Abstract
The dorsolateral striatum is critically involved in movement control and motor learning. Striatal function is regulated by a variety of neuromodulators including acetylcholine. Previous studies have shown that cholinergic activation excites striatal principal projection neurons, medium spiny neurons (MSNs), and this action is mediated by muscarinic acetylcholine subtype 1 receptors (M1) through modulating multiple potassium channels. In the present study, we used electrophysiology techniques in conjunction with optogenetic and pharmacological tools to determine the long-term effects of striatal cholinergic activation on MSN intrinsic excitability. A transient increase in acetylcholine release in the striatum by optogenetic stimulation resulted in a long-lasting increase in excitability of MSNs, which was associated with hyperpolarizing shift of action potential threshold and decrease in afterhyperpolarization (AHP) amplitude, leading to an increase in probability of EPSP-action potential coupling. The M1 selective antagonist VU0255035 prevented, while the M1 selective positive allosteric modulator (PAM) VU0453595 potentiated the cholinergic activation-induced persistent increase in MSN intrinsic excitability, suggesting that M1 receptors are critically involved in the induction of this long-lasting response. This M1 receptor-dependent long-lasting change in MSN intrinsic excitability could have significant impact on striatal processing and might provide a novel mechanism underlying cholinergic regulation of the striatum-dependent motor learning and cognitive function. Consistent with this, behavioral studies indicate that potentiation of M1 receptor signaling by VU0453595 enhanced performance of mice in cue-dependent water-based T-maze, a dorsolateral striatum-dependent learning task.
Keywords: M1 muscarinic receptor, Striatal MSNs, Intrinsic plasticity, AP-EPSP coupling, Dorsolateral striatum-dependent learning
1. Introduction
The striatum is the primary gateway to the basal ganglia and critically involved in movement control, motor learning, habit formation and cognition (Albin et al., 1989; Cools, 2011; DeLong, 1990; Graybiel, 2008; Pisani et al., 2005; Schultz et al., 2003), and its function is highly regulated by multiple neuromodulator systems including the cholinergic system (Goldberg et al., 2012; Pisani et al., 2007). Cholinergic activation plays a critical role in regulating striatal function, including modulation of neuronal excitability of the striatal principal medium spiny neurons (MSNs) (Akins et al., 1990b; Galarraga et al., 1999; Shen et al., 2005, 2007; Xiang et al., 2012), glutamatergic signaling and plasticity at corticostriatal synapses (Bonsi et al., 2008; Calabresi et al., 1992; Hsu et al., 1995; Pakhotin and Bracci, 2007; Pancani et al., 2014; Smolders et al., 1997; Sugita et al., 1991; Wang et al., 2006), GABAergic transmission and inhibitory network activity (English et al., 2012; Koos and Tepper, 2002; Sugita et al., 1991), as well as acetylcholine (ACh) and dopamine (DA) release (Bendor et al., 2010; Foster et al., 2014; Starke et al., 1989; Zhang et al., 2002a). Disturbances of striatal cholinergic signaling have been implicated in multiple neurologic and neuropsychiatric disorders including in Parkinson’s disease, Huntington’s disease, dystonia, Tourette syndrome and schizophrenia (Aosaki et al., 2010; Holt et al., 1999; Kataoka et al., 2010; Lange et al., 1992; Pisani et al., 2007).
Cholinergic regulation of striatal function is primarily mediated through ACh released from tonically active, giant aspiny cholinergic interneurons (ChIs), which have widespread and rich axonal arborizations within the striatum, and activate nicotinic as well as muscarinic acetylcholine receptors (mAChRs) (Aosaki et al., 1995; Bennett et al., 2000; Bolam et al., 1984; Hersch et al., 1994; Kawaguchi et al., 1995; Wilson et al., 1990; Zhou et al., 2002). In addition, recent studies indicate that the striatum also receives cholinergic innervation from the brainstem cholinergic system, particularly the pedunculopontine nucleus that preferentially innervates the dorsolateral striatum (Dautan et al., 2014). The mAChR family includes five highly related but structurally distinct subtypes (M1–M5) with distinct molecular and pharmacological profiles (Bonner et al., 1987; Caulfield and Birdsall, 1998; Hulme et al., 1990). M1, M3 and M5 mAChR subtypes are preferentially coupled to Gq/11 and activate phospholipase C, which initiates phosphoinositide hydrolysis and intracellular Ca2+ mobilization, whereas M2 and M4 subtypes are coupled to Gi/o and associated signaling pathways such as adenylyl cyclase and various ion channels (Caulfield, 1993; Hulme et al., 1990). All mAChR subtypes are expressed in the striatum with M1 and M4 being the most abundant subtypes in this structure (Bernard et al., 1992; Hersch et al., 1994; Levey et al., 1991; Weiner et al., 1990; Yan et al., 2001). The M1 receptor subtype is highly expressed on both dopamine D1 receptor containing striatonigral and D2 receptor containing striatopallidal MSNs (Hersch et al., 1994; Yan et al., 2001). In previous studies, we and others found that cholinergic activation causes acute excitation of MSNs and this action is mediated by M1 receptors through modulating activity of multiple ionic channels, including potassium channels (Akins et al., 1990a; Galarraga et al., 1999; Hsu et al., 1996; Shen et al., 2005, 2007; Xiang et al., 2012) and persistent sodium channels (Carrillo-Reid et al., 2009). In addition, M1 receptor activation also regulates MSN activity by inhibiting voltage-gated calcium channels (Perez-Burgos et al., 2008, 2010; Perez-Rosello et al., 2005), which in turn reduces the afterhyperpolarization (AHP) (Perez-Rosello et al., 2005). Activation of M1-like receptors has also been shown to enhance MNDA receptor-mediated responses (Calabresi et al., 1994) and long-term potentiation in striatal MSNs (Calabresi et al., 1999). However, the long-term impact on MSN excitability following a transient increase in striatal cholinergic activity has not been explored. In the present study, we used electrophysiology techniques in conjunction with optogenetic and pharmacological tools to address this question. We found that optogenetic activation of striatal cholinergic system induces a long-lasting increase in intrinsic excitability of MSNs. The long-lasting response is associated with hyperpolarized action potential threshold and decreased amplitude of AHP following a single action potential, which subsequently lead to a persistent enhancement of excitatory postsynaptic potential-action potential (EPSP-AP) coupling in MSNs without changes in the EPSP slope. The M1 receptor is critically involved in induction of this long-lasting response, as it can be prevented by M1 antagonist VU0255035 and potentiated by M1 positive allosteric modulator (PAM) VU0453595. In addition, administration of the M1 PAM enhances the performance of mice in cue-dependent water-based T-maze, a motor learning task that depends upon the dorsolateral striatum (Balleine et al., 2009; Darvas and Palmiter, 2009).
2. Material and methods
2.1. Animals
Mice with C57BL/J6 background were used in the present studies. All animals were group housed with food and water available ad libitum. Animals were kept under a 12 h light/dark cycle with lights on from 6 a.m. to 6 p.m. and were tested during the light phase. All experimental procedures were approved by the Vanderbilt University Animal Care and Use committee and followed the guidelines set forth by the Guide for the Care and Use of Laboratory Animals. Wild type C57BL/J6 mice and ChAT-ChR2-EYFP line 5 mice [B6.Cg-Tg(Chat-COP4*H134R/EYFP, Slc18a3) 5Gfng/J, ChAT-ChR2 mice] expressing an improved channelrhodopsin-2/EYFP fusion protein (mhChR2:YFP) in the cholinergic neurons via the mouse choline acetyltransferase promoter (Zhao et al., 2011) were obtained from Jackson Laboratory (Bar Harbor, ME). The breeders of Drd1a-tdTomato line 6 mice were kindly provided by Dr. Gregg Stanwood at Vanderbilt University, which were originally developed in the Calakos Laboratory at Duke University (Ade et al., 2011). The ChAT-ChR2-EYFP:Drd1a-tdTomato mice were generated in house by crossbreeding ChAT-ChR2-EYFP line 5 mice with Drd1a-tdTomato line 6 mice.
2.2. Ex vivo brain slice preparation
Mice (26–75 days old) of either sex were deeply anesthetized with isoflurane, and the brains were removed from the skull and submerged in oxygenated (95% O2/5% CO2), ice-cold cutting solution composed of (in mM): 220 glucose, 2.5 KCl, 8 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 D-glucose. Coronal brain slices (300–400 μm) containing the striatum and cortex were cut using a Leica VT1200S microtome (Leica Microsystems Inc., Buffalo Grove, IL), incubated in artificial cerebrospinal fluid (ACSF) at 32 °C for 30 min, and then maintained at room temperature afterward until transferred to a recording chamber. The chamber was continuously perfused with oxygenated ACSF. The ACSF contained (in mM): 126 NaCl, 2.5 KCl, 2.0 CaCl2, 1.3 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 10 D-glucose.
