ABSTRACT
In land plants, plastid and mitochondrial RNAs are subject to post-transcriptional C-to-U RNA editing. T-DNA insertions in the ORGANELLE RNA RECOGNITION MOTIF PROTEIN6 gene resulted in reduced photosystem II (PSII) activity and smaller plant and leaf sizes. Exon coverage analysis of the ORRM6 gene showed that orrm6–1 and orrm6–2 are loss-of-function mutants. Compared to other ORRM proteins, ORRM6 affects a relative small number of RNA editing sites. Sanger sequencing of reverse transcription-PCR products of plastid transcripts revealed 2 plastid RNA editing sites that are substantially affected in the orrm6 mutants: psbF-C77 and accD-C794. The psbF gene encodes the β subunit of cytochrome b559, an essential component of PSII. The accD gene encodes the β subunit of acetyl-CoA carboxylase, a protein required in plastid fatty acid biosynthesis. Whole-transcriptome RNA-seq demonstrated that editing at psbF-C77 is nearly absent and the editing extent at accD-C794 was significantly reduced. Gene set enrichment pathway analysis showed that expression of multiple gene sets involved in photosynthesis, especially photosynthetic electron transport, is significantly upregulated in both orrm6 mutants. The upregulation could be a mechanism to compensate for the reduced PSII electron transport rate in the orrm6 mutants. These results further demonstrated that Organelle RNA Recognition Motif protein ORRM6 is required in editing of specific RNAs in the Arabidopsis (Arabidopsis thaliana) plastid.
KEYWORDS: Arabidopsis thaliana, exon coverage analysis, generally applicable gene-set enrichment for pathway analysis, organelle RNA recognition motif, plastid RNA editing, Sanger sequencing, wholetranscriptome RNA-seq
Introduction
RNA editing is modification of specific nucleotides/nucleosides within RNA molecules. Types of RNA editing include nucleotide insertion/deletion, cytidine-to-uridine (C-to-U) and adenosine-to-inosine (A-to-I) deamination, and uridine-to-cytidine (U-to-C) reverse editing.1,2 RNA editing has been found in viruses, primitive eukaryotes, vertebrates, fungi, and plants, and it occurs in the nucleus, the cytosol, mitochondria, and plastids. RNA editing in plants has been observed in different types of RNAs, but is most abundant in the coding regions of mRNAs.3 RNA editing in the coding regions of mRNAs restores the function of the encoded protein; therefore, it is considered as a mechanism of correction to compensate for defects in the genomes. Organellar (plastid and mitochondrial) RNA editing occurs in almost all land plants.3-6 Two types of RNA editing have been found in plastids and/or mitochondria of flowering plants: C-to-U editing in mRNAs and C-to-U and A-to-I editing in tRNAs.7-10 C-to-U editing in plastid and mitochondrial mRNAs appear to be ubiquitous in land plants.
Prior to the widespread use of next-generation sequencing technologies, detection and quantitation of RNA editing events was laborious and limited by the resolution and strand length of Sanger sequencing as well as a priori knowledge of primer sets. High-throughput methods using PCR technology11 brought the number of discovered plastid editing events up to 34. Of the 34 known events, 2 are within non-coding regions. All editing events within the coding regions result in single amino acid changes, except for one which results in a new translation start site. With next-generation sequencing technologies, we are able to simultaneously detect and quantify editing without a priori knowledge of the sites. The use of RNA-seq technology led to the discovery of a total of 40 plastid editing sites in Columbia (Col-0) wild-type Arabidopsis.12,13 Of the 6 newly discovered sites, only 2 are in the coding region of a gene. The 2 coding region editing events occur within the ndhB gene, change the third base in the corresponding codons, and result in 2 silent mutations. The other 4 sites are within intronic, intergenic, or 3’ UTR regions and are of low-editing-extent (≤12%). The 6 new sites raise questions about the origin of new editing events and the fidelity of editosomes (i.e., editing complexes). Editing events with such low extent and non-obvious biologic implications suggest that some infidelity can exist without detrimental effects and negative selection pressure. It is also possible that the existence of these editing events could be the mechanism that allows for the selection of novel editing events.
C-to-U RNA editing is performed by editosomes of 200 – 400 kDa in size.14,15 Four types of proteins have been identified as C-to-U RNA editing factors in the plastid: PLS-E-DYW subfamily pentatricopeptide repeat proteins (PPR-DYWs), RNA editing interacting proteins / multiple organellar RNA editing factors (RIPs/MORFs), RNA recognition motif-containing proteins (ORRMs), and organelle zinc-finger proteins (OZs).15-20 As described below, each of the 4 types of proteins is considered as a component of the C-to-U RNA editosomes.
