Summary
The Nucleoporin 98 gene (NUP98) is fused to a variety of partner genes in multiple hematopoietic malignancies. Here we demonstrate that NUP98 fusion proteins, including NUP98-HOXA9 (NHA9), NUP98-HOXD13 (NHD13), NUP98-NSD1, NUP98-PHF23, and NUP98-TOP1 physically interact with mixed lineage leukemia 1 (MLL1) and the non-specific lethal (NSL) histone-modifying complexes. ChIP-seq illustrates that NHA9 and MLL1 co-localize on chromatin and are found associated with Hox gene promoter regions. Furthermore, MLL1 is required for the proliferation of NHA9 cells in vitro and in vivo. Inactivation of MLL1 leads to decreased expression of genes bound by NHA9 and MLL1 and reverses a gene expression signature found in NUP98-rearranged human leukemias. Our data reveal a molecular dependency on MLL1 function in NUP98-fusion driven leukemogenesis.
Keywords: NUP98 fusion, mixed lineage leukemia 1, non-specific lethal histone-modifying complexes, leukemia, Hox gene
eTOC
Xu et al. reveal a key role for MLL1 in NUP98 fusion leukemias and show that NUP98 fusion proteins interact with MLL1 and NSL histone modifiers on chromatin to drive expression of both Hoxa and Hoxb cluster genes important for sustaining leukemia.
INTRODUCTION
The nuclear pore complexes (NPCs) span the nuclear envelope and mediate the exchange of macromolecules between the nucleus and cytoplasm (Tran et al., 2006; D’Anfelo et al., 2008). The 98 kD nucleoporin (NUP98) of NPCs was initially identified as a docking protein for cytosol-localized docking of transport substrates (Radu et al., 1995). The NH3-terminus of the NUP98 protein contains 38 nontandem repeats that possess the amino acids phenylalanine (F), leucine (L) and glycine (G) in various orders including FG, FXFG, or GLFG. The Gle2-binding sequence (GLEBS) at the amino terminal of NUP98 is responsible for the binding of Ribonucleic Acid Export 1 (REA1) (Fontoura et al., 1999; Radu et al., 1995). The C-terminal portion of NUP98 encodes the RNA binding domain (RBD) and nuclear localization signal (NLS). NUP98 is mainly localized to the nuclear envelope within the NPC (Radu et al., 1995), however a small fraction of NUP98 has been detected within the nucleus (Radu et al., 1995; Iwamoto et al., 2013), indicating potential dynamic movement of NUP98 between the nuclear interiors and the nuclear pore complex (Griffis et al., 2002). Recent studies (Kalverda et al., 2010) in Drosophila show that nucleoplasm-localized NUP98 functions as a potential transcriptional activator with a preference for promoters of genes with high H3K4 methylation and H4K16 acetylation. Another study in Drosophila demonstrated that the NUP98 protein physically interacts with the non-specific lethal (NSL) and trithorax (Trx)/mixed lineage leukemia (MLL) complexes (Pascual-Garcia et al., 2014).
Similar to the Drosophila NSL complex, the human NSL complex contains the histone acetyltransferase (HAT) males absent on the first (MOF), and other components including NSL1, NSL2, NSL3, MCRS2, plant homeodomain-linked finger-containing protein PHF20, O-linked N-acetylglucosamine transferase isoform 1 (OGT1), host cell factor 1 (HCF1), and the tryptophan-aspartate (WD) repeat domain 5 (WDR5) (Prestel et al., 2010; Raja et al., 2010; Cai et al., 2010). Two components of the human NSL complex WDR5 and HCF1 are shared among members of the mixed-lineage leukemia/set-domain containing (MLL/SET) family of histone 3 lysine 4 (H3K4) methyltransferase complexes (Raja et al., 2010; Cai et al., 2010). MLL1 (also known as KMT2A) is a histone methyltransferase (Milne at al., 2002). Human MOF has been shown to physically associate with MLL1 in a multi-protein complex that catalyzes both histone acetylation and methylation (Dou et al., 2005). These previous studies demonstrate potential physical association and functional links between the NSL and MLL1 complexes.
NUP98 translocations have been detected in patients with various hematopoietic disorders. Although the frequency of NUP98 rearrangements in unselected adult patients with AML is rare (around 1% to 2%), the frequency of NUP98 rearrangements in patients with an 11p15 abnormality is 35% (Gough et al., 2011). However, the frequency of NUP98-rearrangements is greater in childhood AML with between 6–10% of cases harboring this translocation (Bisio et al., 2014). Adults and children with AMLs carrying a NUP98 translocation have a relatively poor prognosis (Hollink et al., 2011; de Rooij et al., 2013; Chou et al, 2009). All the NUP98-translocations produce gene rearrangements that encode fusion proteins which possess a common NH3-terminus which invariably contains the FG/GLFG repeat motifs and GLEBS domain fused in-frame with various COOH-terminal fusion partners. Studies using mouse models expressing NUP98 fusions confirm their leukemogenic potential (Kroon et al., 2001; Pinearlt et al., 2003; Gurevich et al., 2004, Wang et al., 2007, Wang et al., 2009; Gough et al., 2014). However, the molecular mechanisms of NUP98-fusion mediated leukemogenesis and elevated HOX gene expression in this leukemia are unclear.
RESULTS
NUP98 Fusions and The N-terminus of NUP98 Localize to the Nucleus
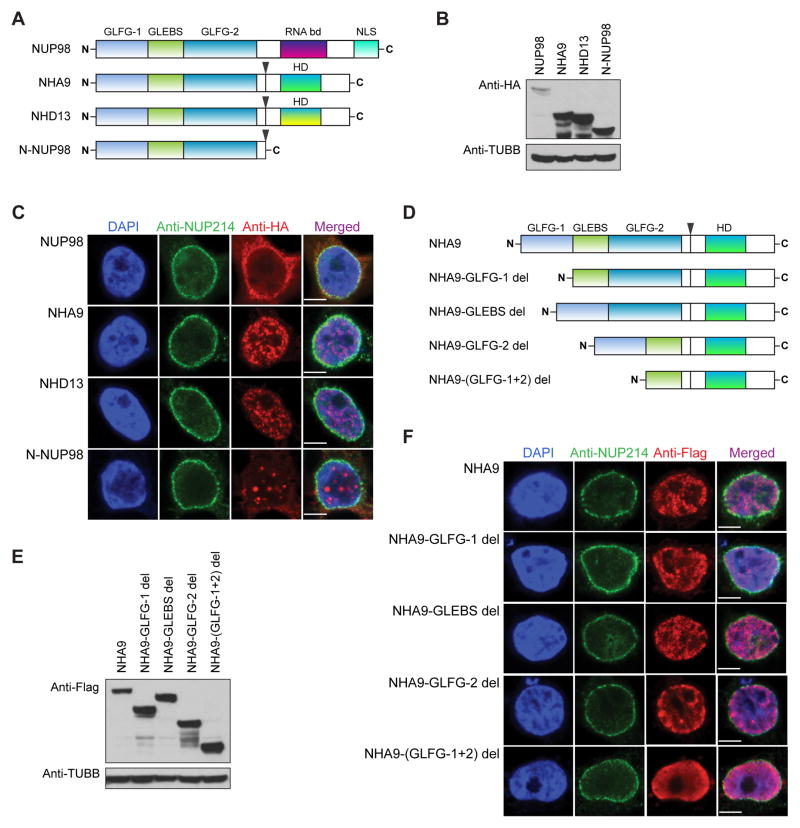
A schematic of wild type (WT) NUP98 is shown in Figure 1A. The first GLFG domain (GLFG-1) contains a total of 17 FG/GLFG repeats; while the second GLFG domain (GLFG-2) contains 21 FG/GLFG repeats. For NHA9 and NHD13 fusions, the HOXA9 or the HOXD13 portion of the fusion protein still preserves the helix-turn-helix (homeobox) DNA-binding domain. To assess the subcellular localization of NUP98 and NUP98 fusions, we overexpressed Human influenza hemagglutinin (HA)-tagged WT full-length NUP98, NHA9 and NHD13 fusion proteins, and the NH3-terminal portion of WT NUP98 (N-NUP98, amino acids 1–469 of the NH3-terminus of NUP98 preserved in NUP98 fusions) in 293T cells by transient transfection. Indeed, we detected expression of the appropriately sized N-NUP98, full-length NUP98, NHA9, and NHD13 fusion proteins by Western blot (Figure 1B). To determine whether HA tagged NUP98 or NUP98 fusions associate with NPCs, cells were double stained for HA and NUP214, a component of NPCs. Immunofluorescence showed that the full-length NUP98 protein is primarily localized to the nuclear envelope with a distinct staining around the rim of the nucleus, and displayed highly overlapping immunolabeling patterns with NUP214 at the nuclear rim (Figure 1C). However, the NUP98 fusion proteins (NHA9 and NHD13) do not co-localize with NUP214 at the nuclear envelope, and are predominantly located in the nucleoplasm with multiple foci (Figure 1C). In contrast to full length NUP98, the N-NUP98 protein, which contains all 38 FG/GLFG repeats, failed to form the typical rim structure at the nuclear envelope and showed more defined FG/GLFG body staining throughout the nucleus (Figure 1C). These data demonstrate that the N-NUP98 exhibits a similar intranuclear localization as the NUP98 fusions NHA9 and NHD13, which is distinct from full-length NUP98.