2.3. Ex vivo electrophysiology
Whole cell or cell-attached recordings were made from visually identified striatal MSNs or cholinergic interneurons under an Olympus BX50WI upright microscope (Olympus, Lake Success, NY). The electrophysiological signal was amplified using an Axon Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Recording electrodes were prepared from borosilicate glass (Sutter Instruments, Novato, CA) using a Narishige puller (model PP-830; Narishige International USA, East Meadow, NY). The electrode resistance was 3–5 MΩ when filled the following pipette solution (in mM): 120 K-MeSO4, 1 MgCl2, 0.1 CaCl2, 10 HEPES, 1 EGTA, 12 phosphocreatine, 0.4 GTP and 2 ATP. The pH of the pipette solution was adjusted to 7.3 with 1 M KOH, and osmolarity was adjusted to 285–295 mOsm. The change of excitability of MSN was assessed in current clamp mode by monitoring the change in the number of spike discharges in response to a near rheobase depolarization current step (1.5 s). The access resistance was checked at the beginning and the end of each experiment, which were compensated using “bridge balance”. The input resistance (RN), action potential threshold and afterhyperpolarization (AHP) amplitude were also monitored. RN was determined using Ohm’s laws by measuring the voltage deviation in response to injection of a hyperpolarization current pulse (−50 pA, 1.5 s). To determine the action potential threshold and AHP amplitude, we applied a depolarization current ramp (100 pA/s) that typically elicited 3–5 spikes in the recorded cell during baseline. The action potential threshold and AHP amplitude of the first spike evoked by the current ramp were measured using MiniAnalysis software (Synaptosoft Inc., Decatur, GA) and averaged over three to four trials. The AHP amplitude was defined as the difference in voltage between spike threshold and the maximal negative deflection after the spike. The hyperpolarization current step, depolarization current step and current ramp were given every 30 s. To determine the effect on excitability of MSNs following increased ACh release by synchronized activation of striatal cholinergic interneurons and processes, blue laser light stimulation (Crystal Laser, 473 nm, 4.7 mW, 4 s prior to the onset of a depolarization pulse applied to the recorded MSN, 10 s in duration, repeated 6 times every 30s) was delivered through an optic fiber (200 μm in diameter, Thorlabs, Newton, NJ) positioned near the slice surface over the recorded cell.
To assess the effect of optical stimulation induced increase in ACh on EPSP-action potential (EPSP-AP) coupling, a pair of EPSPs were evoked in MSNs by electrical stimulation of the corpus callosum at a paired-pulse interval of 50 ms delivered every 30 s using a concentric bipolar tungsten electrode (Frederick Haer Company, Bowdoinham, ME). The EPSPs were recorded at a membrane potential between −87 and −65 mV by injecting a current step between 0 and 170 pA while the intensity of synaptic stimulation was adjusted such that the first EPSPs elicited spikes less than 30% of trials and the second EPSPs elicited spikes over 70% of trials in the paired pulse stimulation protocol. To determine the effect of cholinergic activation on EPSP-AP coupling, blue laser light was applied to the slice for 10s prior to the synaptic stimulation. All drugs were bath applied.
2.4. Electrophysiology data acquisition and analysis
Electrophysiology data were acquired and digitized using a Digidata 1440 A and pClamp 9.2, and analyzed using Clampfit 9.2 (Molecular Devices, Sunnyvale, CA) and MiniAnalysis (Synaptosoft Inc., Decatur, GA). Statistic comparison was performed using t-test or one-way ANOVA with post hoc test, with a p value of <0.05 considered significant. Data were presented as mean ± SEM.
2.5. Visual cue discrimination motor learning
Adult male C57Bl/6 mice were used in this study. The effect of M1 PAM VU0453595 on dorsolateral striatum-dependent, non-spatial discrimination motor learning was assessed using a visual cue-dependent, water based T-maze task as previously described (Balleine et al., 2009; Darvas and Palmiter, 2009; Gorski et al., 2003). The apparatus consisted of a water-filled two-arm T-maze. The stem of the maze was black (35 × 6 ×12 cm) which led to two choice alleys (28 ×6 ×12 cm). One of the choice alleys was covered in a striped pattern and the other was covered in a checkered pattern. Each mouse was assigned a positive arm containing a hidden escape platform, which was randomized between the striped and checkered arm. During testing, the left-right location of the positive arm was semi-randomly specified. Thirty minutes prior to testing, the animals were injected with VU0453595 (1–10 mg/kg, intraperitoneal (i.p.)) or vehicle (20% beta-cyclodextrin (BCD)) and returned to their home cage. Daily testing consisted of placing the mouse in the stem of the maze and allowing it to swim to find the escape platform. If the mouse made an incorrect response in choosing the arm containing the escape platform or did not make a choice (120 s maximum) then it was guided to the escape platform. Eight tests per mouse were performed daily with an inter-trial interval of 10 min. Data are presented as percent correct responses, which was determined by dividing the amount of correct choices made (entering positive arm without entering incorrect arm first) by the amount of total choices made. The number of trials before meeting criteria was also determined for each mouse. A mouse was considered to have met criteria when it made 75 percent correct choices during a testing session. Statistical analysis was performed using a two-way analysis of variance. Comparison of treatment group effects were completed using a Bonferroni multiple comparisons test with a p value of <0.05 considered significant.
2.6. Drugs
All reagents were purchased from Sigma except for DNQX and AP5 (Tocris Bioscience, Ellisville, MO). VU0255035 and VU0453595 were synthesized and characterized in our medicinal chemistry and molecular pharmacology laboratories.
3. Results
3.1. Optogenetic activation of cholinergic interneurons induced long-lasting increase in excitability of MSNs
To determine whether transient activation of cholinergic interneurons (ChIs) and processes in the striatum could induce a persistent change in intrinsic excitability of MSNs, we used ChAT-ChR2-EYFP transgenic mice that expressed channelrhodopsin-2 (ChR2) under the control of the choline acetyltransferase promoter (Zhao et al., 2011) or mice generated by crossbreeding Drd1a-tdTomato BAC transgenic mouse line 6 (Ade et al., 2011) with ChAT-ChR2 transgenic mice. These crossbred transgenic mice allowed for identifying D1 receptor containing striatonigral MSNs with tdTomato-positive somata and D2 receptor containing striatopallidal MSNs with tdTomato-negative somata (Ade et al., 2011) and subsequently determining the effect of optogenetic activation of striatal cholinergic system on excitability of these subsets of MSNs.
Optical stimulation with blue laser light significantly increased the firing rate of ChIs expressing ChR2. As illustrated in Fig. 1, light stimulation (470 nm, 4.7 mW/mm2, 10s) caused ChIs to fire action potentials at higher rate followed by spike-frequency adaptation with no sign of depolarization block (Fig. 1B and D). The mean instantaneous frequency increased from 1.15 ± 0.30 Hz in baseline to 3.75 ± 0.34 Hz during 10s optical stimulation measured in cell-attached mode (n = 6; Fig. 1E). The ChIs were identified by their morphology and subsequent electrophysiology characterization under whole cell current clamp condition. They displayed characteristics of autonomous activity and a voltage- and time-dependent depolarizing sag in membrane potential in response to hyperpolarizing current injection under current clamp condition (Fig. 1B and C) (Bennett and Wilson, 1999; Kawaguchi et al., 1995). In addition, the light stimulation also activated the cholinergic axons, including those innervated from the brainstem cholinergic system (Dautan et al., 2014). First we carried out experiments in ChAT-ChR2 transgenic mice without differentiating D1 and D2 MSNs. The intrinsic neuronal excitability was measured by monitoring the number of spike discharges in response to a near threshold depolarization current pulse (1.5 s in duration) under current clamp condition. The amplitude of the depolarization current pulse was adjusted such that only 2–4 spikes were elicited prior to the optical stimulation. As illustrated in Fig. 2A–1, synchronized activation of striatal ChIs and cholinergic axons using the optical stimulation (470 nm, 4.7 mW/mm2, 10 s, repeated 6 times every 30 s) induced a long-lasting increase in intrinsic excitability of a striatal MSN (Fig. 2A–1). For 4 MSNs, the number of spike in response to a given depolarization pulse was increased by 10.0 ± 3.5 measured at 35 min following the light stimulation. When we recorded from D1 and D2 (non-D1) MSNs in slices taken from mice generated by crossbreeding Drd1a-tdTomato BAC mice with ChAT-ChR2 mice, the light stimulation could induce the persistent increase in excitability of these neurons in a similar way (Fig. 2A2 and 2A3; with increases in number of spike per pulse by 10.6 ± 3.9 for D1 MSNs, n = 3; and 8.2 ± 1.3 for D2 MSNs, n = 2; measured at 35 min after light stimulation). The cell from a ChAT-ChR2 mouse in Fig. 2A–1 seemed to have more prominent maximal response after light stimulation, but this was not consistent observation as there were MSNs from ChAT-ChR2 mice that showed maximal responses comparable to those in D1 and D2 MSNs. We pooled the data together for statistical analysis. Light stimulation elicited an increase in number of spike discharge in response to the depolarization current pulse. The increased excitability developed over time and persisted after termination of the light stimulation with a maximal increase in number of spikes per pulse (change in # of spikes/pulse) of 13.5 (from 3.6 ± 0.4 of baseline to 17.1 ± 1.6, n 9), and a mean increase in number of spikes of 9.9 ± 2.2 measured 35 min after the light stimulation (n = 9; Fig. 2C). In the rest of studies, we used only ChAT-ChR2 transgenic mice without differentiating D1 and D2 MSNs.
Fig. 1. Optogenetic activation of cholinergic interneurons expressing mhChR2::YFP fusion protein.