PPR-DYW proteins contain multiple PLS-type PRR repeats, an extension (E) domain, and a C-terminal DYW domain.18 In silico search of the Arabidopsis genome identified 87 PPR-DYW proteins, 28% of which are targeted to the plastid, 36% of which are targeted to the mitochondrion.21 PLS-type PRR repeats allow PPR-DYW proteins to bind single-stranded RNAs in a sequence-dependent manner.18 The E domain is essential for recruiting a protein with cytidine deaminase activity.22 The DYW domain contains 3 conserved amino acids―DYW, as well as signature amino acids of classic cytidine deaminases, including the HXE motif for pyrimidine protonation and CXXC for zinc coordination.22-24 Although 7 classic cytidine deaminases are encoded by the Arabidopsis genome, none of them are active in organellar RNA editing.3,25 Therefore, PPR-DYW proteins are currently the prime candidate for deaminase activity in plant organelle RNA editing.3,18 For example, LOW PHOTOSYSTEM II ACCUMULATION 66 (LPA66), a plastid-targeted PPR-DYW protein, is specifically required for RNA editing at the psbF-C77 site.26 C77 is the nucleotide number of the cytidine [C] target relative to the nucleotide A of the translation initiation codon ATG in the psbF transcript, which encodes the β subunit of cytochrome b559. A second example is REQUIRED FOR ACCD RNA EDITING 1 (RARE1), another plastid-targeted PPR-DYW protein. RARE1 is essential for RNA editing at cytidine 794 (C794) of the accD transcript,27 which encodes the β subunit of acetyl-coenzyme A carboxylase carboxyl transferase. Proteins with PPR and DYW domains do not necessarily specify sequence and perform deamination simultaneously. The DYW domain of PPR-DYW proteins may be recruited by PPR proteins without a DYW domain to carry out deamination activity.24
RIP/MORF proteins contain conserved RIP/MORF boxes. 16 The Arabidopsis genome encodes 9 functional RIP/MORF proteins: RIP1/MORF8 is dual-targeted to plastids and mitochondria, RIP2/MORF2 and RIP9/MORF9 are targeted to the plastid, and the rest are targeted to the mitochondrion.14,16 Unlike PPR-DYW proteins, which are RNA-sequence specific, RIP/MORF proteins are broadly involved in plastid and/or mitochondrial RNA editing. Fourteen plastid sites and 266 mitochondrial sites are affected in the rip1/morf8 mutant and nearly all plastid sites affected in rip2/morf2 and rip9/morf9 mutants.14,16 RIP/MORF proteins have been found to interact with PPR-DYW proteins via the RIP/MORF box. For example, RIP1/MORF8 interacted with plastid-targeted RARE1 and mitochondrion-targeted MITOCHONDRIAL RNA EDITING FACTOR 10 (MEF10)14,28 RIP/MORF proteins were also found to interact with themselves and other RIP/MORF proteins, suggesting that these proteins may form homo- and hetero-oligomers.16
ORRM proteins contain a RRM; 5 ORRM proteins have been found to be involved in plastid or mitochondrial RNA editing.15,17,19,29 The plastid-targeted ORRM1 is the founding member of this protein family.17 Unlike other ORRMs, ORRM1 contains 2 RIP/MORF boxes, which are required for its interaction with PPR-DYW protein RARE1. The orrm1 mutant showed near complete loss of editing at 12 plastid sites. ORRM2, ORRM3, and ORRM4 are targeted to the mitochondrion and none of them have RIP/MORF boxes. 15,19 The orrm2, orrm3, and orrm4 mutants displayed decreased editing extents at 35, 32, and 262 mitochondrial RNA editing sites, respectively. ORMM6 is targeted to the plastid and it does not have RIP/MORF boxes. 29 The orrm6 mutants demonstrated reduced editing extents at the psbF-C77 and accD-C794 RNA editing sites. ORRM3 was found to interact with RIP1/MORF8, ORRM2, and itself; ORRM4 was found to interact with ORRM3 and itself; and ORRM6 was found to interact with RIP1/MORF8, RIP2/MORF2, RIP9/MORF9, and itself.29 The interactions among ORRMs suggest that ORRMs may form homo- and/or hetero-oligomers.
OZ proteins contain multiple Ran-binding-protein-2 (RanBP2, CXXCX10CXXC) type zinc-finger domains.20 The Arabidopsis genome encodes 4 OZ proteins: OZ1 is targeted to the plastid; OZ2, OZ3, and OZ4 are targeted to the mitochondrion. A loss-of-function mutation in the OZ1 gene resulted in major loss of editing at 14 plastid sites and significant changes in the editing extent at 16 other plastid sites.20 Despite of the large number of editing sites altered in the oz1 mutant, C targets on the same transcripts are differentially affected, suggesting that OZ1 action is site-specific. Consistent with this hypothesis, some RanBP2-type zinc fingers have been shown to bind single-stranded RNAs on a sequence-specific manner.30 OZ1 interacted with itself, ORRM1, RIP1/MORF8, as well as plastid-targeted PPR-DYW proteins CHLORORESPIRATORY REDUCTION 28 (CRR28) and ORGANELLE TRANSCRIPT PROCESSING 82 (OTP82).20 Like RIP/MORF and ORRM proteins, OZ1 interacted with itself in yeast 2-hybrid assays, suggesting that OZ1 may form homooligomers.
In this work, we used a combination of Sanger sequencing and whole-transcriptome RNA-seq to explore the editing site specificity of ORRM6, a unique plastid-targeted RNA editing factor. In addition, we conducted gene set enrichment pathway analysis and identified several Gene Ontology gene sets that were significantly and consistently upregulated in the loss-of-function orrm6 mutants.
Exon coverage analysis confirmed that orrm6–1 and orrm6–2 are loss-of-function mutants with truncated transcripts
Quantitative RT-PCR showed that the functional ORRM6 transcript (i.e., the full length ORRM6 transcript without the T-DNA insertion) cannot be detected in either orrm6 mutant (Fig. 1 in ref.29). This suggests that orrm6–1 and orrm6–2 are loss-of-function mutants. To confirm this, BEDtools and Integrative Genomics Viewer were used to examine the exon coverage of the ORRM6 transcript (Fig. 1) with RNA-seq data from the wild type and the orrm6 mutants. The relative abundance of the first exon in the orrm6 mutants is approximately 60% lower than that in the wild type (Fig. 1B). In the orrm6 mutants, exons 2-4 are almost completely absent (Fig. 1). These data confirmed that T-DNA insertions in the first intron of the ORRM6 gene result in a truncated ORRM6 transcript lacking exons 2–4. The first exon encodes the first 82 amino acids, which correspond to the chloroplast transit peptide and the region between the chloroplast transit peptide and the RRM. Therefore, it is likely that the correspondingly truncated ORRM6 protein does not contain the RRM. T-DNA insertions could also result in early termination codons and the Integrative Genome Viewer detects improper splicing of the orrm6–1 transcript. Both of these could lead to nonsense-mediated decay of the ORRM6 transcript.31
Figure 1.
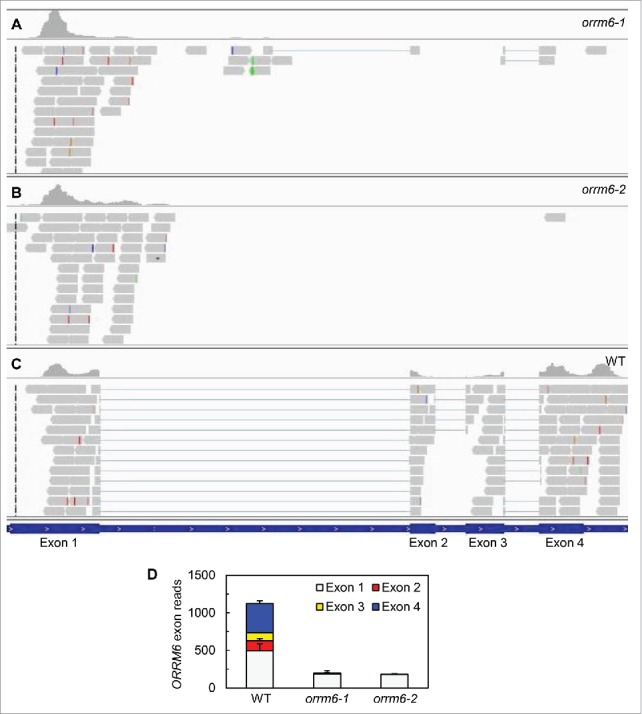
Analysis of ORRM6 transcript levels in the wild-type and orrm6 mutant plants. (A-C) Visual representation of exon reads in RNA samples prepared from orrm6–1, orrm6–2, and wild-type (WT) plants. Integrated Genomic Viewer was used to visually display exon coverage of ORRM6. Each rectangle represents a single 50 bp read and thin lines show reads spanning introns. (D) Numbers of ORRM6 exon reads in RNA samples prepared from the wild-type and orrm6 mutant plants. Total RNA was extracted from mature leaves and analyzed with whole-transcriptome RNA-seq. The values (mean + SE, n = 4) have been normalized by the library size.