Figure 1. The subcellular localization and expression of NUP98 and NUP98 fusions.
(A) Schematic representation of WT full-length NUP98, NUP98-HOXA9 (NHA9), NUP98-HOXD13 (NHD13) and N-NUP98. Fusion break points are indicated with arrows. (B) Western blot analysis of whole cell lysates from transfected 293T cells detected by anti-HA and anti-beta Tubulin (TUBB) antibodies. One representative experiment of three is shown.
(C) Immunofluorescence staining of 293T cells transfected with the Human influenza hemagglutinin (HA)-tagged full-length NUP98, NHA9, NHD13 and N-NUP98 vectors. Cells were stained with an anti-HA and an anti-NUP214 antibody for double-immunofluorescence analysis by confocal microscopy. HA was labeled with Alexa Fluor 568-conjugated secondary antibody (red), and the NUP214 was labeled with Alexa Fluor 488-conjugated secondary antibody (green). Nuclei were counterstained with DAPI (blue). (D) Schematic of the various NHA9 mutant constructs used in this study. (E) Western blot analysis of whole cell lysates from transfected 293T cells detected by anti-Flag and anti-beta Tubulin (TUBB) antibodies. One representative experiment of three is shown.
(F) Immunofluorescence staining of 293T cells transfected with Flag-Avi-tagged NHA9 and NHA9 mutant vectors. Cells were stained with an anti-Flag and an anti-NUP214 antibody for double-immunofluorescence analysis as described in 1C. Flag was labeled with Alexa Fluor 568-conjugated secondary antibody (red).
To define how individual domains of the retained NH3-terminal NUP98 affect the nuclear localization pattern of NUP98 fusion proteins, we generated a series of Flag-Avi-tagged NHA9 mutants. Figure 1D shows the various NHA9 mutants with either deletion of the GLFG-1 domain (Δ2–156, NHA9-GLFG-1 del), deletion of the GLEBS domain (Δ157–213, NHA9-GLEBS del), deletion of the GLFG-2 domain (Δ214–469, NHA9-GLFG-2 del) or deletion of both the GLFG-1 and GLFG-2 domain (all 38 FG/GLFG repeats deleted, NHA9-(GLFG-1+2) del). The expression of these mutant NHA9 proteins in transiently transfected 293Ts was detected by Western blot (Figure 1E) and the subcellular distribution of the proteins was examined by immunofluorescence staining (Figure 1F). The NHA9-GLEBS del mutant showed similar intranuclear localization to intact NHA9 with multiple punctuate foci, whereas the NHA9-(GLFG1+2) del mutant without FG/GLFG repeats localized diffusely throughout the nucleus. The NHA9-GLFG-1 del and the NHA9-GLFG-2 del displayed somewhat less punctuated nuclear foci compared to the staining pattern observed in cells overexpressing NHA9. These data show that the FG/GLFG repeats, and less so the GLEBS domain, are required for NUP98 fusion proteins to form punctuated, intranuclear foci.
NUP98 Fusions Interact With MLL1 and NSL Histone-modifying Complexes
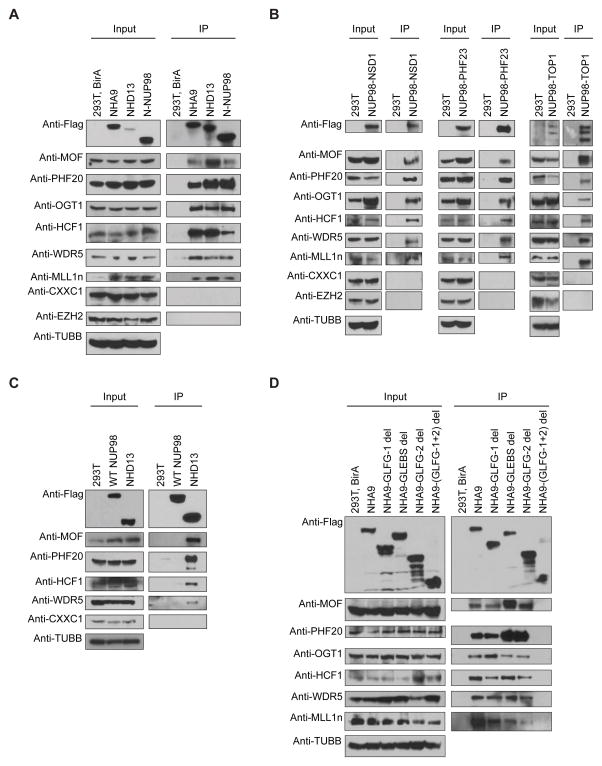
The physical and functional interaction between NUP98 and histone-modifying complexes NSL and Trx, found in Drosophila (Pascual-Garcia et al., 2014), suggests that the intranuclear NUP98 fusion proteins might also interact with the NSL and MLL1 complex. We generated 293T cell lines that stably express bacterial BirA biotin ligase and a COOH-terminal Flag-Avi-Tagged NUP98 fusion protein. Whole cell lysates were prepared and subjected to biotin-mediated affinity purification with streptavidin magnetic beads. As shown in Figure 2A, the known NSL/MLL1 complex components were detected by anti-MOF, anti-PHF20, anti-OGT1, anti-HCF1, anti-WDR5 and anti-MLL1 antibodies in eluates from Flag-Avi-tagged NHA9, NHD13 and N-NUP98 expressing cells, but not from control cells with BirA expression only. Moreover, proteins such as CXXC1 (a unique component in the SETD1 complex) and EZH2 (the catalytic subunit of the Polycomb repressive complex 2), which are not found in the NSL/MLL1 complexes, were not detected in the eluted protein. To test this interaction in a different system, we generated human U937 leukemia cell lines that stably express bacterial BirA biotin ligase and a COOH-terminal Flag-Avi-tagged NHA9 or NHD13 fusion protein (Figure S1A). The interaction between NUP98 fusion proteins and the NSL/MLL1 complex was also detected in U937 cells (Figure S1B).
Figure 2. NUP98 fusions interact with MLL1 and the NSL histone-modifying complex.
