ABSTRACT
Salinity is a serious threat to plant growth and development worldwide reducing agricultural productivity each year. Ethylene is an important phytohormone that affects plants performance under normal and abiotic stress conditions. In this study, role of ethylene was investigated in mitigating salinity stress (100 mM NaCl) effects on photosynthesis in mustard plants subjected to different nitrogen (N; 5 and 10 mM) levels. Plants under salinity stress exhibited marked increase in proline and reduced glutathione (GSH) content and activity of antioxidant enzymes. Nitrogen supplementation at 10 mM was better than 200 µl l−1 ethephon treatment under no stress. However, under salinity stress, both N and ethephon were equally effective. The combined application of 10 mM N and ethephon to salinity stressed plants produced greatest increase in photosynthesis by increasing proline and antioxidant metabolism. Ethylene evolution was high under salinity stress, but treatment of 10 mM N and 200 µl l−1 ethephon greatly decreased ethylene evolution that was equivalent to the 10 mM N treatment alone. This concentration of ethylene decreased the oxidative stress and increased the photosynthetic nitrogen use efficiency (NUE) maximally to increase photosynthesis. The use of ethylene action inhibitor, norbornadiene (NBD) showed reduction in ethylene mediated effects in alleviating salinity. Norbornadiene decreased the photosynthetic-NUE, proline and GSH content that resulted in decrease in photosynthesis under salinity stress. This study indicated that ethylene regulated the proline and antioxidant metabolism under salinity stress to increase photosynthetic functions of mustard grown with low and optimum N. The modulation of ethylene could be adopted in agricultural practices to increase photosynthesis under salinity stress.
KEYWORDS: Ethylene, glutathione, norbornadiene, proline, salinity
Introduction
Soil salinity is a major constrain for agricultural productivity especially in arid and semi-arid regions of the world. Too much of salt disturbs the plant ability to maintain osmotic balance resulting in reduced water uptake and causing ion toxicities. Salinity leads to oxidative stress in plants through the excess production of reactive oxygen species (ROS). These ROS are deleterious to plant metabolism causing lipids peroxidation, oxidation of proteins, damage to nucleic acids, enzyme inhibition and even cell death.1-3 Plants adaptation to salt stress requires ion homeostasis, osmotic adjustment and induction of ROS detoxification mechanisms.4,5 Presence of high Na+ and Cl− ions in the soil restricts the uptake of K+ and other nutrients causing nutritional disorder.6 Salt-stress disturbs nitrogen (N) absorption and decreases the activity of N assimilating enzymes.7 Therefore, if salinity stressed plants are subjected to N deficiency the deleterious effects of salt are aggravated. Supplementation of N to salt stressed plants on the other hand could reduce the salinity-induced decrease in plant growth because N is an important constituent of various metabolites including reduced glutathione (GSH) and proline.8 Proline is an important osmolyte that helps in maintaining water balance and acts as an antioxidant for ROS scavenging.9-11 GSH is another antioxidant involved in plants defense.12
Nitrogen is the main constituent of proteins, enzymes, proline, GSH and pigment system.13 Its' requirement is higher than any other macro- and micro nutrient element. Supplementation of N has been reported to increase free proline content and this depends on N source and its application method.14,15 The interactive effect of salinity and nutrients on plant development may be strictly associated with the availability of nutrients in plant tissues.16 Recently, we have shown that N availability differentially affects plants metabolism under optimum and salt stress conditions and this effect was associated with ethylene and proline.9 Nitrogen played a key role in detoxification of cadmium (Cd) via glutamate and GSH in poplar. Zhang et al.17 reported that application of N to Cd stressed plants increased glutamine synthetase gene expression and Cd tolerance. In a study on mustard, Nazar et al.18 reported that under salinity stress supplementation of salicylic acid increased N and sulfur (S) assimilation that subsequently led to increased GSH synthesis and tolerance.
Ethylene signaling regulates plant growth and development by altering the properties of the growth-repressing DELLA proteins.19 Ethylene has been reported to increase N assimilation and regulate proline production in plants under optimum or stress conditions. 20-22 It may lead to salt tolerance through affecting antioxidant metabolism.23 Iqbal et al.9 reported that N regulates proline production and ethylene formation to alleviate salinity stress. However, detailed studies are needed to explain how ethylene regulates N assimilation and proline production and alleviates salt stress. Ethylene increases N assimilation and N is the important constituent of antioxidants and photosynthetic enzymes, therefore we assumed that plants that receive ethylene will maximally utilize N and show increased photosynthetic-nitrogen use efficiency (NUE) and salt tolerance through increased antioxidants activity. Thus, the present study was conducted to examine how ethephon used as ethylene source regulates photosynthesis under low and optimum N levels in mustard grown under salinity stress. The findings that ethylene regulates photosynthesis under salt stress were substantiated using ethylene action inhibitor, norbornadiene (NBD).
Material and methods
Plant material and growth conditions
Mustard (Brassica juncea L.) cv. Pusa Jai Kisan seeds were surface sterilized with 0.1% HgCl2 and were washed repeatedly with deionized water. The seeds were sown in 15-cm-diameter earthen pots filled with acid-washed sand, purified according to Hewitt24 The pots were kept in the herbal garden of the Department of Botany, Jamia Hamdard, New Delhi, India under natural day/night conditions with day and night temperature of 24/18 ± 3°C and relative humidity of 68 ± 6%. Two plants per pot were maintained. Plants were grown with either 0 or 100 mM NaCl) and 5 mM N (low N) and 10 mM N (optimum N). These plants were sprayed with 0 or 200 µl l−1 ethephon at 20 d after sowing (DAS). The low and optimum N levels were determined from the earlier findings.9 The NaCl concentration and N levels were given along with the modified full strength 300 mL Hoagland's nutrient solution given to each pot on alternate days and 250 mL deionized water daily. Potassium nitrate was used for obtaining 5 and 10 mM NO3− concentrations and K+ was maintained in all treatments including control by the addition of KCl. Plants grown in nutrient solution served as control. The nutrient solution was replaced weekly. The control groups of plants were fed with 300 mL nutrient solution every alternate day and 250 mL of deionized water daily. Ethephon on hydrolysis releases ethylene and phosphorus, therefore uniform phosphorus was also maintained in all pots to ensure the effects of ethylene. In the experiment with the ethylene action inhibitor, 100 µM NBD was applied to plants as foliar spray at 20 DAS together with 0.5% surfactant teepol. The control group of plants was also maintained. The experiment followed a randomized complete block design and the number of replicates for each treatment was 4 (n = 4). Measurements were done at 30 DAS and care was taken to select leaves of the same age for the determinations.
Estimation of leaf Na+ and Cl− content
Content of leaf Na+ and Cl− was determined by digesting leaf tissues (500 mg) with 19 mL of Tri acid mixture; a mixture of 16 mol L−1 concentrated nitric acid (10 mL), 18 mol L−1 concentrated sulfuric acid (5 mL) and 11.65 mol L−1 concentrated perchloric acid (4 mL) prepared in the ratio of 10:5:4 (v/v). The content of Na+ was estimated using a flame photometer, whereas the Cl− content was determined by titration against 0.02 N silver nitrate solution using 5% K2CrO4 as indicator.
Determination of H2O2 and thiobarbituric acid reactive substances (TBARS) content
Content of H2O2 and TBARS was determined adopting the methods of Okuda et al.25 and Dhindsa et al.26 respectively. For determination of H2O2 content, fresh leaf tissues (50 mg) were ground in ice-cold 200 mM perchloric acid. After centrifugation at 1200×g for 10 min, perchloric acid of the supernatant was neutralized with 4 M KOH. The insoluble potassium perchlorate was eliminated by centrifugation at 500×g for 3 min. The reaction was started by the addition of peroxidase and the increase in absorbance was recorded at 590 nm for 3 min. The level of lipid peroxidation in leaves was determined by estimating the content of TBARS. Fresh leaf tissues (0.5 g) were ground in 0.25% 2-thiobarbituric acid in 10% trichloroacetic acid using mortar and pestle. After heating at 95 °C for 30 min, the mixture was quickly cooled on ice bath and centrifuged at 10,000×g for 10 min. The absorbance of the supernatant was read at 532 nm on a spectrophotometer (UV–vis L164, Elico, New Delhi) and corrected for non-specific turbidity by subtracting the absorbance of the same at 600 nm. The content of TBARS was calculated using the extinction coefficient (155 mM−1 cm−1).
Estimation of proline content and assay of P5CS, glutamylkinase activity (GK) and proline oxidase activity
Proline content was determined spectrophotometrically using ninhydrin adopting the method of Bates et al.27 Fresh leaf tissues (300 mg) were homogenized in 3 mL of 3% sulphosalicylic acid. One milliliter each of acid ninhydrin and glacial acetic acid was added to the homogenate filtrate and the reaction was carried for 1 h in a test tube placed in a water bath at 100 °C. The mixture was extracted with toluene and the absorbance was measured on a spectrophotometer at 520 nm using l-proline as a standard.