Sanger sequencing and whole-transcriptome RNA-seq revealed that ORRM6 is required at 2 plastid RNA editing sites: psbF-C77 and accD-C794
Sanger sequencing of reverse transcription-PCR products from the wild-type and orrm6 mutant plants suggested that ORRM6 is required in editing at 2 plastid RNA editing sites: psbF-C77 and accD-C794. To investigate whether ORRM6 plays additional roles, we performed Sanger sequencing of reverse transcription-PCR products of other plastid transcripts (Fig. 2). The results from Sanger sequencing revealed that other plastid RNA editing sites are not substantially affected by the loss-of-function mutations in the ORRM6 gene (Fig. 2).
Figure 2.
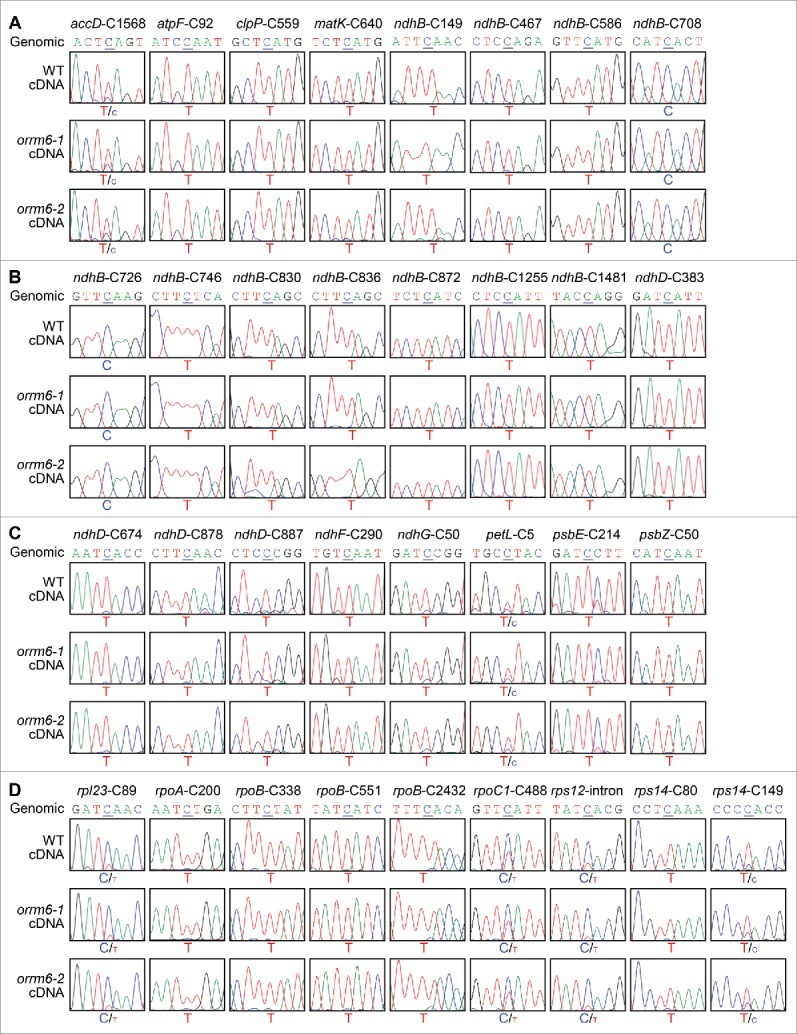
Sanger sequencing of plastid RNA editing sites in the wild-type and orrm6 mutant plants. (A) Analysis of RNA editing at accD-C1568, atpF-C92, clpP-C559, matK-C640, ndhB-C149, ndhB-C467, ndhB-C586, and ndhB-C708. (B) Analysis of RNA editing at ndhB-C726, ndhB-C746, ndhB-C830, ndhB-C836, ndhB-C872, ndhB-C1255, ndhB-C1481, and ndhD-C383. (C) Analysis of RNA editing at ndhD-C674, ndhD-C878, ndhD-C887, ndhF-C290, ndhG-C50, petL-C5, psbE-C214, and psbZ-C50. (D) Analysis of RNA editing at rpl23-C89, rpoA-C200, rpoB-C338, rpoB-C551, rpoB-C2432, rpoC1-C488, rps12-intron, rps14-C80, and rps14-C149. RT-PCR products surrounding the editing sites were directly sequenced. The 7-nucleotide sequences encompassing the cytidine target (underlined) were shown. The corresponding genomic sequences of these 2 sites were displayed as controls.
To quantitatively determine the editing extents at accD-C794, psbF-C77, and other plastid RNA editing sites, we performed whole-transcriptome RNA-seq of Illumina TruSeq libraries made from total mRNAs, as described previously.17,32 In brief, total leaf RNAs were extracted from the wild type and the orrm6 mutants (4 biologic replicates per genotype), rRNAs in the total RNA samples were depleted before mRNA library construction, and the resulting mRNA libraries were sequenced on an Illumina HiSeq 2500. After removal of adaptor sequences and initial checks on sequence quality, sequences (reads) were mapped to a reference genome and assembled into a table with the TopHat software and were visualized with the Integrative Genomics Viewer.32,33 The proportion of edited and unedited mRNAs was calculated for each of the known plastid RNA editing sites. Whole-transcriptome RNA-Seq data identified 2 plastid RNA editing sites that are substantially and consistently reduced in both orrm6–1 and orrm6–2 mutants: psbF-C77 and accD-C794. The editing extent at accD-C794 was 85.8% in the wild type and it was reduced to 30.7% in orrm6–1 and 29.0% in orrm6–2; the editing extent at psbF-C77 was 97.1% in the wild type and it was reduced to 6.8% in orrm6–1 and 6.3% in orrm6–2 (Table 1). In addition to psbF-C77 and accD-C794, whole-transcriptome RNA-seq showed that editing at the intergenic site between ndhK and ndhJ is absent in both orrm6 mutants (Table 1). The biologic significance of this intergenic RNA editing site is not clear. In the wild-type Arabidopsis, only approximately 4.2% of the C target at this RNA editing site was changed to T (Table 1). It is possible that some infidelity exists without detrimental effects on the plant. Taken together, these whole-transcriptome RNA-Seq data are consistent with the results from Sanger sequencing, strand-and-transcript-specific PCR sequencing, and poisoned primer extension.29
Table 1.
