ABSTRACT
Heat shock protein 90 (HSP90) is a highly conserved molecular chaperone that facilitates the maturation of target proteins. Here, we report that the auxin receptor TIR1 is a target of cytosolic HSP90 and that HSP90 and TIR1 form a complex. Inhibition of HSP90 compromised the nuclear localization of TIR1, and abrogated plant responses to the hormone auxin. Our findings suggest that HSP90 positively regulates auxin receptor function. We also propose that HSP90 buffers or hides phenotypic variations in animals and plants by masking mutations in some of its target proteins. Support for this proposal comes from the tir1-1 mutant of Arabidopsis, which showed a root growth defect that was only seen after inhibition of HSP90. We have developed a model in which cytosolic HSP90 works like a capacitor for auxin-related phenotypic variation via regulation of the auxin receptor in response to environmentally and genetically induced perturbations.
KEYWORDS: Auxin signaling, heat shock protein 90, phenotypic variation, stress response, TIR1
Abbreviations
- AFB
auxin signaling F-box
- ARF
auxin-response factor
- BiFC
bimolecular fluorescence complementation
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- HSP
heat-shock protein
- IAA
indole acetic acid
- DEX
dexamethasone
- SCF/Skp/Cullin
F-box–containing complex
- TIR
transport inhibitor response
Heat shock protein 90 (HSP90) aids the folding of nascent proteins into their mature forms. The targets of this molecular chaperone include transcription factors, kinases, phosphatases, and receptors that play important roles in signal transduction pathways in plants, animals, and yeasts.1-3 HSP90 co-operates with co-chaperones such as SGT1 to achieve these effects.4 In addition, HSP90 is involved in the heat shock response by regulating the state of heat shock transcription factors. However, to date, relatively few of the target proteins of HSP90 have been identified in plants compared to animals and yeast. Identifying new target proteins, such as those engaged in important signaling pathways mediated by plant hormones such as auxin, will undoubtedly contribute to our understanding of the functional mechanisms of these proteins.
Auxin has a central role in plant growth and development5 by promoting the degradation of Aux/IAA transcriptional repressors, thereby allowing auxin response factors (ARFs) to activate the transcription of auxin-responsive genes.6 In this process, auxin receptors (TIR1/AFB proteins), which are components of an SCF ubiquitin ligase complex, guide Aux/IAA proteins to the ubiquitin-dependent degradation system.7,8 In our recent publication, we identified the first example of a mutation in the auxin receptor TIR1 that was “buffered” by HSP90. 9
In order to increase our understanding of the role of HSP90 in plant growth, we examined plants with defects in HSP90 chaperone activity. Four genes encoding cytosolic HSP90 homologues (HSP90.1, HSP90.2, HSP90.3, and HSP90.4) are located together on chromosome 5 in Arabidopsis and it is difficult to generate multiple HSP90-knockout mutants. We successfully introduced a D80N and E303K mutation into HSP90.2 (HSP90.2D80N and HSP90.2E303K); these mutations correspond to the D79N mutation in yeast HSP82 and the E313K mutation in Drosophila HSP83, respectively, and both cause dominant-negative effects.10,11 These mutant forms of HSP90.2 are exogenously expressed in Arabidopsis under the control of a dexamethasone (DEX)-inducible system.9 Root growth of transgenic plants expressing HSP90E303K was reduced in the presence of DEX suggesting that inhibition of HSP90 activity severely disturbed plant growth. The number of lateral roots was lower in the mutants than in wild type, and this phenotype was similar to that found in auxin receptor deficient mutants. We therefore speculated that HSP90.2 was probably involved in the auxin response. Treatment of Arabidopsis with the HSP90 inhibitors geldanamycin (GDA) or radicicol (RAD) reduced β-glucuronidase (GUS) expression in Arabidopsis harboring the auxin-responsive marker gene, DR5:GUS. In addition, expression of the IAA5 gene was reduced in plants expressing HSP90.2E303K. These results indicated that HSP90 was required for the expression of auxin-responsive genes.