To determine the activity of P5CS (EC 2.7.2.11/1.2.2.41), GK (EC 2.7.2.11) and proline oxidase (EC 1.5.99.8), enzyme extract was prepared by homogenizing 500 mg leaf sample in 0.1 M Tris–HCl buffer, pH 7.5 at 4 °C. The homogenate was centrifuged at 30,000×g for 30 min. The supernatant was used as the crude extract enzyme preparation for P5CS activity and the pellet was collected and used as extract for the assay of GK and proline oxidase. Activity of P5CS and GK was assayed according to the method of Hayzer and Leisinger28 with a slight modification. The assay mixture of 1.0 mL contained 100 M L-P5C and 100 mM sodium phosphate buffer at pH 7.5. The decrease in absorbance was measured at 340 nm for P5CS. For determining the activity of GK, the extract was kept in freezer at −20 °C. The frozen sample was suspended in 10 mL of 0.1 M Tris–HCl buffer containing 1 mM 1,4-dithiothreitol (DTT) to rupture the cell and centrifuged at 30,000×g for 30 min. The assay mixture contained 50 mM l-glutamate, 10 mM ATP, 20 mM MgCl2, 100 mM hydroxylamine HCl and 50 mM Tris–HCl, pH 7.0 with 200 μL of desalted extract in a final volume of 500 μL. The reaction was started by the addition of enzyme extract. After 30 min of incubation at 37 °C, the reaction was stopped by the addition of 1.0 mL FeCl3.3H2O (2.5%, w/v) and trichloroacetic acid (6%, w/v) in 2.5 M HCl. Protein was precipitated and removed by centrifugation at 12,000×g (4 °C) and absorbance was recorded at 540 nm against a blank identical to the above but lacking ATP. The amount of γ-glutamyl hydroxamate was determined by measuring the absorbance at 540 nm and by comparison with a standard curve of γ-glutamyl hydroxamate. Activity of GK was expressed in U mg−1 protein. One Unit (U) of enzyme activity is defined as μg of γ-glutamyl hydroxamate produced min−1 mg−1 protein.
The method of Huang and Cavalieri29 with slight modification was adopted to determine the activity of proline oxidase. The pellet obtained after centrifugation of enzyme extract was mixed with 1 mL Tricine, KOH buffer (pH 7.5) containing 6 M sucrose. This extract was used for the enzyme assay. The assay mixture contained 1.2 mL of 50 mM Tris–HCl buffer (pH 8.5), 1.2 mL of 5 mM MgCl2, 0.1 mL of 0.5 mM NADP, 0.1 mL of 1 mM KCN, 0.1 mL of 1 mM phenazine methosulfate, 0.1 mL of 0.06 mM 2,6 dichlorophenol indophenol (DCPIP) and 0.1 mL of 0.1 M proline in a final volume of 3 mL. The increase in absorbance was recorded at 600 nm at 25 °C using proline to initiate the reaction. Proline oxidase activity was expressed in U mg−1 protein. One Unit (U) of enzyme activity is defined as mM DCPIP reduced min−1 mg−1 protein.
Activity of antioxidative enzymes
Fresh leaf tissue (200 mg) was homogenized with an extraction buffer containing 0.05% (v/v) Triton X-100 and 1% (w/v) polyvinyl pyrrolidone (PVP) in potassium-phosphate buffer (100 mM, pH 7.0) using chilled mortar and pestle. The homogenate was centrifuged at 15,000×g for 20 min at 4 °C. The supernatant obtained after centrifugation was used for the assay of superoxide dismutase (SOD; EC 1.15.1.1) and GSH reductase (GR; EC 1.6.4.2) enzymes. For the assay of ascorbate peroxidase (APX; EC 1.11.1.11), extraction buffer was supplemented with 2 mM ascorbate. Activity of SOD was determined by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT), according to the methods of Beyer and Fridovich30 and Giannopolitis and Ries.31 A 5-mL reaction mixture containing 5 mM HEPES (pH 7.6), 0.1 mM EDTA, 50 mM Na2CO3 (pH 10.0), 13 mM methionine, 0.025% (v/v) Triton X-100, 63 μmol NBT, 1.3 μmol riboflavin, and the enzyme extract was illuminated for 15 min and a control set was not illuminated to correct for background absorbance. A unit of SOD was defined as the amount of enzyme required to use 50% inhibition of the reaction of NBT at 560 nm.
Activity of APX was determined by adopting the method of Nakano and Asada32 by recording the decrease in absorbance of ascorbate at 290 nm. The assay mixture contained phosphate buffer (50 mM, pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2, and the enzyme extract. Activity of APX was calculated by using the extinction coefficient 2.8 mM−1 cm−1. One unit of enzyme was the amount necessary to decompose 1 μmol of substrate/min at 25 °C. Activity of GR was determined by the method of Foyer and Halliwell33 by monitoring the GSH-dependent oxidation of NADPH at 340 nm. The assay mixture contained phosphate buffer (25 mM, pH 7.8), 0.5 mM oxidized GSH, 0.2 mM NADPH, and the enzyme extract. The activity of GR was calculated by using extinction coefficient at 6.2 mM−1 cm−1. One unit of enzyme was the amount necessary to decompose 1 μmol of NADPH/min at 25 °C.
Glutathione content and redox state
Reduced GSH was assayed by an enzymic recycling procedure34 in which it was sequentially oxidized by 5,5-dithiobis-2-nitrobenzoic acid (DTNB) and reduced by NADPH in the presence of GR. Fresh leaf tissue (500 mg) was ground in liquid nitrogen using mortar and pestle. The ground tissue was suspended in 0.5 mL of 5% sulfosalicyclic acid and centrifuged at 12,000×g for 10 min. A 300 μL aliquot of the supernatant was removed and neutralized by addition of 18-μL of 7.5 M triethanolamine. Aliquot (50 μL) of the sample was mixed with 700 μL of 0.3 mM NADPH, 100 μL DTNB and 150 μL buffer containing 125 mM sodium phosphate, and 6.3 mM EDTA (pH 6.5). A 10 μL aliquot of GR (5 U mL−1) was then added and the change in absorbance at 412 nm was monitored at 30 °C. Redox state was presented as the ratio of GSH to oxidized glutathione (GSSG).
Determination of NR activity and N content
Activity of NR (EC 1.6.6.1) in leaves was measured by preparing enzyme extract using the method of Kuo et al.35 and was assayed spectrophotometrically adopting the procedure of Nakagawa et al.36 as the rate of nitrite production at 28°C. Leaves (1.0 g) were ground to powder with mortar and pestle, and then stored at −80 °C. The powder was thawed for 10 min at 4 °C and homogenized in a blender in 250 mM Tris–HCl buffer, pH 8.5, containing 10 mM cysteine, 1 mM EDTA, 20 μM FAD, 1 mM DTT, and 10% (v/v) glycerol. The homogenate was centrifuged at 10,000×g for 30 min at 4 °C. The assay mixture contained, KNO3 (10 mM), HEPES (0.065 M; pH 7.0), NADH (0.5 mM) in phosphate buffer (0.04 mM; pH 7.2) and enzyme in a final volume of 1.5 mL. The reaction was started by adding NADH. After 15 min the reaction was terminated by adding 1 mL of 1 N HCl solution containing 1% sulfanilamide followed by adding 1 mL of 0.02% aqueous N-1-napthylethylene-di-amine-dihydrochloride (NED). The absorbance was read at 540 nm after 10 min. The method of Lindner37 was used for determining the N content in acid-peroxide leaf samples.
Measurement of ACS activity and ethylene
The methods of Avni et al.39 and Woeste et al.40 were adopted for the determination of the activity of 1-aminocyclopropane carboxylic acid synthase (ACS; EC4.4.1.14). Leaf tissue (5.0 g) was ground in 100 mM HEPES buffer (pH 8.0) containing 4 mM DTT, 2.5 mM pyridoxal phosphate, and 25% PVP. The homogenized preparation was centrifuged at 12,000×g for 15 min. One milliliter of the supernatant was placed in 30 mL tube and 0.1 mL of 5 mM S-adenosylmethionine was added and incubated at 22°C for 2 h. The 1-aminocyclopropane carboxylic acid (ACC) formed was determined by its conversion to ethylene by the addition of 0.1 mL of 20 mM HgCl2 followed by the addition of 0.1 mL of a 1:1 mixture of saturated NaOH/NaCl and placed in ice for 10 min. In the control set, S-adenosyl methionine was not added. For measuring ethylene, 0.5 g of leaf material was cut into small pieces that were placed into 30 mL tubes containing moist paper to minimize evaporation from the tissue and were stoppered with secure rubber caps and placed in light for 2 h under the same condition used for plant growth. One milliliter gas sample from the tubes was withdrawn with a hypodermic syringe and assayed on a gas chromatograph (Nucon 5700, New Delhi) equipped with a 1.8 min Porapack N (80–100 mesh) column, a flame ionization detector and data station. Nitrogen was used as carrier gas. The flow rates of N, hydrogen, and oxygen were 30, 30, and 300 mL min−1, respectively. The detector was set at 150 °C. Ethylene was identified based on the retention time and quantified by comparison with peaks from standard ethylene concentration.