Editing extents at plastid RNA editing sites in wild-type and orrm6 mutant plants.
| Gene | Genome positiona | Edited nucleotideb | Δ amino acid | WT | orrm6–1 | orrm6–2 | orrm6 - WTc |
|---|---|---|---|---|---|---|---|
| accD | 57868 | C794 | S265→L | 85.8 ± 3.2 | 30.7 ± 3.0*** | 29.0 ± 2.0*** | −56.0 |
| accD | 58642 | C1568 | 3’-UTR | 83.5 ± 1.6 | 78.8 ± 2.2 | 78.3 ± 1.3 | −4.9 |
| atpF | 12707 | C92 | P31→L | 94.9 ± 0.2 | 95.1 ± 0.3 | 93.8 ± 0.4 | −0.4 |
| atpH | 13210 | C298 | 3’-UTR | 4.4 ± 0.6 | 4.5 ± 0.3 | 4.9 ± 0.3 | 0.3 |
| clpP | 69942 | C559 | H187→Y | 81.3 ± 0.5 | 82.8 ± 0.7 | 78.0 ± 0.7** | −1.4 |
| matK | 2931 | C640 | H214→Y | 71.9 ± 8.6 | 74.4 ± 4.8 | 79.9 ± 0.3 | 5.2 |
| ndhB | 97016 | C149 | S50→L | 56.9 ± 19.9 | 66.6 ± 11.4 | 67.3 ± 5.6 | 10.1 |
| ndhB | 96698 | C467 | P156→L | 76.6 ± 4.9 | 84.0 ± 0.8 | 86.1 ± 0.5 | 8.5 |
| ndhB | 96579 | C586 | H196→Y | 74.6 ± 3.2 | 78.8 ± 1.3 | 81.9 ± 1.0 | 5.8 |
| ndhB | 96457 | C708 | I236→I | 3.6 ± 0.5 | 3.2 ± 0.2 | 3.2 ± 0.4 | −0.4 |
| ndhB | 96439 | C726 | F242→F | 3.5 ± 0.4 | 3.1 ± 0.3 | 2.7 ± 0.2 | −0.6 |
| ndhB | 96419 | C746 | S249→F | 84.8 ± 5.8 | 72.5 ± 3.3 | 76.6 ± 2.2 | −10.3 |
| ndhB | 95650 | C830 | S277→L | 74.7 ± 1.1 | 75.9 ± 2.0 | 77.5 ± 1.7 | 2.0 |
| ndhB | 95644 | C836 | S279→L | 66.2 ± 2.5 | 61.2 ± 2.3 | 61.0 ± 2.5 | −5.0 |
| ndhB | 95608 | C872 | S291→L | 61.9 ± 4.5 | 64.9 ± 1.0 | 59.3 ± 4.5 | 0.2 |
| ndhB | 95225 | C1255 | H419→Y | 87.8 ± 1.1 | 87.5 ± 1.4 | 88.0 ± 1.4 | 0.0 |
| ndhB | 94999 | C1481 | P494→L | 88.2 ± 1.0 | 84.3 ± 0.7* | 85.2 ± 0.7 | −3.5 |
| ndhD | 117166 | C2 | T1→M | 46.4 ± 4.1 | 36.8 ± 5.7 | 51.2 ± 2.0 | −2.5 |
| ndhD | 116785 | C383 | S128→L | 95.4 ± 1.2 | 96.9 ± 0.3 | 96.7 ± 0.8 | 1.4 |
| ndhD | 116494 | C674 | S225→L | 73.8 ± 2.0 | 74.3 ± 4.9 | 78.3 ± 2.0 | 2.5 |
| ndhD | 116290 | C878 | S293→L | 79.1 ± 2.0 | 83.7 ± 1.3 | 86.2 ± 0.5* | 5.8 |
| ndhD | 116281 | C887 | P296→L | 76.7 ± 5.7 | 87.9 ± 0.6 | 87.9 ± 0.9 | 11.2 |
| ndhF | 112349 | C290 | S97→L | 23.4 ± 4.8 | 21.4 ± 3.6 | 26.8 ± 2.7 | 0.7 |
| ndhG | 118858 | C50 | S17→F | 68.4 ± 6.3 | 81.3 ± 4.0 | 75.9 ± 1.4 | 10.2 |
| ndhK-ndhJ | 49209 | C726d | Intergene | 4.2 ± 0.5 | 0.0 ± 0.0** | 0.0 ± 0.0** | −4.2 |
| petL | 65716 | C5 | P2→L | 35.1 ± 12.3 | 50.4 ± 10.4 | 55.8 ± 11.4 | 18.0 |
| psbE | 64109 | C214 | P72→S | 99.3 ± 0.1 | 99.1 ± 0.2 | 99.2 ± 0.0 | −0.2 |
| psbF | 63985 | C77 | S26→F | 97.1 ± 0.3 | 6.8 ± 0.2*** | 6.3 ± 0.3*** | −90.5 |
| psbZ | 35800 | C50 | S17→L | 61.3 ± 8.6 | 49.6 ± 8.8 | 58.9 ± 5.0 | −7.1 |
| rpl23 | 86055 | C89 | S30→L | 82.3 ± 1.9 | 78.1 ± 0.9 | 78.5 ± 0.3 | −4.0 |
| rpoA | 78691 | C200 | S67→F | 58.9 ± 5.4 | 64.2 ± 4.4 | 61.7 ± 4.6 | 4.0 |
| rpoB | 25992 | C338 | S113→L | 65.9 ± 16.6 | 70.4 ± 17.7 | 65.4 ± 8.3 | 1.9 |
| rpoB | 25779 | C551 | S184→L | 38.1 ± 9.1 | 30.8 ± 7.7 | 27.2 ± 2.5 | −9.2 |
| rpoB | 23898 | C2432 | S811→L | 69.9 ± 7.0 | 70.0 ± 3.3 | 60.3 ± 4.9 | −4.8 |
| rpoC1 | 21806 | C488 | S163→L | 20.8 ± 2.9 | 26.7 ± 1.2 | 27.4 ± 2.9 | 6.2 |
| rps4 | 45095 | C734 | 3’-UTR | 3.2 ± 0.2 | 1.9 ± 0.5 | 2.5 ± 0.2 | −1.0 |
| rps12-intron | 69553 | C(i1 58)e | intron | 19.5 ± 1.1 | 13.5 ± 3.2 | 18.6 ± 4.0 | −3.5 |
| rps14 | 37161 | C80 | S27→L | 96.4 ± 0.4 | 95.1 ± 0.2 | 94.8 ± 0.3* | −1.4 |
| rps14 | 37092 | C149 | P50→L | 87.6 ± 2.3 | 91.0 ± 0.2 | 90.7 ± 0.4 | 3.2 |
| ycf3-intron | 43350 | C(i2 174)f | intron | 7.5 ± 0.9 | 4.5 ± 0.3* | 7.8 ± 0.5 | −1.4 |
The editing extents were determined with the whole-transcriptome RNA-seq method. The values (mean ± SE, n = 4) are given as the ratio of the edited transcript to edited plus unedited transcripts. The asterisk indicates significant difference between the mutant and the wild type (WT) (Student's t-test; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
The Arabidopsis plastid genome reference sequence (NCBI accession number NC_000932.1) was used to indicate the genome positions of the editing sites.
