Abstract
Purpose
To review how PET/MR technology could add value for pediatric cancer patients.
Recent Findings
Since many primary tumors in children are evaluated with MRI and metastases are detected with PET/CT, integrated PET/MR can be a time-efficient and convenient solution for pediatric cancer staging. 18F-FDG PET/MR can assess primary tumors and the whole body in one imaging session, avoid repetitive anesthesia and reduce radiation exposure compared to 18F-FDG PET/CT. This article lists 10 action points, which might improve the clinical value of PET/MR for children with cancer. However, even if PET/MR proves valuable, it cannot enter mainstream applications if it is not accessible to the majority of pediatric cancer patients. Therefore, innovations are needed to make PET/MR scanners affordable and increase patient throughput.
Summary
PET/MR offers opportunities for more efficient, accurate and safe diagnoses of pediatric cancer patients. The impact on patient management and outcomes has to be substantiated by large-scale prospective clinical trials.
Keywords: Pediatric Cancer, Pediatric Lymphoma, Pediatric Sarcoma, Magnetic Resonance, Positron Emission Tomography, PET/MR
Introduction
For children with cancer, accurate staging of the primary tumor and whole body is pivotal for appropriate patient management and optimized outcomes [1–4]. Currently, children with a newly diagnosed solid tumor have to undergo a series of imaging tests, including x-rays, ultrasound, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), methylene diphosphonate (MDP) scintigraphy and meta-iodobenzylguanidine (MIBG) scintigraphy, among others. Decades of experience have demonstrated excellent sensitivities and specificities of these imaging modalities. However, for children with cancer, undergoing a series of imaging tests is stressful, time consuming, can be redundant, expensive and may require repetitive anesthesia. Recent efforts are directed towards the development of comprehensive, patient-tailored “one stop” imaging tests, which can provide a comprehensive evaluation of the primary tumor and metastases in one session. Towards this goal, 18F-FDG PET/CT technologies have been increasingly used for pediatric cancer staging, specifically for staging of malignant lymphomas and sarcomas [1–5]. Several studies have shown excellent agreement between 18F-FDG PET/CT and whole body MR scans for detection of these tumors in children [6–10]. Recently, 18F-FDG PET/MR has been added to the repertoire of clinically available staging techniques, which allow for simultaneous acquisition of 18F-FDG PET and MRI data [11–15] – an advantage for children requiring both tests. Systematic comparisons between these new technologies are critically needed in order to understand and utilize their respective advantages and limitations.
The Children’s Oncology Group (COG) has united pediatric oncologists from hospitals across North America to assess treatment outcomes of specific tumor types. In order to add clinical value, pediatric radiologists need to similarly unite and systematically investigate the impact of new imaging technologies on clinical management and outcomes. Single center investigations of 18F-FDG PET/MR scans for pediatric cancer staging obtained so far cover a limited number of patients with a wide range of different pediatric tumors [11, 12]. These investigations provide limited, often case-based information on clinical impact. To investigate the clinical value of PET/MR in larger and more homogenous pediatric patient populations, the American College of Radiology (ACR) Pediatric Imaging Research Committee (ACR-PIR) has recently formed a consortium of PET/MR investigators at major academic institutions [16]. As these early adopters explore if PET/MR is valuable, a transition to use this technology at a majority of pediatric oncology centers can only occur, if and when this new technology can be provided in a time- and cost-efficient manner to the majority of pediatric cancer patients. Even if PET/MR proves clinically valuable, it cannot enter mainstream applications if it is not accessible to the majority of providers and patients. Robertson et al reported that the capital investment required for initial purchase and infrastructure upgrade, coupled with the lower volume of PET imaging performed in pediatric centers, puts procurement of a new PET/MRI scanner outside the financial capacity of many pediatric centers [17]. Potential solutions to this problem could entail integrating PET technology into 1.5 T MR scanners or the design of MRI coils with integrated PET-detectors, which could be used in existing MRI systems [18]. In addition, technical innovations are needed to accelerate the speed of PET/MR image data acquisition, in order to improve patient acceptance and throughput in oncology centers. The goal would be to provide widely available “one stop” medical imaging solutions with equal or faster acquisition times and improved diagnostic information compared to PET/CT.
1. Focus on Pediatric Patients with Lymphomas and Sarcomas
To date, children and adolescents with cancer are referred to specific staging tests based on the organ of origin and histology of the primary tumor. An overview of current clinical practice for pediatric cancer staging and restaging is provided in Table 1, along with a summary of important clinical questions that need to be addressed for PET/MR in order to impact clinical decisions and and outcomes. Based on the accumulated evidence with 18F-FDG PET/MR imaging studies thus far [11–15], added value is particularly expected for pediatric patients with lymphomas and sarcomas. These patients also represent the main pediatric patient population referred to 18F-FDG PET/CT to date [19–21, 5]. In patients with lymphomas, a main motivation to consider 18F-FDG PET/MR as a potential alternative to PET/CT is a reduction in radiation exposure by 50–70% [11–15]. 18F-FDG PET/MR provides excellent soft tissue contrast (Fig. 1) and has shown equivalent or superior sensitivity compared to traditional 18F-FDG PET/CT for the detection of malignant lymph nodes.[22] Tumor SUV values obtained in the same patients with 18F-FDG PET/MR and 18F-FDG PET/CT showed a high correlation, although SUV values obtained with 18F-FDG PET/MR were systematically lower.[23] In pediatric patients with bone and soft tissue sarcomas, MRI is already the clinical standard for local staging and 18F-FDG PET/CT is often added for whole body staging [24–26, 11, 27]. Initial experiences testify excellent sensitivity of 18F-FDG PET/MR for whole body staging of patients with sarcomas [11, 12]. In these patients, integrating MRI and 18F-FDG PET can create value by providing local and whole body staging in one session. The label “patient convenience” in this context might be underrated. Systematic studies are needed to measure and optimize the time-efficiency of diagnostic tests and the whole process of rendering a cancer diagnosis, counting not only the time a patient spends in a scanner, but the overall time a patient spends in the Imaging Department and Hospital. Results could be used to uncover previously missed opportunities to accelerate and integrate medical tests for comprehensive cancer diagnoses.
Table 1. Pathways for clinical translation of PET/MR studies for pediatric cancer staging.
(Imaging protocol will require *whole body scan or ** whole body scan plus dedicated scan of primary tumor or #customized scan, which could be a local scan only or an extended scan, as deemed appropriate for a given patient)
| Current Clinical Practice | Clinical Translation | Future Clinical Practice |
|---|---|---|
| Lymphoma: 18F-FDG PET/CT before, during and after therapy | Validate equivalence of Deauville and Lugano Criteria for 18F-FDG PET/CT and 18F-FDG PET/MR for staging and therapy response assessment | 18F-FDG PET/MR before, during and after therapy* |
| Soft Tissue Sarcoma: 18F-FDG PET/CT before, during and after therapy | Validate equivalence of 18F-FDG PET/CT and PET/MR-derived SUV and Recist criteria for staging and therapy response assessment | 18F-FDG PET/MR before, during and after therapy** |
| Bone Sarcoma: Local MRI, Chest CT and Bone scan | Validate equivalence of conventional staging and 18F-FDG PET/MR for staging and therapy response assessment | 18F-FDG PET/MR before, during and after therapy** |
| Melanoma and Carcinoma: 18F-FDG PET/CT before, during and after therapy | Validate equivalence of 18F-FDG PET/CT and PET/MR for staging and re-staging | 18F-FDG PET/MR before, during and after therapy** |
| Hepatoblastoma/HCC: MRI and bone scan before, during and after therapy | Evaluate if and for whom 18F-FDG PET/MR may provide additional information. Define indications for a local, extended or whole body scan. | MRI and bone scan before, during and after therapy; 18F- FDG PET/MR in selected patients with multifocal or extrahepatic disease# |
| Wilm’s Tumor: MRI or CT scan | Evaluate if and for whom 18F-FDG PET/MR may provide additional information. Define indications for a local, extended or whole body scan. | MRI before, during and after therapy; 18F-FDG PET/MR in selected patients with extra-renal or recurrent disease# |
| Neuroblastoma: MRI or CT scan, MIBG scan, Bone scan | Compare diagnostic accuracy and efficiency of PET-radiotracers with classical 123I-MIBG scan | 124I-MIBG PET/MR scan before, during and after therapy** |
| Germ Cell Tumors (GCT): MRI or CT scan, rarely 18F-FDG PET/CT for malignant tumors | Validate equivalence of 18F-FDG PET/CT and PET/MR for staging and therapy response assessment of malignant GCT | 18F-FDG PET/MR before, during and after therapy for selected cases of malignant GCT** |
Figure 1. Whole Body 18F-FDG PET/MR of a patient with Hodgkin Lymphoma.