Determination of gas exchange parameters, quantum yield efficiency of PSII, Rubisco activity and photosynthetic-NUE
Leaf gas exchange parameters (net photosynthesis, stomatal conductance and intercellular CO2 concentration) were measured in fully expanded intact leaves with the help of an Infra-Red Gas Analyzer (CI-340 Photosynthesis system, CID Bio-Science, USA). The measurements were made between 10.00 and 11.30 a.m.at light saturating intensity. The atmospheric conditions at the time of measurements were: PAR, <640 mol m−2s−1, air temperature,<22 °C and relative humidity, <70%. Quantum yield efficiency of PSII was measured with the help of chlorophyll fluorometer (OS-30p, USA). After dark adaptation for 30 min, minimal fluorescence (Fo) was measured during the weak measuring pulses and a saturating pulse was used to obtain maximal fluorescence (Fm) and PSII was calculated. Activity of ribulose 1,5-bisphosphate carboxylase (Rubisco; EC 4.1.1.39) was determined spectrophotometrically using the method of Usuda38 by monitoring NADH oxidation at 30°C at 340 nm during the conversion of 3-phosphoglycerate to glycerol 3-phosphate after the addition of enzyme extract to the assay medium. Photosynthetic-NUE was calculated as the ratio of net photosynthesis to N content per unit leaf area, and the leaf area was measured using leaf area meter (LA 211, Systronics, New Delhi).
Statistical analysis
Data were analyzed statistically using analysis of variance (ANOVA) by SPSS statistics (ver. 17.0), and presented as treatment mean ± SE (n = 4). Least significant difference (LSD) was calculated for the significant data at P < 0.05. Data bars with the same letter do not show significant difference by LSD test at P < 0.05.
Results
Ethephon with N decreases salinity induced oxidative stress by lowering the content of ions and TBARS and H2O2 content
Salt stress increased the content of Na+ and Cl− ions and H2O2 and TBARS. Supplementation of N decreased oxidative stress by lowering the content of ions and H2O2 and TBARS both under salt stress and no stress. Nitrogen at 10 mM was more efficient than 5 mM N in lowering oxidative stress under both conditions of stress or no stress. Ethephon application proved better than 5 mM N, but the effect was lesser than 10 mM N under no stress. However, under salt stress both ethephon and 10 mM N exhibited similar effects in lowering oxidative stress. Ethephon applied together with 5 or 10 mM N completely alleviated the negative effect of salt stress by lowering the oxidative stress level below control. The combined application of ethephon and 10 mM N was found superior than ethephon and 5 mM N under stress conditions. The content of H2O2 and TBARS was lowered by 44.4% and 39.6%, respectively with combined application of ethephon and 10 mM N of salt stressed plants (Table 1).
Table 1.
Content of leaf Na+ (mg g−1 DW), Cl− (mg g−1 DW), H2O2 (nmol g −1 FW) and thiobarbituric acid reactive substances (TBARS, nmol g−1 FW) in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were treated either with 5 mM N, 10 mM N, 200 µl l−1 ethephon in different combinations and grown with or without 100 mM NaCl. Data means with the same letter are not different significantly at P < 0.05. DW, dry weight; FW, fresh weight.
| Treatments | Leaf Na+ | Leaf Cl− | H2O2 | TBARS |
|---|---|---|---|---|
| Control | 4.0 ± 0.37c | 4.9 ± 0.18c | 66.6 ± 5.3c | 15.4 ± 0.94c |
| 5 mM N (N5) | 3.3 ± 0.33d | 4.4 ± 0.12d | 55 ± 4.7d | 14.7 ± 0.78d |
| 10 mM N (N10) | 2.7 ± 0.28f | 3.8 ± 0.10f | 39.0 ± 3.6f | 10.6 ± 0.63f |
| 100 mM NaCl | 7.8 ± 0.59a | 8.3 ± 0.44a | 214 ± 11.9a | 49 ± 2.9a |
| 200 µl l−1 ethephon (E) | 3.0 ± 0.2e | 4.0 ± 0.093e | 43.0 ± 3.2e | 11.7 ± 0.57e |
| N5 + NaCl | 6.0 ± 0.45b | 6.7 ± 0.35b | 169 ± 9.6b | 37 ± 1.38b |
| N10 + NaCl | 3.8 ± 0.41c | 5.0 ± 0.14c | 69.3 ± 5.5c | 16.1 ± 0.98c |
| NaCl + E | 3.7 ± 0.39c | 5.1 ± 0.17c | 67.8 ± 5.4c | 15.2 ± 0.94c |
| N5 + E | 1.6 ± 0.1h | 3.0 ± 0.088 g | 30 ± 2.1 g | 8.2 ± 0.42 g |
| N10 + E | 1.0 ± 0.1i | 2.7 ± 0.08h | 25 ± 1.7h | 7.1 ± 0.37h |
| N5 + NaCl + E | 3.1 ± 0.33d | 4.5 ± 0.15d | 53 ± 4.4d | 14.3 ± 0.69d |
| N10 + NaCl + E | 2.4 ± 0.20 g | 3.6 ± 0.11f | 37 ± 3.2f | 9.3 ± 0.58f |
Ethephon with N increased proline and antioxidant metabolism
Proline is an important osmolyte that also acts as an antioxidant to bring salinity tolerance in plants. To study the involvement of N and/or ethephon in proline production and salinity tolerance, activity of GK, P5CS and proline oxidase was determined. It was observed that proline content increased under salt stress and further increased with N and/or ethephon supplementation. Maximum increase in proline was recorded with combined application of ethephon and 10 mM N compared with the control (Table 2). Application of ethephon and 10 mM N increased activity of GK and P5CS by 170% and 128% in the absence of salt stress and by 106% and 81% under salt stress compared with the control.
Table 2.
Proline content (μmol g−1FW), glutamyl kinase (GK; U mg−1protein), proline oxidase (U mg−1protein) and pyrroline-5-carboxylate synthetase activity (P5CS; U min−1 mg−1protein) in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were treated either with 5 mM N, 10 mM N, 200 µl l−1 ethephon in different combinations and grown with or without 100 mM NaCl. Data means with the same letter are not different significantly at P < 0.05. DW, dry weight; FW, fresh weight.
| Treatments | Proline content | GK activity | Proline oxidase | P5CS activity |
|---|---|---|---|---|
| Control | 5.9 ± 0.19h | 0.64 ± 0.0199h | 0.0883 ± 5.5000e-3a | 8.2 ± 0.246h |
| 5 mM N (N5) | 6.7 ± 0.21 g | 0.69 ± 0.022 g | 0.078 ± 3.8000e-3b | 9.1 ± 0.318 g |
| 10 mM N (N10) | 15.4 ± 0.71c | 1.55 ± 0.06c | 0.03 ± 1.9000e-3 g | 16.8 ± 0.84c |
| 100 mM NaCl | 14.0 ± 0.67f | 1.11 ± 0.037f | 0.066 ± 2.9000e-3c | 12.4 ± 0.49f |
| 200 µl l−1 ethephon (E) | 16.1 ± 0.79d | 1.4 ± 0.0613d | 0.0323 ± 2.0000e-3f | 15.6 ± 0.81d |
| N5 + NaCl | 17.6 ± 0.94e | 1.2200 ± 0.04e | 0.051 ± 2.4000e-3d | 14.0 ± 0.63e |
| N10 + NaCl | 12.1 ± 0.61d | 1.34 ± 0.056d | 0.044 ± 2.1000e-3e | 15.2 ± 0.745d |
| NaCl + E | 13.0 ± 0.62d | 1.36 ± 0.0572d | 0.041 ± 2.2000e-3e | 15.4 ± 0.748d |
| N5 + E | 13.9 ± 0.64b | 1.61 ± 0.071b | 0.027 ± 1.3000e-3h | 17.6 ± 0.968b |
| N10 + E | 14.2 ± 0.65a | 1.73 ± 0.083a | 0.021 ± 1.1000e-3 g | 18.7 ± 1.06a |
| N5 + NaCl + E | 15.0 ± 0.731c | 1.51 ± 0.0711c | 0.029 ± 1.6000e-3 g | 16.3 ± 0.848c |
| N10 + NaCl + E | 16.0 ± 0.88b | 1.64 ± 0.0741b | 0.022 ± 1.1000e-3i | 17.2 ± 0.95b |
The proline degrading enzyme, proline oxidase decreased with the application of N and/or ethephon alone in comparison to control, accounting for the observed increased proline content. The combined application of ethephon and 10 mM N maximally decreased the activity of proline oxidase in the absence of salt in comparison with the control. Similarly, under salt stress, ethephon and 10 mM N decreased the activity of proline oxidase by 75% compared with the control (Table 2).
Application of 10 mM N increased GSH content by 57.1% and redox state by 53.5% compared with the control which was higher than both 5 mM N or ethephon under no stress. The combined application of ethephon and 10 mM N maximally alleviated salt stress by increasing GSH content by 78.4% and GSH/GSSG by 75.9% compared with the control.
Application of N and/or ethephon individually increased the activity of antioxidant enzymes to the same extent under salt stress. The combined application of ethephon and 10 mM N maximally increased SOD activity by 2.5-times, APX and GR activity equally by 3.2-times compared with the control (Table 3).