TheC targets are numbered relative to the nucleotide A of the predicted translation initiation codon ATG, where A = 1.
This column shows the average difference in editing extents between the wild type (WT) and orrm6 mutants.
The C target is numbered relative to the nucleotide A of ATG of the ndhK transcript, where A = 1.
The target C is the 58th nucleotide of intron 1 (i1 58).
The target C is the 174th nucleotide of intron 2 (i2 174).
Gene set enrichment pathway analysis revealed that expression of genes involved photosynthesis is significantly upregulated in the orrm6 mutants
It was previously found with quantitative reverse transcription (RT)-PCR that the transcript levels of photosystem II (PSII) genes psbA, psbB, and psbI (where psb refers to PSII) are significantly increased in the orrm6–2 mutant (see Supplemental Fig. S3 in ref.29). To further investigate gene expression changes in orrm6 mutants versus wild type, gene set enrichment analysis was performed using the Generally Acceptable Gene-set Enrichment (GAGE) software and the Gene Ontology biologic process gene sets.34,35 Pathways were discovered by comparing gene sets in the Gene Ontology biologic process pathways for Arabidopsis using normalized counts for the wild type vs. a single mutant line and looking for significance. Significantly upregulated pathways are shared among the 2 orrm6 mutants (Table 2). Of the shared upregulated pathways, 5 of them are gene sets for photosynthetic processes. These data are consistent with the previous quantitative RT-PCR analysis which showed increased transcript levels of PSII related genes despite decreased PSII efficiency.29 The increased expression of photosynthetic genes might be a compensatory response to the reduced PSII electron transport rate in the orrm6 mutants.
Table 2.
Gene sets that were significantly upregulated in the orrm6–1 and orrm6–2 mutants.
| WT vs. orrm6–1 | p value | WT vs. orrm6–2 | p value |
|---|---|---|---|
| DNA-templated transcription, elongation | 0.020 | DNA-templated transcription, elongation | 0.012 |
| Photosynthetic electron transport in PSII | 0.030 | Photosynthesis | 0.021 |
| Photosynthetic electron transport chain | 0.031 | Photosynthetic electron transport chain | 0.025 |
| Electron transport chain | 0.032 | Electron transport chain | 0.025 |
| PSII assembly | 0.033 | PSII assembly | 0.025 |
| Photosynthesis | 0.045 | Photosynthesis, light reaction | 0.026 |
| Photosynthesis, light reaction | 0.049 | Photosynthetic electron transport in PSII | 0.028 |
Gene sets significantly upregulated in the orrm6 mutants were discovered through the Generally Acceptable Gene-set Enrichment pathway analysis of the RNA-seq data, using the Gene Ontology biologic process gene sets.
ORRM6 is a unique C-to-U RNA editing factor in the plastid
ORRM6 differs from ORRM1, the other plastid-targeted ORRM protein, in that it does not contain any RIP/MORF boxes. The 2 RIP/MORF boxes in ORRM1 are required for its interaction with PPR-DYW proteins.17 Consistent with the absence of RIP/MORF boxes in ORRM6, this protein did not interact with PPR-DYW proteins LPA66 and RARE1 in reciprocal bimolecular fluorescence complementation (BiFC) assays.29 LPA66 and RARE1 are required for editing at psbF-C77 and accD-C794, respectively.26,27 Interestingly, ORRM6 was found to interact with RIP1/MORF8, RIP2/MORF2, and RIP9/MORF9, when transiently co-expressed in Nicotiana benthamiana leaves.29 Therefore we hypothesize that the interaction between ORRM6 and RIP/MORF proteins may compensate for the absence of RIP/MORF boxes in ORRM6. ORRM6 was also found to interact with OZ1 and itself in BiFC assays.29 Therefore, we propose that the editosome at psbF-C77 may require LPA66, ORRM6, RIP/MORF proteins (RIP1/MORF8, RIP2/MORF2, and/or RIP9/MORF9), and OZ1, and the editosome at accD-C794 may require RARE1, ORRM6, RIP/MORF proteins (RIP1/MORF8, RIP2/MORF2, and/or RIP9/MORF9), and OZ1 (Fig. 3).
Figure 3.
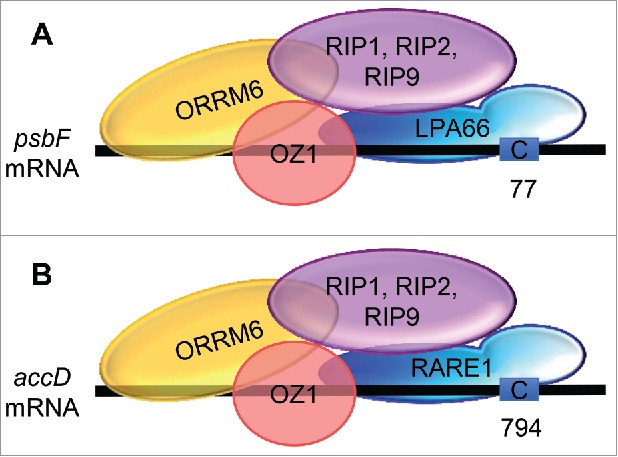
Tentative models for editosomes at the psbF-C77 and accD-C794 RNA editing sites based on protein-protein interaction data. (A) Model for the editosome at psbF-C77. (B) Model for the editosome at accD-C794. For simplicity, only one name is shown for proteins with multiple names (e.g.,"RIP1" for RIP1/MORF8). Black lines represent transcripts; the letter C in blue boxes represents the cytidine target.