(A) The Flag-Avi-tagged NHA9, NHD13 and N-NUP98 proteins were stably expressed in 293T cells with co-expression of the BirA biotin ligase. Whole cell lysates were subjected to immunoprecipitation (IP) using streptavidin magnetic beads. Cell lysates (Input) and proteins eluted from the streptavidin beads (IP) were analyzed by Western blotting with anti-Flag, anti-MOF, anti-PHF20, anti-OGT1, anti-HCF1, anti-WDR5, anti-MLL1n, anti-CXXC1, anti-EZH2 and anti-beta Tubulin (TUBB) antibodies. (B) Co-IP was performed on lysates from Flag-tagged NUP98-NSD1 (left), NUP98-PHF23 (middle) and NUP98-TOP1 (right) transfected 293T cells with an anti-Flag antibody, and analyzed by Western blotting. (C) Immunoblots of Co-IP on lysates from Flag-tagged full-length NUP98 or NHD13 transfected 293T cells with an anti-Flag antibody. (D) Co-IP was performed on lysates from Flag-Avi-tagged NHA9 or NHA9-mutant vectors transfected 293T cells with co-expression of the BirA biotin ligase, and analyzed by Western blotting. Data are representative of three individual experiments. See also Figure S1.
Considering that most NUP98 fusion proteins possess a similar NH3-terminal portion of NUP98, we explored whether other NUP98-fusion proteins also interact with the NSL/MLL1 complex. Consistent with our data above, three other NUP98 fusions, NUP98-NSD1, NUP98-PHF23 and NUP98-TOP1 also interacted with NSL/MLL1 complex proteins (Figure 2B). However, none of these NSL/MLL1 complex proteins were detected in immunoprecipitates from the cells expressing WT full-length NUP98 (Figure 2C). These data suggest that the common NH3-terminus of NUP98 mediates binding of NUP98-fusions to the NSL/MLL1 complex, and that the nuclear envelope associated full-length NUP98 is not available for interaction with the NSL/MLL1 complex, which may occurs inside the nucleus.
To further elucidate the NUP98 fusion domains responsible for the interaction with the NSL/MLL1 complex, we performed biotin-mediated affinity purification on 293T cell lysates expressing BirA biotin ligase and Flag-Avi-tagged NHA9 or one of the NHA9 mutant proteins. Removal of the first 17 FG/GLFG repeats (NHA9-GLFG-1 del) or the GLEBS domain (NHA9-GLEBS del) portion of the NUP98 moiety did not affect binding of NHA9 to NSL/MLL1 complex members (Figure 2D). However, upon deletion of the 21 FG/GLFG repeats (NHA9-GLFG-2 del), the interaction with MLL1 was reduced (Figure 2D). The interaction of NHA9 with NSL/MLL1 complex was completely abolished by deletion of both GLFG domains (NHA9-(GLFG1+2) del) (Figure 2D). These data demonstrate that the FG/GLFG repeat motifs rather than the GLEBS domain of NHA9 are required for the interaction between NHA9 and the NSL/MLL1 complex.
NUP98 Fusion Proteins Bind to Chromatin to Drive Gene Expression
The importance of the NSL and MLL1 complexes in chromatin regulation raises the question of how binding of the NSL/MLL1 complexes to NUP98 fusions might affect the expression of NUP98 fusion target genes. To define the direct binding targets of NUP98-fusion proteins, we immortalized mouse bone marrow (BM) lin−Sca-1+c-Kit+ (LSK) cells by expression of NHA9 or NHD13 through retroviral transduction with vectors carrying C-terminally Flag-Avi-tagged NHA9 or NHD13, and BirA ligase (Figure S2A and S2B). The genome-wide direct binding targets of NHA9 and NHD13 were identified by biotin-mediated chromatin affinity purification coupled with high throughput sequencing using streptavidin beads.
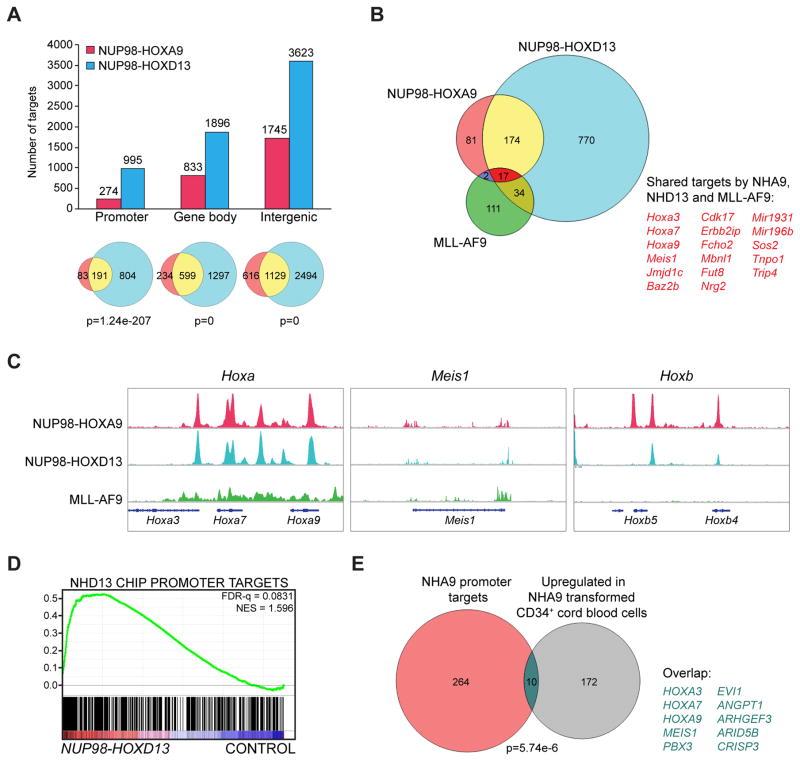
Interestingly, 10% of the NHA9 peaks (274 genes) and 15% of the NHD13 peaks (995 genes) were detected at promoter regions; 29% of the NHA9 peaks (833 genes) and NHD13 peaks (1896 genes) map to the gene body region (containing introns, exons, untranslated regions and transcription termination sites together); while 61% (1745) of the NHA9 peaks and 56% (3623) of the NHD13 peaks are present in intergenic regions (Figure 3A and Table S1). Relative to the background distribution of all such annotated sites in the mouse genome, NUP98 fusion bound regions showed a marked enrichment for promoters (enriched 9.61 fold and 15.27 fold over background for NHA9 and NHD13, respectively) compared to that at gene body and intergenetic regions (Figure S2C). A pairwise comparison between the NHA9 and NHD13 enrichment at all binding loci revealed 191 overlapping binding sites at promoter regions (p=1.24e-207, hypergeometric test), 599 overlapping genes in the gene body regions (p=0) and 1129 overlapping targets at intergenic regions (p=0) (Figure 3A). This significant overlap in binding between NHA9 and NHD13 (hereafter simply referred to as NUP98-HOX) suggests that NUP98-HOX fusions likely use the same transcriptional program to drive leukemia development.
Figure 3. Genome-wide binding of NUP98 fusions in immortalized mouse bone marrow cells and functional correlation of their target genes in mouse and human cells.
(A) Mapping of NHA9 and NHD13 ChIP-seq data identified binding sites as shown in the bar graph. The Venn diagrams illustrate overlapped targets (yellow) between NHA9 (blue) and NHD13 (red) among different genomic regions with a p value calculated by hypergeometric test.
(B) Venn diagram for overlap of NHA9 (red), NHD13 (blue) and previously published MLL-AF9 (green) binding sites at promoter regions. (C) Genome browser tracks representing the shared binding sites of NHA9, NHD13 and MLL-AF9 at the Hoxa cluster loci, the Meis1 locus, and the unique binding of NHA9 and NHD13 at the Hoxb cluster loci. (D) Gene set enrichment analysis (GSEA) illustrates enrichment of NHD13 promoter region binding targets in a published gene expression microarray dataset. NES, normalized enrichment score; FDR, false discovery rate. (E) Venn diagram showing the overlap between NHA9 promoter targets (red) and the upregulated genes in a published gene expression microarray dataset (grey) with a p value calculated by hypergeometric test. See also Figure S2/Table S1.
