Abstract
PKMζ is an autonomously active PKC isoform that is thought to maintain both LTP and long-term memory. Whereas persistent increases in PKMζ protein sustain the kinase’s action in LTP, the molecular mechanism for the persistent action of PKMζ during long-term memory has not been characterized. PKMζ inhibitors disrupt spatial memory when introduced into the dorsal hippocampus from 1 day to 1 month after training. Therefore, if the mechanisms of PKMζ’s persistent action in LTP maintenance and long-term memory were similar, persistent increases in PKMζ would last for the duration of the memory, far longer than most other learning-induced gene products. Here we find that spatial conditioning by aversive active place avoidance or appetitive radial arm maze induces PKMζ increases in dorsal hippocampus that persist from 1 day to 1 month, coinciding with the strength and duration of memory retention. Suppressing the increase by intrahippocampal injections of PKMζ-antisense oligodeoxynucleotides prevents the formation of long-term memory. Thus, similar to LTP maintenance, the persistent increase in the amount of autonomously active PKMζ sustains the kinase’s action during long-term and remote spatial memory maintenance.
Keywords: PKMzeta, PKM-zeta, Memory, Long-term potentiation, LTP
1. Introduction
The persistent action of PKMζ has been proposed to be essential for LTP maintenance and long-term memory storage (Sacktor, 2011). PKMζ is the autonomously active, independent catalytic domain of the atypical PKC isoform PKCζ and is produced in LTP by new protein synthesis from a dedicated PKMζ mRNA (Hernandez et al., 2003; Osten, Valsamis, Harris, & Sacktor, 1996). Increases in the amount of the newly synthesized kinase persist for hours in LTP maintenance (Osten et al., 1996), and the autonomous activity of PKMζ is both necessary and sufficient to enhance synaptic transmission during late-LTP maintenance (Ling, Benardo, & Sacktor, 2006; Ling et al., 2002; Yao et al., 2008). Whereas PKMζ persistently increases in LTP in wild-type mice, null-mutant mice lacking PKMζ (Lee et al., 2013; Volk, Bachman, Johnson, Yu, & Huganir, 2013) compensate for the absence of PKMζ by persistently increasing another atypical PKC isoform, PKCι/λ (Tsokas et al., 2016). In addition to reversing LTP maintenance, long-term memory is disrupted by post-training application of PKMζ inhibitors such as ZIP and chelerythrine and overexpression of a dominant negative mutant form of PKMζ, indicating that memory persistence requires the sustained action of PKMζ (Cai, Pearce, Chen, & Glanzman, 2011; Drier et al., 2002; Pastalkova et al., 2006; Serrano et al., 2008; Shema, Sacktor, & Dudai, 2007; Shema et al., 2011).
The molecular mechanism for the sustained action of PKMζ in memory maintenance has not been investigated in detail, but the most parsimonious notion is that the mechanisms of LTP maintenance and long-term and remote memory storage are the same— a persistent increase of autonomously active PKMζ. PKMζ inhibitors erase spatial memory when introduced into the dorsal hippocampus up to 1 month after training (Pastalkova et al., 2006). Therefore, this hypothesis predicts persistent increases of PKMζ in dorsal hippocampus that last a month in vivo, far longer than the increases of any known learning-induced gene product.
To test this prediction we examined the amount of PKMζ in dorsal hippocampus in two spatial conditioning paradigms: aversive active place avoidance conditioning and appetitive radial arm maze conditioning. PKMζ inhibitors disrupt established long-term memories produced by both types of training (Pastalkova et al., 2006; Serrano et al., 2008). The rapidly acquired active place avoidance paradigm can be used to assess hippocampus-dependent spatial memories, including short-term memory lasting minutes, long-term memory lasting days, and remote memory lasting over a month (Cimadevilla, Fenton, & Bures, 2000; Pastalkova et al., 2006). In addition, the rapid acquisition of active place avoidance allows us to test the effect of acute intracranial injections of PKMζ-antisense during conditioning in order to determine whether an increase in PKMζ is functionally important for the memory. The slowly acquired conditioning on the radial arm maze also produces spatial long-term memory lasting days and remote memory lasting a month.
2. Methods
2.1. Reagents
Reagents were from Sigma unless specified otherwise. The ζ-specific rabbit polyclonal antiserum (1:20,000 for immunoblots) was generated as previously described (Hernandez et al., 2003). The source and concentration of antisera to the other PKC isoforms are α: Gibco #3191SA, rabbit, 1:200; βI: Santa Cruz #sc-8049, mouse, 1:500; βII: antiserum described in (Sacktor et al., 1993), rabbit, 1:100; γ: Santa Cruz #sc-211, rabbit, 1:2000; δ: Santa Cruz #sc-8402, mouse, 1:50; ε: a generous gift from Dr. Robert O. Messing (Univ Texas at Austin, TX), rabbit, 1:1000; η: Santa Cruz #sc-215, rabbit, 1:200; ι/λ: BD transduction #610207, mouse, 1:250). The actin mouse mAb (1:5000) was from Sigma, and the tubulin mouse mAb was from Millipore (1:5000). Protein concentrations were determined by assay using bicinchoninic acid (Pierce) or Bio-Rad RC-DC Protein Assay kit for hippocampal extracts in reducing agents, using bovine serum albumin as standard.
2.2. Preparation of hippocampal extracts
The procedures comply with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the State University of New York, Downstate Medical Center Animal Care and Use Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
After training, dorsal hippocampal extracts were prepared for immunoblots. After decapitation under deep isoflurane anesthesia, hippocampi of 2–3 month-old, male, Long-Evans rats were removed and placed into ice-cold artificial cerebrospinal fluid with high Mg2+ (10 mM) and low Ca2+ (0.5 mM) (Sacktor et al., 1993). The dorsal hippocampus, consisting of 50% of the hippocampus from the septal end, was dissected out, snap-frozen, and stored at −80 °C until lysis. Dorsal hippocampi were homogenized in 200 μl of modified ice-cold RIPA buffer consisting of the following in mM concentrations, unless indicated otherwise: 25 Tris-HCl (pH 7.4), 150 NaCl, 6 MgCl2, 2 EDTA, 1.25% NP-40, 0.125% SDS, 0.625% sodium deoxycholate, 4 p-nitrophenyl phosphate, 25 sodium fluoride, 2 sodium pyrophosphate, 20 dithiothreitol, 10 β-glycerophosphate, 1 μM okadaic acid, phosphatase inhibitor cocktail I & II (2% and 1%, respectively, Calbiochem) or, alternatively, PhosphataseArrest II & III (1%, G-Biosciences), 1 phenylmethylsulfonyl fluoride, 20 μg/ml leupeptin, and 4 μg/ml aprotinin.
