Abstract
Background
Allostatic load (AL) measures the cumulative impact of chronic stress and is associated with adverse health outcomes. A novel scoring system has previously been developed for AL in early pregnancy that is associated with pre-eclampsia. It was hypothesized that AL, as identified by the present model, is associated with psychosocial stressors and, specifically, poor sleep quality.
Methods
Women were selected from a low-risk, community-dwelling study population who enrolled at <15 weeks gestation. Nine physiologic components were divided among the domains of cardiovascular, metabolic, and inflammatory function. Spearman’s rank correlations were used to examine the association of AL with age, income, the Revised Prenatal Distress Questionnaire (NuPDQ), Inventory of Depressive Symptoms (IDS), and Pittsburgh Sleep Quality Index (PSQI). The Wilcoxon rank-sum test was used to compare AL by race and educational attainment.
Results
A total of 103 women were identified, with: a mean age of 29.8 ± 5.0 years, 17.5% black, and mean gestational age 12.2 ± 1.1 weeks. Allostatic load was positively correlated with the PSQI (ρ=0.23, p=0.018). There were no associations with age, income, prenatal distress, race, or depression scores. College-educated women had lower AL compared with those with less education (0.57 ± 0.43 vs 0.81 ± 0.55, p=0.045).
Conclusion
Higher AL, measured by the pregnancy-specific model, was associated with poorer sleep quality and lower educational attainment, both of which were considered to be chronic stressors. These relationships were consistent with previous findings in non-pregnant populations, and suggest that AL may be useful for capturing the physiologic impact of chronic stress in early pregnancy.
Keywords: Allostatic load, Pregnancy, Psychosocial stress, Sleep quality, Pittsburgh Sleep Quality Index (PSQI)
Introduction
Allostatic load (AL) is a measure of the cumulative impact of chronic stress, and represents the ‘wear and tear’ the body experiences when repeated allostatic responses are activated during stressful situations. The allostatic load model incorporates multiple subclinical physiologic parameters into a single index score of risk for AL [1, 2]. It is often represented by changes in primary mediators, such as stress hormones and inflammatory cytokines, and secondary mediators subsequently, including metabolic and cardiovascular parameters. The final stage, allostatic overload, is identified at the culmination of physiological dysregulation resulting in disorder or disease. A caveat of studies using AL models is that not all indices/measures are always available to enter into the model [1]; this limitation may obscure the ability to compare studies [3].
Higher AL is associated with adverse health outcomes, including: cardiovascular disease, impaired cognitive and physical functioning, and 5-year mortality [4]. It is known to be higher among individuals who are considered to experience chronic psychosocial stress, including African Americans and individuals of lower socioeconomic status [5–7]. Allostatic load is also higher in individuals with more subtle chronic stressors, such as individuals living in crowded or substandard housing and those who lack characteristics of resilience [8, 9]. Additionally, sleep is thought to modify the impact of chronic stress, and poor sleep quality and sleep deprivation are considered to be chronic stressors [10, 11]. In many ways, these previous findings demonstrate that AL may provide a biologically plausible mechanism and capture the physiologic cumulative wear and tear of chronic stress that may lead to higher rates of adverse health outcomes.
The majority of studies of AL have focused on non-pregnant populations. However, the allostatic load model is also plausible in the study of adverse pregnancy outcomes. Despite the numerous associations of AL with adverse health outcomes, few studies have examined AL measured during pregnancy and with mixed results [12–15]. Wallace and colleagues, for instance, did not find that AL predicted low birth weight or preterm birth in the models that included race and neighborhood poverty level. Then again, they did not have measures of psychosocial stress in their study [15].
The present authors previously developed a novel model of AL in early pregnancy that demonstrated an association between higher AL scores and increased odds of developing pre-eclampsia; it used data collected in the Prenatal Exposures and Pre-eclampsia Prevention (PEPP) study at the University of Pittsburgh, 1997–2001 [12]. This model included biomarkers that are representative of the metabolic, cardiovascular, and inflammatory domains of AL measured in early pregnancy, defined as <15 weeks gestation. This novel first study of AL was limited both in its size and study design. Specifically, the use of a matched case control did not allow completion of a high-quality analysis of race and socioeconomic status. The assessment of study participants in the PEPP study also did not include detailed survey measures of psychosocial stress. Pregnancy itself is a dynamic state during which typical markers of AL may vary widely from a non-pregnant state and from one time point in gestation to another. A pregnancy-specific model of AL cognizant of the physiologic changes that occur during pregnancy and of the timing of sample collection is essential to the study of AL in pregnancy. Therefore, in order to ensure that this model captured AL and not solely pre-eclampsia risk, further investigation was necessary.