Given that HOX genes are activated in both MLL-AF9 and NUP98-fusion leukemia, we then compared the NHA9 and NHD13 ChIP-seq data with previously reported MLL-AF9 ChIP-seq data (Bernt et al., 2011). As MLL-AF9 has been shown to act on promoters and NUP98-HOX fusion binding sites are enriched at such regions (Figure S2C), we focused our further analyses on areas where NUP98 fusion targets bind promoter regions. 17 NUP98-HOX fusion binding targets overlapped with MLL-AF9 target genes at their promoter regions (Figure 3B, 3C), including Hoxa3, Hoxa7, Hoxa9, Meis1, Jmjd1c, Mir196b, Baz2b and Cdk17 (Figure 3B), suggesting that Hoxa cluster genes and Meis1 may also be important for the NUP98-fusion driven gene expression program. Interestingly, at promoters, NHA9 and NHD13 have unique binding targets such as Hoxb cluster genes, which are not bound by MLL-AF9 (Figure 3C). To further examine whether the NUP98-HOX direct binding targets defined here are important in murine and human cells expressing these fusion proteins, we compared the ChIP-seq results to published gene expression profiling datasets. Gene set enrichment analysis (GSEA) revealed a highly significant enrichment of the NHD13 promoter region binding targets in the upregulated gene sets from murine Lin−Sca-1+(LS) BM cells transduced with NHD13 (Palmqvist et al., 2007) (Figure 3D, NES 1.60; FDR-q=0.08%). Moreover, 10 of the 182 genes whose expression increased ≥2 fold in human cord blood CD34+ cells with NHA9 expression (Takeda et al. 2007) were also direct targets in our system thus showing significant enrichment (p=5.74e-06) (Figure 3E). Among these 10 genes, Hoxa7, Hoxa9 and Pbx3 were significantly upregulated in the before mentioned NHD13 transduced murine BM LS cells (Palmqvist et al., 2007). There was no overlap between the 30 downregulated genes in human cord blood NHA9 transduced cells (Takeda et al., 2006) and the 274 NHA9 promoter targets as defined in Figure 3A. These results show that NUP98-HOX binding is associated with elevated expression of target genes in mouse and human cells.
To determine whether our findings for NUP98-HOX fusions hold true for other NUP98 fusions with fusion partners other than homeobox genes, we immortalized mouse BM LSKs by transduction with vectors carrying Flag-Avi-tagged NUP98-TOP1 and BirA ligase (Figure S2A and S2B). Biotin-mediated ChIP-seq defined the direct binding targets of NUP98-TOP1 (Figure S2D). ChIP-qPCR confirmed the significant enrichment of NUP98-TOP1 at promoter regions of Hoxa7, Hoxa9, Hoxb5, Evi1 and Meis1 (Figure S2E) similar to that of NHA9 (Figure S2F). NUP98-TOP1 binding sites largely overlap with the common NUP98-HOX targets at promoter regions (183 overlapping genes, p=1.27e-269), gene body regions (571 overlapping targets, p=0) and intergenic regions (1067 overlapping sites, p=0) (Figure S2G), indicating that NUP98 fusion proteins with distinct fusion partners share common target genes.
To further dissect the contribution of the NUP98 portion to the DNA binding properties of NUP98 fusion proteins, we retrovirally overexpressed Flag-Avi-tagged N-NUP98, and BirA ligase in mouse BM LSKs (Figure S2A) and performed a Biotin-mediated ChIP-seq (Figure S2H). Interestingly, the 227 N-NUP98 targets at promoter regions included Hoxa7, Hoxa9, Hoxb5, Pbx3, Evi1 and Meis1. ChIP-qPCR validated the significant enrichment of N-NUP98 at these promoter regions (Figure S2I). We then compared the annotated N-NUP98 targets to the common targets between NUP98-TOP1 and NUP98-HOX fusions defined in Figure S2G (NUP98-fusions) and found an overlap at 67 promoter regions (p=1.69e-93), 226 gene body regions (p=2.34e-302) and 310 intergenic regions (p=1.33E-239) (Figure S2J). The significant overlap in binding targets between N-NUP98 and NUP98 fusions, and the co-localization of N-NUP98 and NUP98 fusions at Hoxa, Hoxb and Meis1 loci, as revealed by genome browser tracks (Figure S2K), suggests that the NUP98 moiety of NUP98 fusion proteins contributes to the binding of NUP98 fusions at these target genes.
Colocalization of NHA9 and MLL1 at Hoxa and Hoxb Cluster Gene Loci
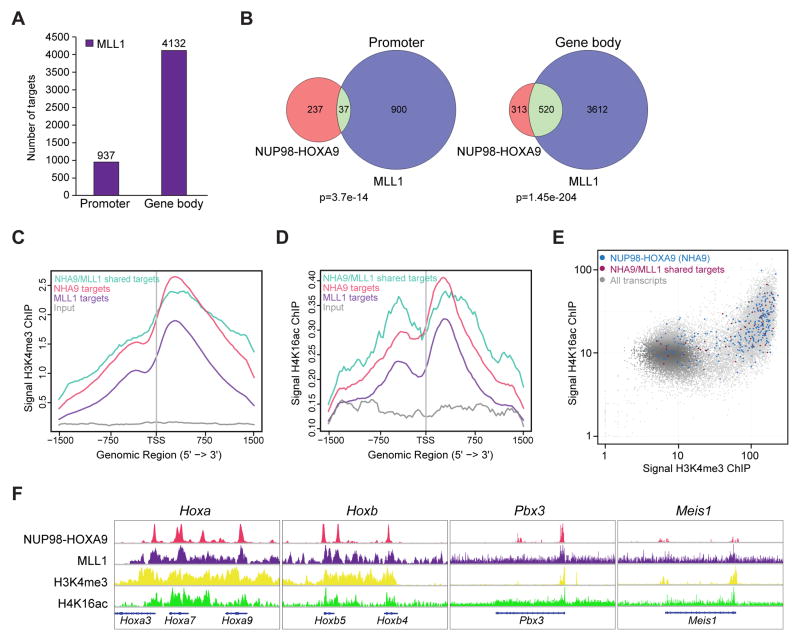
MLL1 has been shown to directly regulate HOX gene expression (Yu et al., 1995; Milne et al., 2002; Ayton et al., 2003; Hsieh et al., 2003; Ernst et al., 2004; Hess et al., 2004; Milne et al., 2005). To test whether NUP98-fusions and MLL1 were recruited to the same regions of Hox gene clusters, MLL1 ChIP-seq was carried out on murine NHA9 cells. Analysis of all bound genomic loci indicated that 937 MLL1 binding sites mapped to promoter regions, while 4132 MLL1 peaks were bound to the gene bodies (Figure 4A and Table S2). We then compared MLL1 and NHA9 binding regions and found a significant overlap at promoter regions (37 genes, p=3.7e-14) and gene body regions (520 genes, p=1.45e-204) (Figure 4B). The 37 overlapping promoter region targets include the Hoxa3, Hoxa7, Hoxa9, Hoxb4, Hoxb5, Meis1, Eya1 and Pbx3 loci (Table S2). The overlapping binding sites between MLL1 and NHA9 show that NHA9 and MLL1 colocalize on chromatin in agreement with our data showing that NHA9 interacts with MLL1 and the extended NSL/MLL1 complexes.
Figure 4. Colocalization of NHA9 and MLL1 at Hoxa and Hoxb cluster gene loci.
(A) The bar graph indicates the number of MLL1-bound targets defined by MLL1 ChIP-seq at promoter and gene body regions in transformed murine NHA9 LSKs.
