Abstract
Recent discoveries on the nature of the activity generated by the reticular activating system (RAS) suggest that arousal is much more involved in perception and movement than previously thought. The RAS is not simply an amorphous, unspecific region but rather a distinct group of nuclei with specific cell and transmitter types that control waking and modulate such processes as perception and movement. Thus, disturbances in the RAS will affect a number of neurological disorders. The discovery of gamma band activity in the RAS determined that high threshold calcium channels are responsible for generating gamma band activity in the RAS. Results showing that waking is mediated by CaMKII modulation of P/Q-type channels and REM sleep is modulated by cAMP/PK modulation of N-type channels points to different intracellular pathways influencing each state. Few studies address these important breakthroughs. Novel findings also show that the same primate RAS neurons exhibiting activity in relation to arousal are also involved in locomotion. Moreover, deep brain stimulation of this region, specifically the pedunculopontine nucleus (PPN DBS), in Parkinson’s disease has salutary effects on movement, sleep, and cognition. Gamma oscillations appear to participate in sensory perception, problem solving, and memory, and coherence at these frequencies may occur at cortical or thalamocortical levels. However, rather than participating in the temporal binding of sensory events, gamma band activity generated in the RAS may help stabilize coherence related to arousal, providing a stable activation state during waking, and relay such activation to the cortex. Continuous sensory input will thus induce gamma band activity in the RAS to participate in the processes of preconscious awareness, and provide the essential stream of information for the formulation of many of our perceptions and actions. Such a role has received little attention but promises to help understand and treat a number of neurological disorders.
Keywords: Calcium channels, deep brain stimulation, gamma oscillations, Parkinson’s disease, pedunculopontine nucleus, waking
Introduction
We spend two thirds of our lives awake. Waking is when we develop ideas, create objects, develop relationships, interact with other people, earn a living, basically, when we do the really important things in life. It is no wonder what an impact neurological disorders have on our quality of life when waking is disturbed. A number of recent publications describe sleep disturbances in certain neurological disorders [1, 2]. This review suggests that it is waking and not sleep that requires attention. Despite the importance of waking, there are very few publications about waking and the process of staying awake. There are many more publications about sleep and sleep dysregulation, about what happens when we have abnormal sleep, and when our vigilance interrupts or pushes aside our sleeping hours [3, 4]. The complaints patients offer are usually that they have “problems sleeping”, hardly ever do they say they have “problems waking”. But the fact is that most neurological disorders involve just that, “problems waking”. These patients are not sleeping enough because waking drive actually is increased, not because sleeping drive is decreased.
That is, hypervigilance and increased REM sleep drive is the factor that cuts down on our sleep, waking us early and often when we suffer from these diseases. For example, we suggested that insomnia is not a “sleep disorder”, but rather a “waking disorder” of excessive waking drive [5]. On the opposite side of the spectrum, hypo-vigilance is rare, most obvious in such disorders as narcolepsy, but also in Alzheimer’s disease (AD) and other neurodegenerative disorders. Below, we will see how the process of waking is begun and how it is maintained. Without a firm understanding of the mechanisms behind normal waking, treating and controlling neurological disorders becomes more difficult. In the following review, we will see how this process is disturbed by neurological disease. In addition, we seem to require a modicum of arousal in order to detect stimuli and perform movements. Perception is based on sufficient arousal. Our motor control also appears to require a level of excitability in order to perform motions accurately. Therefore, arousal is essential to perception and movement. It is obvious that dysregulation in reticular activating system (RAS) output impacts much more than sleep-wake cycles, it affects our ability to perceive and to move around in the world.
Why is this system involved in arousal as well as stimulus detection and motor control? The RAS is a phylogenetically conserved system that modulates fight-or-flight responses. During waking, man’s ability to detect predator or prey is essential to survival. Thus, it is not surprising that the RAS can modulate not only sleep and waking, but also perception, muscle tone, and locomotion. This system is automatically linked to eliciting arousal as well as the control of the motor system in order to optimize attack or escape during waking. Moreover, during REM sleep, atonia keeps us from acting out our dreams. In fact, only our diaphragm and eye muscles appear to be acting out dream content. Therefore, during both waking and REM sleep, the RAS modulates the level of arousal via ascending thalamocortical pathways, and of muscle tone and locomotion via descending reticulospinal systems [6].
The Reticular Activating System (RAS)
After the discovery that the EEG manifested different types of activity during waking vs sleep, Moruzzi and Magoun found that stimulation of the brainstem reticular formation abolished low frequency activity (such as seen during slow wave sleep- SWS) and induced high frequency activity (such as seen during waking) in the cortical EEG [7]. They wrote, “the possibility is considered that a background of maintained activity within the ascending brainstem activating system may account for wakefulness, while reduction of its activity either naturally, by barbiturates, or experimental injury and disease, may respectively precipitate normal sleep, contribute to anesthesia or produce pathological somnolence” [7]. Further transection studies concluded that the, “maintained influence of the ascending brain stem activating system underlies wakefulness, while absence of this influence precipitates sleep” [8]. In later studies, Moruzzi transected the brainstem at the ponto-midbrain junction, a few millimeters caudal to the original cerveau isolé preparation. These transections at mid-pontine pretrigeminal levels produced spontaneous EEG patterns and eye movements, like those observed for the encéphale isolé preparation [9]. Similar mid-pontine transections were performed by Steriade that led to waking EEG signs, while postcollicular-premamillary transections led to SWS EEG [10]. Therefore, nuclei near the pons-midbrain junction were implicated in the generation of high frequency EEG patterns responsible for the generation and maintenance of waking.
Kleitman, Aserinsky and Dement correlated dreams, increased respiration, heart rate, and eye movements to high frequency EEG patterns during REM sleep [11, 12]. They also proposed the dual nature of sleep: REM sleep is a completely different state than SWS, even though they both occur while asleep. Michel Jouvet expanded on these results to show that REM sleep, termed “paradoxical sleep” because of the manifestation of waking EEG patterns, is accompanied by muscle atonia and rostro-pontine transections preserved muscle atonia during REM sleep [13].
