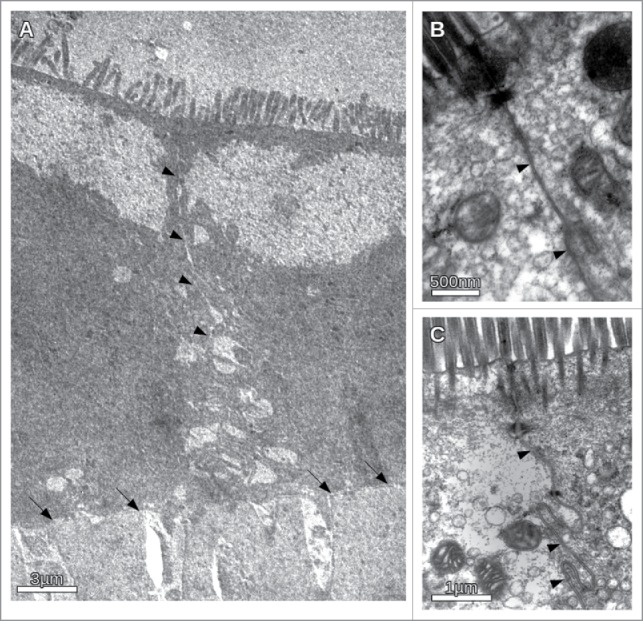
Transmission electron microscopic images of CACO-cells under different osmotic conditions in the presence and absence of the inhibitory peptide. (A) Overview of the intercellular cleft in the presence of the inhibitory peptide under hypertonic conditions. Apically the microvilli could be seen. The basal membrane on the transwell filter is indicated by arrows. The intercellular cleft (indicated by arrowheads) clearly becomes more and more impaired from apical to basal. Irregular widening of the cleft was observed. (B) Apical section of CACO-cells in the vicinity of the junctional complex under hypertonic conditions. Note no obvious morphological change of the junctional complex. (C) CACO-cells in the absence of the inhibitory peptide under hypertonic conditions. The intercellular cleft (arrow heads) is constantly narrow.
Figure 7.
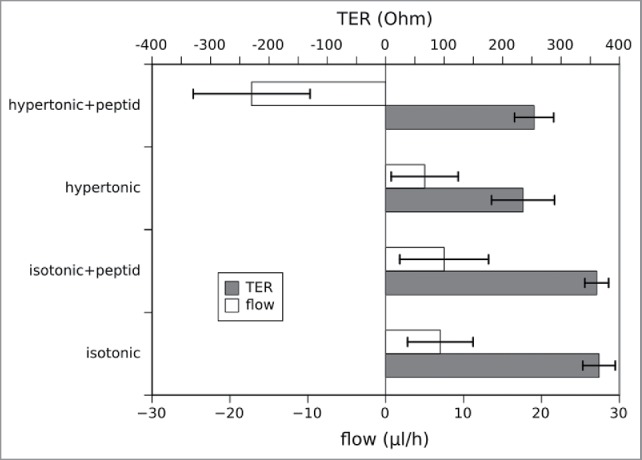
Simultaneous measurement of the water flux and the transepithelial resistance (TER) under different osmotic conditions in the presence and absence of the inhibitory peptide. The data represent mean values of 3 independent measurements, errorbars show the standard deviation. While the flux behaves as in the previous measurements in Figs. 4 and 5, the TER is not affected by the presence or absence of the peptide (2 sided unpaired t-test, p < 0.01). Of course the TER differs dependent on the osmotic conditions and is lower in the hypertonic medium due to the higher ion concentration.
Discussion
In recent years it was often speculated that LI-cadherin (Liver-Intestinal-cadherin) might play a role in the barrier regulation of simple epithelia with respect to osmotically driven water transport, especially under hypertonic conditions in the gut lumen.4-6,12-14,52-55 This regulation of the water transport was supposed to take place due to activation and inactivation of LI-cadherin and thereby changing the width of the lateral intercellular cleft (IC).26 The distribution of LI-cadherin all along the lateral intercellular cleft, which was shown before22,24,24,56,57 and which we also see in CACO2-cells used in this study (not shown) renders LI-cadherin an ideal candidate to regulate the width of the IC. A wide IC gives rise to a low viscous drag but it increases the volume. A high volume on the other hand makes it harder for ATPases (i.e., ion pumps) to increase the osmolarity in the IC to take water out of the gut, either via the tight junctions or via aquaporins in the lateral cell membrane. Although a reasonable hypothesis, no experimental evidence for the influence of LI-cadherin onto water transport could be shown so far.
