ABSTRACT
Human respiratory syncytial virus (RSV) is the leading viral cause of severe lower respiratory disease in young children worldwide. As part of a genome-wide siRNA screen, we recently discovered that actin-related protein 2 (ARP2) is a host factor in the RSV replication cycle. ARP2 is a major constituent of the ARP2/3 complex, which catalyzes actin polymerization involved in cell morphology and motility. In the course of investigating this finding, we also found that RSV infection of human lung epithelial A459 cells induced filopodia formation and stimulated cell motility. The increase in filopodia formation was due, at least in part, to the expression of the RSV fusion F protein. Filopodia formation and increased cell motility appeared to shuttle RSV particles to nearby uninfected cells, facilitating virus cell-to-cell spread. ARP2 depletion did not reduce RSV entry or gene expression early in infection, but reduced subsequent virus production, filopodia formation, cell motility, and viral spread. Thus, the RSV F protein, ARP2-mediated actin nucleation, filopodia formation, and cell mobility all contribute to previously unrecognized mechanisms for RSV cell-to-cell spread that may promote RSV pathogenesis.
KEYWORDS: ARP2, A549 cells, cell motility, filopodia, RSV, virus cell-to-cell spread
Human respiratory syncytial virus (RSV) is an enveloped negative-strand RNA virus of the Pneumoviridae family.1 RSV is estimated to cause more than 3.5 million hospitalizations and 66,000 to 200,000 deaths in children worldwide each year, but lacks a vaccine or effective antiviral drug.2 In a recent study, we searched for host factors involved in RSV gene expression and replication using a whole-genome high-throughput siRNA screen in human airway epithelial A549 cells infected with RSV expressing enhanced green fluorescent protein. Among the top “hits” in this screen were several members of the integrin family with roles in cell motility and membrane dynamics, suggesting that these features of cell biology are important in the RSV replication cycle. Of particular interest was the actin nucleation factor actin-related protein 2 (ARP2), which is highly conserved in most eukaryotes and is a part of the ARP2/3 complex that nucleates branched actin filaments.3
We systematically explored the role of ARP2 in individual steps in the RSV replication cycle in A549 cells, from the early step of virus entry to the late step of budding of progeny virions. Initial experiments indicated that ARP2 did not have a significant role in the initial steps in RSV infection, including virus entry, as well as viral gene transcription and viral protein synthesis early in infection. ARP2-depletion also did not appear to affect syncytium formation. However, ARP2 depletion resulted in a ∼10-fold reduction in the production of progeny RSV virions. In addition, transmission and immune-scanning electron microscopy showed ARP2-depletion resulted in reduced number and altered morphology of progeny virions late in infection. We speculate that the effects of ARP2 depletion on the actin cytoskeleton structure may affect the assembly of RSV ribonucleoprotein complexes, matrix protein, and glycoprotein at the cell membrane. This is under further investigation.
We speculated that ARP2 depletion would affect cell shape and motility, and therefore focused on ultrastructural analysis of the cell surface to study the differential effects of RSV infection and ARP2 knockdown late in infection. Using confocal, electron, and stimulated emission depletion (STED) microscopy, we observed 2 different kinds of protrusions on the surface of infected cells compared with uninfected cells. One was the expected thin filamentous protrusions of about 100 nm in diameter and up to several micrometers in length that were identified by immunostaining as viral particles, which characteristically remain mostly cell-associated. The second type of protrusion had a broader base (0.5–2 µm) and variable length (up to 10 µm and even longer) and fine tips, and were abundantly formed in RSV-infected A549 cells, and were much less abundant in non-infected cells. We identified these structures as filopodia based on their characteristic size and shape, their actin content, and the absence of tubulin within these structures.4-6 Furthermore, filamentous RSV virions frequently were associated with these filopodial protrusions, and RSV particles on the tips of filopodia of infected cells appeared to be shuttled to neighboring uninfected A549 cells (Fig. 1).7,8 Based on these results, we speculate that the increased induction of filopodia facilitated virus spread, and likely also reduced the exposure of RSV to extracellular spaces where neutralization by antibodies would be more efficient.
Figure 1.