(B) Venn diagram shows the overlap between NHA9 (red) and MLL1 (purple) binding targets at promoter and gene body regions with a p value calculated using the hypergeometric test. (C) Shown are the average binding profiles of H3K4me3 defined by H3K4me3 ChIP-seq at a region of ±1.5 kb around the annotated TSSs of NHA9-bound (red), MLL1-bound (purple) and NHA9/MLL1 co-bound targets (turquoise). Tag densities were normalized to the input (grey). (D) Average binding profiles of H4K16ac defined by H4K16ac ChIP-seq experiments are shown. (E) Genome-wide representation of the relation between H3K4me3 and H4K16ac in NHA9 cells at NHA9 promoter targets (blue) and NHA9/MLL1 co-bound promoter targets (dark red) compared to all ~34000 transcripts (gray). The x and y-axis represent binding read numbers per Kb. (F) Genome browser tracks of genomic regions showing colocalization of NHA9, MLL1, H3K4me3 and H4K16ac at four representative, well-known MLL1 targets; Hoxa cluster genes, Hoxb cluster genes, Pbx3 and Meis1. See also Figure S2/Table S2.
Given that that MOF and MLL1 are both part of the NSL/MLL1 complexes and work in concert to modify H4K16ac and H3K4me3 at promoters (Dou et al., 2005), we further tested whether H3K4me3 and H4K16ac modifications are associated with NHA9-bound regions in murine NHA9 cells by anti-H3K4me3 and anti-H4K16ac ChIP-seq. The distribution patterns of H3K4me3 (Figure 4C) and H4K16ac (Figure 4D), shown as the average profile around TSSs (±1.5Kb), indicate high levels of H3K4me3 and H4K16ac in regions of chromatin where NHA9 and MLL1 are found (Figure 4C and 4D). Most of the NHA9-bound targets and NHA9/MLL1 co-bound promoter regions showed a positive correlation between H3K4me3 and H4K16ac levels (Figure 4E). Importantly, specific individual peaks of H3K4me3 and H4K16ac overlapped with NHA9 and MLL1 binding targets, such as Hoxa cluster genes, Hoxb cluster genes, Pbx3 and Meis1, suggesting a tight association between NHA9 and these modifications (Figure 4F). We repeated the MLL1 ChIP-seq using an anti-NH3-terminus MLL1 antibody (MO435) (Liu et al., 2014). This additional experiment confirmed the colocalization of MLL1 with NHA9, H3K4me3 and H4K16ac at NHA9 target gene loci (Figure S2L). These findings demonstrate that NHA9 and MLL1 are associated with the same Hox loci and with chromatin modifications of active gene expression.
MLL1 Is Required for NHA9 Driven Leukemogenesis In Vitro and In Vivo
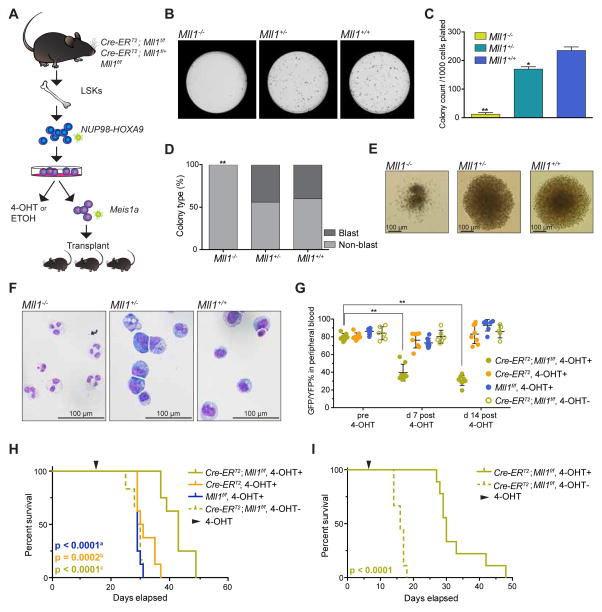
To test the function of MLL1 in NHA9 driven leukemia, we first assessed the impact of Mll1 (Kmt2a) deletion on colony formation from NHA9-transduced mouse BM cells. LSK cells purified from the BM of a well described Mll1 conditional knockout model where the expression of Cre-recombinase is under the control of the ubiquitin C (UBC) promoter and driven by the estrogen receptor (UBC-Cre-ERT2 (referred to as Cre-ERT2)) (Jude et al., 2007; Artinger et al., 2013) were transduced with the NHA9 retrovirus (Figure 5A). The induced excision of the floxed Mll1 by 4-hydroxyl tamoxifen (4-OHT) was confirmed by genomic PCR (Figure S3A). Loss of Mll1 dramatically reduced the number of colonies (Figure 5B, 5C) and reduced cell growth in liquid culture (Figure S3B) compared to those from the Mll1f/f (referred to as Mll1+/+) NHA9 control. Moreover, the Cre-ERT2;Mll1f/f + 4-OHT (referred to as Mll1−/−) NHA9 cells failed to form blast colonies (Figure 5D, 5E). Finally, Mll1−/− NHA9 cells displayed a significant degree of granulocytic differentiation compared to the characteristic blast-like morphology observed in Cre-ERT2;Mll1f/+ + 4-OHT (referred to as Mll1+/−) NHA9 and Mll1+/+ NHA9 cells (Figure 5F). These results indicate that MLL1 is required for maintenance of NHA9-transformed cells in vitro.
Figure 5. The effects of Mll1 loss in a murine NHA9 driven leukemia model.
(A) Schematic for in vitro and in vivo Mll1 knockout experiments.
(B) Representative 35 mm dishes are shown the CFU assay of NHA9 in vitro transformed mouse BM LSKs plated after 48 hr of 4-OHT treatments. (C) Bar graph indicates mean number of colonies per 35 mm dish after 7 days. Data are representative of four individual experiments. (D) Shown is the mean percentage of blast versus non-blast colonies relative to all colonies counted per dish, per genotype. (E) Representative images of colonies at day 7 of CFU assay. (F) Representative images of Wright-Giemsa–stained cytospin preparations of cells harvested at day 7 of CFU assay. (G) Mouse BM LSKs were retrovirally transduced with GFP-NHA9 and YFP-Meis1a, sorted for double positive cells and injected into lethally irradiated C57Bl/6 mice (n=8 per group). Plotted is the GFP+YFP+% of live cells in peripheral (PB) of mice before 4-OHT treatments and at two different time-points post treatments. A dot represents a single mouse in the experiment. (H) Survival curve of mice with primary leukemia. Data are representative of three individual experiments. (I) Primary Cre-ERT2;Mll1f/f NHA9-Meis1a mouse leukemia BM cells were injected into sub-lethally irradiated C57Bl/6 mice. Half of the mice (n=10) were treated with 4-OHT at day 7 after transplant to excise Mll1. See also Figure S3, S4 and S5.
Abbreviations: Cre-ERT2;Mll1f/f + 4-OHT referred to as Mll1−/−, Cre-ERT2;Mll1f/+ + 4-OHT referred to as Mll1+/−, Mll1f/f referred to as Mll1+/+
* p < 0.05, ** p < 0.01. Error bars represent SD of mean.
a.Log-rank test p value comparing survival for Cre-ERT2;Mll1f/f to Mll1f/f
b.Log-rank test p value comparing survival for Cre-ERT2;Mll1f/f to Cre-ERT2
c.Log-rank test p value comparing survival for Cre-ERT2;Mll1f/f to Cre-ERT2;Mll1f/f without 4OHT treatment
To determine the effect of Mll1 loss on NHA9 driven leukemogenesis in vivo, we used a mouse model where co-expression of NHA9 and Meis1a induces rapid AML development based on the rationale that NHA9 function should still be required in this model since Meis1a cannot transform mouse BM by itself (Kroon et al., 2001). NHA9 transformed Cre-ERT2;Mll1f/f, Cre-ERT2 and Mll1f/f BM LSKs were transduced with a retrovirus encoding Meis1a-YFP, and sorted based on the expression of GFP (NHA9) and YFP (Meis1a) by FACS (Figure S3C). The transduced GFP+YFP+ BM cells of individual genotypes were transplanted into lethally irradiated C57BL/6 mice (Figure 5A). Homozygous deletion of Mll1 resulted in significant reduction of the GFP+YFP+ percentage in the peripheral blood (PB) 7 and 14 days post induction of Cre-recombinase (Figure 5G), and led to prolonged survival (Figure 5H) compared to the mice transplanted with either Cre-ERT2 or Mll1f/f NHA9/Meis1a cells. Diseased mice were characterized by extremely elevated peripheral nucleated cell counts, anemia, and splenomegaly (Figure S3D). More than 90% of the cells in the PB (Figure S3E), BM and spleen (data not shown) of leukemic mice were GFP+YFP+ cells. These GFP+YFP+ cells expressed the myeloid cell markers, indicating a myeloid leukemia phenotype (Figure S3F). At time of death, GFP+YFP+ BM cells of Cre-ERT2;Mll1f/f NHA9/Meis1a, 4-OHT+ mice were no longer completely excised (Figure S3G), indicating that the leukemias that eventually form are caused by cells that have escaped Mll1 deletion. Together, these results show that MLL1 is critical for the initiation of NHA9 driven leukemia.