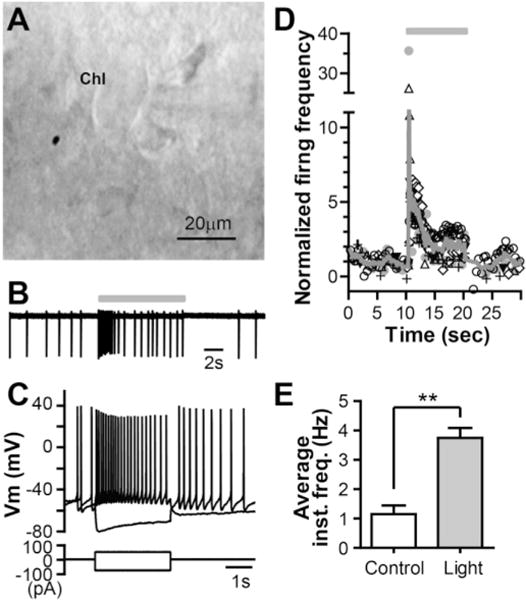
A. Image of a striatal cholinergic interneuron (ChI) under Hoffman modulation contrast microscopy. B. Representative trace of ChI firing in a slice taken from a ChAT-ChR2-EYFP mouse during control and light stimulation (470 nm, 4.7 mW/mm2, 10 s; indicated by the grey bar) under cell-attached configuration. C. Whole-cell current clamp recordings from the same cell as in B after rupturing the membrane, showing the typical voltage responses of ChIs to depolarizing and hyperpolarizing current injections. D. Time course of firing frequency of ChIs in response to light stimulation (indicated by a grey bar). Different symbols represent different cells (n = 6) and each symbol represents instantaneous firing frequency of action potential normalized to the mean value before light stimulation. The solid line represents the average firing frequency across six cells. E. Summary of the effect of light stimulation on the average instantaneous firing frequency of ChIs (**p = 0.0005, n = 6, paired t-test).
Fig. 2. Optogenetic activation of striatal cholinergic system induces long-lasting increase in excitability of MSNs.
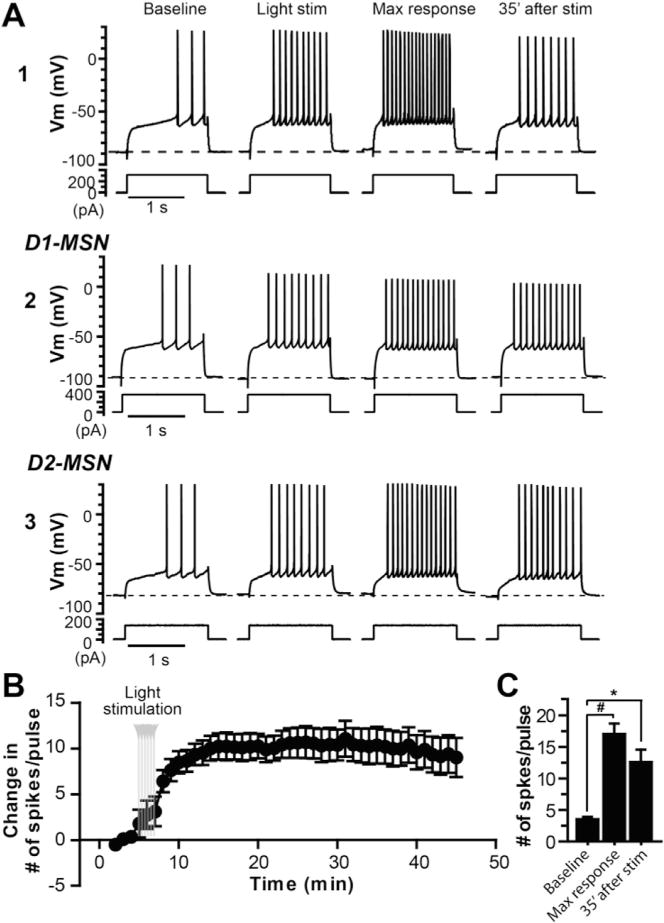
A. Sample traces of membrane potential responses to a depolarization current step from an MSN from a ChAT-ChR2-EYFP mouse (A1), a D1-MSN (A2) and a putative D2-MSN (non-D1, A3) from ChAT-ChR2-EYFP:Drd1a-tdTomato mice before, during and after cholinergic activation by blue laser light stimulation (4.7 mW, 10s in duration, repeated 6 times every 30 s). B. Average time course of change in number of spikes per pulse in response to the depolarization current step from group data pooled from D1, putative D2, and non-discriminated MSNs (n = 9). C. Bar graph summarizing the number of spikes per pulse during baseline, maximal response and 35 min after the light stimulation (one-way ANOVA with repeated measures, F(2,26) = 32.84, p < 0.0001, with Dunnett’s post-test, #p < 0.0005).
3.2. M1 mAChR antagonist VU0255035 inhibits long-lasting increase in excitability induced by optogenetic activation of cholinergic interneurons
Our previous studies and others have demonstrated that M1 mAChR activation can cause acute excitation of MSNs (Akins et al., 1990a; Galarraga et al., 1999; Hsu et al., 1996; Shen et al., 2005, 2007; Xiang et al., 2012). Based on these findings, it is conceivable that the persisting increase in intrinsic excitability of MSNs following the transient elevation in striatal cholinergic activity might be induced by M1 receptor activation. To test the role of M1 in the initiation of this long-lasting response, we applied VU0255035 (10 μM) during the light stimulation and found that the M1 antagonist greatly attenuated the magnitude of long-term increase in MSN intrinsic excitability (Fig. 3). In the presence of VU0255035, the maximal increase in number of spike discharge was significantly lower than that in the control condition (4.4 ± 1.0 in VU0255035, n = 7, compared with 13.5 ± 1.6 in control, n = 9, p = 0.0003, t-test; Fig. 3B), and the number of spikes in response to the current pulse returned to the baseline level 25 min after the light stimulation, which differed significantly from the value in control condition (0.8 ± 1.1, n = 7, compared with 10.3 ± 1.9, n = 9, p = 0.0007, t-test; Fig. 3B). These results suggest that M1 mAChR activation is required for induction of the persistent increase in excitability of MSNs. To test the possibility that optogenetic activation of striatal cholinergic system might cause a persistent increase in ACh tone that acted on M1 receptors and contribute to the long-lasting response, we applied the M1 antagonist VU0255035 (10 μM) 10 min after the light stimulation and found that the M1 antagonist was not able to reverse the increased excitability (data not shown). These data suggested that the expression of long-lasting excitability was not due to persistent increase in ambient ACh level following light stimulation.
Fig. 3. M1 receptor activation is required for the initiation of long-lasting excitation of MSNs induced by endogenous cholinergic activation.
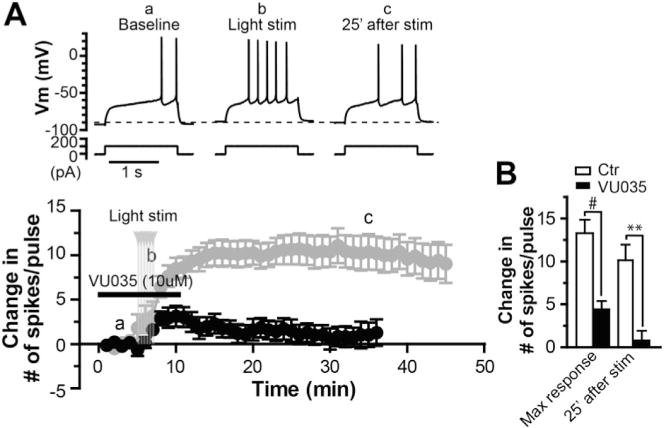
A. Sample traces of membrane potential responses to a depolarization current step (upper) and average time course of change in number of spikes per pulse in response to the depolarization current step (lower, black) before, during and after activation of ChIs by blue laser light stimulation in the presence of M1 antagonist VU0255035 (VU035, 10 μM; n = 7), compared with the average time course in control condition (lower, grey). B. Bar graph summarizing the changes of number of spikes per pulse after light stimulation in presence of VU0255035, compared with those in control (#p = 0.0003, **p = 0.0007, t-test).
3.3. M1 positive allosteric modulator (PAM) VU0453595 potentiates the effect of submaximal optical stimulation of ChIs on MSN excitability
To further verify the role of M1 in the induction of long-lasting increase in MSN excitability, we used novel M1 PAM VU0453595 that displays high selectivity for M1 mAChR over other mAChR subtypes (Ghoshal et al., 2016; Panarese et al., 2014), in conjunction with a submaximal optical stimulation protocol that induced transient but no long-lasting effect on MSN excitability. As illustrated in Fig. 4A, application of M1 PAM VU0453595 (3 μM) induced a transient increase in excitability of MSNs (n = 9), likely due to potentiation of ambient endogenous ACh acting on M1 receptors on MSNs. The number of spikes in response to a given amplitude of current injection measured at 20 min after washout of the M1 PAM was not significantly different from the baseline value (7.0 ± 2.5 compared with 3.8 ± 0.4 in baseline, p = 0.30, n = 9, paired t-test). To moderately elevate striatal cholinergic activity, we applied 20 pulses (50 ms each) of optical stimulation at 2 Hz, which could render cholinergic interneuron firing synchronized at 2 Hz. Fig. 4B showed an example of cell-attached recordings from a cholinergic interneuron in response to this stimulation protocol, demonstrating that action potentials were fairly well time-locked to the light pulses. With this stimulation protocol, we were able to induce a transient but not long-lasting increase in intrinsic excitability of MSNs (Fig. 4C). The number of spike per pulse measured at 20 min after the light stimulation was not significantly differently from the baseline value (6.3 ± 2.5 compared with 3.4 ± 0.3 in baseline, p = 0.27, n = 8, paired t-test). Interestingly, when applied in the presence of VU0453595 (3 μM), the submaximal stimulation protocol induced a robust, long-last increase in MSN excitability with an increase in number of spikes by 12.6 ± 2.6 per depolarization pulse (n = 6; Fig. 4D), significantly higher than that induced by the 2 Hz optical stimulation alone or application of the M1 PAM alone, measured at 20 min after the light stimulation or washout of VU0453595 (2.9 ± 2.5, n = 8; or 3.1 ± 2.3, n = 9; Fig. 4E). These results provided additional support for the important role M1 mAChRs played in the cholinergic activation-induced persistent increase in NSM intrinsic excitability.