ORRM6 is also unique due to the relatively small number of RNA editing sites (psbF-C77 and accD-C794) that are affected in the orrm6 mutants. On the contrary, loss-of-function mutations in the ORRM1 gene resulted in near complete loss of editing at 12 plastid sites,17 and loss-of-function mutations in the ORRM2, ORRM3, and ORRM4 genes resulted in decreased editing at 35, 32, and 262 mitochondrial sites, respectively.15,20 Therefore, it is possible that different ORRM proteins have different editing site specificities.
Materials and methods
Plant materials and growth conditions
The 2 Arabidopsis (Arabidopsis thaliana) T-DNA insertion lines (orrm6–1 [SAIL_763_A05] and orrm6–2 [WiscDsLox485–488P23], both in Columbia ecotype) were obtained from the Arabidopsis Biological Resource Center. Plants were grown in a growth chamber on a 12-h-light/12-h-dark photoperiod, as described previously.36 The light intensity was 150 μmol photons m−2 s−1, the temperature was 20°C, and the relative humidity was 50%.
Analysis of RNA editing by Sanger sequencing
The transcript regions surrounding Arabidopsis plastid RNA editing sites were amplified using Phusion High-Fidelity DNA Polymerase (New England Biolabs) and primers listed in Table 3. The PCR products were sequenced at the Michigan State University Genomics Facility, using the Sanger method and the primers listed in Table 3.
Table 3.
Primers used to amplify and sequence 34 plastid RNA editing sites.
| Primer | Sequence |
|---|---|
| accD_1_F | TTCATTTGTAGTGAAAGCGG |
| accD_1_R | TTTCGCCTACTACGGATCCC |
| accD_2_F | CTACTACCGGTGGAGTGACAGC |
| accD_2_R | AGAATCTGATCTAACAACAGGGAA |
| atpF_1_F | GAGTTTCGGATTTAATACCG |
| atpF_1_R | AGCTCCTTGTAAAGCTTGTTG |
| clpP_1_F | TTGGGTTGACATATACAACCG |
| clpP_1_R | TGAACCGCTACAAGATCAAC |
| matK_2_F | CGTTACCGGGTAAAAGATGC |
| matK_2_R | AGCGGCGTATCCTTTGTTGC |
| ndhB_1–3_F | TTTGCTTCTCTTCGATGGAAG |
| ndhB_1–3_R | ACGACTGGAGTGGGAGATCCTTC |
| ndhB_7–12_F | CGTATACGAAGGATCTCCCAC |
| ndhB_7–12_R | CCTGAGCAATCGCAATAATCG |
| ndhD_1–5_F | TTGAGTACGCGTTCTTTGGAC |
| ndhD_1–5_R | AATAGCTCCATTAAGTCCAGG |
| ndhF_2_F | AAAACCTTCGCCGCATGTGG |
| ndhF_2_R | GCATTCGCTGCAATAGGTCG |
| ndhG_1_F | ATGGATTTGCCTGGACCAATAC |
| ndhG_1_R | TTGATAAATGAATTCCTATTTGTTG |
| petL_2_F | AAATTTGGTAATTAACACGG |
| petL_2_R | ATTTCAATTGAAACTTAGGG |
| psbE_1_psbF_1_F | ACAGGAGAACGTTCTTTTGC |
| psbE_1_psbF_1_R | ATATAATCCATCCGAATGGG |
| psbZ_1_F | ATGAGATACGCGATCCAGTATAC |
| psbZ_1_R | TCAAGAGATAAGAGAATTAAGGATAC |
| rpl23_1_F | AAGAGGTGGAATAGAATAACCCG |
| rpl23_1_R | CAATTCCTACTGGATGCACGC |
| rpoA_1_F | GTAAGCGTCTTTATTATGGACGC |
| rpoA_1_R | CTTGATGAAGTGCTTCTTTAGGAG |
| rpoB_1_3_F | GAAAACCAGTAGGAATATGC |
| rpoB_1_3_R | GTCTCCAATTAATATTTCGGCG |
| rpoB_7_F | GAGGTGGGTTCAGAAAAAGG |
| rpoB_7_R | TATCTGTCCTACATTCATGCG |
| rpoC1_1_F | AGTTTTGTGAACAATGTGGAGTTG |
| rpoC1_1_R | TGAATGATGGGTCTCAACTCGG |
| rps12_1_F | CTTGTACAATTCACATTCTTTGGC |
| rps12_1_R | ACAAGACAGCCAATCCGAAAC |
| rps14_1–2_F | TTATAGGGAGAAGAAGAGGC |
| rps14_1–2_R | TACCAGCTTGATCTTGTTGC |
Analysis of RNA editing extents by whole-transcriptome RNA-Seq
Total leaf RNAs were extracted from the wild type and the orrm6 mutants (4 biologic replicates per genotype) using the RNeasy Plant Mini Kit (QIAGEN), digested with the RNase-Free DNase I (QIAGEN), re-purified with the RNeasy Plant Mini Kit, and submitted to the Michigan State University Genomics Facility for downstream processes. rRNAs were depleted with the Ribo-Zero rRNA Removal kit for plant leaf (Illumina). Sequencing libraries were prepared using the TruSeq Stranded Total RNA Low Throughput Library Prep Kit (Illumina). The libraries were first run on a LabChip GX system (Perkin Elmer) to assess the size distribution of the library fragments and estimate a mean fragment size. The libraries were then quantified using both a Qubit fluorimetric assay and a Kapa Biosystems library quantification qPCR method to determine the molar concentration of each library and adjust each library to a standard concentration. After quality control and quantitation, the 12 libraries (3 genotypes―wild type, orrm6–1, and orrm6–2; 4 biologic replicates per genotype) were pooled on an equal equimolar amount basis and loaded on 2 lanes of an Illumina HiSeq 2500 Rapid Run flow cell (v1). Base calling was done by the Illumina Real Time Analysis (RTA) v1.17.21.3 software and output of RTA was demultiplexed and converted to the FastQ format by the Illumina Bcl2fastq v1.8.4 software.