The present study aimed to validate this previously developed novel model of AL in early pregnancy by evaluating the associations between AL scores and subjective measures of stress by validated questionnaires and proxies of chronic stress, including race/ethnicity, sleep quality, and socioeconomic status. It was hypothesized that higher AL scores would be associated with higher levels of subjectively measured psychosocial stress, as well as well-accepted proxies of chronic stress.
Methods
Study population
A subset of women was identified who were enrolled in the Sleep in Pregnancy Study (SLIP) at the University of Pittsburgh [16, 17]. Women in this study were recruited from the greater Pittsburgh area. Participants were recruited from self-referral, physician referrals, local advertisements or via participation in the University research registries. Women were eligible if they were 10–14 weeks pregnant, having a singleton pregnancy, and between the ages of 18–45 years. Only women intending to deliver were enrolled. Exclusion criteria included self-report of psychopathology, sleep disorders, or current pharmacological/therapeutic treatment for depression. In addition, women with pre-existing diabetes, HIV or uterine abnormalities were excluded. Physiological screening for sleep-disordered breathing or restless legs syndrome/periodic limb movements during sleep was not conducted.
The University of Pittsburgh Institutional Review Board granted approval for this study (# 08010142). All participants provided written, informed consent prior to participation, and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Participants in the parent study completed questionnaires, and provided fasting blood samples at the three times points (~12, 16 and 20 weeks gestation). Eligible participants (N=103) for inclusion in this analysis had available plasma samples collected at approximately 12 weeks gestation and had completed the questionnaires in the first cycle (12 weeks estimated gestational age). Pregnancy and delivery outcomes were ascertained from the medical record following delivery.
Procedures
Measures of stress
Stress was captured using both well-known proxies of stress and subjective measures of stress. Socioeconomic status and self-reported race/ethnicity were included as proxies of chronic stress that are known to be associated with AL [1, 6, 7]. Socioeconomic status was considered to be multidimensional and measured in two ways. A continuous socioeconomic index variable was generated by averaging the component scores of two variables: income and education [18]. Participants first reported income and education as categorical variables. To determine the component score for each variable, a cumulative percentage distribution for each was computed, and the midpoint of the percentage interval for each level of the variable was used as the score for that level [19]. The cumulative distribution for income and education was based on nationally representative data from the pregnant women in the National Health and Nutrition Examination Surveys (NHANES) 1999–2006, and from women giving birth in the state of Pennsylvania in 2011, respectively. The Bureau of Health Statistics and Research, Pennsylvania Department of Health provided the Pennsylvania state birth data. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. The final socioeconomic index score was obtained by these two cumulative percentage distribution scores and dividing by 10, as described by Bodnar et al. [18]. Socioeconomic status was also captured by examining educational attainment as self-reported education level; this was used as a binary variable (college educated vs non-college education) to divide the population.
Several subjective measures of acute and chronic stress, which were administered at the 10–12 week visit, were also included. For acute stress, several validated measures were used: the Inventory of Depressive Symptoms (IDS) [20] rates the nine criterion symptom domains (0–27) needed to diagnose a major depressive episode by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); the Revised Prenatal Distress Questionnaire (NuPDQ) [21] assesses pregnancy-related distress. Participants are asked to indicate if they are currently feeling bothered, upset, or worried about different aspects of pregnancy on a 3-point scale ranging from “not at all” (0) to “very much” (2). An average pregnancy-specific distress score is calculated for each respondent by summing item responses and dividing by the total number of items. Pregnancy-specific distress scores range from 0–2; the Perceived Stress Scale (PSS) [22] assesses the degree to which situations in one’s life are appraised as stressful during the last month. It is one of the most commonly administered subjective stress questionnaires. Scores range from 0 (no stress) to 40 (very stressed). The present study also included the Pittsburgh Sleep Quality Index (PSQI), which is an 18-item questionnaire used to measure sleep quality complaints. Seven component scores assess habitual duration of sleep, nocturnal sleep disturbances, sleep latency, sleep quality, daytime dysfunction, sleep medication usage, and sleep efficiency. The seven components (range 0–3) are summed to yield a measure of global sleep quality with a range of 0 (good sleep quality) to 21 (poor sleep quality) [23]. This was used as a measure of chronic stress, as poor sleep is thought to increase AL [10, 24].