Auxin promotes the degradation of Aux/IAA transcriptional repressors, thereby allowing auxin response factors (ARFs) to activate the transcription of auxin-responsive genes.6 We analyzed IAA7-GFP degradation in response to auxin and found that treatment with RAD inhibited the degradation of IAA7-GFP. This suggests that HSP90 induced the degradation of IAA proteins and induced the expression of auxin-responsive genes during the auxin response. During Aux/IAA degradation, the auxin receptor proteins TIR1/AFB guide Aux/IAA proteins to the ubiquitin-dependent degradation system.7,8 We examined whether HSP90 interacted with TIR1 and found that FLAG-TIR1 and HSP90 formed a complex in an Arabidopsis leaf extract. In addition, the interaction of HSP90.2 and TIR1 was confirmed by a BiFC analysis. We also examined the effect of HSP90 inhibitors on the localization of TIR1-GFP. TIR1-GFP is accumulated in the nuclei under normal conditions; after RAD or GDA treatment most TIR1-GFP remained in the nucleus, but some TIR1-GFP was observed in the cytosol. This suggests that HSP90 aided nuclear localization of TIR1.
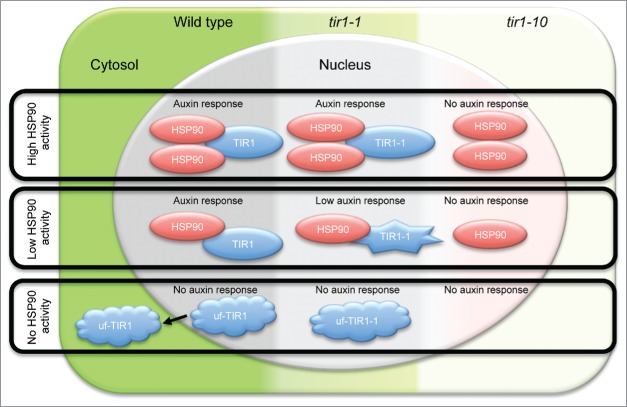
In the nucleus, HSP90 and TIR1 form a complex that appears to function as a chaperone for TIR1. Together with the report of Wang et al.,12 our study9 suggests that HSP90 works with the co-chaperone SGT1b as a chaperone of the SCFTIR1 complex and regulates its auxin receptor function in the nucleus. It is possible that under severe stress conditions when chaperone activity of HSP90 is low, TIR1 can no longer function as an auxin receptor; its protein might be misfolded and a functional SCFTIR1 complex is not formed (Fig. 1).
Figure 1.
Schematic model for the function of Arabidopsis HSP90 as a chaperone for TIR1 in auxin signaling and for its buffering function in wild type (WT) and tir1 mutants. TIR1 needs the chaperone activity of HSP90 to function as a receptor for auxin and to promote an auxin response. HSP90 positively regulates the auxin response by stabilizing TIR1 function. Under conditions when no HSP90 activity occurs, the TIR1 is mostly non-functional and some unfolded TIR1 (uf-TIR1) proteins are exported to the cytosol, causing a severe attenuation in the auxin response. TIR1-1 protein with a point mutation may require much more HSP90 chaperone activity compared to wild type TIR1. Therefore, in low HSP90 activity conditions, mutated TIR1-1 cannot keep its function; as a result, the tir1-1 mutant displays its partially defective auxin response phenotype. In the tir1-10 mutant, the auxin response is severely impaired because the TIR1 protein is absent.