A large number of studies using multiple methods went on to find that the RAS is made up of three specific nuclei: the locus coeruleus nucleus (LC), with norepinephrine/noradrenaline (NE/NA)-containing neurons; the dorsal raphe nucleus (RN), with serotonin (5-HT)-containing neurons; and the pedunculopontine nucleus (PPN), with acetylcholine (ACh)- and glutamate (GLU)-containing neurons. All of these nuclei also contain neurons with the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). The LC and RN inhibit the PPN, and the PPN excites the LC. The LC and RN are most active during waking and SWS, while the PPN is most active during waking and REM sleep [for a detailed description and original references, see 6, 14]. Thus, the PPN is the only RAS nucleus most related to the arousal states of waking and REM sleep.
The Pedunculopontine Nucleus
The PPN is composed of different populations of ACh, GLU, and GABA neurons [15]. Extracellular recordings of PPN neurons in vivo identified six categories of thalamic projecting PPN cells distinguished by their firing properties relative to ponto-geniculo-occipital wave generation [16]. Some of these neurons showed low rates of spontaneous firing (<10 Hz), but most had high rates of tonic firing in the beta/gamma range (20–80 Hz). PPN neurons exhibit beta/gamma frequencies in vivo during active waking and REM sleep, but not during slow wave sleep [16–20]. Similarly, the presence of gamma band activity has been confirmed in the cortical EEG of the cat in vivo when the animal is active [16, 21], and in the region of the PPN in humans during stepping, but not at rest [22]. A recent study showed that PPN neurons fired at low frequencies ~10 Hz at rest, but the same neurons increased firing to gamma band frequencies when the animal awakened, or when the animal began walking on a treadmill [23]. That is, the same cells were involved in both arousal and motor control. Thus, there is ample evidence for gamma band activity during active waking and movement in the PPN in vitro, in vivo, and across species, including man.
Recently, we described the intrinsic membrane mechanisms behind gamma band activity in the PPN [24–29]. Briefly, gamma oscillations are mediated by voltage-dependent, high threshold N- and P/Q-type calcium channels that are present in every PPN neuron, regardless of cell or transmitter type. These channels are distributed along the dendrites of PPN cells [30]. Afferent input traveling through “non-specific” reticular pathways activate PPN dendrites. However, gamma band activity during waking has different mechanisms than gamma band activity during REM sleep. Injections of glutamate into the PPN increased both waking and REM sleep [31], while injections of the glutamatergic receptor agonist N-methyl-D-aspartic acid (NMDA) increased only waking [32], and injections of the glutamatergic receptor agonist kainic acid (KA) increased only REM sleep [33]. Intracellularly, protein kinase C (PKC), which modulates KA receptors, enhances N-type channel activity and has no effect on P/Q-type channel function [34], but CaMKII, which modulates NMDA receptors, was shown to modulate P/Q-type channel function [35].
That is, the two calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. Moreover, there are three cell types in the PPN, those bearing only N-type calcium channels, those with both N- and P/Q-type, and those with only P/Q-type calcium channels [36, 37]. The implications from all of these results is that, a) there is a ‘‘waking’’ pathway mediated by CaMKII and P/Q-type channels and a ‘‘REM sleep’’ pathway mediated by cAMP/PK and N-type channels, and b) different PPN cells fire during waking (those with N+P/Q and only P/Q-type) vs REM sleep (those with N+P/Q and only N-type). Unfortunately, the involvement of high threshold calcium channels and separate intracellular pathways has not been sufficiently studied for their involvement in neurodegenerative and other neurological disorders.
Gamma Band Activity
As far as the cortex is concerned, the difference between gamma band activity during waking vs REM sleep appears to be a lack of coherence [38]. That is, brainstem driving of gamma band activity during waking carries with it coherence across distant cortical regions, while driving of gamma band activity during REM sleep does not include coherence across distant regions [38,39]. Also, carbachol-induced REM sleep with cataplexy is characterized by decreased gamma band coherence in the cortex [40]. These results suggest that, a) brainstem centers drive gamma band activity that is manifested in the cortical EEG, b) during waking brainstem-thalamic projections include coherence across regions, and c) during REM sleep they drive cortical EEG rhythms without coherence. We should note that a critical mediator of coherence is electrical coupling [41], which is present in the PPN [42]. Interestingly, the stimulant modafinil, which is used to treat narcolepsy and excessive daytime sleepiness, is known to increase electrical coupling and thus promote coherence at high frequencies, leading to increased arousal [42, 43]. Below, we will discuss how this unusual agent could be used to increase gamma band activity in conditions in which its generation or maintenance is impaired.
In general, gamma oscillations appear to participate in sensory perception, problem solving, and memory [44–48], and coherence at these frequencies may occur at cortical or thalamocortical levels [49, 50]. Indeed, synchronous gamma band activation among thalamocortical networks [51], and in other neuronal groups is thought to contribute to the merger, or “binding”, of information originating from separate regions [52]. Gamma oscillation deficits have been suggested as a pathophysiologic feature of diseases like AD [21, 53–54]. However, while cortical gamma band activity can be expected to participate in these processes, what is the role of gamma band activity in the RAS? We proposed that activation of the RAS generates the background of gamma activity necessary to support a state capable of reliably assessing the world around us on a continuous basis. That is, these mechanisms may underlie the process of preconscious awareness [29, 41]. Therefore, sensory activation of the RAS provides the background of activation, the level of activity, necessary for perception and voluntary movement [26]. When that level is not met, both perception and motor control are impaired.