Prior to analysis of RNA-Seq data, the adaptor sequences were removed using trimmomatic v 0.32 software (http://www.usadellab.org/cms/?page = trimmomatic).37 The resulting data files were filtered by FASTQ Quality Filter in the FASTX-toolkit v0.0.13 (http://hannonlab.cshl.edu/fastx_toolkit/) so that only reads with at least 85% of base calls with a quality score of 20 were kept. The kept reads were aligned with the Tophat mapping software,32 using the TAIR10 Arabidopsis genome released by Ensembl38 as the reference genome. The Integrative Genomics Viewer (http://software.broadinstitute.org/software/igv/)33 was used to navigate to RNA editing sites and editing extents were calculated by dividing T base calls by C + T base calls.17 The 4 replicates per line were averaged and a student's 2-tailed t-test was performed between the wild type and the orrm6 mutants.
Gene expression and pathway analyses of the RNA-seq data
Following alignment of reads as described above, differential gene expression analysis was performed using a combination of SAMtools (http://samtools.sourceforge.net/),39 htseq-count (http://www-huber.embl.de/HTSeq/doc/overview.html),40 and edgeR ( https://bioconductor.org/packages/release/bioc/html/edgeR.html)41 programs and a previously developed protocol.32 Pathway analysis was performed with the Generally Applicable Gene-set Enrichment (GAGE, http://bioconductor.org/packages/release/bioc/html/gage.html) program,35 using the Gene Ontology for biologic process gene sets. During the GAGE pathway analysis, the “pseudo.counts” expression counts from the RNA-seq data were used because they accounted for the transformations performed to normalize read counts by library size. A paired t-test was performed between the wild type and the orrm6 mutants; the resulting gene sets with statistically significant changes were outputted to text files using the geneData function.
Exon coverage analysis of the ORRM6 gene
The start and end regions of the 4 exons in the ORRM6 gene were defined using the TAIR Ensembl 10 genome annotation file.38 Exon regions were compiled into a BED file for analysis with the program BEDtools.42 The BEDtools coverage tool was used to examine read depth at the defined exons in the wild type, orrm6–1 and orrm6–2 (n = 4 for each genotype). The resulting read counts were combined into an Excel file and the ratios of exon counts to total read counts were compared. Visual inspection of exon coverage was done by navigating Integrative Genomics Viewer to the ORRM6 gene.33
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Ryan L. Wessendorf (Western Michigan University [WMU]) for plant care, Christopher D. Jackson (WMU) for growth chamber management, John Kapenga and Elise de Doncker (WMU) for access to the WMU High Performance Computational Science Laboratory, and Cheng Peng (Michigan State University) for advices on RNA-seq data analysis.
Funding
This work was supported by the US. National Science Foundation under Grant Number MCB-1244008.
References
- 1.Covello PS, Gray MW. On the evolution of RNA editing. Trends Genet 1993; 9(8):265-8; PMID:8379005; https://doi.org/ 10.1016/0168-9525(93)90011-6 [DOI] [PubMed] [Google Scholar]
- 2.Brennicke A, Marchfelder A, Binder S. RNA editing. FEMS Microbiol Rev 1999; 23(3):297-316; PMID:10371035; https://doi.org/ 10.1111/j.1574-6976.1999.tb00401.x [DOI] [PubMed] [Google Scholar]
- 3.Takenaka M, Zehrmann A, Verbitskiy D, Hartel B, Brennicke A. RNA editing in plants and its evolution. Annu Rev Genet 2013; 47:335-52; PMID:24274753; https://doi.org/ 10.1146/annurev-genet-111212-133519 [DOI] [PubMed] [Google Scholar]
- 4.Bock R. Sense from nonsense: How the genetic information of chloroplasts is altered by RNA editing. Biochimie 2000; 82(6–7):549-57; PMID:10946106; https://doi.org/ 10.1016/S0300-9084(00)00610-6 [DOI] [PubMed] [Google Scholar]
- 5.Chateigner-Boutin AL, Small I. Plant RNA editing. RNA Biol 2010; 7(2):213-9; PMID:20473038; https://doi.org/ 10.4161/rna.7.2.11343 [DOI] [PubMed] [Google Scholar]
- 6.Shikanai T. RNA editing in plants: Machinery and flexibility of site recognition. Biochim Biophys Acta 2015; 1847(9):779-85; PMID:25585161; https://doi.org/ 10.1016/j.bbabio.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Fey J, Weil JH, Tomita K, Cosset A, Dietrich A, Small I, Marechal-Drouard L. Role of editing in plant mitochondrial transfer RNAs. Gene 2002; 286(1):21-4; PMID:11943456; https://doi.org/ 10.1016/S0378-1119(01)00817-4 [DOI] [PubMed] [Google Scholar]
- 8.Delannoy E, Le Ret M, Faivre-Nitschke E, Estavillo GM, Bergdoll M, Taylor NL, Pogson BJ, Small I, Imbault P, Gualberto JM. Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation. Plant Cell 2009; 21(7):2058-71; PMID:19602623; https://doi.org/ 10.1105/tpc.109.066654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karcher D, Bock R. Identification of the chloroplast adenosine-to-inosine tRNA editing enzyme. RNA 2009; 15(7):1251-7; PMID:19460869; https://doi.org/ 10.1261/rna.1600609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Karcher D, Bock R. Identification of enzymes for adenosine-to-inosine editing and discovery of cytidine-to-uridine editing in nucleus-encoded transfer RNAs of Arabidopsis. Plant Physiol 2014; 166(4):1985-97; PMID:25315605; https://doi.org/ 10.1104/pp.114.250498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chateigner-Boutin A-L, Small I. A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res 2007; 35(17):e114; PMID:17726051; https://doi.org/ 10.1093/nar/gkm640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentolila S, Oh J, Hanson MR, Bukowski R. Comprehensive high-resolution analysis of the role of an Arabidopsis gene family in RNA editing. PLos Genet 2013; 9(6):e1003584; PMID:23818871; https://doi.org/ 10.1371/journal.pgen.1003584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruwe H, Castandet B, Schmitz-Linneweber C, Stern DB. Arabidopsis chloroplast quantitative editotype. FEBS Lett 2013; 587(9):1429-33; PMID:23523919; https://doi.org/ 10.1016/j.febslet.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 14.Bentolila S, Heller WP, Sun T, Babina AM, Friso G, van Wijk KJ, Hanson MR. RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc Nat Acad Sci U S A 2012; 109(22):E1453-61; PMID:22566615; https://doi.org/ 10.1073/pnas.1121465109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi X, Germain A, Hanson MR, Bentolila S. RNA recognition motif-containing protein ORRM4 broadly affects mitochondrial RNA editing and impacts plant development and flowering. Plant Physiol 2016; 170(1):294-309; PMID:26578708; https://doi.org/ 10.1104/pp.15.01280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takenaka M, Zehrmann A, Verbitskiy D, Kugelmann M, Härtel B, Brennicke A. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc Nat Acad Sci U S A 2012; 109(13):5104-9; PMID:22411807; https://doi.org/ 10.1073/pnas.1202452109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun T, Germain A, Giloteaux L, Hammani K, Barkan A, Hanson MR, Bentolila S. An RNA recognition motif-containing protein is required for plastid RNA editing in Arabidopsis and maize. Proc Nat Acad Sci U S A 2013; 110(12):E1169-78; PMID:23487777; https://doi.org/ 10.1073/pnas.1220162110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkan A, Small I. Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 2014; 65:415-42; PMID:24471833; https://doi.org/ 10.1146/annurev-arplant-050213-040159 [DOI] [PubMed] [Google Scholar]
