Abstract
Pfs25, a Plasmodium falciparum surface protein expressed during zygote and ookinete stages in infected mosquitoes, is a lead transmission-blocking vaccine candidate against falciparum malaria. To enhance immunogenicity, recombinant Pfs25 was chemically conjugated to recombinant nontoxic Pseudomonas aeruginosa ExoProtein A (rEPA) in conformance with current good manufacturing practices (cGMP), and formulated with the alum adjuvant Alhydrogel. In order to meet the regulatory requirements for a phase 1 human clinical trial, the vaccine product was extensively evaluated for stability at an initial time point and through the clinical trial period annually. Because basic quality control methods to characterize alum-based vaccines remain unavailable, a thermal forced degradation study was performed prior to the initial evaluation to identify the methods suitable to detect the quality of vaccine formulations. Our results show that the vaccine product Pfs25-EPA formulated on Alhydrogel is in conformance with regulatory guidelines and suitable for human trials.
Keywords: Malaria, Malaria vaccine, Pfs25, Pfs25-EPA conjugate, Transmission Blocking Vaccine
Introduction
Malaria is one of most deadly infectious diseases in the world, with an estimated 214 million cases and 438,000 deaths occurring in 2015 (1). The transmission of malaria depends on the Anopheles mosquito vector, in which the sexual phase of the parasite life cycle occurs. Vaccines that target sexual stage parasites have been proposed as tools to interrupt transmission and eliminate malaria from communities. Four decades ago, Gwadz and Carter first demonstrated that chickens immunized with sexual stages from the avian parasite Plasmodium gallinaceum generated antibodies that efficiently neutralized parasite gametes in mosquitoes (2, 3). In the ensuing years, a number of proteins targeted by these antibodies were identified, including Pfs25, Pfs28, Pfs48/45 and Pfs230 in P. falciparum, and their orthologues in P. vivax (4-9).
Pfs25 and Pvs25 are the most advanced transmission-blocking vaccine candidates and have been evaluated in clinical trials (8-10). However, the general level of immunogenicity induced by Pfs25 or Pvs25 monomers formulated on Alhydrogel was low, indicating that a more potent preparation is needed to induce higher antibody responses. Recent studies from our laboratory and collaborators demonstrated that the immunogenicity of Pfs25 can be greatly enhanced when conjugated to carrier proteins such as OMPC (the outer-membrane protein complex of Neisseria meningitidis) or rEPA (recombinant nontoxic Pseudomonas aeruginosa ExoProtein A) (11-13).
In order to meet the regulatory requirements for a phase 1 human clinical trial, Pfs25-EPA adjuvanted with Alhydrogel was extensively evaluated at an initial time point and through the clinical trial period annually. Here, we report the results of a thermal forced degradation study, which was designed to identify the methods to assess the quality of vaccine product. We also report the results of long-term stability studies which include testing for the following: appearance, endotoxin content, sterility, general safety (for initial characterization only), strength (protein content tested by o-phthaldialdehyde (OPA) assay and aluminum content determined by atomic absorption), identity (SDS-PAGE and Western blot after desorption of antigen from Alhydrogel), integrity (pH, percent protein bound to Alhydrogel, SDS-PAGE, intrinsic fluorescence CD, Direct Alhydrogel Formulation Immunoassay), and efficacy (mouse potency assay). In addition, we describe the methods used to assess the identity and integrity of the vaccine over time.
Materials and methods
Preparation of non-GMP and clinical grade Pfs25-EPA/Alhydrogel
The clinical grade 78 μg/mL (in Pfs25 content, the percent mass of Pfs25 in the conjugate was 45.4%) Pfs25-EPA consisting of Pfs25H, a recombinant Pichia pastoris expressed Pfs25 protein with a 6-histidine fusion tag (14) adjuvanted on Alhydrogel was manufactured according to current good manufacturing practice (cGMP) at the Walter Reed Army Institute of Research (WRAIR) Pilot Bioproduction Facility (Silver Spring, MD) with methods developed at the Laboratory of Malaria Immunology and Vaccinology (LMIV), National Institute of Allergy and Infectious Diseases, National Institutes of Health (12). Briefly for formulation, 154 mL of cGMP product Pfs25-EPA (Pfs25 content: 0.519 mg/mL) was added to 868 mL of 1,600 μg/mL of Alhydrogel® (Aluminum Hydroxide Gel Adjuvant, Brenntag Biosector, Denmark), mixed by stirring at moderate speed (170 rpm) for 60 min, vialed at 1.0 mL per vial, and stored at 2–8 °C. A non-cGMP grade Pfs25-EPA adjuvanted on Alhydrogel was prepared at LMIV at final concentrations of 100 μg/mL (in Pfs25 content) in 1,600 μg/mL Alhydrogel® by rotating the mixture at 16–24 rpm on a rotary spinner (Appropriate Technical Resources, Laurel, Maryland) for 60 min at room temperature, then aliquoted to 1.0 mL per vial and kept at 2–8 °C until use. The 100 μg/mL non-GMP grade samples for the thermal degradation study were stored at 60°C and removed at day 11 for analysis.