The RAS Perception and Motor Control
A manifestation of ascending RAS output induced by sensory input is the P50 potential that is recorded at the vertex in man [55]. The magnetic equivalent M50 response is also localizable to the region of the vertex [56]. The P50 potential is a click stimulus-induced midlatency auditory evoked response (at a 50–70 msec latency) that follows the brainstem auditory evoked potentials that occur at <10 msec latency, and the primary auditory evoked “Pa” response at a 25 msec latency. The P50 potential is, a) sleep state-dependent, such that it is present during waking and REM sleep, but not during SWS, e.g. is manifested during arousal states when PPN is active, b) blocked by low doses of scopolamine, e.g. it is generated by cholinergic projections of the PPN, and c) rapidly habituating, e.g. reticular in origin with low synaptic security [reviewed in 55]. Animal studies showed that lesions of the PPN or injections of inhibitory agents into the PPN eliminated the equivalent vertex-recorded potential (P13 in the rodent, “wave a” in the feline), emphasizing the origin of the waveform as the PPN [reviewed in 55]. In summary, the P50 potential is an arousal-related waveform in the human. We showed that PD patients manifested decreased habituation of the P50 midlatency auditory evoked potential [57], and that in PD patients who received bilateral pallidotomy that alleviated their motor symptoms, habituation of the P50 potential returned to normal levels [58]. The P50 potential is therefore a valuable noninvasive measure of sensory activation of the RAS in neurological disorders.
As far as locomotion is concerned, stimulation of the PPN at 40–60 Hz (gamma band) was found to elicit locomotion on a treadmill in the decerebrate animal [reviewed in 26], accounting for the effects of stimulating the so-called “mesencephalic locomotor region” (MLR) [59]. The PPN, however, as part of the RAS, is known to modulate posture and locomotion, so that the assignation of the PPN as part of the MLR is inaccurate. A nearby structure, the cuneiform nucleus, was also invoked as the MLR, but recent studies using deep brain stimulation (DBS) of the PPN for PD were found to induce ameliorative effects on posture and locomotion [26], but, DBS of the cuneiform nucleus does not produce such effects on posture or locomotion [60]. In addition, PPN DBS is known to induce glucose utilization in the PPN, the thalamus, and a circumscribed cortical region in the area of the vertex [61]. That is, cortical metabolic and blood flow changes due to PPN DBS are manifested at the cortex in the same region as the P50 potential.
Another potential that is maximally recorded at the vertex is the readiness potential (RP), a negative DC waveform that occurs in advance of a voluntary movement [62, 63]. The RP precedes movement by approximately 600–800 milliseconds [62]. The RP is reduced or absent in PD [64], and is also reduced in Huntington’s disease (HD) [65]. Despite knowledge of the aforementioned, the mechanism underlying this waveform has remained a mystery until recently. Emerging evidence suggests the RP is related to “intentional binding” [66]. Intentional binding is the process whereby a voluntary action is linked with a sensory cue in time. That is, the RP is therefore a valuable noninvasive measure of voluntary motor activation of the RAS in neurological disorders.
In summary, these findings suggest that the RAS is involved in preconscious awareness for sensory perception as well as the intent for voluntary movement, essential processes for the formulation of our sensations and movements. Figure 1 depicts the distribution of the peak amplitude of the P50 auditory evoked potential, which is generated by the PPN, and its overlap with the region of the cortex activated by PPN DBS, as well as the distribution of the RP, all of which signal PPN output to the cortex. In addition, the RP as recorded using DC or long time constant amplifiers is shown, along with spectral analysis of the EEG in relation to an uncued button press. These measures are critical for assessing the role of arousal in perception and movement in neurological disorders.
Figure 1. Sensory and motor manifestations of RAS output in the cortex.
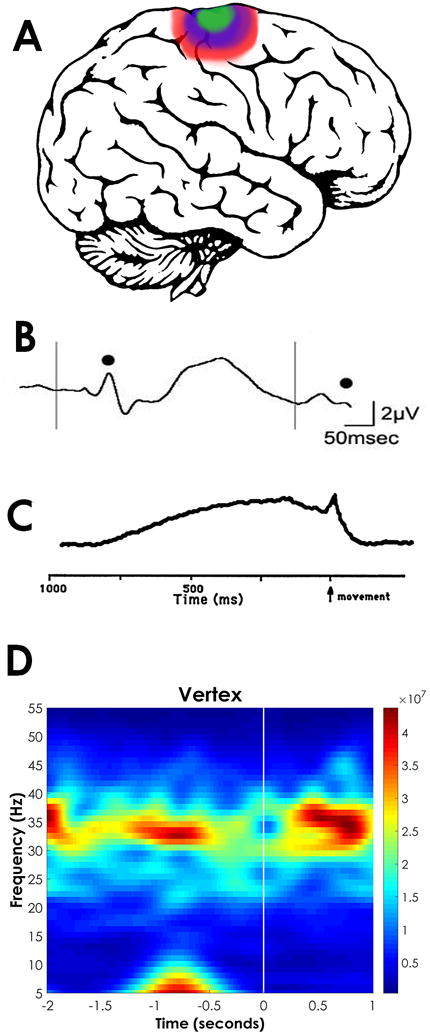
A. Lateral view of the brain showing the distribution of the P50 midlatency auditory evoked potential, which is generated by the PPN [55] and recorded at maximal amplitude at the vertex, as is the magnetic equivalent M50 response [56] (purple region). The RP is also recorded at maximal amplitude in the area of the vertex [62, 63] (blue region). Also, changes in blood flow or metabolic changes during PPN DBS appear in the same region [61]. B. Paired auditory click stimuli induce an evoked response at ~50 msec latency after each stimulus (black dots). If a second stimulus is administered 250 msec after the first, the P50 potential habituates and is of lower amplitude (second black dot). C. A voluntary button press recorded with long time constant elicits a negative DC shift at the vertex known as the RP, which begins 600–800 msec preceding the movement, then shows a peaked motor potential before returning to baseline. D. Event Related Spectral Perturbation (ERSP), which is basically a running power spectrum of an EEG recording at the vertex is shown for 2 sec before and 1 sec after an uncued button press. Note that gamma band activity (30–60 Hz) is present 600–800 msec preceding the movement, presumably representing bottom-up gamma activity.