- 19.Shi X, Hanson MR, Bentolila S. Two RNA recognition motif-containing proteins are plant mitochondrial editing factors. Nucleic Acids Res 2015; 43(7):3814-25; PMID:25800738; https://doi.org/ 10.1093/nar/gkv245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun T, Shi X, Friso G, Van Wijk K, Bentolila S, Hanson MR. A zinc finger motif-containing protein is essential for chloroplast RNA editing. PLos Genet 2015; 11(3):e1005028; PMID:25768119; https://doi.org/ 10.1371/journal.pgen.1005028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lurin C, Andres C, Aubourg S, Bellaoui M, Bitton F, Bruyere C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al.. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 2004; 16(8):2089-103; PMID:15269332; https://doi.org/ 10.1105/tpc.104.022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chateigner-Boutin AL, Colas des Francs-Small C, Fujii S, Okuda K, Tanz SK, Small I. The E domains of pentatricopeptide repeat proteins from different organelles are not functionally equivalent for RNA editing. Plant J 2013; 74(6):935-45; PMID:23521509; https://doi.org/ 10.1111/tpj.12180 [DOI] [PubMed] [Google Scholar]
- 23.Boussardon C, Avon A, Kindgren P, Bond CS, Challenor M, Lurin C, Small I. The cytidine deaminase signature HxE(x)n CxxC of DYW1 binds zinc and is necessary for RNA editing of ndhD-1. New Phytol 2014; 203(4):1090-5; PMID:25041347; https://doi.org/ 10.1111/nph.12928 [DOI] [PubMed] [Google Scholar]
- 24.Hayes ML, Dang KN, Diaz MF, Mulligan RM. A conserved glutamate residue in the C-terminal deaminase domain of pentatricopeptide repeat proteins is required for RNA editing activity. J Biol Chem 2015; 290(16):10136-42; PMID:25739442; https://doi.org/ 10.1074/jbc.M114.631630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faivre-Nitschke SE, Grienenberger JM, Gualberto JM. A prokaryotic-type cytidine deaminase from Arabidopsis thaliana gene expression and functional characterization. Eur J Biochem 1999; 263(3):896-903; PMID:10469156; https://doi.org/ 10.1046/j.1432-1327.1999.00591.x [DOI] [PubMed] [Google Scholar]
- 26.Cai W, Ji D, Peng L, Guo J, Ma J, Zou M, Lu C, Zhang L. LPA66 is required for editing psbF chloroplast transcripts in Arabidopsis. Plant Physiol 2009; 150(3):1260-71; PMID:19448041; https://doi.org/ 10.1104/pp.109.136812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins JC, Heller WP, Hanson MR. A comparative genomics approach identifies a PPR-DYW protein that is essential for C-to-U editing of the Arabidopsis chloroplast accD transcript. RNA 2009; 15(6):1142-53; PMID:19395655; https://doi.org/ 10.1261/rna.1533909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Härtel B, Zehrmann A, Verbitskiy D, van der Merwe JA, Brennicke A, Takenaka M. MEF10 is required for RNA editing at nad2-842 in mitochondria of Arabidopsis thaliana and interacts with MORF8. Plant Mol Biol 2013; 81(4–5):337-46; PMID:23288601; https://doi.org/ 10.1007/s11103-012-0003-2 [DOI] [PubMed] [Google Scholar]
- 29.Hackett JB, Shi X, Kobylarz AT, Lucas MK, Wessendof RL, Hines KM, Bentolila S, Hanson MR, Lu Y. An organelle RNA recognition motif protein is required for photosystem II subunit psbF transcript editing. Plant Physiol 2017; 173:2278-93; PMID:28213559; https://doi.org/ 10.1104/pp.16.01623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen CD, Mansfield RE, Leung W, Vaz PM, Loughlin FE, Grant RP, Mackay JP. Characterization of a family of RanBP2-type zinc fingers that can recognize single-stranded RNA. J Mol Biol 2011; 407(2):273-83; PMID:21256132; https://doi.org/ 10.1016/j.jmb.2010.12.041 [DOI] [PubMed] [Google Scholar]
- 31.Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 2007; 76:51-74; PMID:17352659; https://doi.org/ 10.1146/annurev.biochem.76.050106.093909 [DOI] [PubMed] [Google Scholar]
- 32.Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, Robinson MD. Count-based differential expression analysis of RNA sequencing data using R and bioconductor. Nat Protoc 2013; 8(9):1765-86; PMID:23975260; https://doi.org/ 10.1038/nprot.2013.099 [DOI] [PubMed] [Google Scholar]
- 33.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotech 2011; 29(1):24-6; PMID:21221095; https://doi.org/ 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al.. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25(1):25-9; PMID:10802651; https://doi.org/ 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: Generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 2009; 10:161; PMID:19473525; https://doi.org/ 10.1186/1471-2105-10-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark TJ, Lu Y. Analysis of loss-of-function mutants in aspartate kinase and homoserine dehydrogenase genes points to complexity in the regulation of aspartate-derived amino acid contents. Plant Physiol 2015; 168(4):1512-26; PMID:26063505; https://doi.org/ 10.1104/pp.15.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014; 30(15):2114-20; PMID:24695404; https://doi.org/ 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kersey PJ, Allen JE, Armean I, Boddu S, Bolt BJ, Carvalho-Silva D, Christensen M, Davis P, Falin LJ, Grabmueller C, et al.. Ensembl genomes 2016: More genomes, more complexity. Nucleic Acids Res 2016; 44:D574-80; PMID:26578574; https://doi.org/ 10.1093/nar/gkv1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics 2009; 25(16):2078-9; PMID:19505943; https://doi.org/ 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 2015; 31(2):166-9; PMID:25260700; https://doi.org/ 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26(1):139-40; PMID:19910308; https://doi.org/ 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinlan AR, Hall IM. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010; 26(6):841-2; PMID:20110278; https://doi.org/ 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]