Appearance, endotoxin, sterility and general safety
The appearance, endotoxin level and sterility of clinical grade Pfs25-EPA/Alhydrogel vaccine were assessed by WRAIR. The appearance was performed by visual inspection. The endotoxin level was determined by LAL assay per USP 25 National Formulary (NF) 20, Chapter <85> and the sterility assay was performed per 21 CFR 610.12 and USP 37 Chapter <71>. The general safety analysis for clinical grade Pfs25-EPA/Alhydrogel vaccine was performed by BioReliance (Rockville, Maryland) per 21 CFR, Section 610.11 and USP Chapter <88>. Two guinea pigs were each injected intraperitoneally (ip) with 5.0 mL of the test article, and one guinea pig was injected ip with the negative control HBSS. Two mice were injected ip with 0.5 mL of test article each, and two mice were injected ip with negative control HBSS. The animals were observed every working day, and animal weights were recorded at the end of the study.
Aluminum content
The aluminum content of clinical grade Pfs25-EPA/Alhydrogel vaccine was determined by atomic absorption spectroscopy. The assay was conducted by RTI International (Research Triangle Park, North Carolina).
pH, vaccine desorption, SDS-PAGE and Western blot
The pH of clinical grade Pfs25-EPA/Alhydrogel vaccine was measured using a calibrated pH probe. The non-GMP and clinical grade Pfs25-EPA protein in the test material were desorbed from Alhydrogel by adjusting the formulation pH ≥ 11 using 1N Sodium Hydroxide. The desorbed Pfs25-EPA was immediately neutralized with hydrochloric acid and resolved by SDS-PAGE on tris-acetate gels under non-reduced conditions. The conjugate was visualized with a silver stain and the migration pattern was compared to the reference material.
A Western blot was performed on the desorbed Pfs25-EPA. After resolving the desorbed Pfs25-EPA on a non-reducing tris-acetate SDS-PAGE gel, Western blot analysis was performed using anti-Pfs25 monoclonal antibodies 4B7 and 1G2 (LMIV) and an anti-Exotoxin A polyclonal antibody (List Biological Laboratories, Inc, Campbell, CA).
Protein Folding (Intrinsic Fluorescence)
Intrinsic fluorescence circular dichroism was performed for the non-GMP and clinical grade Pfs25-EPA/Alhydrogel at LMIV. The intensity of a placebo (1,600 μg/mL Alhydrogel without absorption of protein) was subtracted. The λmax and intensity of test samples were compared to the reference material and the freshly made formulations.
o-Phthaldialdehyde assay
The protein content of the non-GMP and clinical grade Pfs25-EPA/Alhydrogel was determined by o-phthaldialdehyde assay (OPA) (15). The % bound was calculated by the following equation:
Direct Alhydrogel Formulation Immunoassay (DAFIA)
Pfs25-EPA content in the non-GMP and clinical grade Pfs25-EPA/Alhydrogel was determined by Direct Alhydrogel Formulation Immunoassay (DAFIA) (16) with anti-Pfs25 monoclonal antibody 4B7, which recognizes a partially concealed linear epitope of Pfs25 (14). Briefly, Alhydrogel formulation composed with standards or test samples (200 μl/well) were added to black, opaque, 96-well U-bottom plates and washed 3 times with phosphate-buffered saline (PBS, pH 7.4) by centrifugation at 1,000 g for 4 min. Supernatants were discarded by quickly inverting the plate for each wash. The plates were then blocked with 200 μl 3% BSA/PBS at room temperature for 1.5 h, followed by 3 washes with PBS. 100 μl of mouse anti-Pfs25 monoclonal antibody 4B7 at 2.1 μg/mL were added and incubated for 1 h at room temperature, followed by 3 washes with PBS. 100 μl of goat anti-mouse IgG fluorescein at 1:200 (Pierce, Rockford, IL) was added to plates and incubated for 1 h at room temperature and washed 3 times with PBS. Following the final wash, pellets were resuspended in 100 μl PBS and read by a fluorometer at 485nm/535 nm.