Table 3.
Activity of superoxide dismutase (SOD; U mg−1 protein), ascorbate peroxidase (APX, U mg−1 protein) and glutathione reductase (GR; U mg−1 protein), reduced glutathione content (GSH; nmol g−1 fresh mass) and redox state (GSH/GSSG) in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were treated either with 5 mM N, 10 mM N, 200 µl l−1 ethephon in different combinations and grown with or without 100 mM NaCl. Data means with the same letter are not different significantly at P < 0.05. DW, dry weight; FW, fresh weight.
| Treatments | SOD | APX | GR | GSH | GSH/GSSG |
|---|---|---|---|---|---|
| Control | 8.7 ± 0.81i | 1.1 ± 0.04j | 0.17 ± 0.01j | 310 ± 15.4i | 17 ± 0.78i |
| 5 mM N (N5) | 9.6 ± 0.86h | 1.3 ± 0.04i | 0.2 ± 0.01i | 372 ± 16.3 g | 20.6 ± 1.03h |
| 10 mM N (N10) | 11.4 ± 0.88f | 1.6 ± 0.09 g | 0.26 ± 0.02 g | 463 ± 18.4e | 26.1 ± 1.69e |
| 100 mM NaCl | 13 ± 0.92e | 2.1 ± 0.08f | 0.3 ± 0.02f | 355 ± 16.4h | 14.8 ± 0.59j |
| 200 µl l−1 ethephon (E) | 10.2 ± 0.82 g | 1.5 ± 0.07h | 0.23 ± 0.02h | 413 ± 18.0f | 24.0 ± 1.44f |
| N5 + NaCl | 14.3 ± 0.98d | 2.4 ± 0.09d | 0.33 ± 0.01e | 410 ± 17.6f | 17.8 ± 0.83i |
| N10 + NaCl | 15.7 ± 1.07c | 2.8 ± 1.11c | 0.44 ± 0.02c | 487 ± 19.5d | 23.1 ± 1.27 g |
| NaCl + E | 16.0 ± 1.1c | 3.0 ± 0.14c | 0.46 ± 0.02c | 490 ± 20.2d | 22.6 ± 1.13 g |
| N5 + E | 14.4 ± 0.99d | 2.3 ± 0.12e | 0.31 ± 0.02e | 494 ± 19.9d | 30.3 ± 2.12b |
| N10 + E | 16.3 ± 1.18c | 2.9 ± 0.14c | 0.36 ± 0.03d | 579 ± 26.3a | 37.6 ± 3.00a |
| N5 + NaCl + E | 19.0 ± 1.23b | 3.3 ± 0.17b | 0.50 ± 0.024b | 527 ± 23.0c | 27.7 ± 1.66d |
| N10 + NaCl + E | 21.6 ± 1.36a | 3.7 ± 0.20a | 0.54 ± 0.029a | 553 ± 25.2b | 29.9 ± 2.09c |
Effect of ethephon with N on N assimilation and ethylene evolution
The N content and NR activity decreased under salt stress. Nitrogen at 10 mM N proved more effective than 5 mM N in increasing N content by 101% and NR activity by 30% compared with the control. The combined application of ethephon and 10 mM N maximally increased N content and NR activity by 166% and 53%, respectively compared with the control (Table 4). Similar results were obtained under salt stress where the combined application of ethephon and 10 mM N maximally alleviated salt stress and increased N content by 03.5% and NR activity by 31.4% compared with the control.
Table 4.
Nitrogen (N) content (mg g−1 DW), nitrate reductase (NR) activity (nmol (NO2) g−1FW h−1), activity of 1-aminocyclopropane carboxylic acid (ACC) (ACS; μg ACC m−2 s−1) and ethylene evolution (μl m−2 s−1) in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were treated either with 5 mM N, 10 mM N, 200 µl l−1 ethephon in different combinations and grown with or without 100 mM NaCl. Data means with the same letter are not different significantly at P < 0.05. DW, dry weight; FW, fresh weight.
| Treatments | N content | NR activity | ACS activity | Ethylene evolution |
|---|---|---|---|---|
| Control | 23.3 ± 1.23h | 353 ± 13.8f | 66.0 ± 3.67e | 35.0 ± 1.7e |
| 5 mM N (N5) | 29.1 ± 1.57 g | 376 ± 17.7e | 55.3 ± 3.48f | 30.6 ± 1.34f |
| 10 mM N (N10) | 46.5 ± 1.73c | 458 ± 20.2c | 43.7 ± 3.27h | 23.5 ± 1.03h |
| 100 mM NaCl | 19.2 ± 0.9j | 261 ± 12.1h | 199.0 ± 6.6a | 65.0 ± 3.74a |
| 200 µl l−1 ethephon (E) | 41.0 ± 1.77d | 432 ± 22.3d | 74.0 ± 4.86c | 41.1 ± 3.28c |
| N5 + NaCl | 20.6 ± 1.16i | 296 ± 14.2 g | 79.4 ± 5.2b | 46.0 ± 3.3b |
| N10 + NaCl | 30.7 ± 1.37f | 369 ± 13.3f | 64.6 ± 3.3e | 34.5 ± 1.82e |
| NaCl + E | 31.2 ± 1.42f | 375 ± 14.6f | 65.3 ± 3.38e | 36.4 ± 1.94e |
| N5 + E | 56.7 ± 2.3b | 507 ± 24.1b | 69.3 ± 4.38d | 38.4 ± 2.28d |
| N10 + E | 61.4 ± 2.41a | 539 ± 28.2a | 53.0 ± 4.22 g | 27.8 ± 1.44 g |
| N5 + NaCl + E | 37.3 ± 1.6e | 443 ± 17.3d | 56.1 ± 3.54f | 29.5 ± 1.33f |
| N10 + NaCl + E | 47.0 ± 1.83c | 464 ± 19.9c | 44.9 ± 3.35h | 24.6 ± 1.24h |
Salinity stress increased evolution of ethylene which was much higher than the control plants. Application of 10 mM N to non-stressed plants decreased ethylene evolution with decrease of 33.8% in ACS activity and 32.9% in ethylene evolution occurred compared with the control. The combined application of ethephon and 10 mM N decreased ACS activity and ethylene evolution by 19.7% and 20.6% under no stress and 32% and 29.7%, respectively under salt stress in comparison to the control (Table 4).
Ethephon with N increased photosynthetic characteristics
Salt stress adversely affected net photosynthesis, stomatal conductance, intercellular CO2 concentration, Rubisco activity and photosynthetic-NUE. Application of N alone to unstressed plants increased photosynthetic traits, but maximal increase in these characteristics were noted with combined application of ethephon and 10 mM N. The individual application of ethephon and N lowered the effects of salt stress and the values became on par with the control plants. The combined treatment of ethephon 10 mM N proved best in alleviating salt stress and improved net photosynthesis, stomatal conductance, intercellular CO2 concentration, quantum yield efficiency of PSII and Rubisco activity by 38.8%, 35.5%, 45.1%, 20.6% and 45.5%, respectively compared with the control (Table 5).
Table 5.
Net photosynthesis (μmol CO2 m−2 s−1), stomatal conductance (mmol CO2 m−2 s−1), intercellular CO2 concentration (μmol CO2 mol−1), quantum yield efficiency of PSII, Rubisco activity (μmol CO2 mg−1protein min−1), photosynthetic nitrogen use efficiency (P-NUE; g m−2) and plant dry mass (g plant−1) in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were treated either with 5 mM N, 10 mM N, 200 µl l−1 ethephon in different combinations and grown with or without 100 mM NaCl. Data means with the same letter are not different significantly at P < 0.05. DW, dry weight; FW, fresh weight.