Clinical Implications
For neurological disorders in which the RAS is overactive, this would mean that alerting stimuli will produce exaggerated responses that would be manifested as exaggerated startle responses or hyperactive reflexes such as the blink reflex. Another property of the RAS is its rapid habituation to repetitive stimuli, which is reflected in its lack of responsiveness to rapidly repeating stimuli. This endows the RAS with its capacity for sensory gating, the property of decreasing responsiveness of repetitive events in favor of novel or different stimuli. For neurological disorders in which this property is affected, we expect a decrease in habituation or a sensory gating deficit. The RAS controls waking and sleep, so that sleep patterns would be dysregulated. If the RAS is down regulated by a disorder, we expect an inability to remain awake, the presence of excessive daytime sleepiness, and an excess of total sleep time, especially an increase in SWS. If, on the other hand, the RAS is up regulated, we expect difficulty in getting to sleep and maintaining sleep. This would be reflected in insomnia or disrupted sleep during the night, as well as increased REM sleep drive, which is characterized by vivid nightmares and frequent awakenings, even hallucinations. The RAS also modulates the maintenance of waking, a property ignored by many, but one that affects a host of functions. The inability to maintain a steady waking state, in the form of maintained gamma band activity, will interfere with attention, learning, and memory, to name a few processes.
What are the EEG, P50 potential, and reflex findings in the most common neurological disorders?
Parkinson’s disease (PD)
In PD, hyperactive reflexes of several kinds have been described [67–71]. PD patients show sleep disturbances that include increased REM sleep drive, decreased SWS, frequent awakenings leading to daytime sleepiness, all resulting in insomnia [72]. Vivid dreams and REM sleep behavior disorder are also common features of PD. These observations suggest that the RAS, especially the PPN that is in charge of waking and REM sleep, is overactive in PD. Recently, the PPN has become a target for deep brain stimulation (DBS) in PD. A number of studies using PPN DBS for the treatment of PD have reported improvements in motor function [73–75], but not all groups reported positive effects [76, 77]. Ferraye et al [76] found that bilateral PPN stimulation at 15–25 Hz improved gait and decreased falls. Moro et al [77] used unilateral stimulation at 50 and 70 Hz to improve falls and motor scores. Stefani et al [78, 79] used PPN stimulation at 10 and 25 Hz, with a significant improvement in sleep patterns and modest improvement in gait. Alessandro et al [80] used 25 Hz stimulation to show a significant amelioration in sleep scores and executive function. Thevanasathan et al [81, 82] showed that PPN stimulation at 20–35 Hz improved reaction time and fall scores. The latter study used double-blind analysis and established that bilateral stimulation was more effective than unilateral. One study performed sleep measures and found that PPN DBS improved not only nighttime sleep, but also daytime sleepiness [83]. Others showed that PPN DBS may improve cognitive function [84], and that low frequency stimulation (5–30 Hz) may improve executive and higher functions [79].
Alzheimer’s disease (AD)
The EEG findings in AD suggest an increase in lower frequencies such as delta, and a decrease in higher frequencies such as beta and gamma [85–88]. However, some studies point to increased gamma band EEG activity in some patients with AD [89, 90]. The blink reflex and startle response are delayed and/or exaggerated in AD [91–93], indicative of decreased sensory gating. The P50 potential was reduced in amplitude as well as decreased in habituation [94]. Together, these findings suggest that the PPN is underactive in AD, accounting for the decreased REM sleep duration [95], decreased high frequencies in the EEG, and decreased P50 potential amplitude. The decreased habituation of the P50 potential may be explained by decreased descending cortical modulation of the RAS. Therefore, both ascending RAS output and descending cortical output are reduced, making it very difficult to reestablish appropriate levels of vigilance.
Huntington’s disease (HD)
The EEG in HD has been reported to show decreases in alpha and beta power [96], but conversely increases in delta and beta power [97]. Surprisingly, changes in gamma band have not been described. Blink, corneal, and jaw reflexes all manifest decreased habituation [98], as does the auditory startle response [99] in HD. We demonstrated decreased amplitude and prolonged latency of the P50 potential (in keeping with decreased arousal levels), as well as a lack of habituation of the P50 potential in a paired click paradigm, consistent with impairment of sensory gating in HD [100]. HD patients also spend less time in REM sleep with increased nighttime arousals and these symptoms can commonly occur as a pre-motor or early manifestation of the disease [101, 102].
Insomnia
The EEG characteristics of insomnia do not show major differences with good sleepers, with some studies reporting an increase in low beta and decrease in high beta frequency power [103], as well as decreases in REM sleep [104]. In general, however, the differences in the EEG are subtle but do suggest intrusion of higher frequency during typically low frequency states, such as the incidence of higher beta activity during SWS [105–107]. Experts in the field agree that primary insomnia patients not only show hyperarousal at night, but also during the day [108, 109]. This particular spectrum suggests that there is high frequency activity during SWS as well as decreased REM sleep output, but the hyperarousal persists during waking. We suggested that at least some insomnia patients may suffer from increased expression of P/Q-type calcium channels, which would preferentially drive the “waking” pathway [110].
Neglect
The EEG in neglect is generally depressed, with overall slowing, increased delta band activity, and inability to generate fast activity [111–113]. In general, reflexes and reaction times are increased in neglect patients [114]. The P50 potential is somewhat reduced in amplitude and habituation but these effects are not significant, perhaps because recordings are done with a single midline electrode and sources in the two hemispheres may be summating algebraically [115]. The fact that a cold pressor test transiently diminishes neglect suggests that there is a lack of arousal in the affected hemisphere, i.e. it is “asleep” [115].