Here, we show for the first time that in fact selective impairment of LI-cadherin function leads to significant changes of the water transport through cultured colon-epithelium cells (CACO2-cells). The key for getting these results was the establishment of an inhibitory peptide for LI-cadherin. The peptide VAALD was found using computer models of the EC1-domain of human LI-cadherin which were based on the structural similarity to E-cadherin. This was necessary since no X-ray structures of LI-cadherin are available so far. We could show on the level of purified proteins using affinity shift chromatography that the developed peptide could in fact inhibit LI-cadherin trans-interaction and by using fluorescently labeled peptides we found, that in fact the inhibitory peptide could approach LI-cadherin in the lateral IC of confluent and differentiated CACO2-cell monolayers. While the inhibitory peptide had no effect in the case of hypotonic or isotonic conditions, water transport from the luminal side to the basal compartment was significantly reduced or even inverted under hypertonic conditions.
The transport rates found here are of similar magnitude as found by others for different epithelia4-6,6,10,13,52,54,55 and the findings confirm that the IC is a key compartment for the transport of ions and water. Due to our measurement technique the absolute values of the water flux might deviate slightly from the measured values. However, as the measurement was performed in the identical way in all cases and as the measurements were done changing the osmotic conditions cyclically, the relative behavior is undoubted altered by the LI-cadherin function as predicted by theoretical considerations.
It has to be emphasized that LI-cadherin might have additional functions for embryonic development, for regeneration, cell recognition etc. However, it appears clear that it plays a fundamental role for keeping the IC narrow to allow the ATPases in the lateral membrane to efficiently built up an osmotic gradient. Our findings are in line with the theoretical predictions published recently.26
The width of the intercellular cleft is dependent on the binding activity of LI-cadherins, which in turn is dependent on the extracellular Ca2+-level. E-cadherin as well as desmocadherins are much less sensitive to extracellular Ca2+ than LI-cadherin, which is active only at physiologic Ca2+-levels and becomes inactive by slight Ca2+-depletion.34 Thus, we would expect, that if Ca2+ is depleted in the case of hypotonic luminal content, the LI-cadherin trans-interactions will be weakened while the adherens junction and the desmosomes are still stable. The hydrostatic pressure that is generated due to the water transport within the cleft will separate the weakened LI-cadherin bounds leading to a widening of the intercellular cleft. The wider cleft provides less viscous friction and thus much higher water flux from the lumen into the interstitium. If now the osmolarity in the lumen is changed to hypertonic, the water and thus the electrolyte flux will be reversed. Therefore the concentrations of electrolytes, including Ca2+ in the IC will be increased to the levels in the interstitium. Under these conditions LI-cadherin will bind, the cleft will become narrow, allowing the ATPases to build up an osmotic gradient out of the lumen re-establishing the water transport into the body.
It has to be emphasized that the main determinant for the selectivity and for resistance any paracellularly transported substance faces are the tight junctions (TJ).11,58 The volume of the intercellular cleft, however, might determine the osmotic gradient which drives the water transport either throught the TJ or partially through the cells via aquaporins. The fact that the transepithelial resistance as well as the transport for small tracer molecules is not significantly altered by our inhibitory peptide indicates that the TJs are not affected. This is in line with the TEM-images showing no visible morphological change of the TJ.
Furthermore we have to keep in mind that the peptide VAALD might have additional effects on the cells than LI-cadherin inhibition. Of course we searched protein databases for similar sequences in other adhesion proteins and the extracellular domains of other proteins and found no match. However, we cannot completely rule out that the peptide does bind to other proteins too.