2.3. Immunoblotting
As previously described (Sacktor et al., 1993), the dissected hippocampal regions or slices removed from the recording chamber (see below) were immediately frozen on glass on dry ice. The CA1 region was excised in a cold room (4 °C) and homogenized in 30 μl of ice-cold modified RIPA lysis buffer. Appropriate volumes of 4× NuPage LDS Sample Buffer (Invitrogen, Carlsbad, CA) and β-mercaptoethanol were added to the homogenates, and samples were boiled for 5 min followed by SDS-PAGE. Following transfer at 4 °C, nitrocellulose membranes (0.2 μm pore size) were blocked for at least 30 min at room temperature with blocking buffer (BB: 5% non-fat dry milk in TBS containing 0.1% Tween 20 [TBS-T]; or Licor Odyssey Blocking Buffer), then probed overnight at 4 °C using primary antibodies dissolved in BB or Licor Odyssey Blocking Buffer with 0.1% Tween 20 and 0.01% SDS. After washing in TBS-T (or phosphate-buffered saline+ 0.1% Tween 20 [PBS-T]; 3 washes, 5 min each), the membranes were incubated with horseradish peroxidase-conjugated (Pierce Biotechnology), alkaline phosphatase-conjugated (Sigma-Aldrich), or IRDye (Licor) secondary antibodies. Proteins were visualized by chemiluminescence (Amersham ECL Western Blotting Analysis System), the Licor Odyssey System, or alkaline phosphatase, developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium. Densitometric analysis of the bands was performed using NIH ImageJ, and values were normalized to actin (Mr = ∼42 kDa, active place avoidance and LTP performed by C.H. and P.T.), or tubulin (Mr = ∼52 kDa, radial arm maze performed by P.S.).
2.4. qRT-PCR
Total RNA from dorsal hippocampus was prepared using the TRIzol Reagent (Invitrogen) according to the manufacture’s instructions. RNA integrity was analyzed by electrophoresis in 1% agarose gels in 1 × MOPS buffer under denatured conditions (2.2 M formaldehyde), stained with ethidium bromide, and visualized under UV light. Nucleic acid was quantified by measuring the absorbance at 260 nm using nanodrop technology. Nucleic acid purity was assessed by quantifying the A260 nm/A280 nm ratio and was acceptable when the ratio was >1.8. A total of 10 μg total RNA was treated with 1 U DNase I (Promega) for 1 h at 37 °C and then heat-inactivated at 65 °C for 10 min before reverse transcription to eliminate genomic DNA contamination. Two μg of total RNA was used to synthesize cDNA with the Superscript II-Reverse Transcriptase (Invitrogen), using random hexamers as primers, under the conditions recommended by the manufacturer. Ten ng of the first strand cDNA was used as template for the qPCR. The following pairs of primers (5′–3′) were used: mGAPDH: TTGTGATGGGTGT-GAACCACGAGA and GAGCCCTTCCACAATGCCAAAGTT; Prkcz exon9: gGCTGCAAGACTTCGACCTCATC and CTGGACGCCTGCT-CAAACACATGT. The Prkcz primers were designed to avoid recognition of mouse PKCζII (Parkinson, Le Good, Whelan, Whitehead, & Parker, 2004).
Reactions were in a total volume of 20 μl containing 5 μl of cDNA, 5 μl of gene-specific primers (final concentration 1 μM each), and 10 μl SYBRGreen I master mix (BioRad). The cycling conditions were: 95 °C for 15 s, 45 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 15 s with a single fluorescence measurement; a final elongation step was carried out at 72 °C for 10 min. PCR was performed using a CFX96 Real-Time System (BioRad). Specificity of the PCR products was confirmed by analysis of the dissociation curves. The melting curve program consisted of temperatures between 60 and 95 °C with a heating rate of 0.1 °C/s and a continuous fluorescence measurement. Additionally, the amplicons’ expected size and the absence of nonspecific products were confirmed by analysis of the real-time PCR products in 1% agarose gels in 1 × TBE, stained with ethidium bromide, and visualized under UV light.
2.5. Hippocampal slice preparation and recording
For LTP experiments, rat hippocampal slices (450 μm) were prepared with a McIlwain tissue slicer as previously described (Sacktor et al., 1993) and were either transferred directly to a submersion recording chamber (32 ± 1 °C) or maintained first in an interface chamber at room temperature for at least 2 h before transfer (see Section 2.6). The superfusate consisted of (in mM) 118 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.25 NaH2PO4, 24 NaHCO3, and 15 glucose, bubbled with 95% O2/5% CO2 and was re-circulated at 4–8 ml/min, using a dual channel peristaltic pump (Masterflex, Cole Parmer, Vernon Hills, IL). In a subset of oligodeoxynucleotide experiments, a custom-made recirculation system employing piezoelectric pumps (Bartels Mikrotechnik GmbH, Dortmund, Germany) was used to perfuse slices with a recirculating volume of 5 ml, as previously described (Tsokas et al., 2016). Field EPSPs (fEPSPs) were recorded with a glass extracellular recording electrode (2–5 MΩ) placed in the CA1 stratum radiatum, and concentric bipolar stimulating electrodes (FHC, Bowdoin, ME) were placed on either side within CA3 or CA1. Pathway independence was confirmed by the absence of paired-pulse facilitation between the two pathways. The high-frequency stimulation consisted of two standard 100 Hz 1-s tetanic trains, spaced 20 s apart, at current intensity producing a slope value that is 70% of spike threshold, which is optimized to produce a relatively rapid onset synthesis of PKMζ and protein synthesis-dependent late-phase LTP (Osten et al., 1996; Tsokas, Ma, Iyengar, Landau, & Blitzer, 2007). The maximum slope of the rise of the fEPSP is analyzed using the WinLTP data acquisition program (Anderson & Collingridge, 2007).
2.6. Antisense oligodeoxynucleotides
We adapted the approach used in Garcia-Osta et al. (2006), in which antisense oligodeoxynucleotides that sterically block the translation site on specific mRNAs are injected into dorsal hippocampus in vivo and then hippocampal slices are rapidly prepared for physiology. In a subset of oligodeoxynucleotide experiments, the antisense was bath-applied for 1 h before tetanization, using a recirculation system. The sequences of the oligodeoxynucleotides were: PKMζ antisense, ctcTTGGGAAGG-CAtgaC; scrambled, aacAATGGGTCGTCtcgG, in which the lower case bases signifies phosphorothioate linkage 5′–3′. We injected the oligodeoxynucleotide in the dorsal hippocampus and 1 h later prepared hippocampal slices within 400 μm of the injection site. The PKMζ-antisense sequence is complementary to the translation start site in the PKMζ mRNA and shows no significant homology to any other sequence in the GenBank database. Scram bled-oligodeoxynucleotide, which also does not match any known sequence, was injected in the contralateral dorsal hippocampus. Both oligodeoxynucleotides are phosphorothioated on the 3-terminal bases at each end to protect against nuclease degradation and were reverse phase cartridge-purified (Gene Link, Hawthorne, NY) (Garcia-Osta et al., 2006). The biotinylated PKMζ antisense (Gene Link, Hawthorne, NY) was labeled by a 5′ biotin modification with a C6 spacer (Garcia-Osta et al., 2006). To be equivalent to applications during massed training in rats, a single dorsal hippocampus injection of biotinylated PKMζ antisense (2 nmol) was given, and the brain was fixed by intracardiac perfusion of 4% paraformaldehyde in PBS (4% PFA) 4 h later, followed by post-fixation in 4% PFA for 48 h. The 40 μm coronal sections were stained by immunohistochemistry using anti-biotin conjugated Cy3 antibody (Jackson ImmunoResearch, West Grove, PA), counterstained with DAPI, and examined by confocal microscopy.