| Treatments | Net photosynthesis | Stomatal conductance | Intercellular CO2 concentration | Quantum yield efficiency of PS II | Rubisco activity | P-NUE | Plant dry mass |
|---|---|---|---|---|---|---|---|
| Control | 13.4 ± 0.81ef | 268 ± 16.4f | 182 ± 9.3f | 0.70 ± 0.03e | 0.99 ± 0.04f | 31 ± 1.58f | 0.83 ± 0.087f |
| 5 mM N (N5) | 14.1 ± 0.9e | 296 ± 20.3e | 204 ± 10.8e | 0.729 ± 0.03e | 1.10 ± 0.06e | 36 ± 2.4e | 0.90 ± 0.089e |
| 10 mM N (N10) | 18.0 ± 1.27c | 351 ± 23.1c | 263 ± 13.6c | 0.838 ± 0.04c | 1.33 ± 0.08c | 56 ± 3.6c | 1.5 ± 0.10c |
| 100 mM NaCl | 8.3 ± 0.41h | 202 ± 11.8h | 131 ± 6.6h | 0.602 ± 0.02 g | 0.47 ± 0.02h | 16 ± 1.3h | 0.6 ± 0.059h |
| 200 µl l−1 ethephon (E) | 16.2 ± 1.15d | 326 ± 22.3d | 224 ± 12.2d | 0.763 ± 0.04d | 1.22 ± 0.07d | 45 ± 3.1d | 1.2 ± 0.098d |
| N5 + NaCl | 9.5 ± 0.52 g | 216 ± 16.9 g | 153 ± 7.5 g | 0.659 ± 0.03f | 0.63 ± 0.04 g | 23 ± 1.5 g | 0.7 ± 0.068 g |
| N10 + NaCl | 12.9 ± 0.7f | 273 ± 17.7f | 175 ± 9.2f | 0.694 ± 0.04e | 1.0 ± 0.05f | 32 ± 1.62f | 0.81 ± 0.08f |
| NaCl + E | 13.2 ± 0.96f | 286 ± 18.4f | 187 ± 10.1f | 0.719 ± 0.03de | 1.03 ± 0.04f | 30 ± 1.44f | 0.87 ± 0.082f |
| N5 + E | 21.3 ± 1.4b | 410 ± 30.4b | 303 ± 16.8b | 0.899 ± 0.05b | 1.59 ± 0.14b | 63 ± 3.8b | 1.9 ± 0.15b |
| N10 + E | 23.4 ± 1.56a | 447 ± 342a | 327 ± 17.9a | 0.947 ± 0.07a | 1.79 ± 0.17a | 69 ± 4.1a | 2.2 ± 0.17a |
| N5 + NaCl + E | 15.0 ± 1.09e | 315 ± 21.1e | 212 ± 11.1e | 0.736 ± 0.04d | 1.18 ± 0.06e | 38 ± 2.2e | 0.99 ± 0.092e |
| N10 + NaCl + E | 18.6 ± 1.29c | 364 ± 25.3bc | 264 ± 15.6c | 0.844 ± 0.05c | 1.44 ± 0.08c | 59 ± 3.4c | 1.4 ± 0.096c |
Photosynthetic-NUE is an important trait to assess the investment of N into photosynthesis. It was observed that salt stress decreased photosynthetic-NUE. In the absence or presence of salt, photosynthetic-NUE was maximally increased with the combined application of ethephon and 10 mM N. Under salt stress maximum increases in photosynthetic-NUE of 90.3% occurred with the combined application of ethephon and 10 mM N (Table 5).
Inhibition of ethylene inhibits proline and GSH content, photosynthetic-NUE and photosynthesis under salt stress
To substantiate the findings that ethylene regulates proline and antioxidant metabolism for protection of photosynthesis under salinity stress, we used NBD as ethylene action inhibitor and monitored these parameters. Ethephon individually increased N-assimilation, content of proline and GSH and photosyntheic-NUE, but the effect was more pronounced when ethephon was given with N. However, treatment with NBD, inhibited the activity of NR and content of N, decreased content of proline and GSH, photosynthetic-NUE, net photosynthesis and Rubisco activity. The inhibition of ethylene resulted in reduced N assimilation recorded as the NR activity and N content, which was reflected in reduced proline and GSH production. As the N was assimilated poorly with the inhibition of ethylene photosynthetic-NUE and Rubisco activity were reduced under normal and salinity stressed plants. This consequently resulted in reduced photosynthesis under salinity stress showing that ethylene has a regulatory function in augmenting photosynthesis through its effect on proline and antioxidant metabolism and N assimilation (Figs. 1-5).
Figure 1.
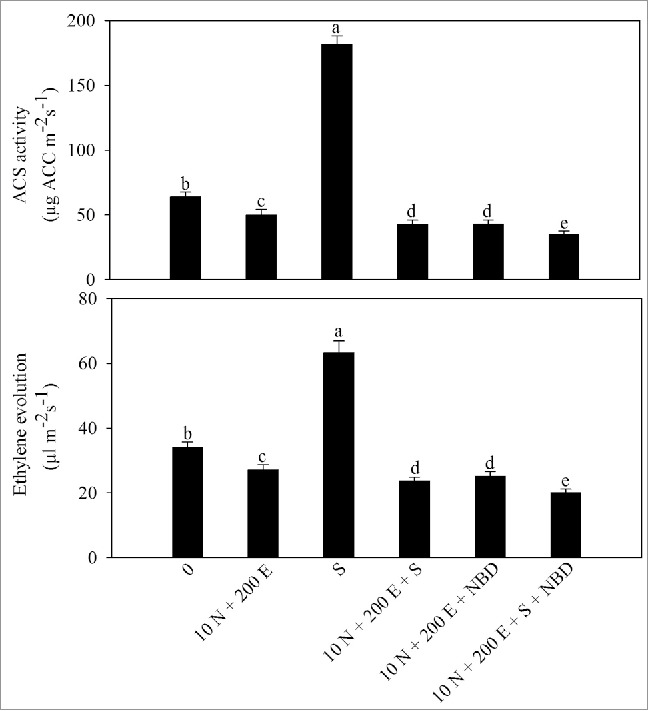
Activity of 1-aminocyclopropane carboxylic acid (ACC) synthase (ACS) and ethylene evolution in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were grown with 100 mM NaCl (S) or 10 mM N plus 200 µl l−1 ethephon (10 N + 200E) individually or in combination, and the plants grown with 10 N + 200 E alone or with NaCl (S) were treated with 100 µM norbornadiene (NBD). Data represent mean values ± SE (n = 4). Means denoted by the same letter are not significantly different at P < 0.05.
Figure 2.
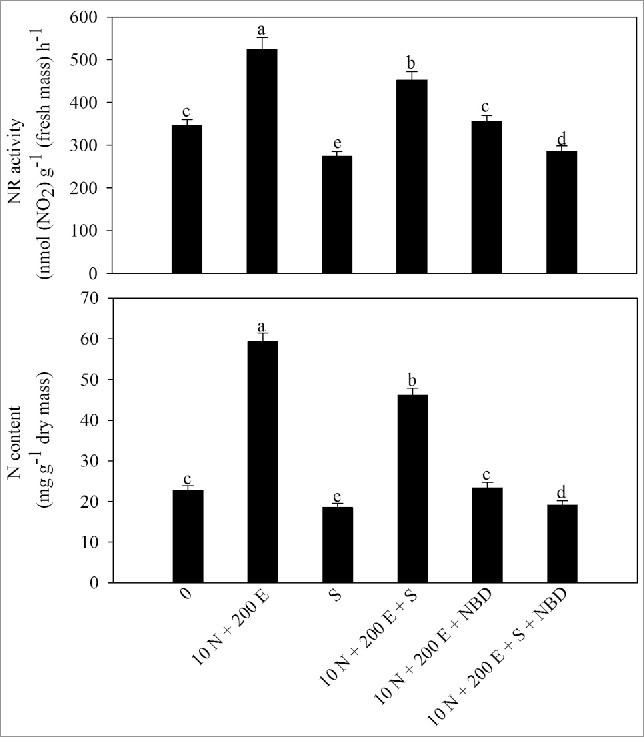
Activity of nitrate reductase (NR) and N content in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were grown with 100 mM NaCl (S) or 10 mM N plus 200 µl l−1 ethephon (10 N + 200E) individually or in combination, and the plants grown with 10 N + 200 E alone or with NaCl (S) were treated with 100 µM norbornadiene (NBD). Data represent mean values ± SE (n = 4). Means denoted by the same letter are not significantly different at P < 0.05.
Figure 3.
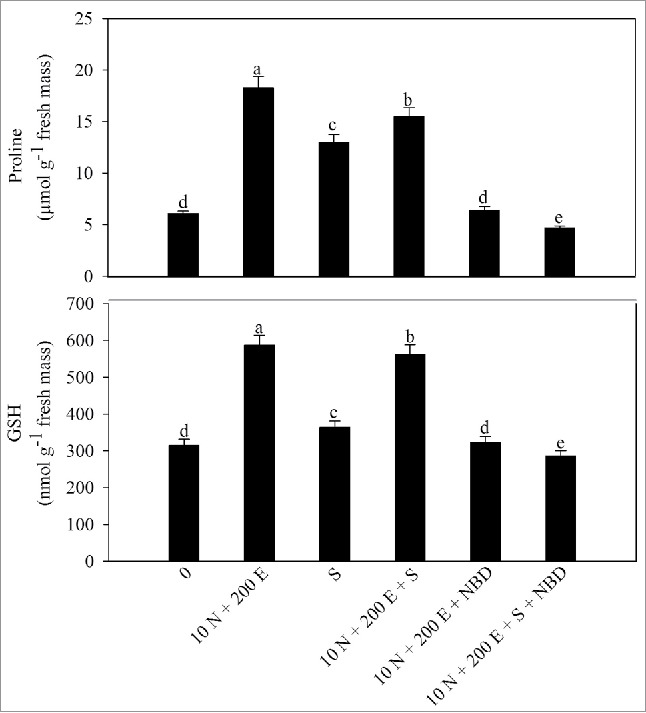
Proline and reduced glutathione (GSH) content in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were grown with 100 mM NaCl (S) or 10 mM N plus 200 µl l−1 ethephon (10 N + 200E) individually or in combination, and the plants grown with 10 N + 200 E alone or with NaCl (S) were treated with 100 µM norbornadiene (NBD). Data represent mean values ± SE (n = 4). Means denoted by the same letter are not significantly different at P < 0.05.
Figure 4.