Fig. 4. M1 PAM VU0453595 potentiates the effect of submaximal optical stimulation of striatal cholinergic system on MSN excitability.
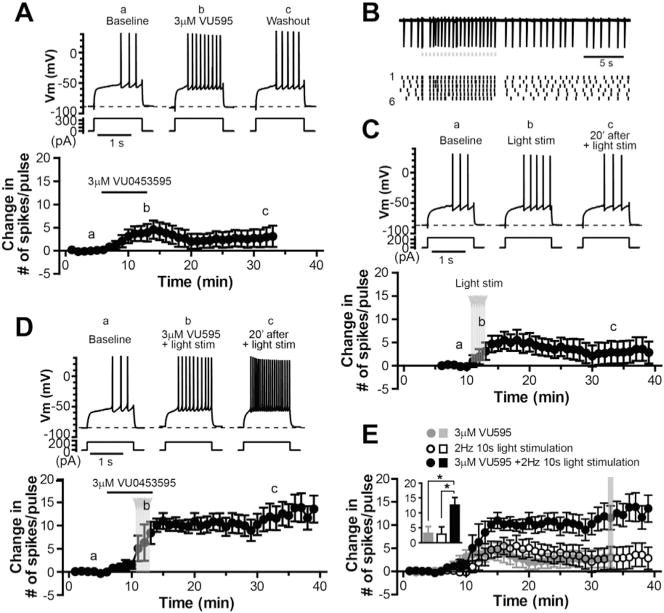
A. Sample traces of membrane potential responses to a given amplitude of depolarization current steps (upper) and average time course of change in number of spike per pulse (lower) before, during and after application of 3 μM VU0453595 (n = 5). B. Sample trace of cell-attached recordings from a ChI in response to a train of 20 light pulse stimuli (grey dots, 50 ms each) at 2 Hz (upper) and raster plot of spike activity from the same ChI as above in response to 6 consecutive trains of light stimulation (lower, the numbers on the left indicate the sweep numbers), showing spikes are fairly well time-locked to the light pulse. C–D. Sample traces (upper) and average time courses of change in number of spike per pulse (lower) before, during and after 6 trains of 2 Hz light stimulation alone (C, n = 8) and in the presence of VU0453595 (D, n = 6). E. Summary time courses and bar graph of change in number of spikes in response to application of 3 μM VU0453595, 2 s Hz 10 s light stimulation, and combination of 3 μM VU0453595 application and the light stimulation, respectively. Data in the bar graph are taken from the time point indicated by the grey line in the time courses (one-way ANOVA, F(2,22) = 4.43, p < 0.05, with Bonferroni’s post-test, *p < 0.05).
3.4. Activation of ionotropic glutamate receptors and DA receptors is not required for induction of the long-lasting increase in MSN excitability following activation of cholinergic interneurons
A previous study indicated that striatal cholinergic interneurons could co-release glutamate, which activates NMDA and AMPA/kainate glutamate receptors in MSNs (Higley et al., 2011). To determine if activation of ionotropic glutamate receptors is required for the induction of the persistent increase in MSN excitability, we included NMDA receptor antagonist AP5 (50 μM) and non NMDA receptor, AMPA/kainate receptor antagonist DNQX (20 μM) in the perfusate during the light stimulation. As illustrated in Fig. 5A–B, the ionotropic glutamate receptor antagonists did not reduce the ability of optical stimulation to induce the persistent increase in MSN excitability. The maximal number of spikes per pulse increased by 11.9 ± 2.2 after the light stimulation (n = 5) with a mean increase by 8.6 ± 2.2 measured at 30 min after the stimulation, which were not significantly different from the data obtained from the experiment in the absence of DNQX and APV (n = 9, p = 0.52 and p = 0.66, respectively, t-test, Fig. 5B). This result indicates that the induction of M1 receptor-dependent long-lasting excitation of MSNs does not require co-activation of ionotropic glutamate receptors.
Fig. 5. Activation of ionotropic glutamate receptors, nAChRs or DA receptors is not required for induction of the long-lasting increase in MSN excitability following striatal cholinergic activation.
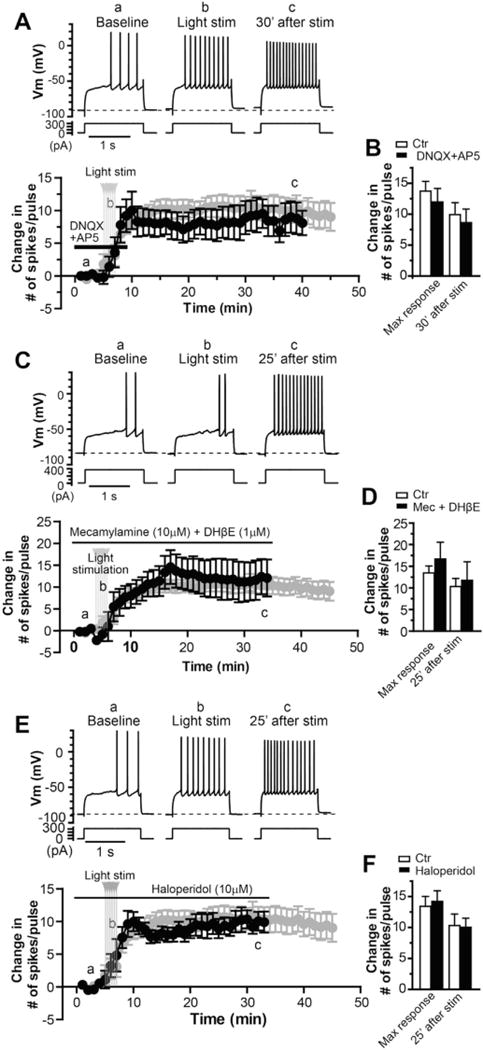
A. Sample traces (upper) and average time course of change in number of spike per pulse (lower, black) before, during and after light stimulation in the presence of 20 μM DNQX and 50 μM AP5 (n = 5), compared with the time course in control (lower, grey, n = 9). B. Bar graph summarizing the effects of optical activation of ChIs on excitability of MSNs in the presence of DNQX and AP5, compared with those in control, presented as maximum change after light stimulation and mean change 25 min after light stimulation (p = 0.52 and p = 0.66, respectively, t-test). C. Sample traces (upper) and average time course of change in number of spike per pulse (lower, black) before, during and after light stimulation in the presence of 10 μM mecamylamine and 1 μM DHβE (n = 5), compared with the time course in control (lower, grey, n = 9). D. Bar graph summarizing the effects of striatal cholinergic activation on excitability of MSNs in the presence of mecamylamine and DHβE, compared with control condition (p = 0.48 and p = 0.77, respectively, t-test). E. Sample traces (upper) and average time course of change in number of spike per pulse (lower, black) before, during and after light stimulation in the presence of 10 μM haloperidol (n = 7), compared with the time course in control (lower, grey, n = 9). F. Bar graph summarizing the effects of striatal cholinergic activation on excitability of MSNs in the presence of haloperidol, compared with control condition (p = 0.79 and p = 0.88, respectively, t-test).
Nicotinic acetylcholine receptors (nAChRs) are present the striatum, mainly at dopaminergic terminals, and dynamically regulate the dopamine neurotransmission (Kaiser et al., 1998; Zhang et al., 2009a, 2009b), which could subsequently modulate intrinsic excitability of MSNs (Hernandez-Lopez et al., 1997; Planert et al., 2013; Surmeier et al., 1992). In addition, nAChRs also express in subsets of striatal interneurons that provided inhibitory controls of MSNs (Faust et al., 2015, 2016; Koos and Tepper, 2002; Luo et al., 2013). To determine if nAChRs play any roles in induction of this form of intrinsic plasticity following cholinergic activation, we included the nAChR antagonists mecamylamine and DHβE in the perfusate. In the presence of 10 μM mecamylamine and 1 μM DHβE, the persistent increase in excitability could still be induced in MSNs by the optical stimulation of striatal cholinergic system (Fig. 5C–D). The maximal number of spikes per pulse increased by 16.7 ± 3.9 after the light stimulation (n = 5) with a mean increase by 11.7 ± 4.3 measured at 25 min after the stimulation, which were not significantly different from the data obtained from the experiment in the control condition (n = 9; p = 0.48 and p = 0.77, respectively, t-test, Fig. 5D), indicating that nAChRs are not involved in the long-term increase in excitability MSNs following striatal cholinergic activation.