Potency studies in mice
All animal studies were performed in compliance with National Institutes of Health guidelines and under the auspices of an Animal Care and Use Committee approved protocol. The efficacy of non-GMP and clinical grade vaccines was evaluated by mouse potency studies. For each study, eighty BALB/c mice were randomly assigned to 8 groups of 10. Four groups were immunized with the freshly prepared formulations by ip injection, and the other four groups were immunized ip with the test vaccines. The mice were immunized at Days 0 and 21 with a volume of 0.5 mL at doses of 0.003, 0.01, 0.1 and 0.3 μg for the stability study, and 0.1, 0.3, 1.0 and 3.0 μg for the thermal degradation study. Mouse sera were collected on Day 35 and the antibody response to Pfs25 was measured by ELISA. By the statistical model for Pfs25-EPA/Alhydrogel developed at LMIV, which support the use of a 30-fold difference in low and high dose groups (0.003 vs 0.1 μg and 0.01 vs 0.3 μg) and a threshold of 6,000 ELISA units, the vaccine was considered potent if no more than three in the low dose group had ELISA units >6,000 and at least seven in the high dose group had ELISA units >6,000 with 10 mice per group. The antibody response of test vaccines was also compared to the freshly prepared formulations at equal doses for informational purposes. Samples for the stability study were carried out at month 3, 9, 15, 21, 27, 35, 40, 46, and 52 to cover the entire clinical trial period.
Results
Thermal degradation study
SDS-PAGE and Western blot
The samples of non-GMP grade Pfs25-EPA/Alhydrogel, formulated at a 100 μg/mL dose and stored at 2-8°C and 60°C for 11 days, were desorbed from Alhydrogel and evaluated by SDS-PAGE and Western blot. Samples stored at 2-8°C for 11 days had a comparable migration pattern and intensity when compared to the reference standard, however, the migration pattern and intensity was changed greatly for the sample stored at 60°C for 11 days (Fig. 1A). The Western blot results demonstrated a similar pattern; the sample stored at 2–8°C for 11 days had a comparable migration pattern and intensity signal with the reference standard when probed with Pfs25 specific monoclonal antibody 4B7, the monoclonal antibody penta-anti-His, and polyclonal antibody anti-Exotoxin A (Fig. 1B). However, the signals markedly decreased for the sample stored at 60°C for 11 days.
Figure 1. Characterization of Pfs25-EPA conjugate desorbed from Alhydrogel formulation following Forced Degradation Study.
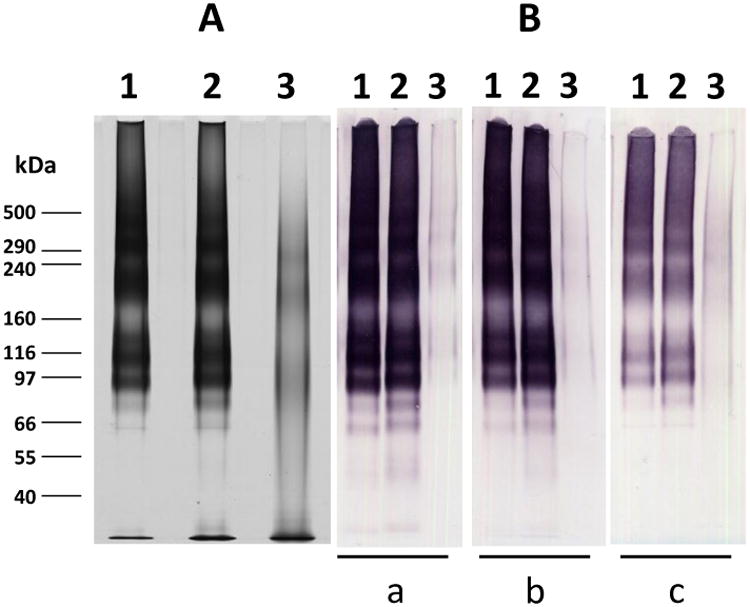
A) SDS-PAGE (silver staining); B) Western blot: (a) probed with monoclonal antibody 4B7; (b) probed with penta-His; (c) probed with anti-Exotoxin A. Lane 1, Pfs25-EPA reference standard (without desorption); Lane 2, Desorption of Pfs25-EPA/Alhydrogel stored at 4°C for 11 days, Lane 3, Desorption of Pfs25-EPA/Alhydrogel stored at 60°C for 11 days. All samples were loaded at 550 ng protein (calculation based on 100% recovery of desorption) per lane. Molecular weight markers are indicated in kDa.