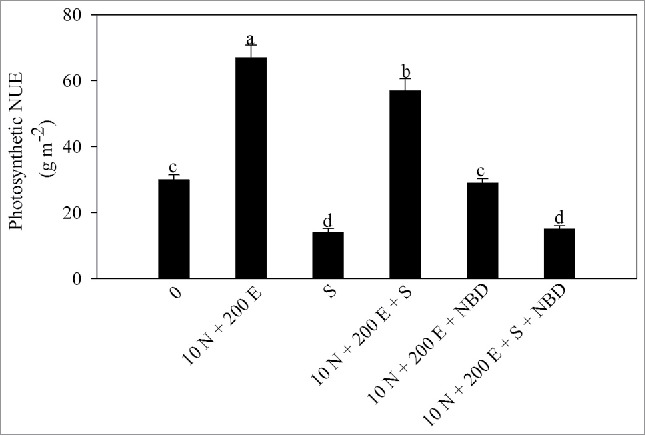
Photosyntheti-nitrogen use efficiency (NUE) in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were grown with 100 mM NaCl (S) or 10 mM N plus 200 µl l−1 ethephon (10 N + 200E) individually or in combination, and the plants grown with 10 N + 200 E alone or with NaCl (S) were treated with 100 µM norbornadiene (NBD). Data represent mean values ± SE (n = 4). Means denoted by the same letter are not significantly different at P < 0.05.
Figure 5.
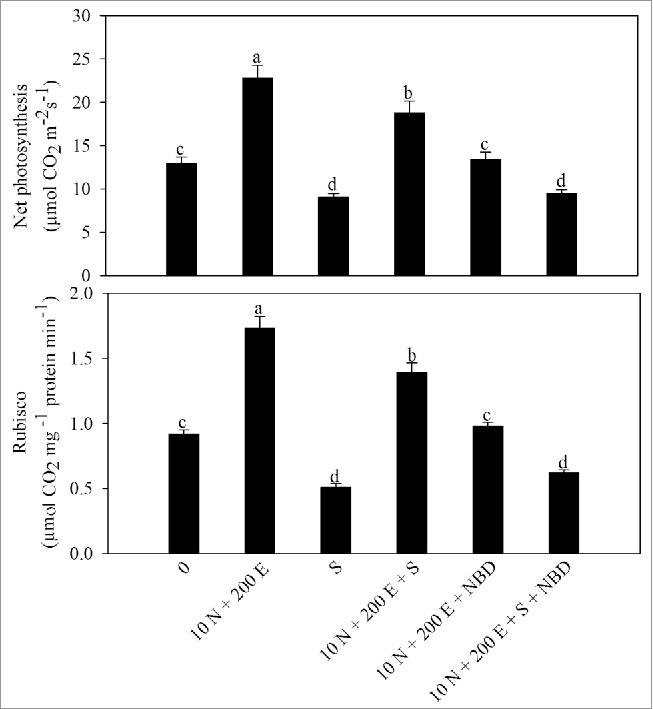
Net photosynthesis and Ribulose 1,5-bisphosphate carboxylase (Rubisco) activity in mustard (Brassica juncea L.) cv Pusa Jai Kisan at 30 DAS. Plants were grown with 100 mM NaCl (S) or 10 mM N plus 200 µl l−1 ethephon (10 N + 200E) individually or in combination, and the plants grown with 10 N + 200 E alone or with NaCl (S) were treated with 100 µM norbornadiene (NBD). Data represent mean values ± SE (n = 4). Means denoted by the same letter are not significantly different at P < 0.05.
Discussion
Salinity tolerance is important in today's context with increasing world population and degrading land due to salt accumulation. To meet the challenges of food security salinity resistant cultivars need to be grown and tolerance strategies must be worked out. Salinity is reported to reduce the yield of crops by affecting plant photosynthetic potential and growth. It causes stomatal closure due to soil water deficit caused by salt-induced osmotic stress, reducing photosynthesis.41 The inhibitory effect of salinity on photosynthesis depends on various factors including gas exchange characteristics, photochemical quenching capacity, reduced Rubisco activity and reduced photosynthetic-NUE.,9,42,43 Salinity-induced nutrient deficiency is yet another important factor for reduced photosynthesis and growth.9,44 The increased accumulation of Na+ and Cl− prevents accumulation of important nutrients causing nutrient deficiency and oxidative stress.45,46 Nutrient enrichment is an important salt tolerance strategy as it reduces the accumulation of toxic Na+ and Cl− ions and increases antioxidants and important metabolites involved in tolerance.9,23,46,47 The roles of S48,Ca49 phosphorus (P).50, K51 in salt tolerance have been reported.
Among the nutrients N is most important and alleviates the negativity of salt stress.9 Besides, phytohormones may regulate salinity tolerance via their effect on nutrient, osmolyte accumulation and antioxidant metabolism. Among phytohormones, ethylene regulates salinity tolerance by inducing several protective mechanisms such as the production of proline9,11 or enhancing the antioxidant system23 through increased nutrient accumulation. Crosstalk of ethylene with N is also available in literature with a synergistic effect of ethylene on N-assimilation.11,21,52 However, till date no report is available regarding the combined effect of N and ethylene in salt tolerance.
The present study focuses on the effect of salinity on plants photosynthetic potential under low and optimum N conditions. Photosynthetic characteristics N, NR activity and photosynthetic-NUE decreased with 100 mM NaCl. However, addition of N significantly alleviated the harmful effect of salinity and increased photosynthetic characteristics, more conspicuously with 10 mM N. The equal increase in photosynthetic characteristics with ethephon or 10 mM N may be associated with the equal ethylene evolution in both the treatments under salt stress. Application of N has been reported to alleviate salt stress by increasing the N-assimilation, photosynthetic-NUE, Rubisco activity and photosynthesis.9 Ethephon, an ethylene releasing compound, is reported to increase Cd stress tolerance in Brassica juncea.53 It increases the N content, NR activity, photosynthetic-NUE, Rubisco activity and thus photosynthesis in plants.21,52 Although, individual effect of both N and ethephon has been reported, however, the combined effect of the two goes unreported. In the present study it was observed that application of ethephon to N treated plants further increased N-assimilation (N content and NR activity) in plants; the maximum increase was with the combined application of ethephon and 10 mM N. The increase in photosynthetic-NUE with ethephon and 10 mM N suggested greater investment of N in the photosynthetic machinery, resulting in higher Rubisco activity and photosynthesis. In our earlier study21 it was found that 200 µl l−1 ethephon with optimum N (80 mg N kg−1soil) maximally increased the NUE in plants and increased stomatal conductance and intercellular CO2 concentration. In the present study, it was observed that in the presence of N, ethephon more efficiently increased the N-assimilation and antioxidant and proline metabolism than their individual effects.
Salinity stressed plants exhibited higher oxidative stress with increased content of Na+ and Cl− and H2O2 and TBARS content. Application of N or ethephon reduced the oxidative stress in stressed plants and the combined application of optimum N and ethephon maximally reduced the oxidative stress. The observed decreased oxidative stress in salinity treated plants upon ethephon and N application might be due to increased activity of antioxidative enzymes, content of proline and GSH and redox state.
Combined application of both ethephon and N increased the APX, GR and SOD activity under salt stress. Antioxidative enzymes and antioxidants are key players in detoxifying the ROS to maintain cellular redox homeostasis within its physiologic limits.54,55 Kachout et al.56 reported that under salt stress a general increase in APX, GR and SOD activity occurred. Dogan et al.57 reported that the balance between ROS production and scavenging decides the degree of damage to the plants. Upon treatment of ethephon to optimum N grown plants maximum increase in antioxidative enzymes activity occurred that destroyed the salinity-induced ROS and enhanced salt tolerance. Öztürk and Demir58 reported that ethephon under salt stress increased catalase and polyphenol oxidase activities and proline content, but decreased peroxidase activity. GSH is an important antioxidant that helps in abiotic stress tolerance. GSH maintains reduced cellular redox environment via metabolizing the varied ROS and their reaction products.59,60 Jozefczak et al.61 reported that GSH plays an important role in Cd chelation as well as in the control of the oxidative challenge. Similarly, Nazar et al.23 reported the role of GSH in salinity tolerance. In a review, Anjum et al.8 reported that both GSH and proline are involved in salinity and metal tolerance. Both GSH and proline are related to ethylene signaling under stress. El-Bassiouny and Bekheta,62 reported that under salt stress, the level of both ethylene and proline content increased in Gimeza 9 cultivar of Triticum aestivum. The treatment of 150 ppm ethrel (ethylene producer) produced highest proline content in Jatropha curcas L. plants.63 Ethylene also regulates GSH level for stress tolerance. Yoshida et al.64 suggested that ethylene and salicylic acid protect against ozone-induced damage in A. thaliana leaves by increasing GSH biosynthesis. Cao et al.65 stated that ethylene signaling mediates lead resistance in A. thaliana seedlings partially in a GSH dependent mechanism. Guan et al.66 suggested that under Cd stress, enhanced LchERF gene expression was responsible partially for the GSH accumulation and the ethylene signal transduction pathways might be involved in GSH accumulation. Nazar et al.18 reported that under salt stress N and S incorporate more efficiently in the thiol (−SH) groups of proteins (cysteine) and its immediate metabolite GSH in salt-tolerant cultivar. Song and Lim67 reported increased GSH accumulation upon N depletion.