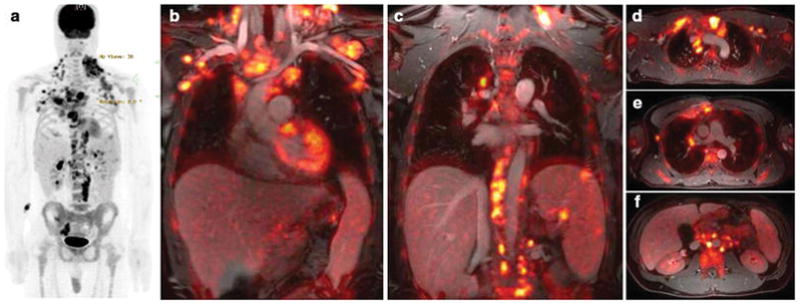
(a) 18F-FDG PET maximum intensity projection shows FDG-avid disease of the neck, chest, axillaries, abdomen and pelvis. (b) Coronal 18F-FDG PET images superimposed on ferumoxytol-enhanced T1-weighted LAVA images clearly show the relation between vessels and multiple hypermetabolic lymph nodes. Three lesions in the spleen are also noted. (c) Axial integrated 18F-FDG PET/LAVA images provide diagnostic information similar to a PET/CT scan, but with improved soft tissue contrast.
2. Reduce Radiation Exposure
Radiation exposure is of higher concern for pediatric patients than adults because children are more susceptible to radiation effects and they live long enough to encounter secondary cancers [28–31]. Cumulative ionizing radiation exposure above 50–100 mSv can increase the risk of secondary cancers later in life [28], such as leukemia or brain cancer [32, 33]. Improved detector technology and longer acquisition times of PET/MR compared to PET/CT, along with replacement of CT by MR for anatomical co-registration, enable reduced radiotracer doses. In accordance with others [11–15], we prescribe an 18F-FDG dose of 3 MBq/kg for four-minute PET data acquisitions per bed position, with excellent tumor-to-background contrast at about 1 hour after 18F-FDG injection (Fig. 1–3). Others calculated that the 18F-FDG dose could be further reduced to 1.5 MBq/kg for four-minute PET data acquisitions [34]. Current data are based on tumor staging results at baseline, when most pediatric tumors show high metabolic activity. Further studies need to clarify, if major reductions in 18F-FDG dose would impact the diagnosis of partial versus complete metabolic response after therapy. If we administer lower radiotracer doses, would we diagnose fewer partial responses and how would this impact patient management and outcomes?
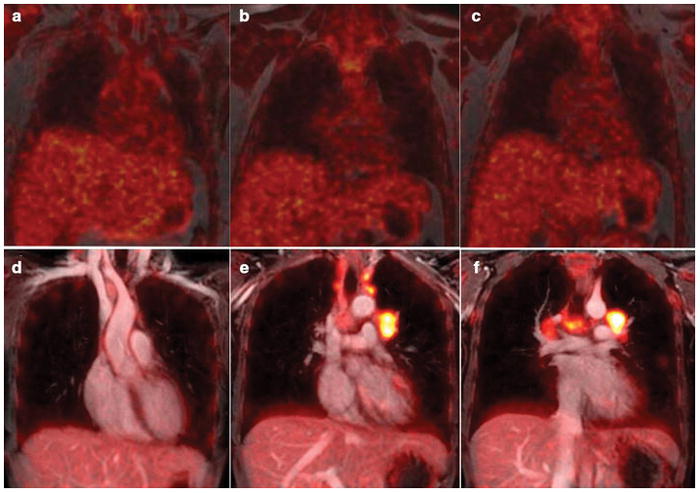
Figure 3. Spatial resolution on 18F-FDG PET/MR is important to render accurate diagnostic information in a young adult with retroperitoneal soft tissue sarcoma.
(a,c) Axial 18F-FDG PET images, superimposed on ferumoxytol-enhanced T1-weighted LAVA images (TR/TE/alpha = 4.1ms/1.7ms/15), obtained for attenuation correction of 18F-FDG PET data. These images with a field of view (FOV) or 50 cm, a matrix of 256 x 128 pixels and a slice thickness of 5.2 mm provide limited anatomical resolution. For example, the superior mesenteric artery is not seen on these scans. (b,d) Axial 18F-FDG PET images, superimposed on ferumoxytol-enhanced T1-weighted LAVA images (TR/TE/alpha = 4.2ms/1.7ms/15), acquired for diagnostic purposes. These images with a field of view (FOV) or 48 cm, a matrix of 320 x 224 pixels and a slice thickness 3.4 mm provide improved tumor delineation from adjacent vessels and bowel. We conclude that current pulse sequences for AC correction do not have sufficient anatomical resolution to be used for diagnostic purposes.
3. Optimize the diagnostic accuracy of MRI scans used for co-registration of PET data
Pediatric staging protocols for lymphoma require a iodinated contrast-enhanced diagnostic CT scan as part of or in addition to a 18F-FDG PET/CT scan [35]. This is different to 18F-FDG PET/CT protocols for adults where unenhanced scans are often sufficient. It has not been established yet, if the same principle applies to PET/MRI, i.e. if pediatric protocols need to include contrast-enhanced scans. A wide variety of T1- and T2-weighted pulse sequences have been proposed for anatomical co-registration of 18F-FDG PET data in children [11–15]. We found in accordance with others that pediatric tumors can be equally well delineated on Gd-enhanced T1-weighted scans and unenhanced T2-weighted scans [36]. However, if the faster T1-weighted scans were applied, intravenous contrast improved vessel and tumor delineation compared to unenhanced T1-weighted scans (Fig. 2). This was particularly useful for accurate tumor measurements and surgical planning.
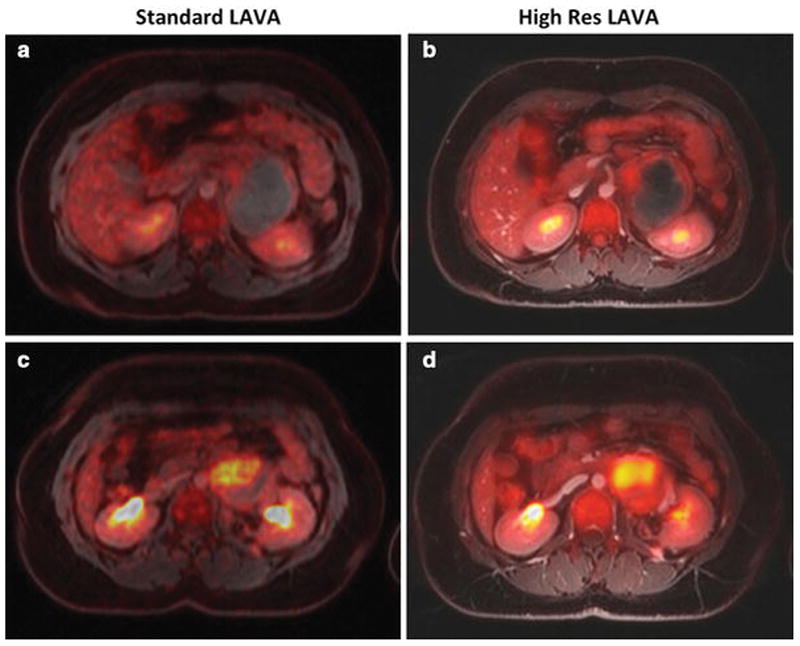
Figure 2. Intravenous MR contrast agent administration improves soft tissue contrast of integrated PET/MR images.
(a–c) Coronally reconstructed 18F-FDG PET images, superimposed on unenhanced T1-weighted LAVA images show limited soft tissue resolution. (d–f) Coronal 18F-FDG PET images, superimposed on ferumoxytol-enhanced T1-weighted LAVA images of a patient with Hodgkin lymphoma show improved soft tissue contrast: The ferumoxytol-enhanced 18F-FDG PET/MR images facilitate the delineation of mediastinal vessels and hypermetabolic lymph nodes. This is important, because current clinical protocols require accurate assessment of the tumor size and metabolic activity before, during and after therapy.