In conclusion, our data strengthen the hypothesis that LI-cadherin might be important for water reabsorption of the gut at intraluminal hypertonic conditions by keeping the intercellular cleft narrow and thus maintaining a high osmotic gradient driving the water transport. Thus, LI-cadherin might represent an interesting target for the development of drugs, for example, for treatment of diarrhea as it alters the osmotic gradient without affecting the tight junctions and therefore not affecting the transport of other molecules. However, additional work has to be done for the further understanding of LI-cadherin and the action of the peptide. It remains to be determined if our cell-culture results can be reproduced in an organ or animal model. Unfortunately these studies were beyond our possibilities. Nevertheless the identification of the sequence VAALD being crucial for the adhesive activity of LI-cadherin might provide the basis for identifying drugs cross-bridging LI-cadherins and thereby strengthening their interaction. Cross bridging by tandem-peptides, i.e., two connected antagonists was found to effectively cross-bridge other cadherins and thus acts as agonistic agent for cadherin mediated cell adhesion. Stabilization of LI-cadherin trans-interaction could be a novel therapeutic approach to positively influence water reabsorption and prevent dehydration of the body.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The DFG (project BA2272/10–1) is acknowledged for financial support.
References
- [1].Koch S, Nusrat A. Dynamic regulation of epithelial cell fate and barrier function by intercellular junctions. Ann N Y Acad Sci 2009; 1165:220-7; PMID:19538310; https://doi.org/ 10.1111/j.1749-6632.2009.04025.x [DOI] [PubMed] [Google Scholar]
- [2].Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci 1999; 4:D286-98; PMID:10077541; https://doi.org/ 10.2741/A426 [DOI] [PubMed] [Google Scholar]
- [3].Takashima S, Gold D, Hartenstein V. Stem cells and lineages of the intestine: a developmental and evolutionary perspective. Dev Genes Evol 2013; 223(1-2):85-102, https://doi.org/ 10.1007/s00427-012-0422-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Spring KR. Routes and mechanism of fluid transport by epithelia. Annu Rev Physiol 1998; 60:105-19; PMID:9558456; https://doi.org/ 10.1146/annurev.physiol.60.1.105 [DOI] [PubMed] [Google Scholar]
- [5].Larsen EH, Willumsen NJ, Møbjerg N, Sørensen JN. The lateral intercellular space as osmotic coupling compartment in isotonic transport. Acta Physiol (Oxf) 2009; 195:171-86; PMID:18983444; https://doi.org/ 10.1111/j.1748-1716.2008.01930.x [DOI] [PubMed] [Google Scholar]
- [6].Larsen EH, Nedergaard S, Ussing HH. Role of lateral intercellular space and sodium recirculation for isotonic transport in leaky epithelia. Rev Physiol Biochem Pharmacol 2000; 141:153-212; PMID:10916425 [DOI] [PubMed] [Google Scholar]
- [7].Singh J, Mlodzik M. Planar cell polarity signaling: Coordination of cellular orientation across tissues. Wiley Interdiscip Rev Dev Biol 2012; 1:479-99; PMID:23066429; https://doi.org/ 10.1002/wdev.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Devriendt B, De Geest BG, Goddeeris BM, Cox E. Crossing the barrier: Targeting epithelial receptors for enhanced oral vaccine delivery. J Control Release 2012; 160:431-9; PMID:22353618; https://doi.org/ 10.1016/j.jconrel.2012.02.006 [DOI] [PubMed] [Google Scholar]
- [9].Assimakopoulos SF, Papageorgiou I, Charonis A. Enterocytes' tight junctions: From molecules to diseases. World J Gastrointest Pathophysiol 2011; 2:123-37; PMID:22184542;https://doi.org/ 10.4291/wjgp.v2.i6.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kiil F. Mechanisms of transjunctional transport of NaCl and water in proximal tubules of mammalian kidneys. Acta Physiol Scand 2002; 175:55-70; PMID:11982505; https://doi.org/ 10.1046/j.1365-201X.2002.00967.x [DOI] [PubMed] [Google Scholar]