Application of ethephon to plants receiving 10 mM N increased proline content in salt treated plants. There is no report regarding the direct effect of ethylene on proline but here we observed that addition of ethephon increases proline accumulation through its regulation on proline metabolizing enzymes both under stress or no stress. Thus, ethephon application increases the N-assimilation in plants that increases proline accumulation. Proline functions as an osmolytes to maintain osmotic balance and as an antioxidant to scavenge the deleterious ROS generated due to salt stress. It maintains cell turgor and cellular redox homeostasis.,68,69 The regulation of proline by ethylene has been reported9,11 but effect of ethylene and N on enzymes related to proline production has not been reported earlier.
Norbornadiene inhibited ethylene action and reduced ethylene sensitivity of plants due to the autocatalytic regulation of ethylene production and inhibited action by binding to the ethylene receptors and suppressed ethylene signaling. Inhibiting ethylene action resulted in decreased N-assimilation, proline and GSH content, photosynthetic-NUE, Rubisco activity and subsequently photosynthesis in both salt stressed plants or without stress plants. Although salt stressed plants suffered a more vigorous reduction in photosynthesis compared with non-stressed plants receiving optimum N and ethephon. This might be due to the fact that inhibition of ethylene action inhibits salt stressed induced ethylene signaling for activating defense process and N in part may also function independent of ethylene.
In conclusion, it may be said that ethylene regulates N-assimilation, proline and antioxidant metabolism and eventually photosynthetic-NUE and photosynthesis under salt stress. The study suggests that there is a regulatory interaction between N and ethylene for salt tolerance and protection of photosynthesis. The use of NBD as action inhibitor of ethylene resulting decrease in N assimilation, proline and antioxidant metabolism, photosynthetic-NUE, Rubisco activity and photosynthesis suggests that ethylene acts as a signaling molecule for regulation of photosynthesis both in presence and absence of salinity stress.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
First author is thankful to the University Grants Commission, New Delhi for DS Kothari Post-Doctoral Fellowship (File No.-No.F.4–2/2006 (BSR)/13–848/2013(BSR)) and to the Head, Department of Botany, Jamia Hamdard, New Delhi for providing research facilities.
References
- 1.Mishra S, Jha AB, Dubey RS. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 2011; 248:565-77; PMID:20857150; https://doi.org/ 10.1007/s00709-010-0210-0 [DOI] [PubMed] [Google Scholar]
- 2.Srivastava S, Dubey RS. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedling. Plant Growth Regul 2011; 64:1-16; https://doi.org/ 10.1007/s10725-010-9526-1 [DOI] [Google Scholar]
- 3.Maheshwari R, Dubey RS. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul 2009; 59:37-49; https://doi.org/ 10.1007/s10725-009-9386-8 [DOI] [Google Scholar]
- 4.Abogadallah GM. Antioxidative defense under salt stress. Plant Signal Behav 2010; 5:369-74; PMID:20118663; https://doi.org/ 10.4161/psb.5.4.10873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munns R, Tester M. Mechanisms of salinity tolerance. Ann Rev Plant Biol 2008; 59:651-81; PMID:18444910; https://doi.org/ 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- 6.Grattan SR, Grieve CM. Salinity-mineral nutrient relations in horticultural crops. Sci Hort 1999; 78:127-57; https://doi.org/ 10.1016/S0304-4238(98)00192-7 [DOI] [Google Scholar]
- 7.Shao QS, Shu S, Du J, Xing WW, Guo SR, Sun J. Effects of NaCl stress on nitrogen metabolism of cucumber seedlings. Russ J Plant Physiol 2015; 62:595-603; https://doi.org/ 10.1134/S1021443715050155 [DOI] [Google Scholar]
- 8.Anjum NA, Aref IM, Duarte AC, Pereira E, Ahmad I, Iqbal M. Glutathione and proline can coordinately make plants withstand the joint attack of metal(loid) and salinity stresses. Front Plant Sci 2014; 5:662; PMID:25484889; https://doi.org/ 10.3389/fpls.2014.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal N, Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard. J Plant Physiol 2015; 178:84-91; PMID:25800225; https://doi.org/ 10.1016/j.jplph.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 10.Filippou P, Bouchagier P, Skotti E, Fotopoulos V. Proline and reactive oxygen/nitrogenspecies metabolism is involved in the tolerant response of the invasiveplant species Ailanthus altissima to drought and salinity. Environ Exp Bot 2014; 97:1-10; https://doi.org/ 10.1016/j.envexpbot.2013.09.010 [DOI] [Google Scholar]
- 11.Iqbal N, Umar S, Khan NA, Khan MIR. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ Exp Bot 2014; 100:34-42; https://doi.org/ 10.1016/j.envexpbot.2013.12.006 [DOI] [Google Scholar]
- 12.Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 1998; 49:249-79; PMID:15012235; https://doi.org/ 10.1146/annurev.arplant.49.1.249 [DOI] [PubMed] [Google Scholar]
- 13.Krapp A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr Opin Plant Biol 2015; 25:115-22; PMID:26037390; https://doi.org/ 10.1016/j.pbi.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 14.Tarighaleslami M, Zarghami R, Boojar MMA, Oveysi M. Effects of drought stress and different nitrogen levels on morphological traits of proline in leaf and protein of corn seed (Zea mays L.). Am-Eurasian J Agric Environ Sci 2012; 12:49-56. [Google Scholar]
- 15.Neuberg M, Pavlíková D, Pavlík M, Balík J. The effect of different nitrogen nutrition on proline and asparagine contentin plant. Plant Soil Environ 2010; 56:305-11. [Google Scholar]
- 16.Hu Y, Schimdhalter U. Interactive effects of salinity and macronutrient level on wheat. II. Composition. J Plant Nutr 1997; 20:1169-82; https://doi.org/ 10.1080/01904169709365325 [DOI] [Google Scholar]
- 17.Zhang F, Wan X, Zhong Y. Nitrogen as an important detoxification factor to cadmium stress in poplar plants. J Plant Interac 2014; 9:249-58; https://doi.org/ 10.1080/17429145.2013.819944 [DOI] [Google Scholar]
- 18.Nazar R, Iqbal N, Syeed S, Khan NA. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol 2011; 168:807-15; PMID:21112120; https://doi.org/ 10.1016/j.jplph.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006; 311:91-4; PMID:16400150; https://doi.org/ 10.1126/science.1118642 [DOI] [PubMed] [Google Scholar]
- 20.Iqbal N, Trivellini A, Masood A, Ferrante A, Khan NA. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol Biochem 2013; 73:128-38; PMID:24095919; https://doi.org/ 10.1016/j.plaphy.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 21.Iqbal N, Nazar R, Syeed S, Masood A, Khan NA. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis and growth under optimal and deficient nitrogen fertilization in mustard. J Exp Bot 2011; 62:4955-63; PMID:21705383; https://doi.org/ 10.1093/jxb/err204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan NA, Mir MR, Nazar R, Singh S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol 2008; 10:534-38; PMID:18761492; https://doi.org/ 10.1111/j.1438-8677.2008.00054.x [DOI] [PubMed] [Google Scholar]
- 23.Nazar R, Khan MIR, Iqbal N, Masood A, Khan NA. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulphur in mustard. Physiol Plant 2014; 152:331-44; PMID:24547902; https://doi.org/ 10.1111/ppl.12173 [DOI] [PubMed] [Google Scholar]
- 24.Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. England: Commonwealth Agricultural Bureaux; 1996. [Google Scholar]
- 25.Okuda T, Matsuda Y, Yamanaka A, Sagisaka S. Abrupt increase inthe level of hydrogen peroxide in leaves ofwinter wheat is caused bycold treatment. Plant Physiol 1991; 97:1265-7; PMID:16668520; https://doi.org/ 10.1104/pp.97.3.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels dismutase and catalase. J Exp Bot 1981; 32:93-101; https://doi.org/ 10.1093/jxb/32.1.93 [DOI] [Google Scholar]
- 27.Bates LE, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil 1973; 39:205-7; https://doi.org/ 10.1007/BF00018060 [DOI] [Google Scholar]
- 28.Hayzer DJ, Leisinger TH. The gene–enzyme relationships of proline biosynthesis in Escherichia coli. J Genet Microbiol 1980; 118:287-93; PMID:6255065; https://doi.org/ 10.1099/00221287-118-2-287 [DOI] [PubMed] [Google Scholar]