Narcolepsy
Narcolepsy is characterized by excessive daytime sleepiness and bouts of cataplexy, in which affective incitement (arousal) leads to a loss of extensor muscle tone. Many patients also have hypnagogic hallucinations, a symptom that emphasizes the likely intrusion of REM sleep into the waking state. During sleep, patients with narcolepsy frequently enter REM sleep within minutes of falling asleep in contrast to the normal latency of 80–100 minutes. That is, both waking and REM sleep are dysregulated in narcolepsy. The P50 potential is reduced in amplitude and habituation in narcolepsy [116]. Almost all narcoleptic patients exhibit human leucocytic antigen (HLA) genotype expression for DQB1 [117], which is quite similar to the HLA expression (DQW1) we found in REM sleep behavior disorder patients [118], many of which develop PD [119].
Novel Therapies
How can we clinically modulate gamma band activity? One way of inducing high frequency activity is with the use of stimulants, and another involves the use of stimulation.
We described the presence of dye and electrical coupling in the RAS through gap junctions, specifically in the PPN [42]. We also found that modafinil decreased the resistance of PPN cells [42], in keeping with results in the cortex, reticular thalamus, and inferior olive [43]. The effects of modafinil are dependent on CaMKII, since its effects are blocked by the CaMKII activation blocker KN-93 [43]. These data suggest that modafinil preferentially promotes high frequency activity through the CaMKII (“waking”) pathway [29,41]. Moreover, studies on cocaine abusers [120], and on an animal model of sleep-disordered breathing [121], suggest that modafinil may also decrease REM sleep. These findings suggest that modafinil may be particularly effective in driving waking without affecting, perhaps decreasing, REM sleep. This agent may thus be effective in increasing the level of arousal, the background of activity, that would improve perception and movement in such disorders as PD, AD, HD, and neglect, as we recently demonstrated [115]. Interestingly, modafinil is quite safe since even the ingestion of massive overdoses led to minor symptoms and no deaths [122].
The results of DBS in the subthalamic nucleus (STN) and PPN show that they are effective for certain measures, are surgically fairly safe, and are well tolerated. As far as the PPN is concerned, stimulation at gamma frequencies in PD appears to improve function in posture and movement, perhaps because the preferred frequency of these cells is being imposed by DBS. Moreover, the use of continuous application of DBS may induce habituation and establish a stable level of activation, essentially helping maintain gamma band frequencies. If this is the case, and appropriate studies on these patients are still necessary, this method may be amenable for the treatment of other disorders involving dysregulation in PPN output, either due to overactivity as in PD, or lack of maintenance of gamma band activity or interrupted gamma activity. That is, increased PPN output may be tractable by DBS in AD, PD, HD, and perhaps even neglect. Obviously, the use of DBS would be called for only in unresponsive and intractable cases, in which all other options have been exhausted. Much more testing in animals and patients is required, along with investigation of physiological mechanisms at the cellular level. The fact is that such physiological measures are absolutely essential in order to demonstrate that manipulations are having a physiologically relevant effect that is indeed altering symptomatology.
Monitoring effectiveness of these therapies using the P50 potential, reflex measures, and the RP would provide established, noninvasive physiological assessments.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P30 GM110702, and the Clinician Scientist Program, University of Arkansas for Medical Sciences.
References
- 1.Jennum P, Santamaria Cano J, Bassetti C, Clarenbach P, Hogl B, Mathis J, Poirrier R, Snka K, Svanborg E, Dolenc Groselj L, Kaynak D, Kruger M, Papavasiliou A, Zahariev Z. In: Sleep disorders in neurogegenerative disorders and stroke, in European Handbook of Neurological management. 2nd. Gilhus NE, Barnes MP, Brainin M, editors. Vol. 1. Blackwell; London: 2011. p. 529. [Google Scholar]
- 2.Overeem S, Reading P, editors. Sleep Disorders in Neurology: A practical approach. Wiley-Blackwell; London: 2010. p. 344. [Google Scholar]
- 3.Kisch D, editor. Sleep Medicine in Neurology. Wiley-Blackwell; London: p. 192. [Google Scholar]
- 4.Lisak RP, Truong DD, Carroll WM, Bhidayashi R. International Neurology A clinical approach. Wiley-Blackwell; London: 2009. p. 695. [Google Scholar]
- 5.Garcia-Rill E, Luster B, Mahaffey S, Bisagno V, Urbano FJ. Pedunculopontine arousal system physiology- implications for insomnia. Sleep Sci. 2015;8:92. doi: 10.1016/j.slsci.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Rill E. Waking and the Reticular Activating System. Academic Press; New York: 2015. p. 330. [Google Scholar]
- 7.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455. [PubMed] [Google Scholar]
- 8.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brainstem activating system. Electroenceph, Clin, Neurophysiol. 1949;1:475. [PubMed] [Google Scholar]
- 9.Moruzzi G. The sleep-waking cycle. Ergeb, Physiol. 1972;64:1. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M, Constantinescu E, Apostol V. Correlations between alterations of the cortical transaminase activity and EEG patterns of sleep and wakefulness induced by brainstem transections. Brain Res. 1969;13:177. doi: 10.1016/0006-8993(69)90152-8. [DOI] [PubMed] [Google Scholar]
- 11.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 12.Aserinsky E, Kleitman N. Two types of ocular motility occurring in sleep. J Appl Physiol. 1955;8:1. doi: 10.1152/jappl.1955.8.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Jouvet M. Research on the neural structures and responsible mechanisms in different phases of physiological sleep. Arch Ital Biol. 1962;100:125. [PubMed] [Google Scholar]
- 14.Garcia-Rill E. In: Sleep and arousal states: reticular activating system, in New Encyclopedia of Neuroscience. Squire LR, Bloom F, Spitzer N, Gage F, Albright T, editors. Vol. 8. Elsevier, Oxford; England: 2009. p. 137. [Google Scholar]