- [11].Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 1998; 60:143-59; PMID:9558458; https://doi.org/ 10.1146/annurev.physiol.60.1.143 [DOI] [PubMed] [Google Scholar]
- [12].van Heeswijk MP, van Os CH. Osmotic water permeabilities of brush border and basolateral membrane vesicles from rat renal cortex and small intestine. J Membr Biol 1986;92:183-93;PMID:3761362; https://doi.org/ 10.1007/BF01870707 [DOI] [PubMed] [Google Scholar]
- [13].Larsen EH, Deaton LE, Onken H, O'Donnell M, Grosell M, Dantzler WH, Weihrauch D. Osmoregulation and excretion. Compr Physiol 2014; 4:405-573; PMID:24715560. [DOI] [PubMed] [Google Scholar]
- [14].Fischbarg J, Diecke FPJ, Iserovich P, Rubashkin A. The role of the tight junction in paracellular fluid transport across corneal endothelium. Electro-osmosis as a driving force. J Membr Biol 2006; 210:117-30; PMID:16868674; https://doi.org/ 10.1007/s00232-005-0850-8 [DOI] [PubMed] [Google Scholar]
- [15].Guo P, Weinstein AM, Weinbaum S. A dual-pathway ultrastructural model for the tight junction of rat proximal tubule epithelium. Am J Physiol Renal Physiol 2003; 285:F241-57; PMID:12670832; https://doi.org/ 10.1152/ajprenal.00331.2002 [DOI] [PubMed] [Google Scholar]
- [16].Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci 2001; 114:629-41; PMID:11171368. [DOI] [PubMed] [Google Scholar]
- [17].Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s). Curr Opin Cell Biol 2013; 25(1):39–46, https://doi.org/ 10.1016/j.ceb.2012.10.010. [DOI] [PubMed] [Google Scholar]
- [18].Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 1997; 13:119-46; PMID:9442870; https://doi.org/ 10.1146/annurev.cellbio.13.1.119 [DOI] [PubMed] [Google Scholar]
- [19].Lechler T. Adherens junctions and stem cells. Subcell Biochem 2012; 60:359-77; PMID:22674079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Levayer R. Regulation of intercellular adhesion during epithelial morphogenesis. Biol Aujourdhui 2012; 206:219-36; PMID:23171844; https://doi.org/ 10.1051/jbio/2012021 [DOI] [PubMed] [Google Scholar]
- [21].Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. J Pathol 2012; 226:158-71; PMID:21989576; https://doi.org/ 10.1002/path.3027 [DOI] [PubMed] [Google Scholar]
- [22].Gessner R, Tauber R. Intestinal cell adhesion molecules. Liver-intestine cadherin. Ann N Y Acad Sci 2000; 915:136-43; PMID:11193569; https://doi.org/ 10.1111/j.1749-6632.2000.tb05236.x [DOI] [PubMed] [Google Scholar]
- [23].Angres B, Kim L, Jung R, Gessner R, Tauber R. LI-cadherin gene expression during mouse intestinal development. Dev Dyn 2001; 221:182-93; PMID:11376485; https://doi.org/ 10.1002/dvdy.1146 [DOI] [PubMed] [Google Scholar]
- [24].Berndorff D, Gessner R, Kreft B, Schnoy N, Lajous-Petter AM, Loch N, Reutter W, Hortsch M, Tauber R. Liver-intestine cadherin: molecular cloning and characterization of a novel Ca(2+)-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol 1994; 125:1353-69; PMID:8207063; https://doi.org/ 10.1083/jcb.125.6.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kreft B, Berndorff D, Böttinger A, Finnemann S, Wedlich D, Hortsch M, Tauber R, Gessner R. LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions. J Cell Biol 1997; 136:1109-21; PMID:9060475; https://doi.org/ 10.1083/jcb.136.5.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ahl M, Weth A, Walcher S, Baumgartner W. The function of 7D-cadherins: a mathematical model predicts physiological importance for water transport through simple epithelia. Theor Biol Med Model 2011; 8:18; PMID:21663598; https://doi.org/ 10.1186/1742-4682-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baumgartner W. Possible roles of LI-Cadherin in the formation and maintenance of the intestinal epithelial barrier. Tissue Barriers 2013; 1:1-0; https://doi.org/ 10.4161/tisb.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wendeler MW, Drenckhahn D, Gessner R, Baumgartner W. Intestinal LI-cadherin acts as a Ca2+-dependent adhesion switch. J Mol Biol 2007; 370:220-30; PMID:17512947;https://doi.org/ 10.1016/j.jmb.2007.04.062 [DOI] [PubMed] [Google Scholar]