Similar to the concept of adding “diagnostic” CT scans to low dose CT scans for attenuation correction (AC) for a PET/CT, whole body PET/MR scans can utilize AC pulse sequences for co-registration of 18F-FDG PET data or use dedicated sequences with increased anatomical resolution [37]. We found significant value in using higher resolution scans (Fig. 3). This currently requires the acquisition of two sequences: a low resolution, dual-echo gradient echo sequence for AC correction (with image matrix matched to the matrix of the PET scan) plus a higher resolution scan for anatomical co-registration of PET data. In principle, it should be possible to acquire one single, high resolution dual-echo gradient echo sequence, from which a lower resolution scan could be reconstructed. However, such technology has not yet been established. The saved acquisition time might be considered negligible for adult patients, but would be valuable for children.
4. Integrate Information about tumor cell density and glucose metabolism
Whole body staging of pediatric cancers can be obtained with classical 18F-FDG PET/CT [4, 38], whole body diffusion weighted MRI [39, 10] or integrated 18F-FDG PET/MR [12, 11]. Whole body MR has been used for screening patients with cancer predisposition syndromes, cancer staging of patients with hereditary increased radiosensitivity, staging of benign disease such as Langerhans Cell Histiocytosis, staging of non 18F-FDG-avid tumors and patients without access to an 18F-FDG PET/CT scan [40, 39, 41]. Since most tumors have a higher cell density than normal organs, the diffusional motion of water protons is more restricted in tumors and displayed by an increased signal intensity on diffusion weighted MR images [8, 42, 43]. Diffusion-weighted MR images can be color-encoded and superimposed on anatomical MR images such that they provide a visual tumor depiction similar to a 18F-FDG PET/MR or 18F-FDG PET/CT scan [10]. Several authors reported comparable sensitivities and specificities of 18F-FDG PET/CT and diffusion weighted MR scans for staging patients with lymphoma and other solid tumors [44–46, 10]. Large scale prospective clinical trials are needed to compare the clinical accuracy and clinical impact of these three imaging technologies for specific pediatric tumor types: Who should get which imaging test at which time and how often?
In addition, we need to evaluate, if and when adding metabolic information to diffusion-weighted MRI or adding diffusion weighted scans to an 18F-FDG PET/MR scan will add clinical value. Either scenario will add time and costs to an already advanced imaging test and therefore, needs to be considered carefully. 18F-FDG PET can add value to diffusion weighted scans by detecting tumor deposits in the spleen and bone marrow. In children, the high cellularity of these organs can mask tumor deposits [44, 47, 48], which can confound tumor detection and carry a risk for under-staging and under-treatment [49, 50]. In pediatric patients who undergo 18F-FDG PET/MR scans, adding diffusion-weighted sequences can be particularly useful for differentiating mediastinal lymphoma and normal thymus [35], as well as benign and malignant abdominal tumors [51]. In brain gliomas, Cuccarini et. al. found that normalized ADC values were directly associated with tumor grade and anaplastic progression [52], which could help to prescribe personalized follow up imaging or interventions. In sarcomas, the degree of restricted diffusion [53, 54] and metabolic activity of the primary tumor at the time of initial diagnosis has been linked to overall survival [55–57]. Patients with soft tissue sarcomas and tumor SUVmax/SUVliver values above 4.6 had significantly decreased survival rates compared to patients with ratios below 4.6 [55]. Since both SUV and ADC are related to tumor grade, but represent different biological tumor characteristics (glucose metabolism and cell density), some investigators proposed the ratio of SUVmax and ADCmin as a combined biomarker for clinical outcomes [58]. Other investigators found that the extent rather than degree of tumor FDG hypermetabolism and diffusion restriction in soft tissue sarcomas is a more robust predictive and prognostic biomarker [59]. This is consistent with the notion that the volume of aggressive tumors is linked to clinical outcomes [60, 61]. Future studies have to show if the volume of high SUVmax/ADCmin tumor parts in heterogenous tumors such as sarcomas is a better predictive biomarker than the overall tumor volume.
5. Increase specificity with nanoparticles
Currently, a wide range of sequences is applied for integrated 18F-FDG PET/MR: T2-weighted sequences require relatively long acquisition times and Gd-enhanced T1-weighted sequences do not provide sufficiently long-lasting vessel enhancement for whole body scans. This problem can be solved with iron oxide nanoparticles, such as the FDA-approved iron supplement ferumoxytol, which can be used “off-label” as a contrast agent. [62] Ferumoxytol is composed of ultrasmall superparamagentic iron oxide nanoparticles, which cause a long lasting positive (bright) signal on T1-weighted MR images and negative (dark) signal on T2-weighted images [63]. Ferumoxytol is currently evaluated for potential FDA-approval as an imaging agent, which would facilitate clinical imaging applications. It should be noted that in rare cases, ferumoxytol can cause severe adverse events or anaphylactic reactions [62]. Therefore, specific FDA guidelines for its administration have to be followed [64]. We found ferumoxytol particularly useful for PET/MRI because this blood pool agent causes long-lasting vascular T1-enhancement for the entire duration of a whole body scan [10]. In addition, ferumoxytol improved the detection of tumors in organs of the reticuloendothelial system, i.e. liver, spleen, bone marrow and lymph nodes, in accordance with previous experiences with first generation nanoparticles [65, 66]. Ferumoxytol can help detect tumors in highly cellular normal marrow in young children and patients after chemotherapy. Reconverted bone marrow is diffusely hypermetabolic on 18F-FDG PET scans and shows restricted diffusion on diffusion weighted MR scans. This can mask tumor deposits. Ferumoxytol nanoparticles lead to differential T2-enhancement of normal, reconverted marrow and tumor (Fig. 4): Macrophages in normal bone marrow phagocytose and retain ferumoxytol nanoparticles, while tumors contain much fewer macrophages and show less ferumoxytol retention. This leads to hypointense (dark) enhancement of normal marrow and relatively hyperintense (bright) signal of tumors on T2-weighted MRI scans [44, 47]. Prospective clinical trials are needed to evaluate if 18F-FDG PET/MR can replace bone marrow biopsies and if the addition of nanoparticles adds clinical value. Do Fe-enhanced 18F-FDG PET/MR scans upstage cancer patients and do these results impact patient management?
Figure 4. Ferumoxytol shows differential enhancement of hypercellular hematopoietic marrow and tumor.
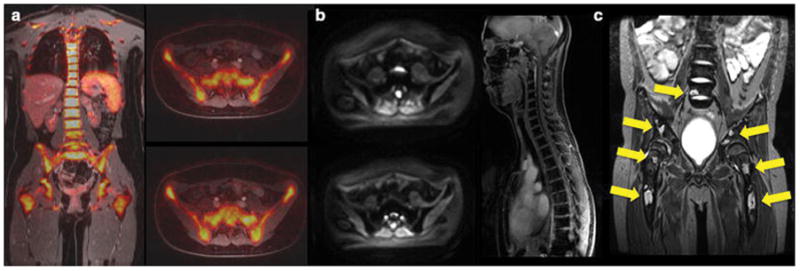
(a) Coronal and axial 18F-FDG PET/MR in a young adult after chemotherapy shows diffusely hypermetabolic hematopoietic marrow, (b) axial diffusion-weighted MR images and coronal LAVA images in the same patient after intravenous injection of ferumoxytol show homogeneous iron oxide uptake in the bone marrow, as indicated by homogenous hypointense (dark) marrow signal. (c) Coronal ferumoxytol-enhanced T2-weighted fast spinecho scan of the lumbar spine and pelvis in an 11 year-old patient with biopsy-proven Hodgkin’s leukemia shows multiple focal tumor lesions in the bone marrow (arrows). The normal marrow shows negative (dark) ferumoxytol enhancement while focal tumors show little or no ferumoxytol uptake and stand out as hyperintense (bright) lesions.
6. Improve the delineation of primary tumors and diagnosis of tissue infiltration
MR imaging is the modality of choice for local staging of bone and soft tissue sarcomas. The extent of bone sarcomas (compartments involved, presence or absence of skip lesions) determines the required surgical procedure. On conventional MR images, the delineation of tumor and peri-lesional edema can be difficult [67]. Various approaches have been tested to solve this problem, such as differential morphological criteria on T2-weighted scans (e.g. “feathery” edema versus mass-like tumor core), differential contrast dynamics (earlier enhancement of the tumor core compared to delayed and often stronger enhancing edema) or differential signal of tumor and edema on diffusion weighted scans (restricted diffusion of the tumor core and prolonged diffusion of the tumor center) [68–72]. All of these criteria yielded limited specificity. Overestimating tumor size can lead to unnecessary surgical resections of too much normal tissue, affect the approach for limb-sparing surgery and lead to unsatisfactory long-term outcomes [70, 73]. Conversely, incomplete tumor resection can impact prognosis and post-surgical care [74–76]: In Ewing sarcomas, a wide surgical margin (R0) with normal tissue around the lesion does not require additional local control while a marginal excision (R1), which includes tumor cells at the cut surface, must be treated by radiotherapy and/or intensified chemotherapy [77]. Recent evidence shows that 18F-FDG-PET can help differentiating tumor tissue and peri-lesional edema: The primary tumor shows marked 18F-FDG uptake while peri-lesional edema shows little or no 18F-FDG uptake [78] (Fig. 5). Prospective clinical trials are needed to evaluate if this information can increase the number of limb-sparing surgeries and thereby, improve long-term outcomes.