DAFIA
The intact Pfs25 content of non-GMP grade Pfs25-EPA/Alhydrogel, formulated at a 100 μg/mL dose and stored at 2-8°C and 60°C for 11 days, was evaluated by DAFIA using anti-Pfs25 monoclonal antibody 4B7. The Pfs25 content remained at the desired concentration after storage at 2-8°C for 11 days, however, the content decreased dramatically and the majority of Pfs25 was not able to be detected by 4B7 after the sample was stored at 60°C for 11 days (Table 1).
Table 1. Protein content by OPA and DAFIA for 100 μg/mL Pfs25-EPA/Alhydrogel vaccine stored at 4°C or 60°C for 11 days.
| Average (μg/mL) ± STDEV | ||
|---|---|---|
|
| ||
| 4°C | 60°C | |
| OPA | 91.82 ± 4.04 | 90.12 ± 0.08 |
| DAFIA | 90.04 ± 19.04 | 3.92 ± 0.44 |
OPA
The total protein of non-GMP grade Pfs25-EPA/Alhydrogel, formulated at a 100 μg/mL dose and stored at 2-8°C and 60°C for 11 days, were evaluated by OPA. The total protein remained at the comparable concentration after storage at 2-8°C and 60°C for 11 days (Table 1).
Protein Folding
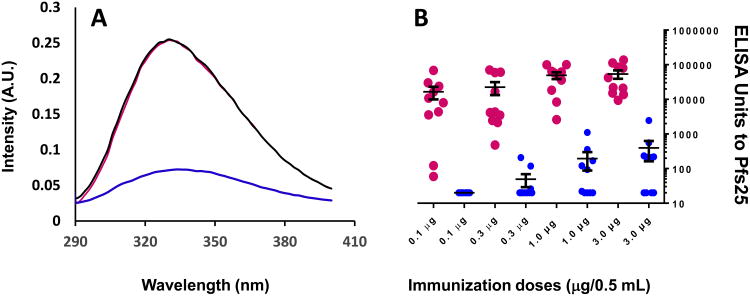
The samples of non-GMP grade Pfs25-EPA/Alhydrogel, formulated at a 100 μg/ML dose and stored at 2-8°C and 60°C for 11 days, were evaluated by intrinsic fluorescence. The sample stored at 2-8°C for 11 days had a comparable λmax and intensity when compared to the reference standard, however, the intensity was markedly decreased for the sample stored at 60°C for 11 days (Fig. 2A).
Figure 2. Characterization of Pfs25-EPA conjugate formulated on Alhydrogel following Forced Degradation Study.
A) Intrinsic fluorescence CD analysis of 100 μg/mL formulation. The red line represents a freshly prepared sample; the black line represents a sample stored at 4°C for 11 days; the blue line represents a sample stored at 60°C for 11 days. B) Mouse potency study. Red solid circles represent a freshly prepared formulation; blue solid circles represent samples stored at 60°C for 11 days. Each bar represents the geometric mean of 10 mice at each dose group.
Potency study
Potency of non-GMP grade Pfs25-EPA/Alhydrogel, formulated at a 100 μg/mL dose and stored at 2-8°C and 60°C for 11 days was determined by antibody responses in mice using ELISA. No ELISA unit exceeded 6,000 in all tested groups (all in high dose group). The non-GMP grade vaccines lost potency after storage at 60°C for 11 days (Fig. 2B). In comparison, the freshly formulated reference vaccine demonstrated potency.