- 29.Huang AHC, Cavalieri AJ. Proline oxidase and water stress induced proline accumulation in spinach leaves. Plant Physiol 1979; 63:531-535; PMID:16660761; https://doi.org/ 10.1104/pp.63.3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 1987; 161:559-66; PMID:3034103; https://doi.org/ 10.1016/0003-2697(87)90489-1 [DOI] [PubMed] [Google Scholar]
- 31.Giannopolitis CN, Ries SK. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 1977; 59:309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 1981; 22:867-80. [Google Scholar]
- 33.Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976; 133:21-5; PMID:24425174; https://doi.org/ 10.1007/BF00386001 [DOI] [PubMed] [Google Scholar]
- 34.Griffith OW. Determination of glutathione disulphide using glutathione reductase and 2 vinylpyridine. Anal Biochem 1980; 106:207-12; PMID:7416462; https://doi.org/ 10.1016/0003-2697(80)90139-6 [DOI] [PubMed] [Google Scholar]
- 35.Kuo TM, Warner RL, Kleinhofs A. In vitro stability of nitrate reductase from barleyleaves. Phytochemistry 1982; 21:531-33; https://doi.org/ 10.1016/0031-9422(82)83134-8 [DOI] [Google Scholar]
- 36.Nakagawa H, Poulle M, Oaks A. Characterization of nitrate reductase from corn leaves (Zea mays cv W64A × W182E). Plant Physiol 1984; 75:285-89; PMID:16663612; https://doi.org/ 10.1104/pp.75.2.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindner RC. Rapid analytical methods for some of the more common inorganic con-stituents of plant tissues. Plant Physiol 1944; 19:76-89; PMID:16653905; https://doi.org/ 10.1104/pp.19.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usuda H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol 1985; 91:455-63. [Google Scholar]
- 39.Avni A, Bailey BA, Mattoo AK, Anderson JD. Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol 1994; 106:1049-55; PMID:7824643; https://doi.org/ 10.1104/pp.106.3.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woeste KE, Ye C, Kieber JJ. Two Arabidopsis mutants that overproduce ethylene are affected in the post transcriptional regulation of 1-aminocyclopropane 1-carboxylic acid synthase. Plant Physiol 1999; 119:521-29; https://doi.org/ 10.1104/pp.119.2.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahnama A, Poustini K, Tavakkol-Afshari R, Tavakoli A. Growth and stomatal responses of bread wheat genotypes in tolerance to salt stress. Int J Biol Life Sci 2010; 6:216-21. [Google Scholar]
- 42.Abbasi GH, Akhtar J, Haq MA, Ali S, Chen ZH, Malik W. Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak J Bot 2014; 46:135-46. [Google Scholar]
- 43.Abbasi GH, Akhtar J, Haq MA, Ahmad N. Screening of maize hybrids for salt tolerance at seedling stage under hydroponic condition. Soil and Environ 2012; 31:83-90. [Google Scholar]
- 44.Parida AK, Das AB. Salt tolerance and salinity effects on plants: A review. Ecotoxicol Environ Saf 2005; 60:324-49; PMID:15590011; https://doi.org/ 10.1016/j.ecoenv.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 45.Khan NA, Syeed S, Masood A, Nazar R, Iqbal N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int J Plant Biol 2010; 1:e1; https://doi.org/ 10.4081/pb.2010.e1 [DOI] [Google Scholar]
- 46.Khorshidi MB, Yarnia M, Hassanpanah D. Salinity effect on nutrients accumulationin alfalfa shoots in hydroponic condition. J Food Agric Environ 2009; 7:787-90. [Google Scholar]
- 47.Song JY, Roe JH. The role and regulation of Trxl, a cytosolic thioredoxin in Schizosac-charomyces pombe. J Microbiol 2008; 46:408-14; PMID:18758731; https://doi.org/ 10.1007/s12275-008-0076-4 [DOI] [PubMed] [Google Scholar]
- 48.Nazar R, Umar S, Khan NA. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal Behav 2015; 10:e1003751; PMID:25730495; https://doi.org/ 10.1080/15592324.2014.1003751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 2006; 141:1653-65; PMID:16798942; https://doi.org/ 10.1104/pp.106.082388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feigin A. Fertilization management of crops irrigated with saline water. Plant Soil 1985; 89:285-99; https://doi.org/ 10.1007/BF02182248 [DOI] [Google Scholar]
- 51.Shirazi MU, Ashraf MY, Khan MA, Naqvi MH. Potassium induced salinity tolerancein wheat. Int J Environ Sci Technol 2005; 2:233-66; https://doi.org/ 10.1007/BF03325881 [DOI] [Google Scholar]
- 52.Iqbal N, Khan NA, Nazar R, Taxievera da Silva J. Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ Exp Bot 2012; 78:84-90; https://doi.org/ 10.1016/j.envexpbot.2011.12.025 [DOI] [Google Scholar]
- 53.Masood A, Iqbal N, Khan NA. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ 2012; 35:524-33; PMID:21950968; https://doi.org/ 10.1111/j.1365-3040.2011.02432.x [DOI] [PubMed] [Google Scholar]
- 54.Teh CY, Mahmood M, Shaharuddin NA, Ho CL. In vitro rice shoot apices as simple model to study the effect of NaCl and the potential of exogenous proline and glutathione in mitigating salinity stress. Plant Growth Regul 2014; 75:1-11. [Google Scholar]
- 55.Kaya C, Tuna AL, Ashraf M, Altunlu H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ Exp Bot 2007; 60:397-403; https://doi.org/ 10.1016/j.envexpbot.2006.12.008 [DOI] [Google Scholar]
- 56.Kachout SS, Hamza KJ, Bouraoui NK, Leclerc JC, Ouerghi Z. Salt-induced changes in antioxidative enzyme activities in shoot tissues of two atriplex varieties. Not Bot Horti Agrobo 2013; 41:115-21. [Google Scholar]
- 57.Dogan M, Tipirdamaz R, Demir Y. Effective salt criteria in callus-cultured tomato genotypes. Z Naturforsch 2010; 65:613-18; PMID:21138065. [DOI] [PubMed] [Google Scholar]
- 58.Öztürk L, Demir Y. Effects of putrescine and ethephon on some oxidative stress enzyme activities and proline content in salt stressed spinach leaves. Plant Growth Regul 2003; 40:89-95; https://doi.org/ 10.1023/A:1023078819935 [DOI] [Google Scholar]
- 59.Kocsy G, Szalai G, Galiba G. Effect of osmotic stress on glutathione and hydroxymethylglutathione accumulation in wheat. J Plant Physiol 2004; 161:785-94; PMID:15310067; https://doi.org/ 10.1016/j.jplph.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 60.Ruiz JM, Blumwald E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002; 214:965-69; PMID:11941474; https://doi.org/ 10.1007/s00425-002-0748-y [DOI] [PubMed] [Google Scholar]
- 61.Jozefczak M, Keunen E, Schat H, Bliek M, Hernández LE, Carleer R, Remans T, Bohler S, Vangronsveld J, Cuypers A. Differential response of arabidopsis leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant PhysiolBiochem 2014; 83:1-9; PMID:25049163; https://doi.org/ 10.1016/j.plaphy.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 62.El-Bassiouny HMS, Bekheta MA. Effect of salt stress on relative water content, lipid peroxidation, polyamines, amino acids and ethylene of two wheat cultivars. Int J Agric Biol 2005; 7:363-8. [Google Scholar]
- 63.Joshi G, Shukla I, Shukla A. Synergistic response of auxin and ethylene on physiology of Jatropha curcas L. Brazilian J Plant Physiol 2011; 23:67-77; https://doi.org/ 10.1590/S1677-04202011000100009 [DOI] [Google Scholar]
- 64.Yoshida S, Tamaoki M, Ioki M, Ogawa D, Sato Y, Aono M, Kubo A, Saji S, Saji H, Satoh S, et al.. Ethylene and salicylic acid control glutathione biosynthesis in ozone-exposed Arabidopsis thaliana. Physiol Plant 2009; 136:284-98; PMID:19453511; https://doi.org/ 10.1111/j.1399-3054.2009.01220.x [DOI] [PubMed] [Google Scholar]
- 65.Cao S, Chen Z, Liu G, Jiang L, Yuan H, Ren G, Bian X, Jian H, Ma X. The arabidopsis ethylene-insensitive 2 gene is required for lead resistance. Plant Physiol Biochem 2009; 47:308-12; PMID:19153049; https://doi.org/ 10.1016/j.plaphy.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 66.Guan C, Jing J, Dianyun W. The glutathione synthesis may be regulated by cadmium-induced endogenous ethylene in Lycium chinense, and overexpression of an ethylene responsive transcription factor gene enhances tolerance to cadmium stress in tobacco. Mol Breeding 2015; 35:1-13; https://doi.org/ 10.1007/s11032-015-0202-z [DOI] [Google Scholar]
- 67.Song SH, Lim CJ. Nitrogen depletion causes up-regulation of glutathione content and gamma-glutamyltranspeptidase in Schizosaccharomyces pombe. J Microbiol 2008; 46:70-74; PMID:18337696; https://doi.org/ 10.1007/s12275-007-0244-y [DOI] [PubMed] [Google Scholar]
- 68.Kishor PBK, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 2014; 37:300-11; PMID:23790054; https://doi.org/ 10.1111/pce.12157 [DOI] [PubMed] [Google Scholar]
- 69.Szabados L, Savouré A. Proline: A multifunctional amino acid. Trend Plant Sci 2010; 15:89-97; PMID:20036181; https://doi.org/ 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]