Figure 5. 18F-FDG PET/MR shows differential enhancement of an osteosarcoma and peri-lesional edema.
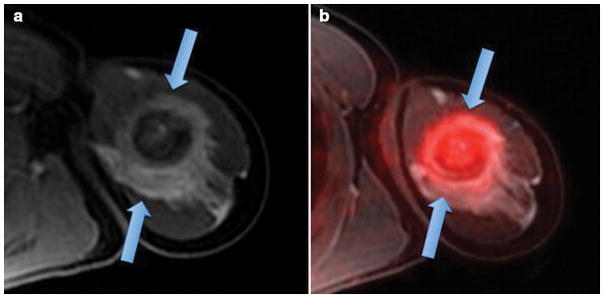
(a) Axial T1-weighted ferumoxytol-enhanced LAVA scan shows an ill defined, aggressive lesion in the proximal left humerus with extensive perilesional contrast-enhanced edema (arrows). (b) Integrated ferumoxytol-enhanced 18F-FDG PET/LAVA scan shows improved delineation of the 18F-FDG avid tumor (arrow) from enhancing peritumoral edema.
For soft tissue sarcomas, standard imaging technologies have a low specificity for the assessment of tumor infiltration of adjacent organs. Incidental evidence suggests that FDG-avid tumor areas may indicate locally infiltrating tumor parts (Fig. 3). In addition, soft tissue sarcomas frequently present with local lymph nodes, which are associated with poor prognosis. The metabolic information from 18F-FDG PET studies is more sensitive (94%–100%) than lymph node size on anatomical images (75%–94%)[5]. Prospective controlled clinical trials are needed to evaluate, if the additional metabolic information up- or downstages patients and if this affects patient management and outcomes.
7. Solve the pulmonary nodule detection dilemma
Recent technological advances have substantially improved the sensitivity of MRI for the detection of pulmonary nodules [79, 80]. However, to date, MRI scans, with or without integrated FDG-PET information, do not yet reach the sensitivity of a CT scan. This is clinically important, because pulmonary disease significantly affects the prognosis and management of pediatric cancer patients: In patients with lymphoma, a pulmonary nodule is considered extra-lymphatic disease, upstages the patient and requires intensified therapy. In patients with sarcomas, pulmonary nodules are usually surgically excised and successful excision significantly affects prognosis. Thus, a missed pulmonary nodule could have severe consequences for patient survival. Of note, current clinical treatment protocols for most pediatric sarcomas suggest surgical management only for one pulmonary nodule above 5 mm or more than three nodules with diameters of more than 3 mm (e.g. COG trial AEWS1221). Most MR imaging sequences have sufficient anatomical resolution to detect such nodules [81]. Recent investigations revealed that the reason for missed pulmonary nodules on MRI is the inability to differentiate pulmonary vessels from small pulmonary nodules (on MRI, vessels can usually not be continuously followed as on a CT scan) [81]. Technical innovations to address this challenge are needed, including breath-hold, data averaging and retrospective respiratory gating schemes. In addition, advanced techniques are needed for improved co-registration of 18F-FDG PET-data and MRI [80]. Clearly, major technical advances are needed until a chest CT scan can be safely replaced by an MRI for the evaluation of pulmonary nodules. In principle, the multi-parametric information of a 18F-FDG PET/MR scan should lend itself to much needed improved specificity: Current CT technologies cannot differentiate pulmonary metastases, which are surgically excised in sarcoma patients, from inflammatory granulomas and intra-pulmonary lymph nodes, which are not excised. Technical innovations that can reliably differentiate benign and malignant nodes would have major impact on patient management.
8. Improve the Accuracy for the Diagnosis of Treatment Response
If 18F-FDG PET/MR should replace 18F-FDG PET/CT as a new “one stop” staging approach with reduced radiation exposure, then this new imaging test would not only have to provide sensitive tumor detection at baseline, but also accurate therapy response assessment.
Systematic tumor-type specific comparative analyses have to analyze if technical and procedural differences between 18F-FDG PET/CT and PET/MR scans can cause differences in tumor therapy response assessments and classifications of responders and non-responders. In pediatric patients with lymphoma, SUVs measured from 18F-FDG PET/MRI indicated good intra-patient reliability [23] and 18F-FDG PET SUVs were strongly correlated between PET/CT and PET/MRI (ρ > 0.72), although PET/MRI showed systematically lower SUV measurements [22]. Technical variables, which may lead to differences in SUV values between PET/CT and PET/MR studies, include differences in AC correction, different radiotracer doses, longer PET acquisition times and more sensitive PET-detectors in some PET/MR scanners [23].
In patients with malignant lymphomas, a decline in tumor glucose metabolism, measured on 18F-FDG PET scans, provided additional information to tumor size measurements for therapy response assessment, which changed patient management in up to 32% [82–84, 21]. Current therapy response assessments of pediatric lymphomas by the St. Jude’s consortium evaluate both changes in tumor size and tumor metabolism, while current protocols of the Children’s Oncology Group solely rely on semi-quantitative measures of tumor metabolism, according to the 5-point Deauville or Lugano criteria [85, 86]. The most frequent management change based on imaging findings is avoiding radiotherapy of residual soft tissue masses at the end of therapy.
In pediatric patients with sarcomas, therapy response is determined by changes in tumor size on imaging studies according to RECIST criteria [85]. The metabolic information from 18F-FDG-PET scans may improve therapy response assessment, especially for tumors with small extraosseous soft tissue components [87, 88]. Due to its high soft tissue contrast, 18F-FDG PET/MR can accurately diagnose hypermetabolic brown fat and hypermetabolic reconverted hematopoietic marrow after chemotherapy [89–91].
Some investigators found concordant changes in glucose metabolism and tumor cell density after chemotherapy in solid tumors of adult patients [85, 53, 54], while other investigators found complementary information of diffusion weighted scans and PET scans [92, 93]. Thus, combining ADC and SUV data might increase diagnostic accuracy in some tumors. [59] It is not clear if chemotherapy-induced normalization of ADC and SUV values occurs at the same time in pediatric cancers.
9. Make 18F-FDG PET/MR useful for radiation planning
To date, CT is the clinical standard method for planning radiation therapy of pediatric cancer patients. CT provides important information about the location of the primary tumor, presence and location of metastases, and linear attenuation coefficients of target tissues for radiotherapy. Linear attenuation coefficients correlate directly with the electron density needed for radiotherapy and therefore, can be used for dose calculations. Due to its higher soft tissue contrast, MRI can add information about the exact delineation and internal composition of target tumors, which can be used for individualized radiotherapy schemes [94]. 18F-FDG PET information has been useful in detecting metastases and determining morphological tumor characteristics, such as internal necrosis and hypoxic areas. A major challenge for the use of PET/MR for radiation planning is that it does not contain direct information about photon attenuation of target tissues. Several investigators are working on solutions to this problem [95, 96]. For example, MR data have been “translated” to pseudo-CT values using deformable image registration algorithms in combination with pattern recognition [96]. In order to make PET/MR data useful for radiation planning, the patient needs to be placed on a flat tabletop in a reproducible manner, e.g. by using specific markers and/or MRI-compatible patient positioning aids. In addition, local radiofrequency coils have to be positioned without deforming the surface of the patient. This can be achieved by using specific RF coil holders [95]. If PET/MR images should be used for radiation planning, a close collaboration between radiologists/nuclear medicine physicians and radiation oncologists is important to ensure clinical value of the acquired scans.