- [29].Wendeler MW, Baumgartner W, Drenckhahn D, Gessner R. Characterization of the homotypic LI-cadherin trans interaction on the single molecule level. Eur J Cell Biol 2004; 83:101-1. [Google Scholar]
- [30].Jung R, Wendeler MW, Danevad M, Himmelbauer H, Gessner R. Phylogenetic origin of LI-cadherin revealed by protein and gene structure analysis. Cell Mol Life Sci 2004; 61:1157-66; PMID:15141301; https://doi.org/ 10.1007/s00018-004-3470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wendeler MW, Jung R, Himmelbauer H, Gessner R. Unique gene structure and paralogy define the 7D-cadherin family. Cell Mol Life Sci 2006; 63:1564-73; PMID:16791429; https://doi.org/ 10.1007/s00018-006-6014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wendeler MW, Praus M, Jung R, Hecking M, Metzig C, Gessner R. Ksp-cadherin is a functional cell-cell adhesion molecule related to LI-cadherin. Exp Cell Res 2004; 294:345-55; PMID:15023525; https://doi.org/ 10.1016/j.yexcr.2003.11.022 [DOI] [PubMed] [Google Scholar]
- [33].Horsfield J, Ramachandran A, Reuter K, LaVallie E, Collins-Racie L, Crosier K, Crosier P. Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development. Mech Dev 2002; 115:15-26; PMID:12049763; https://doi.org/ 10.1016/S0925-4773(02)00094-1 [DOI] [PubMed] [Google Scholar]
- [34].Wendeler MW, Drenckhahn D, Gessner R, Baumgartner W. Intestinal LI-cadherin acts as a Ca2+-dependent adhesion switch. J Mol Biol 2007; 370:220-30; PMID:17512947;https://doi.org/ 10.1016/j.jmb.2007.04.062 [DOI] [PubMed] [Google Scholar]
- [35].Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25:3389-402; PMID:9254694; https://doi.org/ 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Remmert M, Biegert A, Hauser A, Söding J. HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat Methods 2012; 9:173-5; https://doi.org/ 10.1038/nmeth.1818 [DOI] [PubMed] [Google Scholar]
- [37].Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling.Electrophoresis 1997;18:2714-23;PMID:9504803; https://doi.org/ 10.1002/elps.1150181505 [DOI] [PubMed] [Google Scholar]
- [38].Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 1993; 234:779-815; PMID:8254673; https://doi.org/ 10.1006/jmbi.1993.1626 [DOI] [PubMed] [Google Scholar]
- [39].Sali A, Overington JP. Derivation of rules for comparative protein modeling from a database of protein structure alignments. Protein Sci 1994; 3:1582-96; PMID:7833817;https://doi.org/ 10.1002/pro.5560030923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011; 27:343-50; PMID:21134891;https://doi.org/ 10.1093/bioinformatics/btq662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. Determination of the unbinding force of homophilic interaction of vascular endothelial cadherin by atomic force microscopy. Biophys J 1999; 76:A351-A351. [Google Scholar]
- [42].Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci USA 2000; 97:4005-10; PMID:10759550; https://doi.org/ 10.1073/pnas.070052697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baumgartner W, Drenckhahn D. Plasmalemmal concentration and affinity of mouse vascular endothelial cadherin, VE-cadherin. Eur Biophys J 2002; 31:532-8; PMID:12451422; https://doi.org/ 10.1007/s00249-002-0248-9 [DOI] [PubMed] [Google Scholar]