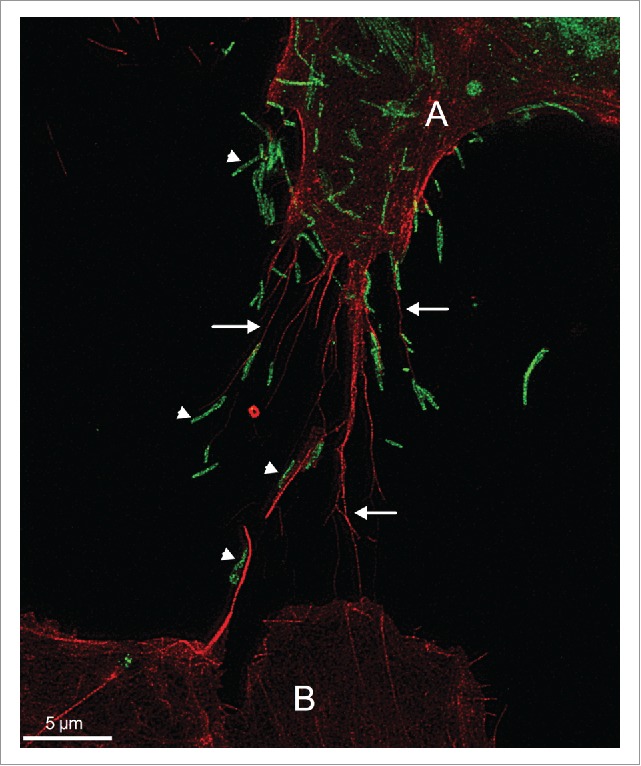
Filopodia-driven RSV cell-to-cell spread. A549 cells were infected with RSV wild type at a multiplicity of infection of 1 for 24 hr. Cells were fixed, permeabilized, and immunostained for RSV F (green) by incubating with mouse monoclonal antibody specific for the RSV F protein (abcam, ab43812) followed by incubating with the secondary antibody anti-mouse AlexaFluor488 (Life Technologies). The cells were further stained with rhodamine phalloidin (Cytoskeleton Inc.) to detect F-actin (red). Images were collected on a Leica TCS SP8 STED 3X system (Leica Microsystems) as described in.7 This image illustrates that the filopodia (indicated by arrows) of the RSV-infected cell (labeled A) appear to convey RSV particles (indicated by arrow heads) to a neighboring cell (labeled B).
We were interested in understanding how RSV induces filopodial structures. In transient expression studies, we found that expression of the RSV fusion (F) protein alone induced filopodia-like cellular protrusions, although the number of filopodia was reduced and their length was shorter compared with those induced by virus infection. Still, the results suggest that expression of a viral protein contributes to this process, similar to results recently described for an Alphavirus9 and for human metapneumovirus (HMPV).10 However, the induction of filopodia was much more robust with RSV than with human parainfluenza virus type 3 (HPIV3) and HMPV, which are members of the Paramyxoviridae and Pneumoviridae families, respectively.
Imaging of live cells yielded a further surprise. In time-lapse movies, we discovered that RSV-infected A549 cells exhibited increased cell motility compared with non-infected cells. Using viral GFP expression as a marker, we observed RSV-infected cells moving dynamically through the monolayer. This increased motility occurred even in confluent monolayers, and appeared to result in a more rapid spread of infection. The motility of RSV-infected cells was inhibited by depletion of ARP2. In addition, increased cell mobility was not observed following infection of A549 cells with HPIV3 or HMPV. Cell motility is regulated by Rho GTPases which are involved in the regulation of the F-actin cytoskeleton and rely on numerous mechanical and biochemical cues [reviewed in ref. 11].
Our study indicates that RSV infection actively modulates cytoskeleton signaling cascades, membrane morphology, and cell motility to increase viral production and spread. A number of viruses are known to manipulate actin dynamics in their replicative cycles.12,13 Some viruses have acquired the ability to hijack cellular actin dynamics, exploiting actin dynamics to support viral intracellular or cell surface-associated movement.14 Furthermore, in a recent study, HMPV was shown to use intercellular extensions for cell-to-cell spread,10 similarly to previous findings for influenza virus15 and, more recently, for Alphaviruses.9 To date, viral interactions with filopodia have been described mostly in viral entry and budding.16 Our results add new variations to the ways that viruses manipulate the cytoskeleton. Specifically, RSV infection induced an increase in filopodia formation that was induced at least in part by expression of the RSV F protein. This identified a new effect of the RSV F protein. RSV infection also induced increased cell motility. Both of these effects occurred late in infection. Both were dependent on APR2, which was identified as a novel cellular host factor in RSV infection. Both effects appeared to be facilitate viral spread, even in fully confluent monolayers. Additionally, ARP2 depletion modestly reduced RSV budding and viral shedding. The ARP2-dependent filopodia signaling cascade provides a possible therapeutic target to reduce RSV disease. Thus, this study identified new factors and mechanisms in RSV spread and pathogenesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Margery Smelkinson, Biological Imaging Section, Research Technologies Branch, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) for her help with STED macroscopy.