Initial and long-term stability study
Aluminum content
The aluminum content of clinical grade 78 μg/mL Pfs25-EPA/Alhydrogel was measured at the initial time point, and the result was 781 ± 24.1 μg/mL, which met the pre-set specification of 675-925 μg/mL (Table 2).
Table 2. Summary of specifications and test results for clinical grade 78 μg/mL Pfs25-EPA/Alhydrogel vaccine stored at 4°C for 4 years.
| Tests | Method | Specification | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Initial | Year 1 | Year 2 | Year 3 | Year 4 | ||||
| Appearance | Visual | White to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) | White to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) | White to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) | White to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) | White to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) | White to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) | |
| Endotoxin(EU/mL) | USP<85> | < 10 EU/mL | < 0.06 | < 0.12 | < 0.12 | < 0.06 | < 0.06 | |
| Sterility | USP<71> | No growth | No growth | No growth | No growth | No growth | No growth | |
| pH | Measure | 6.0 - 8.5 | 7.05 | 7.36 | 7.65 | 8.06 | 7.95 | |
| Protein Folding | λmax (nm) | Intrinisic Fluorescence | Report results | -- | 330 | 329 | 330 | 330 |
| Intensity (AU) | -- | 0.3054 | 0.2604 | 0.2443 | 0.2137 | |||
| Aluminum Content (μg/mL) | Atomic Absorption | 675 -925 | 781 ± 24.1 | -- | -- | -- | -- | |
| General Safety | USP<88> | Satisfactory^ | Satisfactory | -- | -- | -- | -- | |
| Protein Content (μg/mL) | OPA assay | Report results | 85.9 ± 1.6 | 75.1 ± 1.3 | 81.7 ± 6.4 | 87.7 ± 3.3 | 71.5 ± 2.5 | |
| % Bound to Alhydrogel | OPA assay on supernatants | > 90% Bound | >90% | >90% | >90% | >90% | >90% | |
| DAFIA(μg/mL) | Anti-Pfs25 4B7 mAb recognition | Report results | 88.1 ± 9.3 | 87.9 ± 6.0 | 75.4 ± 1.4 | 87.6 ± 2.8 | 90.7±1.9 | |
General safety
The general safety of clinical grade 78 μg/mL Pfs25-EPA/Alhydrogel was evaluated at the initial time point, and the result was satisfactory (Table 2).
Appearance
The appearance of clinical grade 78 μg/mL Pfs25-EPA/Alhydrogel was evaluated at the initial time point and annually. A white to slightly yellow suspension with white particulates (consistent with an Alhydrogel formulation) was observed for all samples evaluated (Table 2).
Endotoxin
The endotoxin level of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel was determined at the initial time point and annually. The vaccine had an endotoxin level <0.12 EU/mL for all time points tested (Table 2).
Sterility
The sterility of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel was determined at the initial time point and annually. No growth was observed for the vaccines at all time points tested (Table 2).
pH
The pH of clinical grade 78μg/mL Pfs25-EPA/alhydrogel was measured at the initial time point and annually. The vaccine had a pH range of 7.05 -8.06 during the four year storage period at 2-8°C. In addition, a pattern of increasing pH was observed over time, although within the allowable range (Table 2).
DAFIA
The integrity of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel was determined at the initial time point and annually by DAFIA. The concentration of the intact protein detected by mAb 4B7 ranged from 75.4 ± 1.4 to 90.7 ± 1.9 μg/mL during the four year storage period at 2-8°C (Table 2).
OPA
The protein content of cli rade 78 μg/mL Pfs25EPA/Alhydrogel was determined at the initial time point and annually. The concentration of protein on Alhydrogel detected by OPA ranged from 71.5 ± 2.5 to 87.7 ± 3.3 μg/mL during the four year storage period at 2-8°C (Table 2). The percentage of protein bound to Alhydrogel of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel was determined at the initial time point and annually by OPA assays on the supernatant. Greater than 90% binding was observed for the vaccine stored at 2-8°C for 4 years.
Protein Folding
The integrity of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel was determined annually by intrinsic fluorescence (the data were not able to be collected due to an instrument malfunction at the initial time point). Only the year 2 sample exhibited a slight difference of λmax (329 nm compared to 330 nm for other time points). However, a pattern of decreasing intensity was observed (Table 2).