To test whether MLL1 is required for the maintenance of NHA9 driven AML, unexcised Cre-ERT2;Mll1f/f NHA9/Meis1a primary leukemia BM cells were injected into sub-lethally irradiated C57BL/6 recipients. Loss of Mll1 induced by treatment with 4-OHT after 7 days of transplantation revealed a significant increase in survival compared to the secondary AML that developed in mice without 4-OHT treatments (Figure 5I, p<0.0001). The secondary leukemias that formed in the Cre-ERT2;Mll1f/f NHA9/Meis1a 4-OHT+ mice were no longer fully excised (data not shown). These data show that MLL1 is critical for the maintenance of NHA9 driven AML.
To further evaluate the role of MLL1 in other NUP98 fusion leukemias, we carried out analogous in vitro experiments for a diverse set of NUP98 fusions. Consistent with our observations for NHA9, homozygous deletion of Mll1 in NHD13 (Figure S4A–D), NUP98-JARID1A (Figure S4E–H) or NUP98-TOP1 (Figure S4I–L) transformed cells, led to a significant reduction in CFU assay colony number and increased granulocytic differentiation. These results demonstrate that MLL1 is indispensable for the maintenance of NUP98 fusion transformed cells in vitro, suggesting that the requirement of MLL1 may be a shared feature for most, if not all, NUP98 fusion driven leukemia.
We next compared the MLL1 dependence of NUP98 fusions to the previously reported MLL1 dependence of MLL-AF9 (Thiel et al., 2010). We found that the deletion of Mll1 had a much more dramatic effect in NUP98-fusion transformed cells than in cells transformed with MLL-AF9, both in CFU assays and in liquid culture (Figure S5A–G). The overexpression of Hoxa9 and Meis1 has been shown to transform primary mouse BM cells in vitro and induce AML in vivo (Kroon et al., 1998). We confirmed that the loss of Mll1 does not inhibit colony formation of BM LSKs transfomed by Hoxa9/Meis1, as described before (Thiel et al., 2010) (Figure S5H–L). In addition, loss of Mll1 did not affect Hox9/Meis1 induced in vivo leukemogenesis (Figure S5M–N). These results demonstrate that the deletion of Mll1 in NUP98-fusion transformed cells had a much more profound effect than did Mll1 deletion in cells transformed with MLL-AF9, and had no effect on cells transformed with Hoxa9/Meis1, indicating relative specificity of MLL1 dependence in NUP98 fusion leukemia.
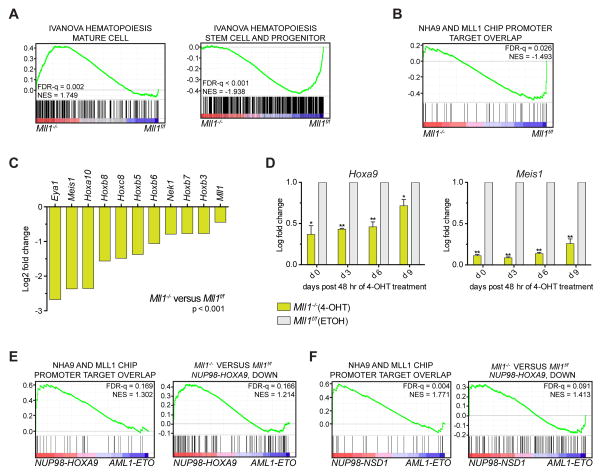
MLL1-dependent Gene Expression Signatures Resemble Human NUP98-NSD1 AML
To identify MLL1 dependent genes involved in the maintenance of NHA9 transformed BM cells, we performed gene expression analysis after Mll1 deletion by RNA-seq and assessed normalized gene expression differences between control and Mll1-deficient NHA9 cells (Table S3). GSEA showed a positive enrichment for gene signatures associated with hematopoietic cell differentiation (Figure 6A, left panel, NES 1.749, FDR-q 0.002), and a negative enrichment for hematopoietic stem cell and progenitor gene signatures in Mll1−/− NHA9 cells (Figure 6A, right panel, NES −1.938, FDR-q <0.001). These data fit with the increased myeloid differentiation phenotype observed in Mll1−/− NHA9 cells. Interestingly, GSEA also demonstrated a significant negative enrichment of genes directly co-bound by NHA9 and MLL1 (Figure 6B, NES −1.493, FDR-q 0.026) in the Mll1−/− NHA9 cells. We confirmed that NHA9 and MLL1 co-bound targets, such as Hoxa cluster, Hoxb cluster, Eya1 and Meis1, were among the most strongly decreased genes upon Mll1 deletion in NHA9 cells (Figure 6C, p<0.001). We validated the decreased expression of Hoxa cluster genes, Hoxb cluster genes and Meis1 by qRT-PCR at various time-points after 4-OHT induced deletion of Mll1 (Figure 6D and Figure S6A). These data indicate that MLL1 is required to sustain expression of oncogenic programs targeted by NHA9. Of note, reduction of Hoxa9, Hoxa10, Hoxb6 and Meis1 gene expression was also detected in NHD13 (Figure S6B) and NUP98-JARID1A (Figure S6C) transformed cells upon homozygous Mll1 deletion. The MLL1 dependent transcriptome in MLL-AF9 cells has previously been characterized (Cao et al., 2014). MLL-AF9 transformed cells show more modest changes of Hoxa7, Hoxa9, Hoxa10 and Meis1 gene expression upon Mll1 loss (Figure S5G), highlighting the dependency of NUP98 fusions on MLL1.
Figure 6. MLL1-dependent gene expression signature in NHA9 transformed mouse LSKs resembles human NUP98-NSD1 leukemia.
(A) GSEA with a differential expression analysis ranked list comparing RNA-seq data from Cre-ERT2;Mll1f/f NHA9 cells at day 3 post 4-OHT treatment (referred to as Mll1−/−) to the cells treated with vehicle control (referred to as Mll1f/f). Enrichment was assessed for genes that are upregulated in mature hematopoietic populations (left) and upregulated in progenitors and stem cells (right) from mouse BM and fetal liver cells. (B) A gene set was created using the list of NHA9/MLL1 co-bound promoter targets from the ChIP-seq analysis. GSEA was generated using the RNAseq differential expression analysis comparing Mll1−/− to Mll1f/f NHA9 cells. (C) Bar graphs indicate log2 fold changes in gene expression as measured by RNAseq when comparing Mll1−/− to Mll1f/f NHA9 cells. (D) NHA9 in vitro transformed Cre-ERT2;Mll1f/f LSKs were plated in liquid culture following 48 hr of 4-OHT or vehicle treatment. Bar graphs illustrate relative expression levels (RT-qPCR) as mean log fold change compared to vehicle control treated cells. The x-axis indicates cells harvested at various time-points post treatment. Error bars represent SD of mean. * p < 0.05, ** p < 0.01. Data are representative of three individual experiments. (E) A gene set was created using the NHA9/MLL1 co-bound promoter targets (left). Another MLL1-dependent gene set was created using the list of significantly downregulated genes revealed by RNA-seq when comparing Mll1−/− to Mll1f/f NHA9 cells with LFC < −1 and adjusted p < 0.05 (right). Enrichment of these MLL1-dependent gene sets in published human gene expression microarray data from NHA9 versus AML1-ETO transfected CD34+ cord blood cells (GSE57194) was assessed. (F) The MLL1-dependent gene sets defined in Figure 6E were used to assess enrichment in published gene expression microarray data from human pediatric de novo AML patients with a NUP98-NSD1 translocation versus patients with an AML1-ETO rearrangement (GSE17855). NES, normalized enrichment score; FDR, false discovery rate. See also Figure S6/Table S3.