Allostatic load
To capture AL in early pregnancy, the methods described by Hux and Roberts were used, as summarized below [12]. The allostatic load index score was determined for each subject using measurements and lab values from stored plasma samples collected at <15 weeks gestation. To calculate AL, nine components representative of three domains of systemic function were used: systolic blood pressure, diastolic blood pressure, and pulse pressure were used for the cardiovascular domain; pre-pregnancy body mass index (BMI), total cholesterol, high-density lipoprotein (HDL), and triglycerides were used for the metabolic domain; and tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were used for the inflammatory domain. For each component, subjects received a single point for each component in the high-risk range. The domain score was then calculated as the proportion of components that were in the high-risk range for that given domain. High-risk ranges for each component were previously defined by Hux and Roberts [12] using nationally representative data and are listed as part of Table 2. Therefore, each individual domain score was a continuous variable ranging from 0–1. The allostatic load score was a continuous measure derived as the sum of the three domain scores and ranged from 0–3 [12].
Table 2.
Allostatic load scores, individual component high cut-off values, and individual component means and standard deviations.
| Mean ± SD | Median (interquartile range) | High-risk cut-off value | Participant meeting risk (%) | |
|---|---|---|---|---|
| Allostatic load | 0.71 ± 0.52 | 0.58 (0.25–1.17) | - | - |
| Systolic blood pressure, mmHg | 107 ± 12 | 104 (100–114) | ≥115 | 24.3 |
| Diastolic blood pressure, mmHg | 67 ± 9 | 64 (60–72) | ≥69 | 37.9 |
| Pulse pressure, mmHg | 40 ± 9 | 40 (34–46) | ≥55 | 6.8 |
| BMI, kg/m2 | 25.7 ± 5.9 | 23.5 (21.6–27.9) | ≥30 | 21.4 |
| Triglycerides, mg/dL | 118 ± 59 | 102 (75–144) | ≥118 | 36.9 |
| Cholesterol, mg/dL | 169 ± 27 | 165 (149–188) | ≥185 | 27.2 |
| HDL, mg/dL | 61 ± 13 | 61 (54–68) | <50 | 16.5 |
| TNF-alpha, μg/mL | 1.8 ± 1.9 | 0.63 (0.27–3.31) | ≥3.316 | 23.3 |
| IL-6, pg/mL | 0.19 ± 0.44 | 0.007 (0.0015–0.125) | ≥0.145 | 22.3 |
High-risk values were determined using empirical and national survey data of values obtained during the first trimester of pregnancy and study-specific data when this information was not available in a national dataset. Excluding inflammatory markers, the same high-risk values as those used in the previous study were used [12]. Briefly, high-risk quartiles were determined using weighted 75th or 25th (HDL only) percentiles from NHANES from 1999–2006 for systolic blood pressure, diastolic blood pressure, pulse pressure, total cholesterol, HDL, and triglycerides. The present study used data in NHANES that was obtained from women with positive urine pregnancy tests who self-reported being between 1–3 months of gestation. These data were analyzed using an appropriate protocol and variables were weighted, as defined in the NHANES tutorial [25]. Briefly, as the NHANES is a cross-sectional study that uses a complex survey design and weighting of participants from data collected in 2-year cycles, the present study generated an 8-year weighting variable from assigned weights that were incorporated into the statistical analyses in STATA. The World Health Organization guidelines define obesity as a BMI ≥30 kg/m2, which was used for the high-risk cut-off of BMI in the present study. Given variability among different assay kits for inflammatory markers (eg, IL-6 and TNF-α), inflammatory marker data from the study population was used to generate a distribution of values for IL-6 and TNF-α, and used the 75th percentile values for each marker as the high-risk quartile cut-off for calculating AL. These cut-offs are also described alongside results in Table 2.