SDS-PAGE and Western blot
The identity and integrity of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel were evaluated by SDS-PAGE with silver stain at the initial time point and annually. The vaccine desorption had a comparable migration pattern to the reference material under non-reducing conditions. However, for samples at year 2 and after, a stronger faint smear in the molecular weight range of approximately 66 kDa to the bottom of the gel was observed (Fig. 3A).
Figure 3. Characterization of Pfs25-EPA conjugate desorbed from Alhydrogel formulation following 4 years storage at 2-8°C.
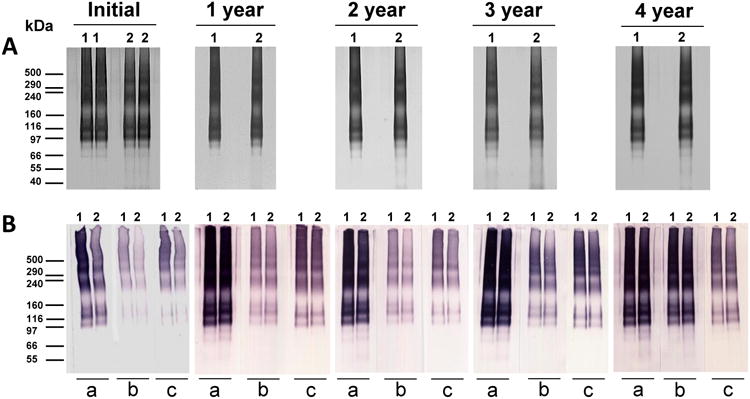
A) SDS-PAGE (silver staining); B) Western blot: (a) probed with monoclonal antibody 4B7; (b) probed with penta-His; (c) probed with anti-Exotoxin A. Lane 1, Pfs25-EPA reference standard (without desorption); Lane 2, Pfs25-EPA/Alhydrogel desorption. All samples were loaded at 550 ng protein (calculation based on 100% recovery of desorption) per lane. Molecular weight markers are indicated in kDa.
The identity and integrity of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel were evaluated by Western blot at the initial time point and annually. Overall, the vaccine desorption had a comparable migration pattern and intensity signal with the reference material under non-reducing conditions. However, slight degradation bands approximately below 66 kDa were observed for samples at year 2 and when probed with mAb 4B7. In addition, slight degradation bands approximately below 66 kDa were observed for samples at year 4 when probed with penta-His (Fig. 3B).
Potency study
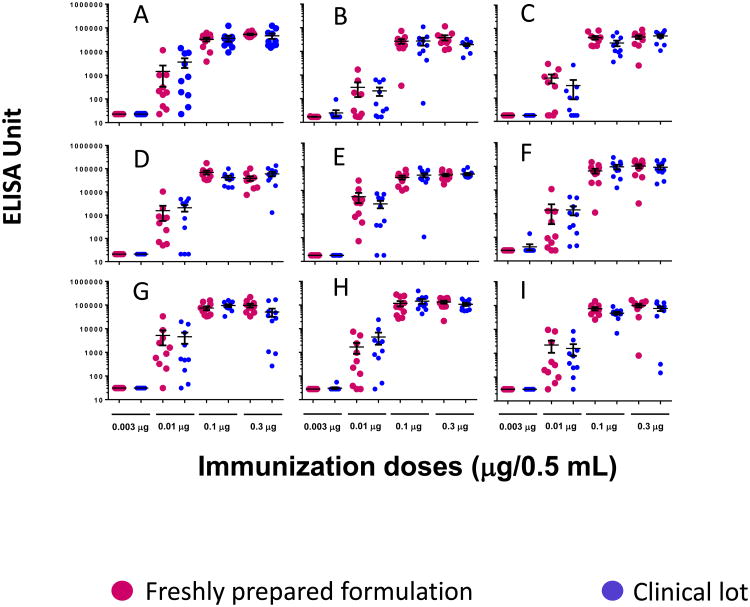
The stability of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel was evaluated by a mouse potency study at an initial (3 month) time point and at 9, 15, 21, 27, 35, 40, 46, and 52 months. As expected, with 10 mice per group, no more than three in the low dose (0.003 and 0.01 μg) groups had ELISA units >6,000 and at least seven in the high dose (0.1 and 0.3 μg) groups had ELISA units >6,000. The potency results of clinical grade 78μg/mL Pfs25-EPA/Alhydrogel met the pre-established statistical model for the two high and low ranges tested (0.003 vs 0.1 μg and 0.01 vs 0.3 μg) and the vaccine was considered potent (Fig. 4). An additional set of potency data was generated with the freshly formulated vaccine at the four different delivery doses. The ELISA data also met the pre-established statistical model for the potency assay (Fig. 4).