10. Detect chemotherapy-induced tissue injuries
Continuous improvements in cancer therapies lead to a growing number of cancer survivors, with currently more than 14.5 million cancer survivors in the United States.[97] Up to 95% of these patients will develop morbidities due to cancer therapy-induced tissue injuries.[98, 99] While pediatric patients and young adults comprise a minority among cancer survivors, cancer therapy can have more severe effects on their growing and developing tissues.[97, 98] For example, close to 50% of patients with ALL who are treated with intravenous and intrathecal methotrexate (MTX) develop neurological problems, such as chronic headaches, seizures, motor problems and cognitive impairment, which severely limit a child’s social re-integration and academic development. [100–102] In contrast to transient acute toxicities of cytotoxic drugs, these late effects develop slowly over time due to an impaired growth and regeneration of the affected organ.[98] At the end of cancer therapy, patients typically have no or minor clinical symptoms. However, months to years later, they experience debilitating functional impairments.[97, 98, 100, 103–106],[107] There is a window of time between exposure to cancer therapy and future morbidity, which could be used for corrective actions. Unfortunately, once cancer survivors present with clinical symptoms, it is often too late for health-preserving interventions. To prevent morbidities, it is important to develop diagnostic tools, which can detect early stages of tissue damage that are still reversible. Finding on conventional MRI studies showed a poor correlation between white matter abnormalities and neurologic deficits in children with ALL.[108] T2 prolongation in the deep cerebral white matter, noted in 15–75% of patients, were not consistently associated with neurologic deficits.[109, 110] Cheung et al speculated that there is a threshold effect, such that differences in neurobehavioral problems do not become apparent until white matter damage to the brain is extensive enough to result in clinically detected leukoencephalopathy [111]. This might explain the high number of asymptomatic MRI findings in our cohort. Accordingly, Bhojwani et al. found asymptomatic leukoencephalopathy in 73 out of 355 (20.6%) ALL patients [112]. FDG PET can provide additional information about cognitive reserve and its modulation.[113–115] Chiaravalloti et al. [116, 117] reported FDG PET brain results in a cohort of 74 adult patients with lymphoma before, during and after ABVD chemo-therapy. In a groupwise analysis, FDG activity was reduced on the mid-treatment PET in Brodmann areas 10, 11, and 32 bilaterally. In addition, Ponto et al. reported long-term frontal FDG hypometabolism in asymptomatic breast cancer patients undergoing cyclophosphamide, MTX, and 5-FU/doxorubicin therapy.[118] Our team found a significant reduction in cerebral blood flow in specific brain areas and significantly lower mean SUV values in the hippocampus of pediatric cancer survivors compared to normal controls [119]. These initial studies suggest potential value of 18F-FDG PET/MR as a biomarker for chemotherapy induced brain injury. Larger prospective clinical trials are needed to evaluate, if early detection and rescue interventions can improve the long-term health of cancer survivors.
In summary, 18F-FDG PET/MR can provide safer, more specific and more efficient cancer staging for pediatric patients than currently available. A number of open questions specific to the pediatric oncology population have to be elucidated through tumor-type tailored prospective clinical trials. Study quality, cost, length of total anesthesia time for young children, and time to complete the entire staging evaluation have to be compared with the current clinical standard. This might show that PET/MR is superior in terms of quality, cost, anesthesia time, and/or time efficiency. However, even if PET/MR proves valuable, it cannot enter mainstream applications if it is not accessible to the majority of pediatric cancer patients. Therefore, innovations are needed to make PET/MR scanners affordable and increase patient throughput. The availability of novel radiotracers will likely be limited to few teritary centers, such as 18F-FDG-DOPA PET/CT for evaluation of persistent hyperinsulinaemic hypoglycemic of infancy and detection of insulinomas, 18F-fluoride PET for staging of osteosarcoma, 124I PET for iodine-positive thyroid cancer, 18F-FDOPA for medullary thyroid carcinoma, 68Ga-DOTATOC for neuroendocrine tumors and 124I MIBG for neuroblastoma, among others. PET/MR imaging protocols need to be homogenized across centers to facilitate centralized data analyses by the Children’s Oncology Group and other stakeholders. Efforts are under way to generate a consensus regarding pediatric 18F-FDG PET/MR procedures at major academic institutions in North America and Europe.
Acknowledgments
This work was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant number R01 HD081123-01A1.
Footnotes
Conflict of Interest
Heike Daldrup-Link declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Federman N, Feig SA. PET/CT in evaluating pediatric malignancies: a clinician’s perspective. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48(12):1920–2. doi: 10.2967/jnumed.107.046045. [DOI] [PubMed] [Google Scholar]
- 2.Tatsumi M, Miller JH, Wahl RL. 18F-FDG PET/CT in evaluating non-CNS pediatric malignancies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48(12):1923–31. doi: 10.2967/jnumed.107.044628. [DOI] [PubMed] [Google Scholar]
- 3.Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48(12):1932–9. doi: 10.2967/jnumed.107.045286. [DOI] [PubMed] [Google Scholar]
- 4.Kleis M, Daldrup-Link H, Matthay K, Goldsby R, Lu Y, Schuster T, et al. Diagnostic value of PET/CT for the staging and restaging of pediatric tumors. Eur J Nucl Med Mol Imaging. 2009;36(1):23–36. doi: 10.1007/s00259-008-0911-1. [DOI] [PubMed] [Google Scholar]
- 5.Uslu L, Donig J, Link M, Rosenberg J, Quon A, Daldrup-Link HE. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. 2015;56(2):274–86. doi: 10.2967/jnumed.114.146290. [DOI] [PubMed] [Google Scholar]
- 6.Punwani S, Taylor SA, Bainbridge A, Prakash V, Bandula S, De Vita E, et al. Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology. 2010;255(1):182–90. doi: 10.1148/radiol.09091105. 255/1/182 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Krohmer S, Sorge I, Krausse A, Kluge R, Bierbach U, Marwede D, et al. Whole-body MRI for primary evaluation of malignant disease in children. Eur J Radiol. 2010;74(1):256–61. doi: 10.1016/j.ejrad.2009.01.037. S0720-048X(09)00061-8 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Kwee TC, van Ufford HM, Beek FJ, Takahara T, Uiterwaal CS, Bierings MB, et al. Whole-body MRI, including diffusion-weighted imaging, for the initial staging of malignant lymphoma: comparison to computed tomography. Invest Radiol. 2009;44(10):683–90. doi: 10.1097/RLI.0b013e3181afbb36. [DOI] [PubMed] [Google Scholar]
- 9.Kwee TC, Takahara T, Ochiai R, Katahira K, Van Cauteren M, Imai Y, et al. Whole-body diffusion-weighted magnetic resonance imaging. Eur J Radiol. 2009;70(3):409–17. doi: 10.1016/j.ejrad.2009.03.054. S0720-048X(09)00181-8 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Klenk C, Gawande R, Uslu L, Khurana A, Qiu D, Quon A, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. The lancet oncology. 2014;15(3):275–85. doi: 10.1016/S1470-2045(14)70021-X. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013;43(7):860–75. doi: 10.1007/s00247-012-2570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer JF, Gatidis S, Schmidt H, Guckel B, Bezrukov I, Pfannenberg CA, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273(1):220–31. doi: 10.1148/radiol.14131732. [DOI] [PubMed] [Google Scholar]
- 13.Ponisio MR, McConathy J, Laforest R, Khanna G. Evaluation of diagnostic performance of whole-body simultaneous PET/MRI in pediatric lymphoma. Pediatr Radiol. 2016;46(9):1258–68. doi: 10.1007/s00247-016-3601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezrukov I, Schmidt H, Gatidis S, Mantlik F, Schafer JF, Schwenzer N, et al. Quantitative Evaluation of Segmentation- and Atlas-Based Attenuation Correction for PET/MR on Pediatric Patients. J Nucl Med. 2015;56(7):1067–74. doi: 10.2967/jnumed.114.149476. [DOI] [PubMed] [Google Scholar]