The detailed procedure for implanting and subsequently performing intrahippocampal injections has been published (Tsokas et al., 2016). Briefly, in preparation for stereotaxic surgery to implant the injection cannula hardware, the rats were anesthetized by 50 mg/kg i.p. Nembutal. The rats were mounted in a Kopf stereotaxic frame to implant a pair of guide cannulae with the tip above the injection target in the dorsal hippocampus (relative to Bregma AP −3.5 mm, ML ± 2.6 mm, DV −2.0 mm). The injection hardware implanted were manufactured by Plastics One, Roanoke, VA (Part Numbers: C313G, C313GDC/1, 303DC/1, C313I). A week after surgery, the rats received active place avoidance training. Before testing the effect of the antisense oligodeoxynucleotide injection on place avoidance, the rats received a bilateral injection of saline (1 μl/side) and were left in the home cage to habituate to the procedure. The day after the initial pretraining exposure to the place avoidance apparatus, the rats were injected bilaterally with 2 nmol/μl oligodeoxynucleotide in PBS (1 μl/side) 20 min before the start of active place avoidance training. The animals were restrained, the cannula cap and dummy cannula were removed, and the injection needle was inserted into the guide cannula so that it protruded 0.75 mm from the end of the guide. The other end of the needle was connected to a 1 μl Hamilton syringe via Tygon tubing. The oligodeoxynucleotide solution was infused for 2 min. After the infusion, the needle was left in place for 3 min before it was removed. The animals were returned to their home cage to recover from any acute effects of the injection and to allow diffusion of the antisense oligodeoxynucleotide before training began.
For LTP experiments, we tested the effects of antisense on activity-dependent PKMζ synthesis by two methods: (1) assaying PKMζ changes 30 min posttetanization after intrahippocampal injections as described above to confirm the efficacy of the oligodeoxynucleotide injections on LTP, and (2) after applying equivalent concentrations of oligodeoxynucleotides (20 μM, based upon a 1:100 dilution in the hippocampus postinjection) after preparation of slices, in order to optimize the exposure of the oligodeoxynucleotides to the slice (Tsokas et al., 2016). For the first method, 1 h after intracranial injections of oligodeoxynucleotides in naïve rats, hippocampal slices are prepared and, after ∼2 h incubation in an interface chamber, tested for LTP in a submersion chamber. In the second method, slices obtained from naïve rats were perfused with a recirculating volume of 5 ml superfusate for 30 min before antisense- or scrambled-oligodeoxynucleotide was dissolved in the superfusate and recirculated for 1 h before tetanization and for the duration of the experiment thereafter (30 min post-tetanus), using a custom-made recirculation submersion system involving piezoelectric pumps (Bartels Mikrotechnik GmbH, Dortmund, Germany). The activity-dependent increase in PKMζ in the presence of scrambled oligodeoxynucleotide and the inhibition of this increase by PKMζ-antisense measured by immunoblot 30 min posttetanization were indistinguishable by the two methods, and therefore the changes in the amounts of the proteins were combined (injected: scrambled, tetanized [% scrambled, untetanized]: 150 ± 23%, n = 11; bath-applied: scrambled, tetanized [% scrambled, untetanized]: 158 ±17%, n = 7; t16 = 0.26, P = 0.8; injected: antisense, tetanized [% antisense, untetanized]: 104 ± 16%, n = 7; bath-applied, antisense, tetanized [% antisense, untetanized]: 110 ± 7%, n = 11; t16 = 0.40, P = 0.7).
2.7. Active place avoidance
The place avoidance procedures have been described in detail (Cimadevilla, Kaminsky, Fenton, & Bures, 2000). Briefly, the rat was placed on an 82-cm diameter metal disk that is elevated 78 cm from the floor and rotates at 1 rpm within a room with numerous visual landmarks off of the disk. Prior to training, the rat was implanted with a subcutaneous shock electrode between the shoulders, through which a constant current (0.3 mA, 60 Hz, 500 ms) electrical foot shock is delivered whenever the rat enters an unmarked shock zone. The impedance between the shock electrode and the skin was approximately 1000 times less than the impedance between the rat’s feet and the metal disk, which is grounded, so the major voltage drop is across the feet. The shock zone was an unmarked 60° sector that is defined by distal visual landmarks in the room. The location of the rat was determined from an overhead television camera each 33 ms by a PC-controlled tracking system (Bio-Signal Group). When the system detects the rat in the shock zone, the shock is delivered and repeated every 1500 ms until the rat leaves the shock zone.
Place avoidance training began with a pretraining trial. The rat was placed on the rotating disk to explore the environment with the shock turned off for 10 min. After resting in the home cage for 10 min, the rat received eight 10-min training trials with the shock turned on. There was a 10-min rest in the home cage between trials. The extended training protocol consisted of two sessions of the eight 10-min training trials with an inter-session interval of 1 week. Retention of the 1-day or 1-month place avoidance memory was tested by returning the rat to the rotating disk with the shock off. The time to first enter the shock zone estimated retention of memory. Short-term memory was established by turning on the shock for 10 min, and then, without removing the rat from the environment, retention was tested during a 10-min test period with the shock off.
2.8. Eight-arm radial maze
Spatial reference memory is distinguished from spatial working memory in the eight-arm radial maze because in reference memory, information about which arm locations are consistently baited is valid across trials, whereas working memory requires spatial information for which arm locations were visited within a trial, information that is only useful for the specific trial. We used the standard four-arms baited, four-arms unbaited task variant (Olton, Becker, & Handelmann, 1979). In this task, lesions of the hippocampus increase working memory errors, but not reference memory errors (Olton et al., 1979). This basic result (Niewoehner et al., 2007; Potvin, Allen, Thibaudeau, Dore, & Goulet, 2006) contrasts, however, with many studies indicating that the hippocampus is critical for spatial reference memory in water maze tasks and other tests of spatial reference memory (Morris, Garrud, Rawlins, & O’Keefe, 1982).
The rats were food deprived to 85–90% of their free-feeding weight prior to training on the eight-arm radial maze. The maze was 220 cm in diameter with a 60-cm diameter central platform. Each arm was 16-cm wide and radiated 80 cm from the center. The maze was wiped with 70% ethanol between trials and rotated 90° every day to discourage the use of internal maze cues. The day before formal training began, each rat received two 10-min shaping trials with all arms baited by placing approximately 0.05 g of a sweetened oatmeal cereal mash (Maypo; International Home Foods) in the sunken food well at the end of each arm. Two rats were on the maze for the first shaping trial; and 1 h later, each rat received a second shaping trail by itself. On training trials, four arms were baited, and the food cups at the ends of the unbaited arms had inaccessible mash to control for odor cues. The locations of baited and unbaited arms were constant for a subject and balanced across subjects. There were 10 training trials on each day. The rat was confined to the center of the maze by a large, overturned transparent bowl prior to each trial. Once released, the rat was free to forage until it consumed all the accessible food, or until 3 min had elapsed. Entry to an arm was scored when the rat crossed the halfway point of an arm. A trial was scored for correct entries, reference memory errors (visits to unbaited arms), and working memory errors (return visits to an arm) (Olton, 1987). Training continued for 6 d (60 trials) to establish a strong memory, or 3 d (30 trials) to produce a memory lasting 1 day but not 1 month. One day or 30 days after training ceased, a single reinforced trial was given to test memory.