Biological measures
Systolic blood pressure, diastolic blood pressure, and pulse pressure were obtained from a single measurement at <15 weeks estimated gestational age obtained at the first visit and the time of first blood sample collection. The standard for measure at <15 weeks was previously used for criteria in the first pregnancy-specific model of AL described by Hux and Roberts, and thus has been duplicated in the present study [12]. Pulse pressure was calculated as the difference between systolic and diastolic blood pressures. Pre-pregnancy BMI was determined from self-reported weight and measured height. If pre-pregnancy weight could not be recalled, weight at enrollment was used. Morning blood samples (20 mL) were obtained from participants during the physical examination between 07:00 to 10:00 for assay of IL-6 and TNF-α. The mean time of collection in this study was at 12.2 ± 1.1 weeks. Samples were centrifuged, aliquoted, and stored at −80 ºC until assay. Plasma levels of IL-6 and TNF-α were determined by high-sensitivity enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, Minnesota), according to the manufacturer’s instructions. The range for IL-6 was 0.156–10 pg/mL; and for TNF-α, the range was 0.5–32 pg/mL. All samples were run in duplicate, and the coefficient of variation between samples was 10%. Cholesterol, HDL, and triglyceride were measured by colorimetric assays (Pointe Scientific, Canton, MI). Coefficients of variation (COV) were 6.5%, 4.7%, and 2.3%, respectively.
Statistical analyses
Allostatic load was analyzed as a continuous variable. The distribution for AL in this population was not normally distributed; therefore, using non-parametric approaches in these analyses was used with caution. Spearman’s rank correlations were used to examine the association of AL to age, income, the NuPDQ, socioeconomic index score, the IDS, the PSS and the PSQI. Furthermore, given the well-reported associations between race and AL, and educational status and AL, partial correlations were determined for the NuPDQ, IDS, PSS, and PSQI, adjusting for black race and attainment of a college degree. Spearman’s rank correlations were used, as the distribution of the data was not normal. The AL scores of blacks were compared to those of non-blacks using the Wilcoxon rank-sum test. For educational attainment, the AL scores of women who achieved a college degree or greater were compared to those that did not, using the Wilcoxon rank-sum test. Significance was defined as a p-value of <0.05. All statistical analyses were performed using STATA, Version 12 (College Station, TX).
Results
A total of 103 eligible women were idnetified for inclusion in the study. Women were aged 29.8 ± 5.0 years and were 12.2 ± 1.1 weeks pregnant at the time of the collection of plasma samples. Most women identified their race/ethnicity as white (75.7%), while 17.5% identified as black and 6.8% identified as other races/ethnicities. More than half of subjects had attained at least a college degree. Overall, there were low rates of complications in this cohort with only two diagnoses of pre-eclampsia, two of gestational diabetes, and no preterm birth. The complete demographic description of participants included in this study is in Table 1.
Table 1.
Demographics of study participants (N=103).
| Mean age, years ± SD | 29.8 ± 5.0 |
| Race | |
| Black, % (N) | 17.5 (18) |
| White, % (N) | 75.7 (78) |
| Other, % (N) | 6.8 (7) |
| Income, % (N) | |
| (<$20,000) | 15.5 (16) |
| ($20,000–50,000) | 21.7 (22) |
| ($50,000–100,000) | 35.9 (37) |
| (>$100,000) | 23.3 (24) |
| Do not know/wish to answer | 3.9 (4) |
| Education, % (N) | |
| ≤high school graduate | 8.7 (9) |
| Technical or trade school/some college | 16.5 (17) |
| College degree | 34.0 (35) |
| Some post-graduate work | 11.7 (12) |
| Post-graduate degree | 29.1 (30) |
| Mean Socioeconomic Index Score ± Standard Deviation | 6.0 ± 2.3 |
| Primiparious*, % (N) | 63 (63) |
| Mean gestational age at sample collection, weeks ± SD | 12.2 ± 1.1 |
| Mean gestational age at delivery, weeks ± SD | 39.8 ± 1.2 |
| Preterm birth, % (N) | 0 (0) |
| Pre-eclampsia, % (N) | 2 (2) |
| Gestational diabetes, % (N) | 2 (2) |
Missing data from three women
Mean AL in this study was 0.71 ± 0.52. The mean values and standard deviations for each of the individual components of AL were listed alongside the high-risk cut-offs for each in Table 2. Descriptive data on AL as well as each survey measure of psychosocial stress is provided in Table 3.
Table 3.