HSP90 can mask genetic or epigenetic mutations in eukaryotes to create cryptic phenotypes in a phenomenon known as buffering. The cryptic phenotypes can be made visible by perturbing HSP90 activity, such as by increasing the temperature or adding a low concentration of HSP90 inhibitor.13-18 In the tir1-1 mutant, the TIR1 protein has an amino acid substitution (G147D); in the T-DNA insertion mutant, tir1-10, TIR1 is absent. The tir1-1 mutation causes a cryptic auxin response defect.9 We found that the induction of IAA1 and IAA5 genes in the tir1-1 mutant was almost the same after auxin treatment as the wild type, while expression of the genes was strongly reduced in the tir1-10 mutant.9 Interestingly, the expression level of auxin-inducible genes were decreased with low concentration of HSP90 inhibitors in tir1-1 suggesting that HSP90 cover the cryptic mutation of TIR1-1 function.9 These findings suggest that HSP90 can buffer the effects of a point mutation in its target protein TIR1. We hypothesize that HSP90 may assist the mutated TIR1G147D protein to recover receptor function in the tir1-1 mutant; TIR1G147D may require more HSP90 chaperone activity compared to wild-type TIR1, and the tir1-1 mutant is therefore hypersensitive to inhibition of HSP90 (Fig. 1). In the presence of a low concentration of HSP90 inhibitor, as in low HSP90 activity conditions, HSP90 could not buffer the mutation of TIR1 and the phenotype of the tir1-1 mutant was exposed (Fig. 1). The auxin response phenotype was not cryptic in the tir1-10 mutant because it is a null allele of TIR1 (Fig. 1). Our findings support the concept that HSP90 can produce cryptic phenotypes following mutation in a target protein such as auxin receptor TIR19.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci 2002; 59:1640-8; PMID:12475174; https://doi.org/ 10.1007/PL00012491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, et al.. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the HSP90 chaperone. Cell 2005; 120:715-27; PMID:15766533; https://doi.org/ 10.1016/j.cell.2004.12.024 [DOI] [PubMed] [Google Scholar]
- 3.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 2010; 11:515-28; PMID:20531426; https://doi.org/ 10.1038/nrm2918 [DOI] [PubMed] [Google Scholar]
- 4.Takahashi A, Casais C, Ichimura K, Shirasu K. HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc Natl Acad Sci USA 2003; 100:11777-82; PMID:14504384; https://doi.org/ 10.1073/pnas.2033934100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mockaitis K, Howell SH. Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J 2000; 24:785-96; PMID:11135112; https://doi.org/ 10.1111/j.1365-313X.2000.00921.x [DOI] [PubMed] [Google Scholar]
- 6.Reed JW. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci 2001; 6:420-5; PMID:11544131; https://doi.org/ 10.1016/S1360-1385(01)02042-8 [DOI] [PubMed] [Google Scholar]
- 7.Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 2005; 9:109-19; PMID:15992545; https://doi.org/ 10.1016/j.devcel.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 8.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature 2005; 435:441-5; PMID:15917797; https://doi.org/ 10.1038/nature03543 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe E, Mano S, Nomoto M, Tada Y, Hara-Nishimura I, Nishimura M, et al.. HSP90 stabilizes auxin-responsive phenotypes by masking a mutation in the auxin receptor TIR1. Plant Cell Physiol 2016; 57:2245-54; PMID:27816945; https://doi.org/ 10.1093/pcp/pcw170 [DOI] [PubMed] [Google Scholar]
- 10.van der Straten A, Rommel C, Dickson B, Hafen E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J 1997; 16:1961-9; PMID:9155022; https://doi.org/ 10.1093/emboj/16.8.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panaretou B, Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J 1998; 17:4829-36; PMID:9707442; https://doi.org/ 10.1093/emboj/17.16.4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Zhang Y, Kieffer M, Yu H, Kepinski S, Estelle M. HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat Commun 2016; 7:10269; PMID:26728313; https://doi.org/ 10.1038/ncomms10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangster TA, Lindquist S, Queitsch C. Under cover: causes, effects and implications of Hsp90-mediated genetic capacitance. Bioessays 2004; 26:348-62; PMID:15057933; https://doi.org/ 10.1002/bies.20020 [DOI] [PubMed] [Google Scholar]
- 14.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature 1998; 396:336-42; PMID:9845070; https://doi.org/ 10.1038/24550 [DOI] [PubMed] [Google Scholar]
- 15.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature 2002; 417:618-24; PMID:12050657; https://doi.org/ 10.1038/nature749 [DOI] [PubMed] [Google Scholar]
- 16.Sollars V, Lu X, Xiao L, Wang X, Garfinkel MD, Ruden DM. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat Genet 2003; 33:70-4; PMID:12483213; https://doi.org/ 10.1038/ng1067 [DOI] [PubMed] [Google Scholar]
- 17.Jarosz DF, Lindquist S. Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science 2010; 330:1820-4; PMID:21205668; https://doi.org/ 10.1126/science.1195487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohner N, Jarosz DF, Kowalko JE, Yoshizawa M, Jeffery WR, Borowsky RL, et al.. Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 2013; 342:1372-5; PMID:24337296; https://doi.org/ 10.1126/science.1240276 [DOI] [PMC free article] [PubMed] [Google Scholar]