2.9. Statistics
Two-population or paired Student’s t tests were performed to compare protein levels as appropriate. For LTP experiments the responses to test stimuli were averaged across 5 min for statistical comparisons. Multi-factor comparisons were performed using ANOVA with repeated measures, as appropriate. The degrees of freedom for the critical t values of the t tests and the F values of the ANOVAs are reported as subscripts. Post-hoc multiple comparisons were performed by Tukey tests as appropriate. Statistical significance was accepted at P < 0.05.
3. Results
3.1. PKMζ persistently increases in spatial long-term memory, but not short-term memory
We first examined changes in the amount of PKMζ in the dorsal hippocampus following active place avoidance conditioning that produces short-term and long-term memory (Fig. 1A). A single 10-min active place avoidance training session produces spatial short-term memory (Supplementary Fig. 1) that requires intact functioning of the dorsal hippocampus (Cimadevilla, Fenton, et al., 2000) and is not disrupted by the PKMζ inhibitor ZIP (Pastalkova et al., 2006). The single training session does not increase PKMζ protein in rat hippocampus (Fig. 1B). In contrast, eight 10-min training trials produce long-term memory that is disrupted by PKMζ inhibitors 1 day after training (Pastalkova et al., 2006; Serrano et al., 2008) (Fig. 1A). The eight training trials induce an increase in PKMζ protein by the last training session that persists for at least 1 day (Fig. 1B). The long-term memory training protocol produces variable memory retention 1 day after training, allowing us to compare memory retention with the increase in PKMζ (Fig. 1B, inset above right). The increases in PKMζ and memory retention significantly correlate, indicating that the increase relates to memory strength, rather than to the retrieval experience itself. Animals trained but not tested for retrieval also show increases in PKMζ 1 day after training (Fig. 1B). In contrast, when the same shock sequence that the trained animals received was delivered independently of the animals’ position (“unavoidable shock controls”), the increases in hippocampal PKMζ were not observed (Fig. 1B), as expected in the absence of the expression of conditioned fear or learned helplessness (as shown in behavioral data presented in Video 1, Supplementary Fig. 2). As in late-LTP (Hernandez et al., 2003; Kelly, Crary, & Sacktor, 2007), the increase of hippocampal PKMζ after learning occurs by translation of PKMζ protein from unchanging amounts of hippocampal PKMζ mRNA, as measured 6 h after training by qRT-PCR (controls, set at 100 ± 3%; trained, 99 ± 4%; n’s = 9; t16 = 0.17; P = 0.87).
Fig. 1.
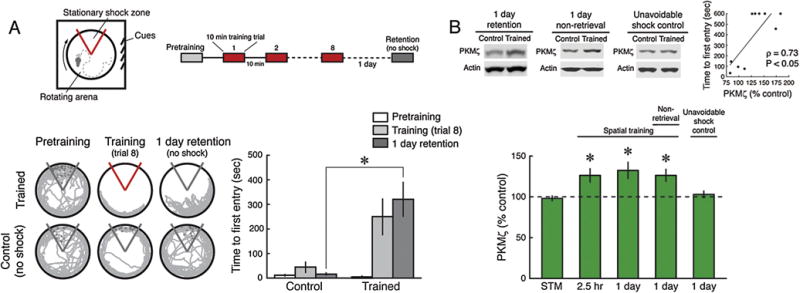
Persistent increased PKMζ in spatial long-term memory. (A) Inset above left, schematic of the active place avoidance training apparatus. The animal is placed on a slowly rotating disk (circle) with a stationary shock zone (red sector), which the animal learns to avoid by attending to cues in the room (square). Inset above right, schematic representation of the 8-trial training protocol with 1-day memory retention testing. Below left, representative 5 min paths. Control animals are placed in the apparatus without conditioning. Below right, mean ± SEM measure of active place avoidance behavior for rats. There is a significant effect of training phase (pretraining, training, retention) (F2,34 = 10.18, P < 0.001), treatment (control and trained) (F1,17 = 10.25, P < 0.01), as well as their interaction (control, n = 8, trained, n = 11, F2,34 = 8.42, P < 0.001). The 1-day retention performance is significantly different (*, Tukey post hoc test, P < 0.01). (B) PKMζ in dorsal hippocampus increases 1 day after 8-trial training. Insets above left, representative immunoblots of PKMζ (Mr = ∼55 kDa); below, mean ± SEM, compared to controls set at 100%. Short-term memory (STM) induced by a single 10 min training session does not increase PKMζ (n’s = 6, t10 = 0.42, P = 0.68). Massed training of eight 10-min sessions, lasting 2.5 h, increases PKMζ by the end of training (n’s = 6, t10 = 2.57, P < 0.05). The increase persists 1 day with retention testing (control, n = 8, trained, n = 10, t16 = 2.13, P < 0.05) and without retention testing (n’s = 8, t14 = 2.62, P < 0.05). The same pattern of unavoidable shocks produces no increase in PKMζ (n’s = 8, t14 = 0.45, P = 0.66). Inset above right, PKMζ protein correlates with 1-day memory strength (100% is mean PKMζ in untrained controls; Spearman’s correlation ρ and P values are given in the figure).
Analysis of the complete cohort of PKC isoforms expressed in hippocampus indicates persistent increases of two additional isoforms 1 day after training—the other atypical PKC, PKCι/λ, and the conventional PKC, PKCβI (Supplementary Fig. 3). In contrast to PKMζ, the increases in PKCι/λ and PKCβI do not correlate with long-term memory retention (Supplementary Fig. 3).
3.2. PKMζ-antisense blocks the persistent increase in PKMζ and spatial long-term memory
If the persistent increase in PKMζ is essential for long-term memory, then blocking the increase should prevent the formation of long-term memory. To test this prediction, we examined the effect of acute applications of PKMζ-antisense oligodeoxynucleotides (Fig. 2A, above) (Tsokas et al., 2016). We first validated the efficacy of the PKMζ-antisense during LTP in rat hippocampal slices. The PKMζ-antisense blocks the increase of PKMζ in LTP and not other LTP-induced gene products: the other atypical PKC isoform, PKCι/λ, which increases transiently in LTP (Kelly et al., 2007; Osten et al., 1996; Tsokas et al., 2016), and eukaryotic elongation factor 1A (eEF1A) (Tsokas et al., 2005, 2016) (Fig. 2A). The efficacy of PKMζ-antisense in rat is thus similar to its effects in mice (Tsokas et al., 2016), as expected because the sequence of the targeted translational start site is identical in rat and mouse PKMζ mRNAs (Hernandez et al., 2003). The PKMζ-antisense but not the control scrambled oligodeoxynucleotide prevents formation of late-LTP (Fig. 2B).
Fig. 2.