- [44].Baumgartner W, Golenhofen N, Grundhöfer N, Wiegand J, Drenckhahn D. Ca2+ dependency of N-cadherin function probed by laser tweezer and atomic force microscopy. J Neurosci 2003; 23:11008-14; PMID:14657157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baumgartner W, Osmanagic A, Gebhard M, Kraemer S, Golenhofen N. Different pH-dependencies of the two synaptic adhesion molecules N-cadherin and cadherin-11 and the possible functional implication for long-term potentiation. Synapse 2013; 67:705-15; PMID:23649972; https://doi.org/ 10.1002/syn.21679 [DOI] [PubMed] [Google Scholar]
- [46].Baumgartner W, Schütz GJ, Wiegand J, Golenhofen N, Drenckhahn D. Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. J Cell Sci 2003; 116:1001-11; PMID:12584244; https://doi.org/ 10.1242/jcs.00322 [DOI] [PubMed] [Google Scholar]
- [47].Heiliger E, Osmanagic A, Haase H, Golenhofen N, Grabrucker AM, Weth A, Baumgartner W. N-cadherin-mediated cell adhesion is regulated by extracellular Zn(2+). Metallomics 2015; 7:355-62; PMID:25579424; https://doi.org/ 10.1039/c4mt00300d [DOI] [PubMed] [Google Scholar]
- [48].Heupel WM, Baumgartner W, Laymann B, Drenckhahn D, Golenhofen N. Different Ca2+ affinities and functional implications of the two synaptic adhesion molecules cadherin-11 and N-cadherin. Mol Cell Neurosci 2008; 37:548-58; PMID:18201900; https://doi.org/ 10.1016/j.mcn.2007.12.003 [DOI] [PubMed] [Google Scholar]
- [49].Heupel W-M, Efthymiadis A, Schlegel N, Müller T, Baumer Y, Baumgartner W, Drenckhahn D, Waschke J. Endothelial barrier stabilization by a cyclic tandem peptide targeting VE-cadherin transinteraction in vitro and in vivo. J Cell Sci 2009; 122:1616-25; PMID:19420236; https://doi.org/ 10.1242/jcs.040212 [DOI] [PubMed] [Google Scholar]
- [50].Williams G, Williams E-J, Doherty P. Dimeric versions of two short N-cadherin binding motifs (HAVDI and INPISG) function as N-cadherin agonists. J Biol Chem 2002; 277:4361-7; PMID:11726665; https://doi.org/ 10.1074/jbc.M109185200 [DOI] [PubMed] [Google Scholar]
- [51].Skaper SD, Facci L, Williams G, Williams EJ, Walsh FS, Doherty P. A dimeric version of the short N-cadherin binding motif HAVDI promotes neuronal cell survival by activating an N-cadherin/fibroblast growth factor receptor signalling cascade. Mol Cell Neurosci 2004; 26:17-23; PMID:15121175; https://doi.org/ 10.1016/j.mcn.2003.12.015 [DOI] [PubMed] [Google Scholar]
- [52].Curran PF. Na, Cl, and water transport by rat ileum in vitro. J Gen Physiol 1960; 43:1137-48; PMID:13813357; https://doi.org/ 10.1085/jgp.43.6.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Diamond JM. The mechanism of isotonic water transport. J Gen Physiol 1964; 48:15-42; PMID:14212146; https://doi.org/ 10.1085/jgp.48.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 2010; 72:463-93; PMID:20148685; https://doi.org/ 10.1146/annurev-physiol-021909-135833 [DOI] [PubMed] [Google Scholar]
- [55].Wright EM, Loo DDF. Coupling between Na+, sugar, and water transport across the intestine. Ann N Y Acad Sci 2000; 915:54-66; PMID:11193601; https://doi.org/ 10.1111/j.1749-6632.2000.tb05223.x [DOI] [PubMed] [Google Scholar]
- [56].Bartolmäs T, Hirschfeld-Ihlow C, Jonas S, Schaefer M, Geßner R. LI-cadherin cis-dimerizes in the plasma membrane Ca(2+) independently and forms highly dynamic trans-contacts. Cell Mol Life Sci 2012; 69:3851-62; PMID:22842778; https://doi.org/ 10.1007/s00018-012-1053-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weimann A, Zimmermann M, Gross M, Slevogt H, Rieger A, Morawietz L. CDX2 and LI-cadherin expression in esophageal mucosa: use of both markers can facilitate the histologic diagnosis of Barrett's esophagus and carcinoma. Int J Surg Pathol 2010; 18:330-7; PMID:20444732 [DOI] [PubMed] [Google Scholar]
- [58].Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol 1986; 102:2125-36; PMID:3711143; https://doi.org/ 10.1083/jcb.102.6.2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.