Striatal mAChR activation is known to strongly modulate dopamine release (Foster et al., 2014; Threlfell et al., 2010; Tzavara et al., 2004; Zhang et al., 2002b) that, in turn, plays an important role in modulating intrinsic excitability of MSNs (Hernandez-Lopez et al., 1997; Planert et al., 2013; Surmeier et al., 1992). To directly assess if increase in MSN excitability following activation of ChIs is -mediated by dopaminergic system, we performed the experiment similar to those described above in presence of haloperidol at concentration of 20 μM that antagonized both D1 and D2 dopamine receptors (Seeman and Van Tol, 1994). As illustrated in Fig. 5E–F, the optical stimulation was still able to induce the persistent increase in MSN excitability in the presence of antagonism of D1 and D2 receptors. The maximal number of spikes per pulse increased by 14.1 ± 1.7 after the light stimulation (n = 7) with a mean increase by 10.0 ± 1.5 measured at 25 min after the stimulation, which were not significantly different from the control experiment (n = 9, p = 0.79 and p = 0.88, respectively, t-test, Fig. 5F). Because haloperidol is primarily a D2 preferring antagonist and might not completely antagonize D1 activity at the concentration of 20 μM, we repeated the experiment in the presence of D1 antagonist SCH23390. The long-lasting response could still be induced in MSNs by the optical stimulation in the presence of 3 μM SCH23390. The maximal number of spikes per pulse increased by 16.1 ± 1.9 after the light stimulation (n = 3) with a mean increase by 13.1 ± 13.3 measured at 35 min after the stimulation, which were not significantly different from the control experiment (n = 9; p = 0.37 and p = 0.38, respectively, t-test; Fig. S1). These data suggest that activity of striatal dopamine system is not required for induction of M1 mediated persistent increase in MSN excitability.
3.5. Hyperpolarized action potential threshold and decreased afterhyperpolarization are associated with persistent increases in MSN excitability
Next, we sought to determine the mechanism underlying the increased intrinsic excitability of MSNs following cholinergic activation. We monitored the resting membrane potential (RMP), input resistance (RN), action potential threshold and afterhyperpolarization (AHP) following a single action potential before and after induction of the persistent increase in intrinsic excitability. We found no significant changes in RMP and RN associated with the long lasting increased excitability (−88.7 ± 2.4 mV and 53.5 ± 7.1 MΩ during baseline, compared with −86.8 ± 2.8 mV and 54.3 ± 5.7 MΩ measured at 35 min after light stimulation, respectively, n = 9; Fig. 6A–B). The changes of action potential threshold and AHP were monitored by measuring the threshold and AHP of the first spike in response to a depolarization current ramp (100 pA/s) with an amplitude that typically elicited 3–5 spikes in baseline condition. We found that the increased excitability was concomitant with hyperpolarized action potential thresholds and reduced AHP amplitudes. As illustrated in Fig. 6C–F, increase in ACh release by light stimulation caused negative shifts of the action potential threshold and decrease in AHP amplitude, which persisted along with long-lasting increase in excitability (AP threshold: −50.5 ± 1.2 mV during baseline, −53.1 ± 1.5 mV during light stimulation and −54.3 ± 1.4 mV at 35 min after light stimulation [Fig. 6D; n = 7]; AHP: 8.1 ± 0.8 mV during baseline, 7.5 ± 0.7 mV during light stimulation and 6.5 ± 0.5 mV at 35 min after light stimulation [Fig. 6E; n = 7]). The changes of AP threshold and AHP amplitude were also readily appreciated in the phase-plot of APs before and after light stimulation (Fig. 6F). There were no statistically significant changes in maximal dv/dt and minimal dv/dt after induction of the long-lasting increase in excitability (Fig. 6G; 157.9 ± 11.1 V/s in baseline, 163.9 ± 12.3 V/s during light stimulation, and 163.0 ± 16.0 V/s 35 min after light stimulation for maximal dv/dt; −53.5 ± 3.9 V/s in baseline, −53.9 ± 3.4 V/s during light stimulation, and −51.7 ± 3.8 V/s 35 min after light stimulation for minimal dv/dt). These data suggest that overall there are no significant changes in AP depolarizing and repolarizing kinetics associated with persistent increase in excitability.
Fig. 6. Hyperpolarized action potential threshold and decrease in AHP are associated with persistent increase in MSN excitability.
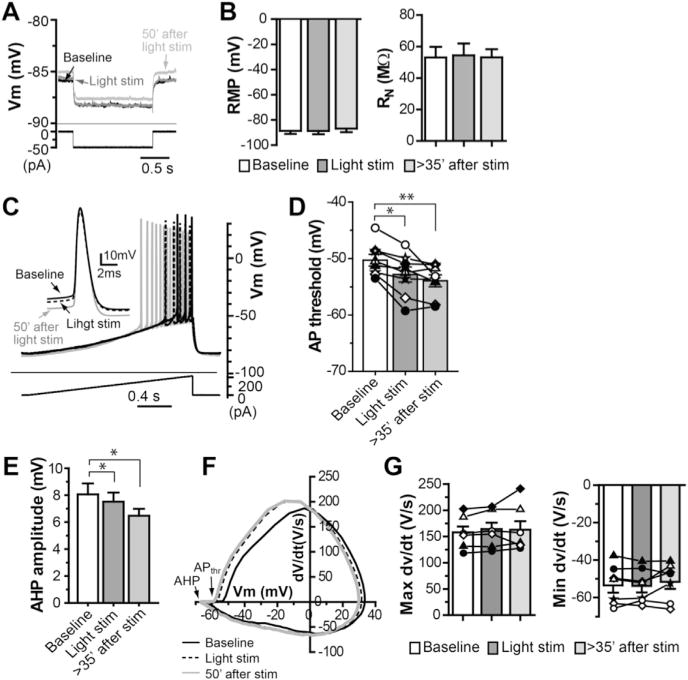
A. Sample traces of membrane potential in response to a hyperpolarization current injection (−50 pA) at resting membrane potential (RMP) to assess input resistance (RN) during baseline, light stimulation and 50 min after light stimulation. B. Summary of RMP and RN during baseline, light stimulation and over 35 min after light stimulation (one-way ANOVA with repeated measures, F(2,26) = 2.64, p > 0.1 for RMP; F(2,20) = 0.11, p > 0.5 for RN). C–E. Sample traces of action potentials (APs) elicited by depolarizing ramp current injection (C, inset: the first APs superimposed under different conditions as indicated), summary of AP threshold (D; one-way ANOVA with repeated measures, F(2,20) = 13.89, p < 0.001, with Dunnett’s post-test, **p < 0.005) and summary of AHP amplitude (E; one-way ANOVA with repeated measures, F(2,20) = 7.27, p < 0.01, with Dunnett’s post-test, *p < 0.01) during baseline, light stimulation and over 35 min after light stimulation. F. The rate of membrane potential change (dV/dt) plotted against membrane potential (phase plot) of APs from a typical experiment, demonstrating the changes of AP threshold (APthr) and AHP amplitude after light stimulation. G. Summary of maximal dv/dt and minimal dv/dt of APs during baseline, light stimulation and over 35 min after light stimulation (one-way ANOVA with repeated measures, F(2, 20) = 0.53, p > 0.5 for maximal dv/dt; F(2, 20) = 1.809, p > 0.1 for minimal dv/dt).
3.6. Cholinergic activation enhances EPSP-action potential coupling in MSNs
To assess the consequence of increase the excitability of MSNs on excitatory synaptic signaling in the striatum, we tested the long-term impact of cholinergic activation on EPSP-action potential (EPSP-AP) coupling in MSNs. A pair of EPSPs were evoked in MSNs by electrical stimulation of the corpus callosum at inter-stimulation interval of 50 ms every 30 s and recorded at a membrane potential between −87 and −65 mV while the intensity of synaptic stimulation was adjusted such that the first EPSPs elicited spikes less than 30% of trials and the second EPSPs elicited spikes over 70% of trials. In such recording conditions, optical stimulation caused a long lasting increase in EPSP-AP coupling (1.9 ± 0.1 spikes/2 EPSPs 30 min after light stimulation, compared with 0.9 ± 0.2 spikes/2 EPSPs during baseline, p = 0.011, n = 5; Fig. 7A and B). The increase in EPSP-AP coupling was associated with hyperpolarized shifts of AP threshold with no changes in the EPSP slope (AP threshold: −52.6 ± 1.2 mV during baseline, compared with −55.0 ± 1.7 mV 30 min after light stimulation, n = 5, Fig. 7C; EPSP slope: 2.64 ± 0.82 mV/ms during baseline, compared with 2.59 ± 0.96 mV/ms 30 min after light stimulation, n = 5; Fig. 7D).
Fig. 7. Persistent increase in EPSP-AP coupling at glutamatergic synapses in MSNs following striatal cholinergic activation.
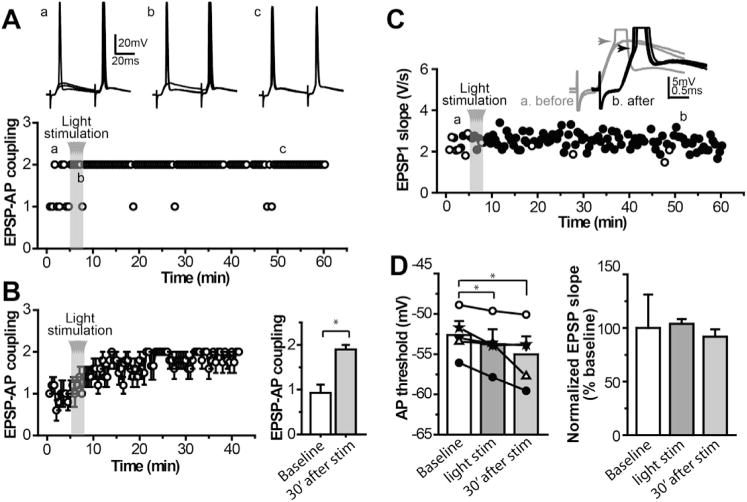
A. Sample traces of subthreshold and supra-threshold EPSPs (upper) and time course of EPSP-action potential coupling (lower) from a typical MSN in response to a pair of electrical stimulation applied to the corpus callosum before, during and after light stimulation, showing the increased EPSP-spike coupling after optogenetic activation of ChIs. B. Average time course and bar graph of EPSP-AP coupling for group data (*p < 0.05, n = 5, paired t-test). C. Time course of EPSP slope from the same cell as in A, showing no apparent change in EPSP slope. Inset: sample traces of subthreshold and suprathreshold EPSPs before and after light stimulation. D. Bar graphs summarizing action potential threshold (left) and normalized EPSP slope (right) before, during and after light stimulation (one-way ANOVA with repeated measures, F(2,14) = 7.503, p < 0.05, with Dunnett’s post-test, **p < 0.01, for AP threshold; F(2,14) = 0.125, p > 0.5, for normalized EPSP slope).