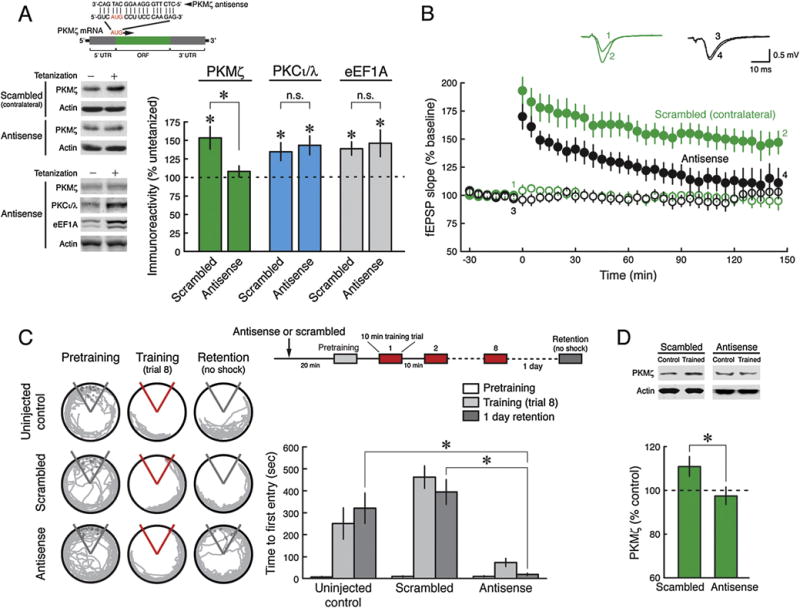
Antisense blockade of new PKMζ synthesis prevents late-LTP and spatial long-term memory. (A) PKMζ-antisense selectively blocks activity-dependent PKMζ synthesis. Inset above, diagram of PKMζ mRNA shows the 5′- and 3′-untranslated regions (UTRs), the open reading frame (ORF), and translation initiation site (AUG). PKMζ-antisense sequence is displayed hybridized with its complementary sense sequence, located between positions −3 and +15 from the translation start site. PKMζ-antisense or scrambled oligodeoxynucleotides are introduced into slices by intracranial hippocampal injection or in bath as described in Section 2. Immunoblots of CA1 extracts from hippocampal slices are probed with antisera to PKMζ, PKCι/λ, eEF1A, and actin as loading control. LTP slices are frozen 30 min after tetanization, and controls from adjacent slices within each hippocampus (set at 100%) receive only test stimulation. Left, representative immunoblots; right, mean ± SEM. PKMζ: scrambled, tetanized vs. untetanized, n’s = 18, t17 = 3.49, P < 0.005; PKMζ-antisense, tetanized vs. untetanized, n’s = 18, t17 = 1.07, P = 0.30; PKMζ-antisense vs. scrambled, t34 = 2.70, P < 0.02; PKCι/λ: scrambled, tetanized vs. untetanized, n’s = 14, t13 = 2.76, P < 0.02; PKMζ-antisense, tetanized vs. untetanized, n’s = 6, t5 = 3.41, P < 0.02; PKMζ-antisense vs. scrambled, t18 = 0.42, P = 0.68; eEF1A: scrambled, tetanized vs. untetanized, n’s = 14, t13 = 4.04, P< 0.002; PKMζ-antisense, tetanized vs. untetanized, n’s = 14, t13 = 2.52, P<0.05; PKMζ-antisense vs. scrambled, t26 = 0.37, P = 0.71. (B) Late-LTP is blocked by PKMζ-antisense, but not by scrambled oligodeoxynucleotide injected in the contralateral hippocampus. Above, numbered, color-coordinated representative field excitatory postsynaptic potential (fEPSP) traces correspond to time points noted below. Below, filled black circles, antisense (n = 7); filled green circles, scrambled (n = 6); color-coordinated open circles are untetanized control pathways recorded within each slice (for average of fEPSPs at 145–150 post-tetanization, two-way ANOVA, oligodeoxynucleotide: F1,28 = 16.2, P = 0.002; stimulation: F1,28 = 39.2, P< 0.0001; interaction: F1,28 = 10.0, P< 0.05). (C) PKMζ-antisense blocks long-term memory formation. Left, representative paths. Right, mean ± SEM of active place avoidance training and 1-day memory retention. There is a significant effect of training phase (pretraining, training, retention) (F2,64 = 38.26, P < 0.001), treatment (uninjected, scrambled, antisense) (F2,32 = 21.29, P < 0.001), as well as their interaction (F4,64 = 9.02, P< 0.001). Retention in the antisense group is worse than each of the other groups (uninjected control, n = 11, scrambled, n = 13, antisense, n = 11, * indicates significance by Tukey post hoc test, both P’s < 0.01). (D) PKMζ antisense blocks training-induced PKMζ synthesis measured 1 day after 8-trial training. Rats were treated in trained and untrained pairs, and PKMζ in each trained rat was normalized by the amount in the untrained animal. Above, representative immunoblots; below, mean ± SEM. Scrambled, untrained vs. trained, n’s = 11, t10 = 2.56, P<0.05; PKMζ-antisense, untrained vs. trained, n’s = 7, t6 = 0.65, P = 0.54; PKMζ-antisense vs. scrambled, t16 = 2.16, P < 0.05.
To determine the effects of PKMζ-antisense on the persistent increase in PKMζ induced by spatial conditioning and long-term memory, bilateral intrahippocampal injections of PKMζ-antisense or scrambled oligodeoxynucleotides were made in separate animals (Fig. 2C and D; Supplementary Fig. 4A). PKMζ-antisense but not the control scrambled oligodeoxynucleotide blocks the increase of PKMζ observed during long-term memory at 1 day and disrupts the formation of 1-day long-term memory (Fig. 2C and D). The acute infusion of PKMζ-antisense does not affect the basal amounts of PKMζ, consistent with the relatively long half-life of the basal kinase (Osten et al., 1996; Tsokas et al., 2016) (Supplementary Fig. 4B). In an additional control for nonspecific effects, we found the intrahippocampal injections of PKMζ-antisense do not disrupt hippocampus-dependent shortterm memory for active place avoidance (Supplementary Fig. 5B).
3.3. PKMζ persistently increases in remote spatial memory
We next examined whether spatial memories are accompanied by persistent increases of hippocampal PKMζ for very long periods of time, recognizing that the alterations may be complex due to systems-level consolidation. Spaced training by two 8-trial training sessions separated by 1 week produces remote memory that is disrupted by PKMζ inhibition in the hippocampus 1 month after training (Pastalkova et al., 2006) (Fig. 3A). We found that, like the dependence on persistent increased PKMζ activity, the increase in PKMζ protein in the hippocampus induced by spaced training persists for 1 month (Fig. 3B). In contrast, a single 8-trial training session produces memory that lasts for 1 day (Fig. 1A), but not 1 month (Fig. 3C), and the initial persistent increase in PKMζ observed at 1 day (Fig. 1B) returns to baseline by 1 month (Fig. 3D).
Fig. 3.