- 15.Purz S, Sabri O, Viehweger A, Barthel H, Kluge R, Sorge I, et al. Potential Pediatric Applications of PET/MR. J Nucl Med. 2014;55(Supplement 2):32S–9S. doi: 10.2967/jnumed.113.129304. [DOI] [PubMed] [Google Scholar]
- 16.Daldrup-Link H, Voss S, Donig J. ACR Committee on Pediatric Imaging Research. Pediatr Radiol. 2014;44:1193–4. doi: 10.1007/s00247-013-2850-7. [DOI] [Google Scholar]
- 17.Robertson MS, Liu X, Plishker W, Zaki GF, Vyas PK, Safdar NM, et al. Software-based PET-MR image coregistration: combined PET-MRI for the rest of us! Pediatr Radiol. 2016;46(11):1552–61. doi: 10.1007/s00247-016-3641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olcott P, Kim E, Hong K, Lee BJ, Grant AM, Chang CM, et al. Prototype positron emission tomography insert with electro-optical signal transmission for simultaneous operation with MRI. Phys Med Biol. 2015;60(9):3459–78. doi: 10.1088/0031-9155/60/9/3459. [DOI] [PubMed] [Google Scholar]
- 19.Amthauer H, Furth C, Denecke T, Hundsdoerfer P, Voelker T, Seeger K, et al. FDG-PET in 10 children with non-Hodgkin’s lymphoma: initial experience in staging and follow-up. Klinische Padiatrie. 2005;217(6):327–33. doi: 10.1055/s-2005-872517. [DOI] [PubMed] [Google Scholar]
- 20.Bakhshi S, Radhakrishnan V, Sharma P, Kumar R, Thulkar S, Vishnubhatla S, et al. Pediatric nonlymphoblastic non-Hodgkin lymphoma: baseline, interim, and posttreatment PET/CT versus contrast-enhanced CT for evaluation--a prospective study. Radiology. 2012;262(3):956–68. doi: 10.1148/radiol.11110936. 262/3/956 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Furth C, Steffen IG, Amthauer H, Ruf J, Misch D, Schonberger S, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol. 2009;27(26):4385–91. doi: 10.1200/JCO.2008.19.7814. [DOI] [PubMed] [Google Scholar]
- 22.Sher AC, Seghers V, Paldino MJ, Dodge C, Krishnamurthy R, Krishnamurthy R, et al. Assessment of Sequential PET/MRI in Comparison With PET/CT of Pediatric Lymphoma: A Prospective Study. AJR Am J Roentgenol. 2016;206(3):623–31. doi: 10.2214/AJR.15.15083. [DOI] [PubMed] [Google Scholar]
- 23.Lyons K, Seghers V, Sorensen JI, Zhang W, Paldino MJ, Krishnamurthy R, et al. Comparison of Standardized Uptake Values in Normal Structures Between PET/CT and PET/MRI in a Tertiary Pediatric Hospital: A Prospective Study. AJR Am J Roentgenol. 2015;205(5):1094–101. doi: 10.2214/AJR.15.14304. [DOI] [PubMed] [Google Scholar]
- 24.Balyasnikova S, Lofgren J, de Nijs R, Zamogilnaya Y, Hojgaard L, Fischer BM. PET/MR in oncology: an introduction with focus on MR and future perspectives for hybrid imaging. American journal of nuclear medicine and molecular imaging. 2012;2(4):458–74. [PMC free article] [PubMed] [Google Scholar]
- 25.Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez-Moller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53(6):845–55. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 26.Herzog H, Van Den Hoff J. Combined PET/MR systems: an overview and comparison of currently available options. Q J Nucl Med Mol Imaging. 2012;56(3):247–67. [PubMed] [Google Scholar]
- 27.Platzek I, Beuthien-Baumann B, Langner J, Popp M, Schramm G, Ordemann R, et al. PET/MR for therapy response evaluation in malignant lymphoma: initial experience. MAGMA. 2013;26(1):49–55. doi: 10.1007/s10334-012-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100(24):13761–6. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. The British journal of radiology. 2008;81(965):362–78. doi: 10.1259/bjr/01948454. 81/965/362 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Robbins E. Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer. 2008;51(4):453–7. doi: 10.1002/pbc.21599. [DOI] [PubMed] [Google Scholar]
- 31.Board on Radiation Effects Research (BRER) The National Academic Press; 2006. Health Risks from Exposure to Low Level of Ionizing Radiation: BEIR VII Phase 2. [Google Scholar]
- 32.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. Bmj. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatidis S, Schmidt H, la Fougere C, Nikolaou K, Schwenzer NF, Schafer JF. Defining optimal tracer activities in pediatric oncologic whole-body 18F-FDG-PET/MRI. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3503-5. [DOI] [PubMed] [Google Scholar]
- 35.Gawande RS, Khurana A, Messing S, Zhang D, Castaneda RT, Goldsby RE, et al. Differentiation of normal thymus from anterior mediastinal lymphoma and lymphoma recurrence at pediatric PET/CT. Radiology. 2012;262(2):613–22. doi: 10.1148/radiol.11110715. [DOI] [PubMed] [Google Scholar]
- 36.Klenk C, Gawande R, Tran VT, Leung JT, Chi K, Owen D, et al. Progressing Toward a Cohesive Pediatric 18F-FDG PET/MR Protocol: Is Administration of Gadolinium Chelates Necessary? J Nucl Med. 2016;57(1):70–7. doi: 10.2967/jnumed.115.161646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aghighi M, Pisani LJ, Sun Z, Klenk C, Madnawat H, Fineman SL, et al. Speeding up PET/MR for cancer staging of children and young adults. Eur Radiol. 2016 doi: 10.1007/s00330-016-4332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alessio AM, Kinahan PE, Manchanda V, Ghioni V, Aldape L, Parisi MT. Weight-based, low-dose pediatric whole-body PET/CT protocols. J Nucl Med. 2009;50(10):1570–7. doi: 10.2967/jnumed.109.065912. 50/10/1570 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Siegel MJ, Acharyya S, Hoffer FA, Wyly JB, Friedmann AM, Snyder BS, et al. Whole-body MR imaging for staging of malignant tumors in pediatric patients: results of the American College of Radiology Imaging Network 6660 Trial. Radiology. 2013;266(2):599–609. doi: 10.1148/radiol.12112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daldrup-Link HE, Franzius C, Link TM, Laukamp D, Sciuk J, Jurgens H, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol. 2001;177(1):229–36. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 41.Daldrup-Link HE, Link TM, Moller HE, Wiedermann D, Bonnemain B, Corot C, et al. Carboxymethyldextran-A2-Gd-DOTA enhancement patterns in the abdomen and pelvis in an animal model. Eur Radiol. 2001;11(7):1276–84. doi: 10.1007/s003300000699. [DOI] [PubMed] [Google Scholar]
- 42.Koh DM, Blackledge M, Collins DJ, Padhani AR, Wallace T, Wilton B, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009;19(11):2728–38. doi: 10.1007/s00330-009-1469-4. [DOI] [PubMed] [Google Scholar]
- 43.Padhani AR, Koh DM, Collins DJ. Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology. 2011;261(3):700–18. doi: 10.1148/radiol.11110474. 261/3/700 [pii] [DOI] [PubMed] [Google Scholar]
- 44.van Ufford HM, Kwee TC, Beek FJ, van Leeuwen MS, Takahara T, Fijnheer R, et al. Newly diagnosed lymphoma: initial results with whole-body T1-weighted, STIR, and diffusion-weighted MRI compared with 18F-FDG PET/CT. AJR American journal of roentgenology. 2011;196(3):662–9. doi: 10.2214/AJR.10.4743. [DOI] [PubMed] [Google Scholar]
- 45.Gu J, Chan T, Zhang J, Leung AY, Kwong YL, Khong PL. Whole-body diffusion-weighted imaging: the added value to whole-body MRI at initial diagnosis of lymphoma. AJR American journal of roentgenology. 2011;197(3):W384–91. doi: 10.2214/AJR.10.5692. [DOI] [PubMed] [Google Scholar]
- 46.Abdulqadhr G, Molin D, Astrom G, Suurkula M, Johansson L, Hagberg H, et al. Whole-body diffusion-weighted imaging compared with FDG-PET/CT in staging of lymphoma patients. Acta radiologica. 2011;52(2):173–80. doi: 10.1258/ar.2010.100246. [DOI] [PubMed] [Google Scholar]
- 47.Ording Muller LS, Avenarius D, Olsen OE. High signal in bone marrow at diffusion-weighted imaging with body background suppression (DWIBS) in healthy children. Pediatr Radiol. 2011;41(2):221–6. doi: 10.1007/s00247-010-1774-8. [DOI] [PubMed] [Google Scholar]
- 48.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. Journal of digital imaging : the official journal of the Society for Computer Applications in Radiology. 2004;17(3):205–16. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deak PD, Smal Y, Kalender WA. Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology. 2010;257(1):158–66. doi: 10.1148/radiol.10100047. [DOI] [PubMed] [Google Scholar]
- 50.Pepe M, Longton G, Janes H. Estimation and Comparison of Receiver Operating Characteristic Curves. The Stata journal. 2009;9(1):1. [PMC free article] [PubMed] [Google Scholar]