- 15.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contan distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steriade M, Paré D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 18.Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:79. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 20.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 22.Fraix V, Bastin J, David O, Goetz L, Ferraye M, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLOS ONE. 2013;8:e83919. doi: 10.1371/journal.pone.0083919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, et al. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm. 2016;123:667. doi: 10.1007/s00702-016-1577-7. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, et al. Gamma band activity in the RAS-intracellular mechanisms. Exp Brain Res. 2014;232:1509. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, et al. Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm. 2015;123:655. doi: 10.1007/s00702-015-1485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Rill E, D’Onofrio S, Luster B, Mahaffey S, Urbano FJ, et al. The 10 Hz Frequency: a fulcrum for transitional brain states. Translat Brain Rhyth. 2016;1:7. [PMC free article] [PubMed] [Google Scholar]
- 28.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urbano FJ, D’Onofrio SM, Luster BR, Hyde JR, Bosagno V, et al. Pedunculopontine nucleus gamma band activity- preconscious awareness, waking, and REM sleep. Frontiers in Sleep and Chronobiol. 2014;5:210. doi: 10.3389/fneur.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyde JR, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013;115:1402. doi: 10.1152/japplphysiol.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer J Physiol Reg Integ Comp Physiol. 2001;280:R752. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 32.Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopotine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001;66:109. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 33.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 34.Stea A, Soomg TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15:929. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, et al. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Nat Acad Sci USA. 2008;105:341. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2015;3:e12431. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep. 2016:e12787. doi: 10.14814/phy2.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro S, Falconi A, Chase M, Torterolo P. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur J Neurosci. 2013;37:1330. doi: 10.1111/ejn.12143. [DOI] [PubMed] [Google Scholar]
- 39.Cavelli M, Castro S, Schwartzkopf N, Chase M, Falconi A, Torterolo P. Coherent cortical oscillations decrease during REM sleep in the rat. Behav Brain Res. 2015;281:318. doi: 10.1016/j.bbr.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 40.Torterolo P, Castro-Zaballa S, Cavelli M, Chase M, Falconi A. Neocortical 40 Hz oscillations during carbachol-induced rapid eye movement sleep and cataplexy. Eur J Neurosci. 2015;281:318. doi: 10.1111/ejn.13151. [DOI] [PubMed] [Google Scholar]
- 41.Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P, Garcia-Rill E. Gamma band activity in the reticular activating system (RAS), Frontiers Neurol. Sleep Chronobiol. 2012;3:6. doi: 10.3389/fneur.2012.00006. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbano FJ, Leznik E, Llinas RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Nat Acad Sci USA. 2007;104:12554. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual system? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 45.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Nat Acad Sci USA. 1989;86:1698. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient than efficient visual search in human EEG. Int J Psychophysiol. 2009;73:350. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Palva S, Monto S, Palva JM. Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage. 2009;49:3257. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 48.Voss U, Holzmann R, Tuin I, Hobson JA. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep. 2009;32:1191. doi: 10.1093/sleep/32.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Nat Acad Sci USA. 1991;88:897. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singer W. Synchronization of cortical activity and its putative role in informtion processing and learning. Annu Rev Physiol. 1993;55:349. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 51.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llinás RR, Paré D. Of dreaming and wakefulness. Neurosci. 1991;44:521. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 53.Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Nat Acad Sci USA. 1991;88:11037. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Rill E, Skinner RD. In: The sleep state-dependent P50 midlatency auditory evoked potential, in Sleep Medicine. Lee-Chiong TL, Carskadon MA, Sateia MJ, editors. Hanley & Belfus; Philadelphia: 2001. p. 697. [Google Scholar]
- 56.Garcia-Rill E, Moran K, Garcia J, Findley WM, Walton K, Strotman B, et al. Magnetic sources of the M50 response are localized to frontal cortex. Clin Neurophysiol. 2008;119:388. doi: 10.1016/j.clinph.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teo C, Rasco Al, Al-Mefty K, Skinner RD, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease. Movement Disord. 1997;12:655. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- 58.Teo C, Rasco AI, Skinner RD, Garcia-Rill E. Disinhibition of the sleep state-dependent P1 potential in Parkinson’s disease- improvement after pallidotomy. Sleep Res Online. 1998;1:62. [PubMed] [Google Scholar]
- 59.Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659. [PubMed] [Google Scholar]
- 60.Mazzone P, Sposato S, Insola A, Scarnati E. The clinical effects of deep brain stimulation of the pedunculopontine tegmental nucleus in movement disorders may not be related to the anatomical target, leads location, and setup of electrical stimulation. Neurosurg. 2013;73:894. doi: 10.1227/NEU.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 61.Ballanger B, Lozano AM, Moror E, van Elmeren T, Hamani C, Chen R, Cilia R, Houle S, Poon YY, Lang AE, Strafella P. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patienst with advanced Parkinson’s disease: A [15O] H2O PET study. Human Brain Mapping. 2009;30:3901. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kornhuber HH, Deecke L. Changes in the Brain Potential in Voluntary Movements and Passive Movements in Man: Readiness Potential and Reafferent Potentials. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;284:1. [PubMed] [Google Scholar]