Distributions of survey measures.
| Mean ± SD | Range | Median (interquartile range) | N | |
|---|---|---|---|---|
| Prenatal Distress Score | 0.38 ± 0.29 | 0–1.22 | 0.33 (0.22–0.56) | 91 |
| Perceived Stress Scale | 13.3 ± 6.3 | 1–33 | 12 (9–17) | 101 |
| Inventory of Depressive Symptoms | 6.8 ± 3.0 | 2–22 | 6 (5–8) | 102 |
| Pittsburgh Sleep Quality Index | 5.6 ± 2.5 | 1–14 | 5 (4–7) | 103 |
| Allostatic load | 0.71± 0.52 | 0–2 | 0.583 (0.25–1.17) | 103 |
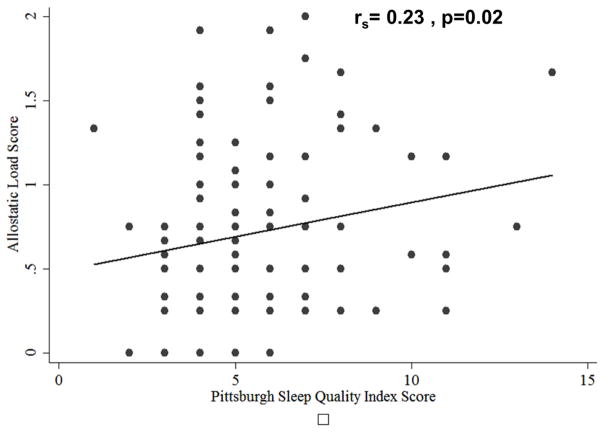
Allostatic load was positively correlated with the Pittsburgh Sleep Quality Index Score (ρ=0.2321, p=0.018) (Table 4). There were no significant associations of AL with age, income, the NuPDQ, PSS, or DS (Table 4). In an adjusted partial correlation analysis of subjective measures of stress, the correlation between the PSQI became non-significant (ρ=0.1928, p=0.0510), and there was still no significant association between AL and the NuPDQ, PSS, and IDS. Allostatic load tended to be higher among blacks than non-blacks (0.97 ± 0.62 vs 0.66 ± 0.48, p=0.054), although this was not a statistically significant finding. Allostatic load was lower among college-educated women in comparison with those who were not (0.57 ± 0.43 vs 0.81 ± 0.55, p=0.045).
Table 4.
Correlation of allostatic load with demographics and subjective measures of stress.
| Unadjusted coefficent | Race- and education-adjusted coefficient | |||
|---|---|---|---|---|
| Spearman Rho | p | Spearman Rho | p | |
| Age | −0.0055 | 0.96 | - | - |
| Income | 0.0853 | 0.39 | - | - |
| Socioeconomic Index Score | −0.1035 | 0.30 | - | - |
| Prenatal Distress Questionnaire Score | 0.031 | 0.77 | 0.0387 | 0.72 |
| Perceived Stress Scale | 0.0593 | 0.56 | −0.0007 | 0.99 |
| Inventory of Depressive Symptoms Score | 0.0281 | 0.78 | 0.0260 | 0.80 |
| Pittsburgh Sleep Quality Index | 0.2321 | 0.018 | 0.1928 | 0.051 |
Discussion
This study demonstrated significant associations between a novel model of AL in early pregnancy and poor sleep quality, which is a known chronic stressor. Additionally, it demonstrated higher allostatic load scores in early pregnancy among those with lower educational attainment, which is a proxy for socioeconomic status and yet again another association observed in the non-pregnant population. Notably, these relationships were not observed in other subjective measures of stress. The demonstrated associations between the model of AL and socioeconomic status, as measured through education and sleep quality, were consistent with previous well-accepted associations in the literature [5, 7, 26]. These findings validate this model, which was previously associated with increased pre-eclampsia risk in a different study population [12], and it is believed that this is the first study, to date, to report an association between AL and these measures that are detectable in early pregnancy.