3.7. M1 PAM VU0453595 enhances dorsolateral striatum-dependent, non-spatial discrimination learning
The dorsolateral striatum is critically involved in motor learning, particularly stimulus-response learning and non-spatial discrimination motor learning (Darvas and Palmiter, 2009; Devan and White, 1999; Featherstone and McDonald, 2004; Graybiel, 2008; Yin and Knowlton, 2004). Previous studies indicated that in rats a substantial number of MSNs and ChIs in the dorsolateral striatum have increased activity during acquisition of auditory cue-dependent T-maze learning (Barnes et al., 2005) and during the trial onset in performing an auditory cue-dependent behavioral task (Barnes et al., 2005; Yarom and Cohen, 2011), respectively. These data raised an interesting possibility that an increase in activity of MSNs following M1 mAChR activation could enhance the performance of dorsolateral striatum-dependent learning task. To test this hypothesis, we determined the ability of M1 PAM VU0453595 to enhance non-spatial discrimination motor learning by using a visual cue-dependent, water based T-maze task that has been shown to depend on functional integrity of the dorsolateral striatum (Balleine et al., 2009; Darvas and Palmiter, 2009; Gorski et al., 2003). In the task, the mice swim down the maze to an intersection point where they choose between two arms with different patterns on the walls (intra-maze cues). A correct response consists of entering the arm with the escape platform without previously entering an incorrect arm. The mice were tested daily 30 min after treatment with VU0453595 (1–10 mg/kg; n = 9–10) or vehicle (20% BCD; n = 9). Mice in all treatment groups increased the percent of correct choices to approximately 80% by the end of testing (effect for time; F(9,351) = 14.63, p < 0.0001) (Fig. 8A). More specifically, the number of correct choices significantly increased from day 1–7 (F(6,249) = 7.159, p < 0.0001) and remained unchanged from days 8–10 (F (2,102) = 0.38, p > 0.05). Pretreatment with VU0453595 dose-dependently led to a significant increase in correct choices during the tests on days 1–7 (F(3,249) = 5.375, p = 0.0013). However, on days 8–10, vehicle animals performed the task at a similar level as VU0453595 treated animals (F(3,102) = 0.86, p > 0.05). All mice met criteria (75 percent correct choices in a single testing session) by day 10 of testing. Animals pretreated with VU0453595 significantly reduced the number of trials needed to meet criteria when compared to vehicle treated animals (F(3,36) = 3.287, p = 0.0316). Vehicle treated animals reached criteria in 36 ± 7.4 trials while animals treated with 1, 3, or 10 mg/kg VU0453595 met criteria in 37.6 ± 6.3, 23.2 ± 3.9, and 17.6 ± 2.6 trials, respectively (Fig. 8B). Thus, daily VU0453595 administration appears to enhance the acquisition of this version of non-spatial discrimination learning as evident by reducing the time required to achieve the correct choice criteria.
Fig. 8. M1 PAM VU0453595 enhances cue-dependent non-spatial learning.
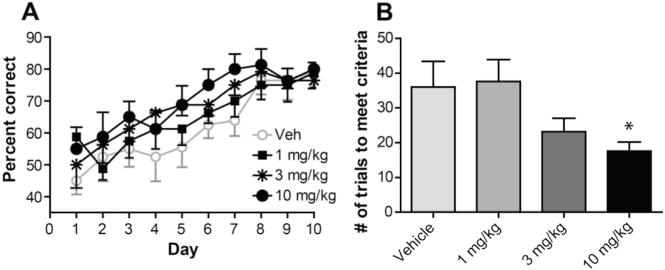
A. All mice learned the task to about 80 percent efficiency by day 10 of testing. Pretreatment with VU0453595 significantly increased the percent of correct choices made during testing days 1–7 when compared to vehicle treated animals. Data represent mean percent correct ± S.E.M.; p = 0.0013. There were no significant differences between vehicle and VU0453595 treated mice from day 8–10 (p > 0.05). B. All mice met criteria (75 percent correct during one testing session) by day 10 of testing. Mice treated with VU0453595 (10 mg/kg) reached criteria in significantly less trials than vehicle treated mice. Data represent mean number of trials to criteria ±SEM; *p = 0.0316.
4. Discussion
In the present study, we used optogenetic techniques in combination with novel pharmacological tools to investigate long-term effect of transient increase in striatal cholinergic activity on intrinsic excitability of striatal projection neurons. We focused on MSNs in the dorsolateral striatum, part of a sensorimotor circuit that is critically involved in stimulus-response controlled habits and general performance of learned behavioral tasks (Featherstone and McDonald, 2004; Graybiel, 2008). We found that striatal cholinergic activation by optogenetic stimulation results in persistent increase in intrinsic excitability of both direct and indirect pathway MSNs. The induction of this long-lasting increase in excitability is mediated by M1 mAChR activation. A hyperpolarized action potential threshold and decreased AHP amplitude are associated with the long-lasting increase in excitability, which subsequently lead to an increase in EPSP-AP coupling in MSNs.
Muscarinic ACh receptor mediated signaling plays an important role in regulating activity-dependent long-term synaptic plasticity in various brain regions, including hippocampus, cortex, striatum and amygdala (Calabresi et al., 1992, 1999; Huerta and Lisman, 1993; McCoy et al., 2008; McCoy and McMahon, 2007; Shinoe et al., 2005; Watanabe et al., 1995; Zheng et al., 2012). In addition, mAChR activation itself can induce long-term synaptic depression or potentiation in the hippocampus and cortex (Fernandez de et al., 2008; Scheiderer et al., 2006). However, the long-term impact of muscarinic receptor activation on neuronal excitability has been elusive. Now we provide evidence that a transient increase in activity of striatal cholinergic system can induce a long-lasting increase in intrinsic excitability of both striatonigral and striatopallidal MSNs. The induction of this long-lasting response is dependent on activation of M1 receptors, as the magnitude of the long-term response could be attenuated or potentiated by M1 antagonist VU0255035 or M1 PAM VU0453595, respectively (Figs. 3 and 4).
Striatal cholinergic interneurons can co-release glutamate that acts on NMDA and AMPA/kainate receptors on MSNs, as indicated in a previous study (Higley et al., 2011) as well as our unpublished observation using optogenetic approaches where activation of ChIs and processes in the striatum produces postsynaptic responses in MSNs that can be reduced by NMDA and AMPA/kainate receptor antagonists. It has been shown that NMDA receptor activation is required for induction of the persistent increase of intrinsic excitability in CA1 pyramidal cells, accompanying the long-term potentiation (LTP) induced by pairing of pre- and postsynaptic activity at Shaffer collateral-CA1 synapses (Xu et al., 2005). One possibility is that the long-lasting increase in MSN excitability observed in the present studies could be through the activation of glutamate receptors, particularly NMDA receptors in MSNs, owing to co-release of glutamate by ChIs. However, in the presence of ionotropic glutamate receptor antagonists DNQX and AP5, the persistent increase in intrinsic excitability still occurred following the transient increase in striatal cholinergic activity (Fig. 5A), suggesting that NMDA receptor activation is not required. Cholinergic activation also regulates striatal dopamine release via nAChRs as well as mAChRs (Foster et al., 2014; Threlfell et al., 2010, 2012; Tzavara et al., 2004; Zhang et al., 2002b), and dopaminergic signaling could, in turn, modulate MSN excitability (Hernandez-Lopez et al., 1997; Planert et al., 2013; Surmeier et al., 1992). Activation of D1-like receptor has been reported to induce prolonged increase in intrinsic excitability in other neuronal population, such as layer V pyramidal cells in the prefrontal cortex (Chen et al., 2007). However, the present studies demonstrate that the long-lasting response can still be induced in the presence of DA receptor antagonism (Fig. 5 and Fig. S1). Taken together, these results strongly suggest that a transient elevation in striatal cholinergic activity can induce a long-lasting intrinsic plasticity in MSNs that is dependent on M1 mAChR activation, but does not require activation of ionotropic glutamate receptors nor does it require dopamine receptor activation.
Recent studies suggest that striatal cholinergic activation could drive inhibitory responses in MSNs (Nelson et al., 2014; Witten et al., 2010) via GABA release from DA terminals (Nelson et al., 2014) or from subsets of interneurons mediated by nAChRs (English et al., 2012; Faust et al., 2015, 2016; Luo et al., 2013). In the present studies, we also observed transient depolarization followed by hyperpolarization in response to light stimulation in a small subset of MSNs, which might reflect the co-release of glutamate from cholinergic terminals (Higley et al., 2011) and co-release of GABA from DA terminals (Nelson et al., 2014) and/or release of GABA from interneurons in response to nAChR activation (English et al., 2012; Faust et al., 2015, 2016; Luo et al., 2013). Nevertheless, these MSNs also exhibited long-lasting increase in intrinsic excitability following the optogenetic activation of striatal cholinergic system regardless of the presence of seemingly inhibitory responses during the light stimulation. This observation indicates that the transient, seemingly inhibitory response during the light stimulation did not interfere with the induction of long-lasting increase in MSN intrinsic excitability by the cholinergic activation.