Figure 4. Mouse potency study.
Red solid circles represent a freshly prepared formulation while blue solid circles represent a clinical formulation stored at 2-8°C for: (A) 3 months, (B) 9 months, (C) 15 months, (D) 21 months, (E) 27 months, (F) 35 months, (G) 40 months, (H) 46 months, and (I) 52 months. Each bar represents the average and standard deviation of 10 mice at each dose group.
Discussion
One of the major challenges facing the quality control evaluation process for alum-based vaccines is to develop a set of stability-indicating methods (SIMs) which may include one or several assays essential to the quality control process. Although aluminum containing adjuvants have been used since 1926 (17) and are the currently most frequently used adjuvants in the USA (18-21), basic quality control methods used for characterizing alum-based vaccines remain unavailable. According to the regulatory guidance, a SIM is:
“A validated quantitative analytical procedure that can detect the changes with time in the pertinent properties of drug substance and drug product. A stability-indicating assay accurately measures the active ingredients, without interference from degradation products, process impurities, excipients, or other potential impurities.”(22)
To develop a SIM to be used for assessing the quality of the Pfs25-EPA/Alhydrogel vaccine, a thermal stress study (also referred to as thermal forced degradation study) was designed and qualified. This stress test is not required by the regulatory agencies at the early stage of clinical development; however, it can provide valuable information for selecting the needed analytical methods (23-25). Our results indicate that after dry-heat storage for 11 days at 60°C, the secondary/tertiary structure of Pfs25-EPA changes as demonstrated by Western blotting and intrinsic fluorescence, with a loss of epitopes as detected by DAFIA. Over 90% of Pfs25-EPA was degraded when evaluated by SDS-PAGE, Western blot and DAFIA. In addition, vaccine potency was lost when evaluated by a mouse potency study. As expected, the total protein remained unchanged when assayed by OPA; this was likely due to the fact that the OPA reagent detected the N-terminal amine group or amine groups on the sidechains of lysine, which were still present in the sample despite the degradation of Pfs25-EPA on Alhydrogel. In conclusion, SDS-PAGE with silver staining and Western blot can be used to determine the vaccine identity and integrity when antigen is desorbed from Alhydrogel, intrinsic fluorescence can be used to measure the change of vaccine tertiary or quaternary structures in the vaccine formulation, DAFIA can be used to determine the amount of intact antigen bound to Alhydrogel and mouse potency assays can be used to measure the biological activities of vaccine. These methods can be used as a set of SIMs for evaluating Alhydrogel-based vaccines.
Aluminum hydroxide adjuvant (Alhydrogel) exhibits broad x-ray diffraction bands that indicate a low degree of order, and corresponds to a mineral that is known as poorly crystalline boehmite (26). As the adjuvant ages, it is likely that further order develops by sequential deprotonation and dehydration; aluminum ions are complexed by forming double hydroxide bridges which releases protons. As a result, the pH and surface area decrease as Alhydrogel ages (26, 27). However, our results show that the pH of vaccine increased during storage for 4 years at 4°C, suggesting that the stability of the protein-conjugate bound to Alhydrogel plays a role in the overall pH of vaccine. We speculate that the degraded protein products may have reacted with the free protons released by the aging Alhydrogel and depleted free protons in solution. In comparison, the pH of Pfs25-EPA conjugate alone remained unchanged during the storage period of 3.5 years at -80°C (data are not shown).
In addition to the pH increase, a slight increase in lower molecular weight bands as detected by SDS-PAGE and a decrease in intrinsic fluorescence signal were also observed. These results indicate that the structure of Pfs25-EPA conjugates formulated on Alhydrogel changed slightly during the four years of storage at 4°C. Based on the amount of lower molecular weight bands present, the changes in structure did not affect the overall quality of the conjugate; therefore, the vaccine specifications set prior to manufacture were met for the entire testing period of four years.