These findings represent a valuable contribution to the limited literature on AL and pregnancy. Although many have proposed an association between adverse pregnancy outcomes, psychosocial stressors, and even AL [27–29], few studies have examined AL in a pregnant population and replicated findings in the pregnant population similar to those in the non-pregnant population. Wallace and Harville studied AL at 26–28 weeks gestation and found that black women actually had lower AL [15]. Likewise, Morrison et al. studied AL in pregnant women in the National Health and Nutrition Examination Survey (NHANES), with lower income or age [13]. It is believed that the inconsistency in these results may be attributable to dynamic physiologic adaptations that occur throughout pregnancy. At 26–28 weeks gestation, the placenta is well established and many of these changes that make pregnancy physiologically different from the non-pregnant state have occurred [30]. Additionally, since Morrison et al. combined data from all pregnant women, irrespective of their gestational age, differences in AL may have been masked in the statistical analyses. By focusing on a narrow and early time point in gestation, this study examined AL prior to more dynamic changes in a state more closely resembling non-pregnant physiology.
These findings align with previous work on the physiologic impact of stress among pregnant women. Psychosocial and prenatal stress are associated with higher levels of inflammatory markers and cytokines throughout pregnancy [31, 32] and the present findings are consistent, given that AL is a composite measure of the physiologic impact of stress. Particularly interesting was the positive correlation of AL and worsening sleep quality. Previous work has shown poorer sleep quality among pregnant women, particularly those of lower socioeconomic status, and increased risk for both preterm birth and post-partum major depression, with the strongest effect being in early pregnancy [16, 33, 34]. Furthermore, poor sleep quality and chronic deprivation is considered to be a chronic stressor with known physiologic impact and increased risk for disease [35]. It is believed that the present findings may contribute to a physiologic rationale for how stress and sleep may contribute to adverse pregnancy outcomes.
Although these findings demonstrate an association between AL measured in pregnancy and the known stressors, this study had several limitations. Many of these limitations came from the study population. This particular population was a healthy, community dwelling population, as evidenced by their low number of pregnancy complications. It is uniquely different from the PEPP study population used in the development of the pregnancy-specific allostatic load model described by Hux and Roberts [12]. This population was unique for a relatively high socioeconomic status, small number of non-white participants, higher but still small distribution of age, and low frequency of pregnancy complications. These demographics specifically limited ability to detect some associations that were otherwise expected, notably in age, income, race, and composite socioeconomic status index score. Further work building upon these findings should include a more diverse population with larger number of participants.
A peculiar finding was also the lack of association between AL and other subjective survey measures of stress. This was initially a surprising finding; however, the included subjective stress measures – the Inventory of Depressive Symptoms, Perceived Stress Scale, and Prenatal Distress Questionnaire – capture ongoing depressive symptomatology and more acute stress events. That said, although these measures capture psychological stress, they do not capture or characterize long-lasting stressors. The allostatic load concept models allostatic load as a measure of cumulative physiologic wear and tear. Although few studies have explored the relationship between subjective measures of acute stress and allostatic load, the lack of a strong correlation between measures of acute stress and AL is unsurprising and consistent with the conceptual model [1, 3, 36]. Thus, the AL model is positively correlated with chronic stressors, such as low socioeconomic status or poor sleep quality, but not acute stressors.
Conclusion
This study reinforces the model of AL in early pregnancy and also demonstrates a clear association between AL and sleep quality, a known chronic stressor evident in early pregnancy. Importantly, it was also able to replicate a well-established relationship of AL to educational attainment, which is one indicator of socioeconomic status. These findings suggest that this model indeed captures AL, as it has been previously understood in the stress-disease literature, and further supports that AL may be a useful tool for studying the physiologic impact of chronic stress in pregnancy and adverse outcomes. The finding of a correlation between sleep quality and AL also provides a possible point of intervention between a readily modifiable risk factor for adverse pregnancy outcomes.
Supplementary Material
Fig 1.
Allostatic load score versus Pittsburgh Sleep Quality Index score for study participants in early pregnancy (N=103)
Highlights.
Higher allostatic load is associated with adverse health outcomes.
Allostatic load is also higher in individuals with more subtle chronic stressors.
An association was observed between allostatic load and various stressors that are detectable in early pregnancy.
There was a positive correlation of allostatic load and worsening sleep quality.
This model captures allostatic load, as it has previously been understood in stress-disease literature.
Acknowledgments
Sources of funding
This work was supported by a grant from the Doris Duke Charitable Foundation to the University of Pittsburgh to fund Clinical Research Fellow Vanessa Hux, an NIMH Grant 2R25 MH054318 (PI: Gretchen Haas, PhD), NIH grant NICHD P01 HD030367 (PI: Carl Hubel, PhD), and NINR R00 NR010813 (PI: Michele Okun, PhD).