One possible mechanism underlying the persistent increased excitability of MSNs might be the prolonged elevation of ambient ACh caused by a persistent increase in ChI discharges following optogenetic stimulation. We reasoned that it is unlikely, because optogenetic stimulation did not produce an apparent increase in spontaneous firing of ChIs after the stimulation terminated (Figs. 1B and 4B). Furthermore, while the M1 antagonist VU0255035 inhibited the long-term increase in MSN excitability when applied during light stimulation (Fig. 3), it had no effect when applied 10 min after the light stimulation (data not shown). These results suggest that the long-lasting response could not be attributed to persistent increase in ambient ACh following optogenetic stimulation of striatal cholinergic system.
We and others have shown that muscarinic agonists can inhibit glutamatergic synaptic transmission in striatal MSNs (Hernandez-Echeagaray et al., 1998; Higley et al., 2009; Pakhotin and Bracci, 2007; Pancani et al., 2014), and this effect is mediated by M4 receptors (Pancani et al., 2014). In contrast, we did not observe the change of EPSP slope during the optical activation of striatal cholinergic system (Fig. 7C–D). It could be due to the timing of optical and electrical stimulations, and the amount of ACh available when EPSPs were evoked. Our previous studies showed that increase in ChI activity by optical stimulation (500 ms) applied 25 ms prior to the electrical stimulation of the glutamatergic afferents could only cause a small (~10%), transient, not significant inhibition of EPSCs in MSNs in striatal slices taken from ChAT-ChR2 mice (Pancani et al., 2014), suggesting that M4 receptors that mediate this action are not activated substantially by the amount of ACh release upon the optical stimulation. Additionally, in the present studies the EPSPs were evoked after the termination of 10s optical stimulation (see method section), in which there might not be sufficient ACh available to activate M4 receptors when EPSPs were evoked, due to the fact that the increase in ChI firing induced by 10s optical stimulation decays considerably during the late part of the stimulation (Fig. 1B) and the amount of residual, increased ACh might not be sufficient to activate M4 receptors.
M1 receptor activation can acutely regulate intrinsic neuronal excitability in various brain regions, including striatal MSNs, through modulating multiple ionic channels (Brown and Passmore, 2009; Carr and Surmeier, 2007; Chiang et al., 2010; Fisahn et al., 2002; Krnjevic, 2004; Shen et al., 2005, 2007; Xiang et al., 2012). One of the most prominent channels that are modulated by M1 receptor activation is the low-threshold, non-inactivating voltage-gated M-type potassium channels (also known as the Kv7 channels or KCNQ channels) (Brown and Passmore, 2009; Delmas and Brown, 2005; Marrion, 1997; Shen et al., 2005). It has been shown that the Kv7.2/KCNQ2 and Kv7.3/KCNQ3 subtypes are expressed in striatal MSNs and M1 receptor activation inhibits the activity of these channels (Shen et al., 2005). Interestingly, in the infralimbic prefrontal cortex, brief block of M-channels with XE-991, an M-channel inhibitor, resulted in a prolonged increase in neuronal excitability that was associated with a decrease in AHP amplitude following a single action potential (Santini and Porter, 2010). Thus, it is likely that the long-lasing increase in MSN excitability and the associated decrease in AHP amplitude observed in the present studies are initiated by M1 mediated inhibition of M-channels. In addition, M1 mediated modulation of M-channels might also contribute to the decrease in AP threshold accompanying the long-lasting increase in excitability. In line with this, it has been indicated that M-channels in the axon initial segment can control AP threshold in CA1 pyramidal cells, and reduction of the M-channel function results in a decreased AP threshold (Shah et al., 2008). It remains to be determined whether a similar mechanism operates in the M1 receptor activation-initiated long-term decrease in AP threshold observed in the present studies. While the mechanism by which M1 activation induces a lasting decrease in Kv7 currents is not known, similar persistent changes in currents through voltage-dependent channels have been observed in other neuronal populations (Brager and Johnston, 2007; Fan et al., 2005; Frick et al., 2004; Nelson et al., 2005; Schreurs et al., 1998; Wang et al., 2003; Xu et al., 2005; Yang and Santamaria, 2016), and could be mediated by multiple potential mechanisms, including persistent changes in channel phosphorylation states or internalization of functional channels.
Striatal ChIs are tonically active and the prominent component of ChI activity is thought to be a burst-pause pattern in response to salient stimuli, particularly in primates (Aosaki et al., 1995; Apicella, 2007; Kimura et al., 1984), which might be engaged through thalamic inputs (Ding et al., 2010). More recent studies indicated that ChIs in rodent dorsolateral striatum displayed robust excitation during performing movement in an auditory cue-dependent behavioral task, and particularly the increased activity was associated with the trial onset (Benhamou et al., 2014; Yarom and Cohen, 2011). The increased cholinergic activity associated with movement onset might play a central role in facilitating striatum-dependent motor learning through activation of muscarinic receptors.
The finding that M1 mAChR activation results in intrinsic plasticity that leads to increased EPSP-AP coupling in dorsolateral striatal MSNs is particularly interesting in view of mounting body of studies indicating the critical role of the dorsolateral striatum in non-spatial stimulus-response learning or habit learning (Balleine et al., 2009; Darvas and Palmiter, 2009; Devan and White, 1999; Featherstone and McDonald, 2004; Graybiel, 2008; McDonald and White, 1994; Yin et al., 2004). In particular, inactivation of dorsolateral striatum impaired the acquisition of response learning in an intra-maze cue-dependent T-maze task (Chang and Gold, 2004), and changes in spike activity patterns of neurons in the dorsolateral striatum were highly correlated with changes in behavioral performance in auditory cue-dependent T-maze task (Barnes et al., 2005). In addition, a gradual increase in dorsolateral ACh level was found to be associated with the development of the response learning in a cross maze task and the animals with the highest ratio of basal ACh in the dorsolateral striatum verses hippocampus were the first to switch from a place to response strategy in this learning task (Chang and Gold, 2003), indicating the important involvement of striatal cholinergic system in stimulus-response learning. In the present studies, we demonstrated that M1 PAM is able to facilitate cue-dependent response learning in a water based T-maze, a task that depends upon intact dorsolateral striatum function. This finding, together with the results from previous studies, highlights the importance of M1 receptors in striatum-dependent learning.
Moreover, muscarinic receptor activation also plays an important role in regulating long-term synaptic plasticity at corticostriatal synapses (Calabresi et al., 2000; Pisani et al., 2007). Particularly, activation of M1 mAChRs has been suggested to promote induction of activity-dependent LTP and suppress long-term depression at corticostriatal synapses in the dorsolateral striatum (Bonsi et al., 2008; Calabresi et al., 1992, 2000; Wang et al., 2006). Thus, the persistent increase in EPSP-AP coupling dorsolateral striatum resulting from M1-dependent intrinsic plasticity in MSNs, together with M1-mediated facilitation of activity dependent long-term synaptic plasticity at corticostriatal synapses, might provide the cellular mechanisms by which M1 receptor activation promote striatum-dependent learning.
Supplementary Material
Acknowledgments
The authors would like to thank Weimin Peng for excellent assistant in maintaining colonies and genotyping for transgenic mice and Dr. Gregg Stanwood at Vanderbilt University to provide breeders of Drd1a-tdTomato line 6 mice.
Funding
This work was supported by grants from National Institutes of Health: 2RO1MH082867 (C.W.L.), RO1MH073676 (P.J.C), P50NS071669 (P.J.C.), RO1NS065867 (Z.X).
Abbreviations
- ACh
acetylcholine
- ACSF
artificial cerebrospinal fluid
- AHP
afterhyperpolarization
- AP
action potential
- AP5
DL-2-amino-5-phosphono-pen-tanoic acid
- ChI
cholinergic interneuron
- ChR2
channelrhodopsin-2
- D1
subtype-1 dopaminergic receptor
- D2
subtype-2 dopaminergic receptor
- DA
dopamine
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- EPSP
excitatory postsynaptic potential
- GABA
gamma-aminobutyric acid
- LTP
long-term potentiation
- M1
subtype-1 muscarinic acetylcholine receptor
- mAChR
muscarinic acetylcholine receptor
- MSN
medium spiny neuron
- nAChR
nicotinic acetylcholine receptor
- PAM
positive allosteric modulator
- RMP
resting membrane potential
- RN
input resistance
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2017.03.017.
Footnotes
Conflict of interest
J.M.R., C.W.L. and P.J.C. are inventors on multiple composition of matter patents protecting allosteric modulators of M1.