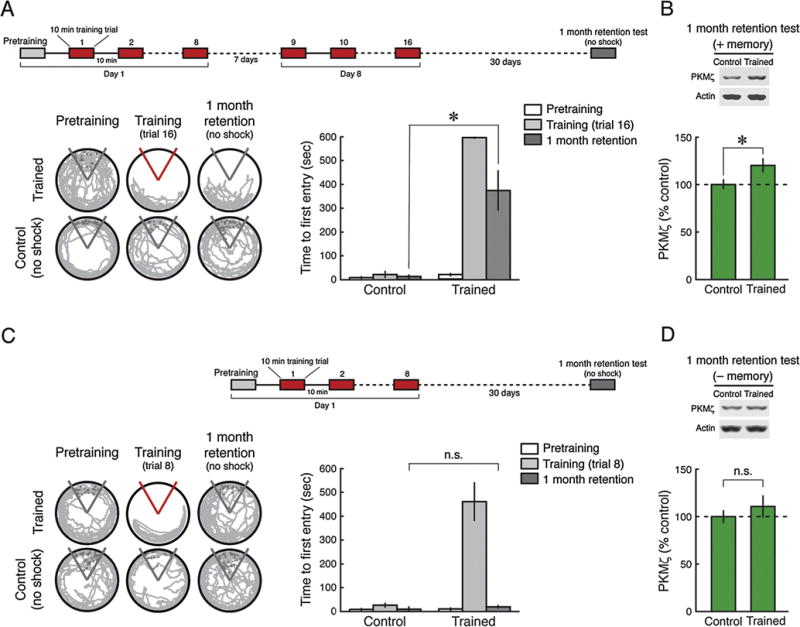
Increased PKMζ persists in dorsal hippocampus 1 month after spaced active place avoidance training. (A) Inset above, schematic representation of training protocol consisting of two 8-trial training sessions spaced 1 week apart with 30-day memory retention testing. Left, representative 5 min paths before training, at the end of training, and during retention testing with the shock off 1 month after spaced training. Control animals are placed in the apparatus without conditioning. Right, mean ± SEM, measure of active place avoidance behavior 1 month after spaced training. There is a main effect of treatment (control, trained) (F1,10 = 125.56, P < 0.0001), training phase (pretraining, training, retention) (F2,20 = 37.63, P < 0.0001), as well as their interaction (F2,20 = 34.03, P < 0.0001). The retention performance differs (*, significant post hoc Tukey HSD test, n’s = 6, P < 0.01). (B) Above, representative immunoblots; below, mean ± SEM, showing increases in dorsal hippocampal PKMζ 1 month after spaced training (n’s = 6, t10 = 2.38, P < 0.05). (C and D) After 1 month, memory induced by a single 8-trial massed training session fades, and the amount of PKMζ in dorsal hippocampus is indistinguishable from that of untrained controls. (C) Left, representative 5 min paths before training, at the end of training, and during retention testing with the shock off 1 month after massed training. Control rats are placed in the apparatus without conditioning. Right, mean ± SEM measure of active place avoidance behavior 1 month after massed training. There is a main effect of treatment (control, trained) (F1,13 = 31.46, P < 0.0001), training phase (pretraining, training, retention) (F2,26 = 40.71, P < 0.0001), and interaction between treatment and training phase (pretraining, training, retention) (F2,26 = 35.41, P < 0.0001). There is no significant difference between the two groups on 1-month retention testing (control, n = 8, trained, n = 7, P = 0.99; n.s., no significance). (D) Immunoblots shows no significant difference in the amount of dorsal hippocampal PKMζ between control and 1 month after massed training. Above, representative immunoblots. Below, mean ± SEM (control, n = 8, trained, n = 7, t13 = 0.87, P = 0.40).
We then examined remote spatial memory produced by radial arm maze conditioning, a slowly acquired appetitive spatial task. PKMζ inhibition in the hippocampus disrupts reference memory in the radial arm maze, which requires information about which arm locations are consistently baited across trials, but does not affect working memory, which requires information relevant to which arms are visited within a specific trial (Serrano et al., 2008). Radial arm maze conditioning over 6 days produces reference memory that lasts from 1 day to at least 1 month (Fig. 4A). Likewise, 6 days of conditioning induces an increase in hippocampal PKMζ that persists from 1 day to 1 month (Fig. 4B). In contrast, 3 days of radial arm maze conditioning produces long-term memory that persists for 1 day but not for 1 month (Fig. 4C). The 3 days of training produce increases in PKMζ that persist for 1 day but not for 1 month (Fig. 4D), coinciding with the persistence of the memory.
Fig. 4.
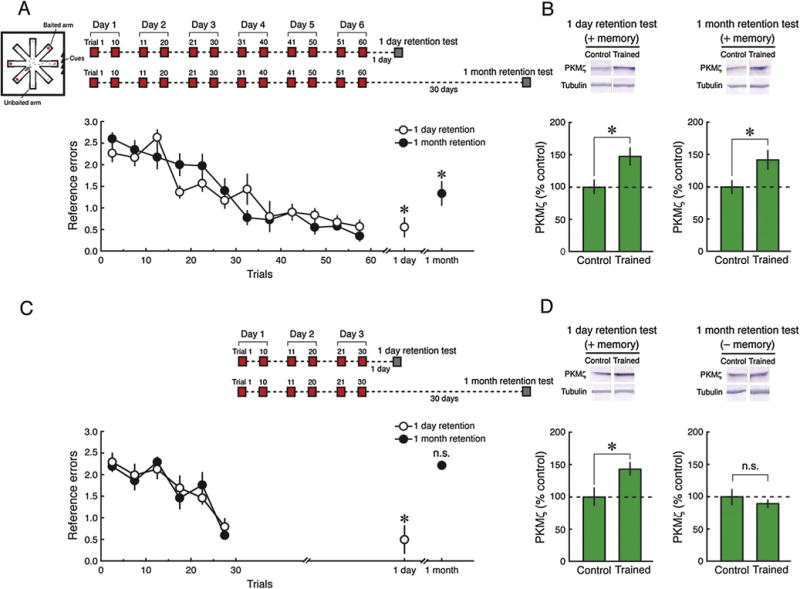
Increased PKMζ persists in dorsal hippocampus 1 month after radial arm maze conditioning. (A) Inset above left, schematic representation of radial arm maze with 4 of 8 arms baited. The baited and empty arms remain the same throughout the training and retention testing. Inset above right, schematic diagram of 6-day training protocol producing 1-month memory. Training consists of 10 trials per day for 6 days, and retention testing is either 1 day or 1 month later. Below, decrease of reference memory errors over 6 days of training and for both 1 day and 1 month after training; mean ± SEM. There is a main effect of training phase (beginning of training, end of training, retention) (F2,24 = 62.83, P < 0.0001), and interaction between group (1 day retention, 1 month retention) and training phase (F2,24 = 3.68, P < 0.05). Tukey post hoc tests reveal that the reference errors in the retention test are significantly lower than at the beginning of training (1 day retention, n = 6, P < 0.001; 1 month retention, n = 8, P < 0.001). (B) Above, representative immunoblots; below, mean ± SEM, showing increases in dorsal hippocampal PKMζ both 1 day and 1 month after 6 days of training (1 day post-training: n’s = 6, t10 = 2.76, P < 0.05; 1 month post-training: control, n = 6, trained, n = 8, t12 = 2.23, P < 0.05). (C and D) Memory and increased dorsal hippocampal PKMζ induced by 3 days of radial arm training persist for 1 day but not for 1 month. (C) Inset above, schematic diagram of 3-day training protocol producing memory retention for 1 day but not 1 month. Training consists of 10 trials per day for 3 days, and retention testing is either 1 day or 1 month later. Below, decreases of reference memory errors during training and for 1 day but not 1 month memory after training; mean ± SEM. There is a main effect of group (1 day retention, 1 month retention) (F1,10 = 10.74, P < 0.01), training phase (beginning of training, end of training, retention) (F2,20 = 34.08, P < 0.0001), and interaction between group and training phase (F2,20 = 16.49, P < 0.0001). Tukey post hoc tests reveal that reference errors are significantly lower than at the beginning of training in the 1-day retention test (n = 6, P < 0.001), but not in the 1-month retention test (n = 6, P = 0.99). (D) Above, representative immunoblots; below, mean ± SEM, showing increases in dorsal hippocampal PKMζ at 1 day after 3-day training, but no significant change at 1 month (1 day post-training: control, n = 6, trained, n = 5, t9 = 2.42, P < 0.05; 1 month post-training: control, n = 5, trained, n = 6, t9 = 0.82, P = 0.44).