Footnotes
Conflicts of interest
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and biobehavioral reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism: clinical and experimental. 2003;52:10–6. doi: 10.1016/s0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 4.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation: allostatic load and its health consequences. MacArthur studies of successful aging. Archives of internal medicine. 1997;157:2259–68. [PubMed] [Google Scholar]
- 5.Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkin SS, Basurto-Davila R, Karlamangla A, Bird CE, Lurie N, Escarce J, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Annals of epidemiology. 2009;19:194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96:826–33. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosomatic medicine. 2012;74:178–86. doi: 10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental psychology. 2007;43:341–51. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism: clinical and experimental. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hux VJ, Roberts JM. A potential role for allostatic load in preeclampsia. Maternal and Child Health Journal. 2015;19:591–7. doi: 10.1007/s10995-014-1543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison S, Shenassa ED, Mendola P, Wu T, Schoendorf K. Allostatic load may not be associated with chronic stress in pregnant women, NHANES 1999–2006. Annals of Epidemiology. 2013;23:294–7. doi: 10.1016/j.annepidem.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace M, Harville E, Theall K, Webber L, Chen W, Berenson G. Neighborhood poverty, allostatic load, and birth outcomes in African American and white women: findings from the Bogalusa Heart Study. Health and Place. 2013;24:260–6. doi: 10.1016/j.healthplace.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace ME, Harville EW. Allostatic Load and Birth Outcomes Among White and Black Women in New Orleans. Maternal and Child Health Journal. 2012 doi: 10.1007/s10995-012-1083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okun ML, Tolge M, Hall M. Low socioeconomic status negatively affects sleep in pregnant women. Journal of Obstetric, Gynecologic, and Neonatal Nursing: JOGNN / NAACOG. 2014;43:160–7. doi: 10.1111/1552-6909.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okun ML, Kline CE, Roberts JM, Wettlaufer B, Glover K, Hall M. Prevalence of sleep deficiency in early gestation and its associations with stress and depressive symptoms. J Womens Health (Larchmt) 2013;22:1028–37. doi: 10.1089/jwh.2013.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. The Journal of Nutrition. 2010;140:999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myrianthopoulos NC, French KS. An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2:283–99. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 20.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological medicine. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 21.Lobel M. The Revised Prenatal Distress Questionnaire. State University of New York; Stony Brook: 1996. [Google Scholar]
- 22.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Haney A, Buysse DJ, Rosario BL, Chen YF, Okun ML. Sleep disturbance and cardiometabolic risk factors in early pregnancy: a preliminary study. Sleep medicine. 2014;15:444–50. doi: 10.1016/j.sleep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prevention CfDCa. Continuous NHANES Web Tutorial. Center for Disease Control and Prevention; Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- 26.Seeman T, Gruenewald T, Karlamangla A, Sidney S, Liu K, McEwen B, et al. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. American Journal of Human Biology : the official journal of the Human Biology Council. 2010;22:463–72. doi: 10.1002/ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Maternal and Child Health Journal. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 28.Hogue CJ, Bremner JD. Stress model for research into preterm delivery among black women. American Journal of Obstetrics and Gynecology. 2005;192:S47–55. doi: 10.1016/j.ajog.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 29.Latendresse G. The interaction between chronic stress and pregnancy: preterm birth from a biobehavioral perspective. Journal of Midwifery and Women’s Health. 2009;54:8–17. doi: 10.1016/j.jmwh.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackburn ST. Maternal, Fetal, & Neonatal Physiology: a clinical perspective. 3. St. Louis, Mo: Saunders Elsevier; 2007. [Google Scholar]
- 31.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic medicine. 2005;67:625–31. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 33.Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. Journal of Affective Disorders. 2011;130:378–84. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okun ML, Schetter CD, Glynn LM. Poor sleep quality is associated with preterm birth. Sleep. 2011;34:1493–8. doi: 10.5665/sleep.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Annals of the New York Academy of Sciences. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 36.Nugent KL, Chiappelli J, Rowland LM, Hong LE. Cumulative stress pathophysiology in schizophrenia as indexed by allostatic load. Psychoneuroendocrinology. 2015;60:120–9. doi: 10.1016/j.psyneuen.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.