References
- Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci. 2011;5:32. doi: 10.3389/fnsys.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins PT, Surmeier DJ, Kitai ST. M1 muscarinic acetylcholine receptor in cultured rat neostriatum regulates phosphoinositide hydrolysis. J Neurochem. 1990a;54:266–273. doi: 10.1111/j.1471-4159.1990.tb13310.x. [DOI] [PubMed] [Google Scholar]
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990b;344:240–242. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M. Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. 2010;10(Suppl. 1):S148–S157. doi: 10.1111/j.1447-0594.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Bendor J, Lizardi-Ortiz JE, Westphalen RI, Brandstetter M, Hemmings HC, Jr, Sulzer D, Flajolet M, Greengard P. AGAP1/AP-3-dependent endocytic recycling of M5 muscarinic receptors promotes dopamine release. EMBO J. 2010;29:2813–2826. doi: 10.1038/emboj.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou L, Kehat O, Cohen D. Firing pattern characteristics of tonically active neurons in rat striatum: context dependent or species divergent? J Neurosci. 2014;34:2299–2304. doi: 10.1523/JNEUROSCI.1798-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum, a combination of choline acetyltransferase immunocytochemistry, golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J, Pisani A. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci. 2008;28:6258–6263. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Bernardi G. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology. 1999;38:323–326. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Post-receptor mechanisms underlying striatal long-term depression. J Neurosci. 1994;14:4871–4881. doi: 10.1523/JNEUROSCI.14-08-04871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Tecuapetla F, Vautrelle N, Hernandez A, Vergara R, Galarraga E, Bargas J. Muscarinic enhancement of persistent sodium current synchronizes striatal medium spiny neurons. J Neurophysiol. 2009;102:682–690. doi: 10.1152/jn.00134.2009. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International union of pharmacology. XVII classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Inactivation of dorsolateral striatum impairs acquisition of response learning in cue-deficient, but not cue-available, conditions. Behav Neurosci. 2004;118:383–388. doi: 10.1037/0735-7044.118.2.383. [DOI] [PubMed] [Google Scholar]
- Chen L, Bohanick JD, Nishihara M, Seamans JK, Yang CR. Dopamine D1/5 receptor-mediated long-term potentiation of intrinsic excitability in rat prefrontal cortical neurons: Ca2+-dependent intracellular signaling. J Neurophysiol. 2007;97:2448–2464. doi: 10.1152/jn.00317.2006. [DOI] [PubMed] [Google Scholar]
- Chiang PH, Yeh WC, Lee CT, Weng JY, Huang YY, Lien CC. M(1)-like muscarinic acetylcholine receptors regulate fast-spiking interneuron excitability in rat dentate gyrus. Neuroscience. 2010;169:39–51. doi: 10.1016/j.neuroscience.2010.04.051. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol. 2011;21:402–407. doi: 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc Natl Acad Sci USA. 2009;106:14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, Mena-Segovia J. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci. 2014;34:4509–4518. doi: 10.1523/JNEUROSCI.5071-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Faust TW, Assous M, Shah F, Tepper JM, Koos T. Novel fast adapting interneurons mediate cholinergic-induced fast GABAA inhibitory postsynaptic currents in striatal spiny neurons. Eur J Neurosci. 2015;42:1764–1774. doi: 10.1111/ejn.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, Assous M, Tepper JM, Koos T. Neostriatal GABAergic interneurons mediate cholinergic inhibition of spiny projection neurons. J Neurosci. 2016;36:9505–9511. doi: 10.1523/JNEUROSCI.0466-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a simple discrimination task. Behav Brain Res. 2004;150:15–23. doi: 10.1016/S0166-4328(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Fernandez de SD, Nunez A, Borde M, Malinow R, Buno W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci. 2008;28:1469–1478. doi: 10.1523/JNEUROSCI.2723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J Neurosci. 2014;34:3253–3262. doi: 10.1523/JNEUROSCI.4896-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C, Bargas J. Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci. 1999;19:3629–3638. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal A, Rook JM, Dickerson JW, Roop GN, Morrison RD, Jalan-Sakrikar N, Lamsal A, Noetzel MJ, Poslusney MS, Wood MR, Melancon BJ, Stauffer SR, Xiang Z, Daniels JS, Niswender CM, Jones CK, Lindsley CW, Conn PJ. Potentiation of M1 muscarinic receptor reverses plasticity deficits and negative and cognitive symptoms in a schizophrenia mouse model. Neuropsychopharmacology. 2016;41:598–610. doi: 10.1038/npp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hernandez-Echeagaray E, Galarraga E, Bargas J. 3-Alpha-chloro-imperia-line, a potent blocker of cholinergic presynaptic modulation of glutamatergic afferents in the rat neostriatum. Neuropharmacology. 1998;37:1493–1502. doi: 10.1016/s0028-3908(98)00131-2. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS ONE. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Herman MM, Hyde TM, Kleinman JE, Sinton CM, German DC, Hersh LB, Graybiel AM, Saper CB. Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience. 1999;94:21–31. doi: 10.1016/s0306-4522(99)00279-1. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Gean PW. Muscarinic depression of excitatory synaptic transmission mediated by the presynaptic M3 receptors in the rat neostriatum. Neurosci Lett. 1995;197:141–144. doi: 10.1016/0304-3940(95)11915-j. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Yang CH, Huang CC, Gean PW. Carbachol induces inward current in neostriatal neurons through M1-like muscarinic receptors. Neuroscience. 1996;73:751–760. doi: 10.1016/0306-4522(96)00066-8. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by alpha-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurons: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci USA. 1984;81:4998–5001. doi: 10.1073/pnas.81.15.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K. Synaptic mechanisms modulated by acetylcholine in cerebral cortex. Prog Brain Res. 2004;145:81–93. [PubMed] [Google Scholar]
- Lange KW, Javoy-Agid F, Agid Y, Jenner P, Marsden CD. Brain muscarinic cholinergic receptors in Huntington’s disease. J Neurol. 1992;239:103–104. doi: 10.1007/BF00862983. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Janssen MJ, Partridge JG, Vicini S. Direct and GABA-mediated indirect effects of nicotinic ACh receptor agonists on striatal neurones. J Physiol. 2013;591:203–217. doi: 10.1113/jphysiol.2012.241786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV. Control of m-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- McCoy P, Norton TT, McMahon LL. Layer 2/3 synapses in monocular and binocular regions of tree shrew visual cortex express mAChR-dependent long-term depression and long-term potentiation. J Neurophysiol. 2008;100:336–345. doi: 10.1152/jn.01134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy PA, McMahon LL. Muscarinic receptor dependent long-term depression in rat visual cortex is PKC independent but requires ERK1/2 activation and protein synthesis. J Neurophysiol. 2007;98:1862–1870. doi: 10.1152/jn.00510.2007. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du LS. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC. Striatal cholinergic interneurons drive GABA release from dopamine terminals. Neuron. 2014;82:63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panarese DJ, Rook JM, Poslusney MS, Melancon BJ, Bridges TM, Cho HP, Dickerson J, Hopkins CR, Wood MR, Xiang Z, Morrison R, Stauffer SR, Daniels JS, Niswender C, Jones CK, Conn PJ, Lindsley CW. Development of non-isatin M1 positive allosteric modulators. P MEDI. 2014;130 [Google Scholar]
- Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ, Xiang Z. M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci. 2014;5:318–324. doi: 10.1021/cn500003z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Burgos A, Perez-Rosello T, Salgado H, Flores-Barrera E, Prieto GA, Figueroa A, Galarraga E, Bargas J. Muscarinic M(1) modulation of N and L types of calcium channels is mediated by protein kinase C in neostriatal neurons. Neuroscience. 2008;155:1079–1097. doi: 10.1016/j.neuroscience.2008.06.047. [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A, Prieto GA, Galarraga E, Bargas J. CaV2.1 channels are modulated by muscarinic M1 receptors through phosphoinositide hydrolysis in neostriatal neurons. Neuroscience. 2010;165:293–299. doi: 10.1016/j.neuroscience.2009.10.056. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson’s disease. Mov Disord. 2005;20:395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- Planert H, Berger TK, Silberberg G. Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine. PLoS One. 2013;8:e57054. doi: 10.1371/journal.pone.0057054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30:12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiderer CL, McCutchen E, Thacker EE, Kolasa K, Ward MK, Parsons D, Harrell LE, Dobrunz LE, McMahon LL. Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor-dependent long-term depression at CA3-CA1 synapses. J Neurosci. 2006;26:3745–3756. doi: 10.1523/JNEUROSCI.5507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 2008;105:7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25:11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders I, Bogaert L, Ebinger G, Michotte Y. Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J Neurochem. 1997;68:1942–1948. doi: 10.1046/j.1471-4159.1997.68051942.x. [DOI] [PubMed] [Google Scholar]
- Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci U S A. 1991;88:2608–2611. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. Faseb J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu NL, Wu CP, Duan S, Poo MM. Bidirectional changes in spatial dendritic integration accompanying long-term synaptic modifications. Neuron. 2003;37:463–472. doi: 10.1016/s0896-6273(02)01189-3. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Ikegaya Y, Saito H, Abe K. Roles of GABAA, NMDA and muscarinic receptors in induction of long-term potentiation in the medial and lateral amygdala in vitro. Neurosci Res. 1995;21:317–322. doi: 10.1016/0168-0102(94)00867-f. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Thompson AD, Jones CK, Lindsley CW, Conn PJ. Roles of the m1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2012;340:595–603. doi: 10.1124/jpet.111.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- Yang Z, Santamaria F. Purkinje cell intrinsic excitability increases after synaptic long term depression. J Neurophysiol. 2016;116:1208–1217. doi: 10.1152/jn.00369.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom O, Cohen D. Putative cholinergic interneurons in the ventral and dorsal regions of the striatum have distinct roles in a two choice alternative association task. Front Syst Neurosci. 2011;5:36. doi: 10.3389/fnsys.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009a;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009b;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002a;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple musca-rinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002b;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Wess J, Alzheimer C. M2 muscarinic acetylcholine receptors regulate long-term potentiation at hippocampal CA3 pyramidal cell synapses in an input-specific fashion. J Neurophysiol. 2012;108:91–100. doi: 10.1152/jn.00740.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