4. Discussion
PKMζ was first identified as an autonomously active, atypical PKC isoform that persistently increases in LTP maintenance (Osten et al., 1996; Sacktor et al., 1993). Because atypical PKC inhibitors reverse both LTP maintenance and long-term memory (Cai et al., 2011; Drier et al., 2002; Ling et al., 2002; Pastalkova et al., 2006; Serrano, Yao, & Sacktor, 2005; Serrano et al., 2008; Shema et al., 2007, 2011), we hypothesized that PKMζ might also persistently increase in the maintenance of memory. Here, we find that spatial training induces an increase of PKMζ in the dorsal hippocampus that persists from the end of conditioning to at least one month, the longest time point tested and to our knowledge far longer than any other known learning-induced gene product.
The persistent increases in PKMζ during LTP maintenance and memory storage have similar properties. Activity-dependent, de novo synthesis of PKMζ is critical for formation of the persistent increases in both LTP and memory, and this new synthesis is required for both the sustained synaptic potentiation and the behavioral modification (Tsokas et al., 2016) (Fig. 2). The increases of PKMζ occur specifically during long-term but not short-term forms of synaptic potentiation (Osten et al., 1996), and likewise the increases of PKMζ in dorsal hippocampus occur specifically during long-term but not short-term forms of memory (Fig. 1B, Supplementary Fig. 5B). These data, together with evidence that short-term memory is not affected by the PKMζ-antisense (Supplementary Fig. 5A), indicate new synthesis of PKMζ does not contribute to short-term memory. But because the relatively brief exposure of the PKMζ-antisense does not affect basal levels of the kinase in hippocampus (Supplementary Fig. 4B), these experiments do not exclude the possible functioning of pre-existing PKMζ in short-term memory. In LTP maintenance the persistent increase of PKMζ correlates with the degree of synaptic potentiation (Osten et al., 1996). Likewise, in spatial memory maintenance the persistent increase in PKMζ correlates with the extent of memory retention (Fig. 1B). No testing of the memory is required for the sustained increase in PKMζ, consistent with the putative role of the persistent kinase in information storage and not retrieval (Fig. 1B). Similar to LTP (Kelly et al., 2007), PKMζ protein increases in long-term memory without changes in mRNA levels. Therefore, we speculate that spatial conditioning causes PKMζ increases through new synthesis from pre-existing dendritic PKMζ mRNA (Muslimov et al., 2004) located at or near the specific synapses that are strongly activated during conditioning (Hernandez, Oxberry, Crary, Mirra, & Sacktor, 2014; Hernandez et al., 2003). The persistent local increases in the autonomously active kinase then maintain increased postsynaptic AMPAR-mediated synaptic transmission at these specific synapses by, for example, increasing the trafficking of GluA2 to postsynaptic sites through the action of N-ethylmaleimide sensitive factor (NSF) (Yao et al., 2008; Migues et al., 2010), so as to persistently modify the networks of neurons activated during learning to facilitate memory storage (Sacktor, 2011). The ability to measure persistent increases in hippocampal PKMζ after only a brief period of training suggests the possibility that the basal state of the dorsal hippocampus of naïve, caged-reared animals contains relatively low levels of PKMζ and few long-term memories stored by the kinase prior to our experimental conditioning.
When repeated training produces remote memories lasting 1 month, the persistent increases in PKMζ induced by the training also last 1 month. Both aversive active place avoidance and appetitive radial arm maze conditioning produce spatial memories that persist at least 1 month and parallel month-long increases in PKMζ, indicating that the persistent increases are not specific to the type of memory nor the valence of the reinforcement during the conditioning. These increases in PKMζ during remote memory maintenance persist far longer than the increases of c-Fos (Kovacs, 2008) or Arc (Shepherd & Bear, 2011), which last for only hours after experience, and may equal or exceed even the long-lived increase of ΔFosB, a transcription factor that persistently increases for at least 1 day after training (Eagle et al., 2015) and for several weeks after chronic exposure to drugs of abuse (Nestler, 2008). Active place avoidance training modifies dentate gyrus responses to neocortical stimulation of the perforant path measured 1 day after training in vivo (Park, Burghardt, Dvorak, Hen, & Fenton, 2015), and extended active place avoidance training produces persistent modifications in synaptic circuitry in the CA3-CA1 pathway that can last 1 month (Pavlowsky, Wallace, Fenton, & Alarcon, 2017). Like the persistent increases in PKMζ (Fig. 1B), these persistent alterations in synaptic function do not require memory retrieval (Pavlowsky et al., 2017). These very long-term alterations in synaptic function coincide with the storage of remote memory because they are not detected in animals with poor memory recall at 1 month (Pavlowsky et al., 2017). Likewise, we find that following training protocols that produce 1-day long-term memory that decays by 1 month, the initial increase in PKMζ observed at 1 day returns to baseline by 1 month (Figs. 1, 3, and 4).
These results suggest that distinct molecular mechanisms might maintain PKMζ for different lengths of time to sustain long-term memories of varying durations. Several putative mechanisms for maintaining PKMζ have been proposed, including positive feedback loops at the levels of PKMζ mRNA transcription (Chen et al., 2014; Hernandez et al., 2014; Ko et al., 2016), translation of the PKMζ message (Fiumara et al., 2015; Jalil, Sacktor, & Shouval, 2015; Westmark et al., 2010), and PKMζ protein stability (Sacktor, 2011). How these mechanisms become engaged during memory formation and thus sustain PKMζ during memory maintenance are fundamental questions for elucidating how memories are stored.
5. Conclusions
Persistent increases of the autonomously active PKMζ sustain the kinase’s action during long-term and remote spatial memory maintenance.
Supplementary Material
Acknowledgments
TCS is supported by United States NIH funding 2R37 MH057068, RO1 MH53576, RO1 DA034979 (with HS), and the Lightfighter Trust. AAF is supported by United States NIH grants R01 MH084038, R01 MH099128, R01 AG043688, and United States NSF IOS-1146822. PT is an Alexander S Onassis Public Benefit Foundation Scholar.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nlm.2016.07.008.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Authors’ contributions
All authors shared in the conception and design, acquisition of data, analysis and interpretation of data, and drafting or revising the article.