In conclusion, the methods developed in this study are valuable in evaluating Alhydrogel-based vaccines, including Pfs25-EPA. The Pfs25-EPA conjugates formulated on Alhydrogel retain acceptable quality with regards to purity, identity, integrity, and efficacy after four years of storage at 4°C.
Acknowledgments
The authors would like to thank J. Patrick Gorres for assisting in the writing of the manuscript. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- EPA
ExoProtein A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Malaria Report. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 2.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature. 1976;263:57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 3.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 4.Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Malaria transmission-blocking vaccines--how can their development be supported? Nat Med. 2000;6:241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- 5.Duffy PE, Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 7.Saul A. Mosquito stage, transmission blocking vaccines for malaria. Curr Opin Infect Dis. 2007;20:476–481. doi: 10.1097/QCO.0b013e3282a95e12. [DOI] [PubMed] [Google Scholar]
- 8.Stowers A, Carter R. Current developments in malaria transmission-blocking vaccines. Expert Opin Biol Ther. 2001;1:619–628. doi: 10.1517/14712598.1.4.619. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, Stowers A, Miller LH, Saul A. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine. 2005;23:3131–3138. doi: 10.1016/j.vaccine.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, Lynn L, Song G, Zhang Y, Reiter K, MacDonald N, Narum DL, Long CA, Miller LH, Saul A, Mullen GE. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25:3923–3933. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimp RL, Jr, Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, Rausch KM, Kumar K, Wu Y, Jin AJ, Jones DS, Narum DL. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine. 2013;31:2954–2962. doi: 10.1016/j.vaccine.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, Dobrescu G, Lambert L, Keister D, Rippeon Y, Long CA, Shi L, Caulfield M, Shaw A, Saul A, Shiver J, Miller LH. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci U S A. 2006;103:18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai CW, Duggan PF, Shimp RL, Jr, Miller LH, Narum DL. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J Biotechnol. 2006;121:458–470. doi: 10.1016/j.jbiotec.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Zhu D, Saul A, Huang S, Martin LB, Miller LH, Rausch KM. Use of o-phthalaldehyde assay to determine protein contents of Alhydrogel-based vaccines. Vaccine. 2009;27:6054–6059. doi: 10.1016/j.vaccine.2009.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D, Huang S, Gebregeorgis E, McClellan H, Dai W, Miller L, Saul A. Development of a Direct Alhydrogel Formulation Immunoassay (DAFIA) J Immunol Methods. 2009;344:73–78. doi: 10.1016/j.jim.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenny AT, Pope CG, Waddington H, Wallace V. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;29:38–45. [Google Scholar]
- 18.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines--US perspective. Vaccine. 2002;20(3):S18–23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 20.Peek LJ, Martin TT, Elk Nation C, Pegram SA, Middaugh CR. Effects of stabilizers on the destabilization of proteins upon adsorption to aluminum salt adjuvants. J Pharm Sci. 2007;96:547–557. doi: 10.1002/jps.20762. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, O'Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002;19:715–728. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 22.FDA. Administration FaD; 2000. FDA Guidance for Industry, Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls Documentation. http://www.msk.nclinnovations.org/medregulations/v1/html/Guidance/Guidance_Analytical%20Method%20&%20Validation.pdf. [Google Scholar]
- 23.Blessy M, Patel RD, Pajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs-A review. J Pharm Analysis. 2014;4:159–165. doi: 10.1016/j.jpha.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakole RD, Charde MS, Kumar J, Welankiwar AS. Review: Development of forced degradation studies of drugs. International Journal of Advances in Pharmaceutics. 2013;2 [Google Scholar]
- 25.Singh S, Junwal M, Modhe G, Tiwari H, Kurmi M, Parashar N, Sidduri P. Forced degradation studies to assess the stability of drugs and products. TrAC Trends in Analytical Chemistry. 2013;49:71–88. [Google Scholar]
- 26.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Review of Vaccines. 2007;6:685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 27.Burrell LS, White JL, Hem SL. Stability of aluminium-containing adjuvants during aging at room temperature. Vaccine. 2000;18:2188–2192. doi: 10.1016/s0264-410x(00)00031-1. [DOI] [PubMed] [Google Scholar]