To examine the potential clinical relevance of the MLL1-dependent gene expression signature found in NHA9 cells, we first compared this gene signature to the microarray dataset of human cord blood CD34+ cells retrovirally infected with NHA9 or t(8;21) (AML1-ETO) vector (Abdul-Nabi et al., 2010). GSEA exhibits enrichment of NHA9/MLL1 co-bound promoter targets (Figure 6E, left panel, NES 1.302, FDR-q 0.169), and enrichment of the MLL1-dependent gene expression signature (Figure 6E, right panel, NES1.214, FDR-q 0.166) in the upregulated genes from human CD34+ NHA9 vs AML1-ETO cells. We further compared our RNA-seq data to published human microarray data from pediatric de novo AML patients with a NUP98-NSD1 translocation versus a t(8;21) rearrangement (Hollink et al., 2011). GSEA analysis on this dataset illustrates significant enrichment of NHA9/MLL1 co-bound promoter targets (Figure 6F left panel, NES 1.771, FDR-q 0.004), and enrichment of the MLL1-dependent gene expression signature (Figure 6F right panel, NES 1.413, FDR-q 0.091) in NUP98-NSD1 vs AML1-ETO AML patient gene expression data. These results demonstrate strong similarity between the MLL1-dependent gene expression signature in our murine NHA9 AML model and the expression profile of human NUP98 fusion AML, indicating that our findings in murine AML models can be extended to human leukemia, and suggesting that MLL1 is likely an important determinant of human NUP98 fusion driven AML.
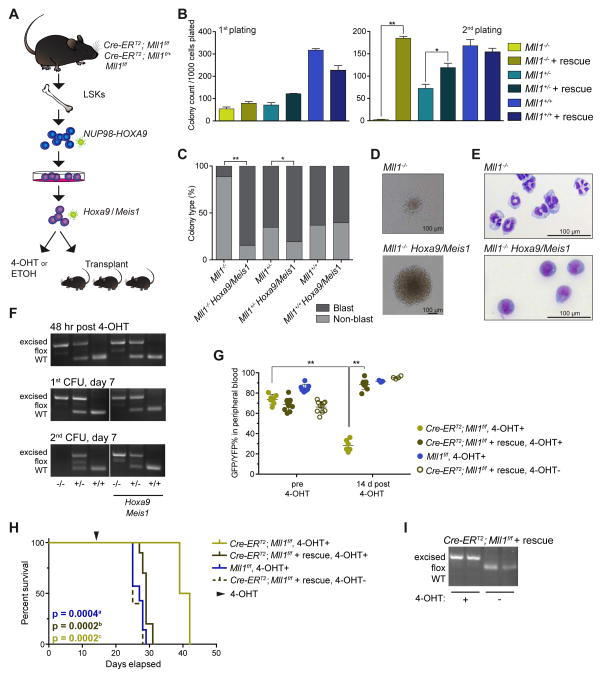
Hoxa9 and Meis1 Overexpression Rescues NUP98-HOXA9 Transformed Cells From MLL1 Dependence
The downregulation of Hoxa9 and Meis1 in Mll1−/− NHA9 cells (Figure 6D) suggests a role for Hoxa9 and Meis1 in NHA9-induced leukemia. To test whether the overexpression of Hoxa9/Meis1 can rescue NHA9 cells from their dependence on MLL1, NHA9 transduced Cre-ERT2;Mll1f/f, Cre-ERT2;Mll1f/+ and Mll1f/f BM cells were transduced with a Hoxa9/Meis1-expressing retrovirus (Figure 7A). The overexpression of Hoxa9/Meis1 restored the clonogenic capacity of Mll1−/− NHA9 cells in the second round of the CFU replating assay (Figure 7B). Strikingly, more than 80% of the colonies formed in Hoxa9/Meis1 overexpressed Mll1−/− NHA9 cells exhibited a typical blast colony shape (Figure 7C, 7D) and displayed blast morphology (Figure 7E). As a control, effective Mll1 excision was observed by PCR in 4-OHT treated cells (Fig 7F). These data show that upregulation of Hoxa9 and Meis1 by MLL1 in NHA9 cells contributes to the maintenance of NHA9 transformed cell proliferation in vitro. The overexpression of Hoxa9 or Meis1 alone did not rescue the clonogenic defect in Mll1−/− NHA9 cells (data not shown), suggesting that the interplay between Hoxa9 and Meis1 is required for NHA9 induced leukemia. To further examine whether Hoxa9/Meis1 overexpression rescues the leukemogenicity of NHA9 cells from MLL1 dependence, NHA9 transformed Cre-ERT2;Mll1f/f BM LSKs overexpressing Hoxa9 and Meis1, were transplanted into lethally irradiated C57BL/6 mice (Figure 7A). Mice transplanted with Mll1−/− NHA9 + Hoxa9/Meis1a cells displayed a significant increase in leukemia burden as measured by percentage of GFP+YFP+ cells in PB (Figure 7G) and had a significantly shortened overall survival (Figure 7H) compared to the non-rescue Mll1−/− NHA9/Meis1a control. PCR analysis confirmed that both floxed Mll1 alleles were excised at time of death in the 4-OHT treated Cre-ERT2;Mll1f/f NHA9 + Hoxa9/Meis1a mice (Figure 7I). These results illustrate that the upregulation of Hoxa9 and Meis1 by MLL1 contributes to NHA9 driven leukemia.
Figure 7. Hoxa9 and Meis1 overexpression rescues murine NHA9 driven leukemia from MLL1 dependence.
(A) Schematic for in vitro and in vivo Mll1 knockout rescue experiments.
(B) CFU assay of NHA9 in vitro transformed mouse BM LSKs, plated after 48 hr of 4-OHT treatments. Samples stating “+rescue” were infected with virus containing Hoxa9 and Meis1 and selected by hygromycin treatment, prior to excision. Bar graphs display mean number of colonies per 35 mm dish. Day 7 of the 1st plating (left) and 2nd plating (right). Data are representative of three individual experiments. (C) Shown is the mean percentage of blast versus non-blast colonies relative to all colonies counted per dish, per sample after the 1st plating. (D) Representative images of colonies formed at day 7 of the 1st plating. (E) Representative images of Wright-Giemsa–stained cytospin preparations of cells harvested at day 7 of the 1st plating. (F) PCR analysis illustrates excision throughout the duration of a colony forming experiment. Representative gel images are shown. (G) Mouse BM LSKs were retrovirally transduced with NHA9 and Meis1a or both Meis1a and Hoxa9 (“+ rescue”). Sorted cells were injected into lethally irradiated C57Bl/6 mice. Plotted is the GFP+YFP+% of live cells in PB of mice before 4-OHT treatment and 14 days post treatment. A dot represents a single mouse in the experiment. (H) Survival curve. Mice were treated with 4-OHT at day 15 after transplant. (I) PCR analysis illustrates Mll1 excision in the murine BM of Cre-ERT2;Mll1f/f + Hoxa9/Meis1 recipient mice at time of death. Representative gel images are shown. See also Figure S7.