- 51.Gawande RS, Gonzalez G, Messing S, Khurana A, Daldrup-Link HE. Role of diffusion-weighted imaging in differentiating benign and malignant pediatric abdominal tumors. Pediatr Radiol. 2013;43(7):836–45. doi: 10.1007/s00247-013-2626-0. [DOI] [PubMed] [Google Scholar]
- 52.Cuccarini V, Erbetta A, Farinotti M, Cuppini L, Ghielmetti F, Pollo B, et al. Advanced MRI may complement histological diagnosis of lower grade gliomas and help in predicting survival. Journal of neuro-oncology. 2015 doi: 10.1007/s11060-015-1960-5. [DOI] [PubMed] [Google Scholar]
- 53.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–25. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afaq A, Andreou A, Koh DM. Diffusion-weighted magnetic resonance imaging for tumour response assessment: why, when and how? Cancer Imaging. 2010;10(Spec no A):S179–88. doi: 10.1102/1470-7330.2010.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baum SH, Fruhwald M, Rahbar K, Wessling J, Schober O, Weckesser M. Contribution of PET/CT to prediction of outcome in children and young adults with rhabdomyosarcoma. J Nucl Med. 2011;52(10):1535–40. doi: 10.2967/jnumed.110.082511. jnumed.110.082511 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Franzius C, Bielack S, Flege S, Sciuk J, Jurgens H, Schober O. Prognostic significance of (18)F-FDG and (99m)Tc-methylene diphosphonate uptake in primary osteosarcoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2002;43(8):1012–7. [PubMed] [Google Scholar]
- 57.Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. European journal of nuclear medicine and molecular imaging. 2004;31(2):189–95. doi: 10.1007/s00259-003-1353-4. [DOI] [PubMed] [Google Scholar]
- 58.Rakheja R, Chandarana H, DeMello L, Jackson K, Geppert C, Faul D, et al. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. AJR Am J Roentgenol. 2013;201(5):1115–9. doi: 10.2214/AJR.13.11304. [DOI] [PubMed] [Google Scholar]
- 59.Jackson T, Crawley A, Klenk C, Rubin D, Quon A, Daldrup-Link H. Correlation of 18F-FDG activity and diffusion restriction of rhabdomyosarcomas on PET/MR: Potential additional prognostic factors. Clin Nucl Med. under review. [Google Scholar]
- 60.Strongin A, Yovino S, Taylor R, Wolf J, Cullen K, Zimrin A, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. International journal of radiation oncology, biology, physics. 2012;82(5):1823–30. doi: 10.1016/j.ijrobp.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 61.Rodeberg DA, Stoner JA, Garcia-Henriquez N, Randall RL, Spunt SL, Arndt CA, et al. Tumor volume and patient weight as predictors of outcome in children with intermediate risk rhabdomyosarcoma: a report from the Children’s Oncology Group. Cancer. 2011;117(11):2541–50. doi: 10.1002/cncr.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. American journal of hematology. 2010;85(5):315–9. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 63.Simon GH, von Vopelius-Feldt J, Fu Y, Schlegel J, Pinotek G, Wendland MF, et al. Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Invest Radiol. 2006;41(1):45–51. doi: 10.1097/01.rli.0000191367.61306.83. 00004424-200601000-00007 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Muehe AM, Feng D, von Eyben R, Luna-Fineman S, Link MP, Muthig T, et al. Safety Report of Ferumoxytol for Magnetic Resonance Imaging in Children and Young Adults. Invest Radiol. 2016;51(4):221–7. doi: 10.1097/RLI.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H, Yu JS, Kim DJ, Chung JJ, Kim JH, Kim KW. Diffusion-weighted MR imaging before and after contrast enhancement with superparamagnetic iron oxide for assessment of hepatic metastasis. Yonsei medical journal. 2012;53(4):825–33. doi: 10.3349/ymj.2012.53.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naganawa S, Sato C, Nakamura T, Kumada H, Ishigaki T, Miura S, et al. Diffusion-weighted images of the liver: comparison of tumor detection before and after contrast enhancement with superparamagnetic iron oxide. Journal of magnetic resonance imaging : JMRI. 2005;21(6):836–40. doi: 10.1002/jmri.20346. [DOI] [PubMed] [Google Scholar]
- 67.Masrouha KZ, Musallam KM, Samra AB, Tawil A, Haidar R, Chakhachiro Z, et al. Correlation of non-mass-like abnormal MR signal intensity with pathological findings surrounding pediatric osteosarcoma and Ewing’s sarcoma. Skeletal radiology. 2012;41(11):1453–61. doi: 10.1007/s00256-012-1383-8. [DOI] [PubMed] [Google Scholar]
- 68.Hoffer FA, Nikanorov AY, Reddick WE, Bodner SM, Xiong X, Jones-Wallace D, et al. Accuracy of MR imaging for detecting epiphyseal extension of osteosarcoma. Pediatr Radiol. 2000;30(5):289–98. doi: 10.1007/s002470050743. [DOI] [PubMed] [Google Scholar]
- 69.Brisse H, Ollivier L, Edeline V, Pacquement H, Michon J, Glorion C, et al. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34(8):595–605. doi: 10.1007/s00247-004-1192-x. [DOI] [PubMed] [Google Scholar]
- 70.Kaste SC. Imaging pediatric bone sarcomas. Radiol Clin North Am. 2011;49(4):749–65. vi–vii. doi: 10.1016/j.rcl.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erlemann R, Reiser MF, Peters PE, Vasallo P, Nommensen B, Kusnierz-Glaz CR, et al. Musculoskeletal neoplasms: static and dynamic Gd-DTPA--enhanced MR imaging. Radiology. 1989;171(3):767–73. doi: 10.1148/radiology.171.3.2717749. [DOI] [PubMed] [Google Scholar]
- 72.James SL, Panicek DM, Davies AM. Bone marrow oedema associated with benign and malignant bone tumours. Eur J Radiol. 2008;67(1):11–21. doi: 10.1016/j.ejrad.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 73.McDonald DJ. Limb-salvage surgery for treatment of sarcomas of the extremities. AJR Am J Roentgenol. 1994;163(3):509–13. doi: 10.2214/ajr.163.3.8079835. discussion 14–6. [DOI] [PubMed] [Google Scholar]
- 74.Rainusso N, Wang LL, Yustein JT. The adolescent and young adult with cancer: state of the art -- bone tumors. Current oncology reports. 2013;15(4):296–307. doi: 10.1007/s11912-013-0321-9. [DOI] [PubMed] [Google Scholar]
- 75.Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacology & therapeutics. 2013;137(1):89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Barr RD, Wunder JS. Bone and soft tissue sarcomas are often curable--but at what cost?: a call to arms (and legs) Cancer. 2009;115(18):4046–54. doi: 10.1002/cncr.24458. [DOI] [PubMed] [Google Scholar]
- 77.Daldrup-Link HE, Mohanty A, Cuenod C, Pichler B, Link T. New perspectives on bone marrow contrast agents and molecular imaging. Semin Musculoskelet Radiol. 2009;13(2):145–56. doi: 10.1055/s-0029-1220885. [DOI] [PubMed] [Google Scholar]
- 78.Littooij AS, Torigian DA, Kwee TC, de Keizer B, Alavi A, Nievelstein RA. Potential Clinical Applications of PET/Magnetic Resonance Imaging. PET Clinics. 2013;8(3):367–84. doi: 10.1016/j.cpet.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Hochhegger B, Marchiori E, Irion K, Moreira J, Zanetti G. MRI in assessment of lung cancer. Thorax. 2011;66(4):357. doi: 10.1136/thx.2011.159111. [DOI] [PubMed] [Google Scholar]
- 80.Chandarana H, Heacock L, Rakheja R, DeMello LR, Bonavita J, Block TK, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology. 2013;268(3):874–81. doi: 10.1148/radiol.13130620. [DOI] [PubMed] [Google Scholar]
- 81.Muehe A, Theruvath A, Lai L, Quon A, Aghighi M, Holdsworth S, et al. PET/MR Cancer Staging of Children and Young Adults: The Stanford Approach. Eur Radiology under review. 2017 [Google Scholar]
- 82.Wegner EA, Barrington SF, Kingston JE, Robinson RO, Ferner RE, Taj M, et al. The impact of PET scanning on management of paediatric oncology patients. European journal of nuclear medicine and molecular imaging. 2005;32(1):23–30. doi: 10.1007/s00259-004-1645-3. [DOI] [PubMed] [Google Scholar]
- 83.Montravers F, McNamara D, Landman-Parker J, Grahek D, Kerrou K, Younsi N, et al. [(18)F]FDG in childhood lymphoma: clinical utility and impact on management. European journal of nuclear medicine and molecular imaging. 2002;29(9):1155–65. doi: 10.1007/s00259-002-0861-y. [DOI] [PubMed] [Google Scholar]
- 84.Depas G, De Barsy C, Jerusalem G, Hoyoux C, Dresse MF, Fassotte MF, et al. 18F-FDG PET in children with lymphomas. European journal of nuclear medicine and molecular imaging. 2005;32(1):31–8. doi: 10.1007/s00259-004-1604-z. [DOI] [PubMed] [Google Scholar]