- 63.Deecke L, Grozinger B, Kornhuber HH. Voluntary finger movement in man: cerebral potentials and theory. Biol Cybern. 1976;23:99. doi: 10.1007/BF00336013. [DOI] [PubMed] [Google Scholar]
- 64.Simpson JA, Khuraibet AJ. Readiness potential of cortical area 6 preceding self paced movement in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1987;50:1184. doi: 10.1136/jnnp.50.9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner LM, Croft RJ, Churchyard A, Looi JC, Apthorp D, Georgiou-Karistianis N. Abnormal Electrophysiological Motor Responses in Huntington’s Disease: Evidence of Premanifest Compensation. PLoS One. 2015;10:e0138563. doi: 10.1371/journal.pone.0138563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo HG, Wittmann M, Hinterberger T, Schmidt S. The readiness potential reflects intentional binding. Front Hum Neurosci. 2014;8:421. doi: 10.3389/fnhum.2014.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson IT, Lenman JAR, Johnston BB. Habituation of the orbi- cularis oculi reflex in dementia and dyskinetic states. J Neurol Neurosurg Psychiat. 1978;41:824. doi: 10.1136/jnnp.41.9.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimura J. Disorder of interneurons in parkinsonism: the orbicularis oculi reflex to paired stimuli. Brain. 1973;96:87. doi: 10.1093/brain/96.1.87. [DOI] [PubMed] [Google Scholar]
- 69.Nakashima K, Shimoyama R, Yokoyama Y, Takahashi K. Auditory effects on the electrically elicited blink reflex in patients with Parkinson’s disease. Electroenceph Clin Neurophysiol. 1993;89:108. doi: 10.1016/0168-5597(93)90092-4. [DOI] [PubMed] [Google Scholar]
- 70.Penders CA, Delwaide PJ. Blink reflex studies in patients with parkinsonism before and during surgery. J Neurol Neurosurg Psychiat. 1971;34:674. doi: 10.1136/jnnp.34.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothwell JC, Obeso JA, Traub MM, Marsden CD. The behavior of the long-latency stretch reflex in patients with Parkinson’s disease. J Neurol Neurosurg Psychiat. 1983;46:35. doi: 10.1136/jnnp.46.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiat. 2007;79:368. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 73.Mazzone PSP, Lozano A, Sposato S, Scarnati E, Stefani A. Proceedings of 14th Meeting of the World Society of Stereotactic and Functional Neurosurgery (WSSFN) Monduzzi; Bologna: 2005. Brain stimulation and movement disorders: where we going? p. 345. [Google Scholar]
- 74.Mazzone P, Insola A, Sposato S, Scarnati E. The deep brain stimulation of the pedunculopontine tegmental nucleus. Neuromod. 2009;12:191. doi: 10.1111/j.1525-1403.2009.00214.x. [DOI] [PubMed] [Google Scholar]
- 75.Mazzone P, Sposato S, Insola A, Dilazzaro V, Scarnati E. Stereotactic surgery of nucleus tegmenti pedunculopontine [corrected] Brit J Neurosurg. 2008;22:S33. doi: 10.1080/02688690802448327. [DOI] [PubMed] [Google Scholar]
- 76.Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardes S, Pollak P. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133:205. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- 77.Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 78.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Troppei D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 79.Stefani A, Peppe A, Galati S, Stampanoni A, Bassi M, D’Angelo V, Pierantozzi M. The serendipity case of the pedunculopontine nucleus low-frequency brain stimulation: chasing a gait response, finding sleep, and cognitive improvement. Frontiers Neurol. 2013;4:68. doi: 10.3389/fneur.2013.00068. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, Placidi F, Romigi A, Iani C, Marzetti F, Peppe A. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive problems. J Neurol Sci. 2010;289:44. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 81.Thevanasathan W, Silburn PA, Brooker H, Coyne TJ, Kahn S, Gill SS, Aziz TZ, Brown P. The impact of low-frequency stimulation of the pedunculopontine nucleus region on reaction time in Parkinsonism. J Neurol Neurosurg Psychiat. 2010;81:1099. doi: 10.1136/jnnp.2009.189324. [DOI] [PubMed] [Google Scholar]
- 82.Thevanasathan W, Cole MH, Grapel CL, Hyam JA, Jenkinson N, Brittain JS, Coyne TJ, Silburn PA, Aziz TZ, Kerr G, Brown P. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain. 2012;135:1446. doi: 10.1093/brain/aws039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, Stanzione P, Stefani A. Deep brain stimulation of pedunculopontine tegmental nucleus: role of sleep modulation in advanced Parkinson disease patients- one-year follow-up. Sleep. 2012;35:1637. doi: 10.5665/sleep.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyckoki T, Mandat T, Nauman P. Pedunculopontine nucleus deep brain stimulation in parkinson’s disease. Arch Med Sci. 2011;7:555. doi: 10.5114/aoms.2011.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horie T, Koshino Y, Murata T, Omori M, Isaki K. EEG analysis in patients with senile dementia and Alzheimer’s disease. Jpn J Psychiat Neurol. 1990;44:91. doi: 10.1111/j.1440-1819.1990.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 86.Kim JS, Lee SH, Park G, Kim S, Bae SM, Kim DW, Im CH. Clinical implications of quantitative electroencephalography and current source density in patients with Alzheimer’s disease. Brain Topog. 2012;25:461. doi: 10.1007/s10548-012-0234-1. [DOI] [PubMed] [Google Scholar]