References
- Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. Journal of Neuroscience Methods. 2007;162:346–356. doi: 10.1016/j.jneumeth.2006.12.018. http://dx.doi.org/10.1016/j.jneumeth.2006.12.018, pii: S0165-0270(07)00002-7. [DOI] [PubMed] [Google Scholar]
- Cai D, Pearce K, Chen S, Glanzman DL. Protein kinase M maintains long-term sensitization and long-term facilitation in Aplysia. Journal of Neuroscience. 2011;31:6421–6431. doi: 10.1523/JNEUROSCI.4744-10.2011. http://dx.doi.org/10.1523/JNEUROSCI.4744-10.2011, pii: 31/17/6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Cai D, Pearce K, Sun PY, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife. 2014;3:e03896. doi: 10.7554/eLife.03896. http://dx.doi.org/10.7554/eLife.03896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Fenton AA, Bures J. Functional inactivation of dorsal hippocampus impairs active place avoidance in rats. Neuroscience Letters. 2000;285:53–56. doi: 10.1016/s0304-3940(00)01019-3. [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Kaminsky Y, Fenton A, Bures J. Passive and active place avoidance as a tool of spatial memory research in rats. Journal of Neuroscience Methods. 2000;102:155–164. doi: 10.1016/s0165-0270(00)00288-0. [DOI] [PubMed] [Google Scholar]
- Drier EA, Tello MK, Cowan M, Wu P, Blace N, Sacktor TC, Yin JC. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nature Neuroscience. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- Eagle AL, Gajewski PA, Yang M, Kechner ME, Al Masraf BS, Kennedy PJ, Robison AJ. Experience-dependent induction of hippocampal DeltaFosB controls learning. Journal of Neuroscience. 2015;35:13773–13783. doi: 10.1523/JNEUROSCI.2083-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.2083-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumara F, Rajasethupathy P, Antonov I, Kosmidis S, Sossin WS, Kandel ER. MicroRNA-22 gates long-term heterosynaptic plasticity in aplysia through presynaptic regulation of CPEB and downstream targets. Cell Reports. 2015;11:1866–1875. doi: 10.1016/j.celrep.2015.05.034. http://dx.doi.org/10.1016/j.celrep.2015.05.034. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. Journal of Neuroscience. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1674-06.2006, pii: 26/30/7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Sacktor TC. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. Implications for the molecular mechanism of memory. Journal of Biological Chemistry. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. http://dx.doi.org/10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Oxberry WC, Crary JF, Mirra SS, Sacktor TC. Cellular and subcellular localization of PKMzeta. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2014;369:20130140. doi: 10.1098/rstb.2013.0140. http://dx.doi.org/10.1098/rstb.2013.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalil SJ, Sacktor TC, Shouval HZ. Atypical PKCs in memory maintenance: The roles of feedback and redundancy. Learning and Memory. 2015;22:344–353. doi: 10.1101/lm.038844.115. http://dx.doi.org/10.1101/lm.038844.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MT, Crary JF, Sacktor TC. Regulation of protein kinase Mζ synthesis by multiple kinases in long-term potentiation. Journal of Neuroscience. 2007;27:3439–3444. doi: 10.1523/JNEUROSCI.5612-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.5612-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HG, Kim JI, Sim SE, Kim T, Yoo J, Choi SL, Kaang B-K. The role of nuclear PKMζ in memory maintenance. Neurobiology of Learning and Memory. 2016;135:50–56. doi: 10.1016/j.nlm.2016.06.010. http://dx.doi.org/10.1016/j.nlm.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. Journal of Neuroendocrinology. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. http://dx.doi.org/10.1111/j.1365-2826.2008.01734.x, pii: JNE1734. [DOI] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, Messing RO. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–419. doi: 10.1038/nature11803. http://dx.doi.org/10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mζ enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. http://dx.doi.org/10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nature Neuroscience. 2002;5:295–296. doi: 10.1038/nn829. http://dx.doi.org/10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMζ maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nature Neuroscience. 2010;13:630–634. doi: 10.1038/nn.2531. http://dx.doi.org/10.1038/nn.2531, pii: nn.2531. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muslimov IA, Nimmrich V, Hernandez AI, Tcherepanov A, Sacktor TC, Tiedge H. Dendritic transport and localization of protein kinase Mζ mRNA: Implications for molecular memory consolidation. Journal of Biological Chemistry. 2004;279:52613–52622. doi: 10.1074/jbc.M409240200. http://dx.doi.org/10.1074/jbc.M409240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, Sacktor TC. Distribution of protein kinase Mζ and the complete protein kinase C isoform family in rat brain. Journal of Comparative Neurology. 2000;426:243–258. doi: 10.1002/1096-9861(20001016)426:2<243::aid-cne6>3.0.co;2-8. http://dx.doi.org/10.1002/1096-9861(20001016)426:2<243::AID-CNE6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: Role of DeltaFosB. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. http://dx.doi.org/10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner B, Single FN, Hvalby O, Jensen V, Meyer Zum Alten Borgloh S, Seeburg PH, Bannerman DM. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. European Journal of Neuroscience. 2007;25:837–846. doi: 10.1111/j.1460-9568.2007.05312.x. http://dx.doi.org/10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physiology & Behavior. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space and memory. Behavioral and Brain Sciences. 1979;2:313–365. [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC. Protein synthesis-dependent formation of protein kinase Mζ in long-term potentiation. Journal of Neuroscience. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA. Experience-dependent regulation of dentate gyrus excitability by adult-born granule cells. Journal of Neuroscience. 2015;35:11656–11666. doi: 10.1523/JNEUROSCI.0885-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.0885-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson SJ, Le Good JA, Whelan RD, Whitehead P, Parker PJ. Identification of PKCzetaII: An endogenous inhibitor of cell polarity. EMBO Journal. 2004;23:77–88. doi: 10.1038/sj.emboj.7600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. http://dx.doi.org/10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pavlowsky A, Wallace EJ, Fenton AA, Alarcon JM. Persistent modifications of hippocampal synaptic function during remote spatial memory. Neurobiology of Learning and Memory. 2017;138:182–197. doi: 10.1016/j.nlm.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin O, Allen K, Thibaudeau G, Dore FY, Goulet S. Performance on spatial working memory tasks after dorsal or ventral hippocampal lesions and adjacent damage to the subiculum. Behavioral Neuroscience. 2006;120:413–422. doi: 10.1037/0735-7044.120.2.413. http://dx.doi.org/10.1037/0735-7044.120.2.413. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMζ maintain long-term memory? Nature Reviews Neuroscience. 2011;12:9–15. doi: 10.1038/nrn2949. http://dx.doi.org/10.1038/nrn2949, pii: nrn2949. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. http://dx.doi.org/10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Fenton AA. PKMζ maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biology. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. http://dx.doi.org/10.1371/journal.pbio.0060318, pii: 08-PLBI-RA-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. Journal of Neuroscience. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. http://dx.doi.org/10.1126/science.1200215, pii: 331/6021/1207. [DOI] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nature Neuroscience. 2011;14:279–284. doi: 10.1038/nn.2708. http://dx.doi.org/10.1038/nn.2708, pii: nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. Journal of Neuroscience. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. http://dx.doi.org/10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Hsieh C, Yao Y, Lesburgueres E, Wallace EJ, Tcherepanov A, Sacktor TC. Compensation for PKMzeta in long-term potentiation and spatial long-term memory in mutant mice. Elife. 2016;5 doi: 10.7554/eLife.14846. http://dx.doi.org/10.7554/eLife.14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. Journal of Neuroscience. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4548-06.2007, pii: 27/22/5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–423. doi: 10.1038/nature11802. http://dx.doi.org/10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NE, O’Brian CA. Kinetic analysis of protein kinase C inhibition by staurosporine: Evidence that inhibition entails inhibitor binding at a conserved region of the catalytic domain but not competition with substrates. Molecular Pharmacology. 1992;41:387–392. [PubMed] [Google Scholar]
- Westmark P, Cj W, Wang S, Levenson J, Kj OR, Burger C, Malter J. Pin1 and PKMζ sequentially control dendritic protein synthesis. Science Signaling. 2010;3:ra18. doi: 10.1126/scisignal.2000451. http://dx.doi.org/10.1126/scisignal.2000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Sacktor TC. PKMζ maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. Journal of Neuroscience. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0223-08.2008, pii: 28/31/7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