- 85.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. 50/Suppl_1/122S [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, et al. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54(5):683–90. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- 87.Gupta K, Pawaskar A, Basu S, Rajan MG, Asopa RV, Arora B, et al. Potential role of FDG PET imaging in predicting metastatic potential and assessment of therapeutic response to neoadjuvant chemotherapy in Ewing sarcoma family of tumors. Clin Nucl Med. 2011;36(11):973–7. doi: 10.1097/RLU.0b013e31822f684b. [DOI] [PubMed] [Google Scholar]
- 88.McKinley ET, Bugaj JE, Zhao P, Guleryuz S, Mantis C, Gokhale PC, et al. 18FDG-PET predicts pharmacodynamic response to OSI-906, a dual IGF-1R/IR inhibitor, in preclinical mouse models of lung cancer. Clin Cancer Res. 2011;17(10):3332–40. doi: 10.1158/1078-0432.CCR-10-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metz S, Lohr S, Settles M, Beer A, Woertler K, Rummeny EJ, et al. Ferumoxtran-10-enhanced MR imaging of the bone marrow before and after conditioning therapy in patients with non-Hodgkin lymphomas. Eur Radiol. 2006;16(3):598–607. doi: 10.1007/s00330-005-0045-9. [DOI] [PubMed] [Google Scholar]
- 90.Daldrup-Link HE, Rummeny EJ, Ihssen B, Kienast J, Link TM. Iron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin’s lymphoma: differentiation between tumor infiltration and hypercellular bone marrow. Eur Radiol. 2002;12(6):1557–66. doi: 10.1007/s00330-001-1270-5. [DOI] [PubMed] [Google Scholar]
- 91.Daldrup-Link HE, Henning T, Link TM. MR imaging of therapy-induced changes of bone marrow. Eur Radiol. 2007;17(3):743–61. doi: 10.1007/s00330-006-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ippolito D, Fior D, Trattenero C, Ponti ED, Drago S, Guerra L, et al. Combined value of apparent diffusion coefficient-standardized uptake value max in evaluation of post-treated locally advanced rectal cancer. World J Radiol. 2015;7(12):509–20. doi: 10.4329/wjr.v7.i12.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Byun BH, Kong CB, Lim I, Choi CW, Song WS, Cho WH, et al. Combination of 18F-FDG PET/CT and diffusion-weighted MR imaging as a predictor of histologic response to neoadjuvant chemotherapy: preliminary results in osteosarcoma. J Nucl Med. 2013;54(7):1053–9. doi: 10.2967/jnumed.112.115964. [DOI] [PubMed] [Google Scholar]
- 94.Leibfarth S, Monnich D, Welz S, Siegel C, Schwenzer N, Schmidt H, et al. A strategy for multimodal deformable image registration to integrate PET/MR into radiotherapy treatment planning. Acta Oncol. 2013;52(7):1353–9. doi: 10.3109/0284186X.2013.813964. [DOI] [PubMed] [Google Scholar]
- 95.Paulus DH, Thorwath D, Schmidt H, Quick HH. Towards integration of PET/MR hybrid imaging into radiation therapy treatment planning. Med Phys. 2014;41(7):072505. doi: 10.1118/1.4881317. [DOI] [PubMed] [Google Scholar]
- 96.Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med. 2008;49(11):1875–83. doi: 10.2967/jnumed.107.049353. [DOI] [PubMed] [Google Scholar]
- 97.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 98.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA : the journal of the American Medical Association. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cullen J. Because statistics don’t tell the whole story: a call for comprehensive care for children with cancer. CA: a cancer journal for clinicians. 2014;64(2):79–82. doi: 10.3322/caac.21215. [DOI] [PubMed] [Google Scholar]
- 100.Goldsby RE, Liu Q, Nathan PC, Bowers DC, Yeaton-Massey A, Raber SH, et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(2):324–31. doi: 10.1200/JCO.2009.22.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahoney DH, Jr, Shuster JJ, Nitschke R, Lauer SJ, Steuber CP, Winick N, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1712–22. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 102.Halsey C, Buck G, Richards S, Vargha-Khadem F, Hill F, Gibson B. The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients; results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI. J Hematol Oncol. 2011;4:42. doi: 10.1186/1756-8722-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown TR, Vijarnsorn C, Potts J, Milner R, Sandor GG, Fryer C. Anthracycline induced cardiac toxicity in pediatric Ewing sarcoma: a longitudinal study. Pediatr Blood Cancer. 2013;60(5):842–8. doi: 10.1002/pbc.24404. [DOI] [PubMed] [Google Scholar]
- 104.Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA : the journal of the American Medical Association. 2010;304(2):172–9. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 105.Karimova EJ, Wozniak A, Wu J, Neel MD, Kaste SC. How does osteonecrosis about the knee progress in young patients with leukemia?: a 2- to 7-year study. Clinical orthopaedics and related research. 2010;468(9):2454–9. doi: 10.1007/s11999-010-1358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18(18):3262–72. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 107.Miettunen PM, Lafay-Cousin L, Guilcher GM, Nettel-Aguirre A, Moorjani V. Widespread osteonecrosis in children with leukemia revealed by whole-body MRI. Clinical orthopaedics and related research. 2012;470(12):3587–95. doi: 10.1007/s11999-012-2579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paakko E, Vainionpaa L, Pyhtinen J, Lanning M. Minor changes on cranial MRI during treatment in children with acute lymphoblastic leukaemia. Neuroradiology. 1996;38(3):264–8. doi: 10.1007/BF00596544. [DOI] [PubMed] [Google Scholar]
- 109.Mahoney D, Shuster JJ, Nitschke R, Lauer SJ, Steuber CP, Winick N, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. Journal of Clinical Oncology. 1998;16(5):1712–22. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 110.Pääkkö E, Harila-Saari A, Vanionpää L, Himanen S, Pyhtinen J, Lanning M. White matter changes on MRI during treatment in children with acute lymphoblastic leukemia: correlation with neuropsychological findings. Medical and pediatric oncology. 2000;35(5):456–61. doi: 10.1002/1096-911x(20001101)35:5<456::aid-mpo3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 111.Cheung YT, Sabin ND, Reddick WE, Bhojwani D, Liu W, Brinkman TM, et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol. 2016;3(10):e456–e66. doi: 10.1016/S2352-3026(16)30110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):949–59. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perneczky R, Diehl-Schmid J, Drzezga A, Kurz A. Brain reserve capacity in frontotemporal dementia: a voxel-based 18F-FDG PET study. European journal of nuclear medicine and molecular imaging. 2007;34:1082–7. doi: 10.1007/s00259-006-0323-z. [DOI] [PubMed] [Google Scholar]
- 114.Rasgon NL, Geist CL, Kenna HA, Wroolie TE, Williams KE, Silverman DHS. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PloS one. 2014;9:e89095. doi: 10.1371/journal.pone.0089095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rubnitz J, Relling M, Harrison P, Sandlund J, Ribeiro R, Rivera G, et al. Transient encephalopathy following high-dose methotrexate treatment in childhood acute lymphoblastic leukemia. Leukemia. 1998;12(8):1176–81. doi: 10.1038/sj.leu.2401098. [DOI] [PubMed] [Google Scholar]
- 116.Chiaravalloti A, Pagani M, Di Pietro B, Danieli R, Tavolozza M, Travascio L, et al. Is cerebral glucose metabolism affected by chemotherapy in patients with Hodgkin’s lymphoma? Nucl Med Commun. 2013;34(1):57–63. doi: 10.1097/MNM.0b013e32835aa7de. [DOI] [PubMed] [Google Scholar]
- 117.Chiaravalloti A, Pagani M, Cantonetti M, Di Pietro B, Tavolozza M, Travascio L, et al. Brain metabolic changes in Hodgkin disease patients following diagnosis and during the disease course: An 18F-FDG PET/CT study. Oncology Letters. 2015;9(2):685–90. doi: 10.3892/ol.2014.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponto LLB, Menda Y, Magnotta VA, Yamada TH, Denburg NL, Schultz SK. Frontal hypometabolism in elderly breast cancer survivors determined by [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET): a pilot study. International journal of geriatric psychiatry. 2014 doi: 10.1002/gps.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Theruvath A, Ilivitzki A, Muehe A, Theruvath J, Luna-Fineman S, Sakamoto S, et al. PET/MRI of Chemotherapy-induced Brain, Heart and Bone Injuries. Radology. 2017 doi: 10.1148/radiol.2017170073. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]