- 87.Koponen H, Partanen J, Paakonen A, Mattila E, Riekkinen PJ. EEG spectral analysis in delirium. J Neurol Neurosurg Psychiat. 1989;52:980. doi: 10.1136/jnnp.52.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park JY, Lee SK, An SK, Lee SJ, Kim JJ, Kim KH, Namkoong K. Gamma oscillatory activity in relation to memory ability in older adults. Int J Psychophysiol. 2012;86:58. doi: 10.1016/j.ijpsycho.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, Jelic V. Decresaed EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005;26:165. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Van Deusen JA, Vuurman EFMP, Verhey FRJ, van Kranen-Mastenbroek VHJM, Riedel WJ. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm. 2008;115:1301. doi: 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonanni L, Anzellotti F, Varanese S, Thomas A, Manzoli L, Onofij M. Delayed blink reflex in dementia with Lewy bodies. J Neurol Neurosurg Psychiat. 2007;78:1137. doi: 10.1136/jnnp.2006.113746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Green JB, Burba A, Freed DM, Elder WW, Xu W. The P1 component of the middle latency auditory potential may differentiate a brainstem subgroup of Alzheimer’s disease. Alz Dis Assoc Disord. 1997;11:153. doi: 10.1097/00002093-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 93.Ueki A, Goto K, Sato N, Iso H, Morita Y. Prepulse inhibition of acoustic startle response in mild cognitive impairment and mild dementia of Alzheimer type. Psychiat Clin Neurosci. 2006;60:55. doi: 10.1111/j.1440-1819.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 94.Buchwald JS, Erwin R, Read S, Van Lancker J, Cummings JL. Midlatency auditory evoked responses: differential abnormality of P1 in Alzheimer’s disease. Electroenceph Clin Neurophyiol. 1989;74:378. doi: 10.1016/0168-5597(89)90005-1. [DOI] [PubMed] [Google Scholar]
- 95.Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6:S16. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 96.Painold A, Anderer P, Holl AK, Saleu-Zhylarz GM, Saletu B, Bonelli RM. Comparative EEG mapping studies in Huntington’s disease patients and controls. J Neural Transm. 2010;117:1307. doi: 10.1007/s00702-010-0491-7. [DOI] [PubMed] [Google Scholar]
- 97.Bylsma FW, Peyser CE, Folstein SE, Ross C, Brandt J. EEG power spectra in Huntington’s disease: clinical and neuropsychological correlates. Neuropsychol. 1994;32:137. doi: 10.1016/0028-3932(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 98.Bollen E, Arts RJ, Roos RA, van der Velde EA, Buruma OJ. Brainstem reflexes and brainstem auditory evoked responses in Huntington’s chorea. J Neurol Neurosurg Psychiat. 1986;49:313. doi: 10.1136/jnnp.49.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swanson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiat. 1995;58:192. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uc EY, Skinner RD, Rudnitzky RL, Garcia-Rill E. The midlatency auditory evoked potential P50 is abnormal in Huntington’s disease. J Neurol Sci. 2003;212:1. doi: 10.1016/s0022-510x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 101.Arnulf I, Nielson J, Lohmann E, Schieffer J, Wild E, Jennum P, Konofal E, Walker M, Oudiette D, Tabrizi S, Durr A. Rapid Eye Movement sleep disturbances in Huntington Disease. Arch Neurol. 2008;65:482. doi: 10.1001/archneur.65.4.482. [DOI] [PubMed] [Google Scholar]
- 102.Goodman AOG, Rogers L, Pilsworth S, McAllister CJ, Shneerson JM. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington’s Disease. Curr Neurol Neurosci Rep. 2010;11:211. doi: 10.1007/s11910-010-0163-x. [DOI] [PubMed] [Google Scholar]
- 103.Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 104.Baglioni C, Regen W, Teghen A, Spiegelhalder K, Feige B, Nissen C, Riemann D. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18:195. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Feige B, Baglioni C, Spiegelhalder K, Hirscher V, Nissen C, Riemann D. The microstructure of sleep in primary insomnia: an overview and extension. Int J Psychophysiol. 2013;89:171. doi: 10.1016/j.ijpsycho.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 107.Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C, Riemann D, Nissen C. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Bonnet MH, Arand DI. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Riemann D, Spiegelhalder K, Feige B, Vodeholzer U, Berger M, Perils M, Nissen M. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Garcia-Rill E, Luster B, Mahaffey S, Bisagno V, Urbano FJ. Pedunculopontine arousal system physiology- implications for insomnia. Sleep Sci. 2015;8:92. doi: 10.1016/j.slsci.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coslett HB, Bowers D, Heilman KM. Reduction of cerebral activation after right hemisphere stroke. Neurol. 1987;37:957. doi: 10.1212/wnl.37.6.957. [DOI] [PubMed] [Google Scholar]
- 112.Demeurisse G, Hublet C, Paternot J. Quantitative EEG in subcortical neglect. Neurophysiol Clin. 1988;28:259. doi: 10.1016/S0987-7053(98)80116-0. [DOI] [PubMed] [Google Scholar]
- 113.Watson RT, Andriola M, Heilman KM. The electroencephalogram in neglect. J Neurol Sci. 1977;34:343. doi: 10.1016/0022-510x(77)90151-4. [DOI] [PubMed] [Google Scholar]
- 114.Ptak R, Schnider A. Reflexive orienting in spatial neglect is biased towards behaviorally salient stimuli. Cereb Cortex. 2006;16:337. doi: 10.1093/cercor/bhi111. [DOI] [PubMed] [Google Scholar]
- 115.Woods AJ, Mennemeier M, Garcia-Rill E, Meythaler J, Mrak VW, Jewel GR, Murphy H. Bias in magnitude estimation following left hemisphere injury. Neuropsychol. 2006;44:1406. doi: 10.1016/j.neuropsychologia.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boop FA, Garcia-Rill E, Dykman R, Skinner RD. The P1: Insights into attention and arousal. J Pediat Neurosurg. 1993;20:57. doi: 10.1159/000120765. [DOI] [PubMed] [Google Scholar]
- 117.Olerup O, Schaffer M, Hillert J, Sachs C. The narcolepsy-associated DRw15, DQw6, Qw2 haplotype has no unique HLA-QDA or –DQB restriction fragments and does not extend to the HLA-DP region. Immunogenics. 1990;32:41. doi: 10.1007/BF01787327. [DOI] [PubMed] [Google Scholar]
- 118.Schenck C, Garcia-Rill E, Segall M, Noreen H, Mahowald MW. HLA class II genes associated with REM sleep behavior disorder. Ann Neurol. 1996;39:261. doi: 10.1002/ana.410390216. [DOI] [PubMed] [Google Scholar]
- 119.Schenck CH, Bundlie SR, Mahowald M. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement disorder. Neurol. 1996;46:1787. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 120.Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effect of modafinil on sleep in chronic cocaine users. Amer J Psychiat. 2010;167:331. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panckeri KA, Schotland HM, Pack AI, Hendricks JC. Modafinil decreases hypersomnolence in the English bulldog, a natural animal model of sleep-disordered breathing. Sleep. 1996;19:626. doi: 10.1093/sleep/19.8.626. [DOI] [PubMed] [Google Scholar]
- 122.Carstairs SD, Urquhart A, Hoffman J, Clark RF, Cantrell FL. J Med Toxicol. 2010;6:307. doi: 10.1007/s13181-010-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