Funding
This study was supported by the Intramural Research Program of NIAID, NIH.
References
- [1].Afonso CL, Amarasinghe GK, Banyai K, Bao Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand FX, Briese T, et al.. Taxonomy of the order Mononegavirales: update 2016. Arch Virol 2016; 161(8):2351-60; PMID:27216929; https://doi.org/ 10.1007/s00705-016-2880-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al.. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375(9725):1545-55; PMID:20399493; https://doi.org/ 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 2006; 7(10):713-26; PMID:16990851; https://doi.org/ 10.1038/nrm2026 [DOI] [PubMed] [Google Scholar]
- [4].Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011; 12(7):413-26; PMID:21697900; https://doi.org/ 10.1038/nrm3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 2003; 40(2):209-27; PMID:14556705; https://doi.org/ 10.1016/S0896-6273(03)00633-0 [DOI] [PubMed] [Google Scholar]
- [6].Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev 2008; 88(2):489-513; PMID:18391171; https://doi.org/ 10.1152/physrev.00021.2007 [DOI] [PubMed] [Google Scholar]
- [7].Mehedi M, McCarty T, Martin SE, Le Nouen C, Buehler E, Chen YC, Smelkinson M, Ganesan S, Fischer ER, Brock LG, et al.. Actin-Related Protein 2 (ARP2) and Virus-induced Filopodia facilitate human respiratory syncytial virus spread. PLoS Pathog 2016; 12(12):e1006062; PMID:27926942; https://doi.org/ 10.1371/journal.ppat.1006062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mehedi M. PLoS pathogens issue image. PLoS Pathog 2016; 12(12):ev12.i12; https://doi.org/ 10.1371/image.ppat.v12.i12 [DOI] [Google Scholar]
- [9].Martinez MG, Kielian M. Intercellular extensions are induced by the Alphavirus structural proteins and mediate virus transmission. PLoS Pathog 2016; 12(12):e1006061; PMID:27977778; https://doi.org/ 10.1371/journal.ppat.1006061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].El Najjar F, Cifuentes-Munoz N, Chen J, Zhu H, Buchholz UJ, Moncman CL, Dutch RE. Human metapneumovirus induces reorganization of the actin Cytoskeleton for direct cell-to-cell spread. PLoS Pathog 2016; 12(9):e1005922; PMID:27683250; https://doi.org/ 10.1371/journal.ppat.1005922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 2010; 26:315-33; PMID:19575647; https://doi.org/ 10.1146/annurev.cellbio.011209.122036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Newsome TP, Marzook NB. Viruses that ride on the coat-tails of actin nucleation. Semin Cell Dev Biol 2015; 46:155-63; PMID:26459972; https://doi.org/ 10.1016/j.semcdb.2015.10.008 [DOI] [PubMed] [Google Scholar]
- [13].Haglund CM, Welch MD. Pathogens and polymers: microbe-host interactions illuminate the cytoskeleton. J Cell Biol 2011; 195(1):7-17; PMID:21969466; https://doi.org/ 10.1083/jcb.201103148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 2009; 6(1):10-21; PMID:19616762; https://doi.org/ 10.1016/j.chom.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roberts KL, Manicassamy B, Lamb RA. Influenza A virus uses intercellular connections to spread to neighboring cells. J Virol 2015; 89(3):1537-49; PMID:25428869; https://doi.org/ 10.1128/JVI.03306-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang K, Baginski J, Hassan SF, Volin M, Shukla D, Tiwari V. Filopodia and Viruses: An analysis of membrane processes in entry mechanisms. Front Microbiol 2016; 7:300; PMID:27014223 [DOI] [PMC free article] [PubMed] [Google Scholar]