Abbreviations: Cre-ERT2;Mll1f/f + 4-OHT referred to as Mll1−/−, Cre-ERT2;Mll1f/+ + 4-OHT referred to as Mll1+/−, Mll1f/f referred to as Mll1+/+
* p < 0.05, ** p < 0.01. Error bars represent SD of mean.
a.Log-rank test p value comparing survival for Cre-ERT2;Mll1f/f to Mll1f/f
b.Log-rank test p value comparing survival for Cre-ERT2;Mll1f/f to Cre-ERT2;Mll1f/f + Hoxa9/Meis1
c.Log-rank test p value comparing survival for Cre-ERT2;Mll1f/f to Cre-ERT2;Mll1f/f + Hoxa9/Meis1 without 4OHT treatment
To determine whether the MLL1 dependence in NHA9 is not only dependent on Hoxa, but also Hoxb genes, analogous in vitro rescue experiments were performed using NHA9 transformed Cre-ERT2;Mll1f/f cells. The overexpression of Hoxb6 alone or in combination with Meis1, both rescued the clonogenic defect of Mll1−/− NHA9 cells in a serial CFU replating assay (Figure S7A–B), and altered the colony morphology (Figure S7C–D). Together these data provide strong evidence that NUP98 fusion proteins drive leukemogenesis, at least in part through dysregulation of both Hoxa and Hoxb gene expression by MLL1.
DISCUSSION
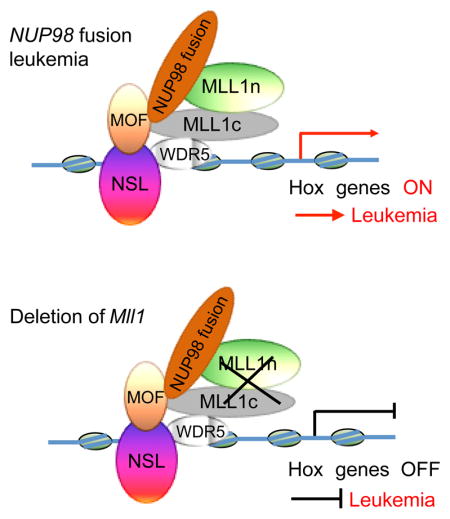
We identified an interaction between NUP98 fusions and the NSL and MLL1 complexes, and indicated that this interaction is likely mediated by the FG/GLFG repeats in the NUP98-moiety of the NUP98 fusion proteins. A study in Drosophila demonstrates a component of the NSL complex, MBD-R2, is required for the recruitment of NUP98 to chromatin (Pascual-Garcia et al., 2014). These data suggest that the association of NUP98 fusions with NSL/MLL1 complexes may be through the direct binding of the NUP98 fusion FG/GLFG repeats to PHF20 (the human homologue of Drosophila MBD-R2). However the specific site of FG/GLFG repeats mediating the binding with PHF20 remains to be determined.
NUP98 fusion proteins with distinct fusion partners share common target genes, suggesting that the NUP98 moiety of these fusion proteins may contribute to the DNA binding properties of NUP98 fusions. The significant overlap in binding targets between N-NUP98 and NUP98 fusions, further support this hypothesis. Since N-NUP98 does not contain any recognizable DNA-binding or chromatin-binding motif itself, the recruitment of N-NUP98 to these specific gene loci is likely due to the interaction between N-NUP98 and other proteins or protein complexes with DNA or chromatin binding capacity, including the NSL/MLL1 complex. Our results suggest that this NUP98 moiety-mediated recruitment of NUP98 fusions to certain regions of the chromatin by the interaction with NSL/MLL1 complexes may be a shared general pathogenic mechanism of NUP98 fusions in AML.
The colocalization of NHA9 and MLL1 at Hoxa and Hoxb cluster gene loci, as revealed by ChIP-seq, not only supports the identified interaction between NHA9 and NSL/MLL1 complexes, but also indicates that NHA9 utilizes this interaction to be recruited to chromatin, at least to some of the NHA9 binding targets, including Hox gene loci. A recent publication showed chromatin-prebound CRM1 influences localization of the NHA9 fusion to induce expression of Hox cluster genes (Oka et al., 2015). However, CRM1 targets are generally associated with H3K27me3 repressive modifications, thus the mechanisms of CRM1 action are unclear. Of note, this study was performed in murine embryonic stem (ES) cells, in which the chromatin modifications associated with HOX loci are quite different from those in other cell types. Therefore, these findings in ES cells need to be assessed in leukemia cells.
The role of endogenous MLL1 in other AML subtypes, such as MLL-fusion driven leukemia has previously been reported (Milne et al., 2010; Thiel et al., 2010), but the NUP98-fusion dependence on MLL1 appears to be even more absolute than for MLL-AF9. These data are of particular interest given the recent publication showing that the histone methyltransferase activity of MLL1 is dispensable for MLL-AF9 leukemogenesis (Mishra et al., 2014). Therefore our findings indicate a somewhat unique, and more dramatic requirement for MLL1 in NUP98-fusion driven leukemia. The deletion of Mll1 in NHA9 cells resulted in significant downregulation of not only Hoxa, but also Hoxb cluster genes. However, the Hoxb cluster genes are not activated in MLL-AF9 leukemia cells, and as expected, Mll1 deficient MLL-AF9 cells did not show any change in Hoxb gene expression (Cao et al., 2014). This differential Hox gene expression pattern between NHA9 and MLL-AF9 leukemia upon Mll1 deletion might explain part of the more dramatic dependency of NUP98 fusion leukemia on MLL1. The capability of Hoxb6 alone to rescue the clonogenic capacity of Mll1−/−NHA9 cells further supports the idea that this unique Hoxb gene expression pattern is dependent on MLL1 and functionally important for NUP98-fusion driven leukemia.
Our results suggest a more general role for MLL1 complex and a potential role for the NSL complex in NUP98-fusion driven leukemia. Previous studies show that NUP98-homeobox fusions promote self-renewal and aberrant gene expression to a significantly greater extent than NUP98 fusions with non-homeobox partners (Saw et al., 2013), suggesting that the COOH-terminal fusion partner may influence the oncogenic potency of NUP98 fusions. Whether and how MLL1 differentially affects this aspect of NUP98 fusions remains to be explored. In this study, we focus our attention on the role of MLL 1 in HOX gene regulation and leukemogenesis driven by NUP98 fusions. However, there is likely some contribution from the NSL complex components, such as MOF, to NUP98-fusion driven leukemia. Indeed, we found the enrichment of H4K16ac marks on NHA9 binding targets at promoters, suggesting the activation of these genes may be also regulated by the HAT activity of MOF. It will be interesting to further examine whether and how MOF affects the gene expression and oncogenic program in NUP98 fusion driven leukemia and whether MOF might also represent a therapeutic opportunity.
In conclusion, our findings support an emerging model that various fusion oncoproteins hijack histone modifying complexes to drive leukemogenic transformation. The discovery of this common leukemogenic pathway for NUP98 fusion proteins, provides a rationale for the search of potential common therapeutic approaches in the treatment of leukemia patients carrying various NUP98-rearrangements. The role of MLL1 in NUP98 fusion AML as established here, suggests that inhibition of the function of MLL1 or its interaction with Menin, as recently described by others (Borkin et al., 2015) or other methods to disrupt the MLL1 complex, may be an effective approach for patients with NUP98 fusion leukemia. Further studies will determine whether modulation of MOF HAT activity or interruption of the association between NUP98 fusions and the NSL/MLL1 complexes with small molecules would represent an approach in treating poor prognosis pediatric AMLs with NUP98 fusions.
EXPERIMENTAL PROCEDURES
Mice
The generation of the Mll1 conditional knockout mouse has been described previously (Jude et al., 2007). Wild-type C57BL/6 mice were purchased from Taconic, Hudson, NY. All animal experiments described in this study were approved by and adhered to the guidelines of the Memorial Sloan-Kettering Cancer Center Animal Care and Use Committee.
Plasmids
NUP98-HOXA9 and full-length NUP98 cDNAs with NH3-terminal HA tags in an MSCV-IRES-GFP vector were provided by Dr. Nabeel R. Yaseen (Ghannam et al., 2004); NUP98-HOXD13 cDNA by Dr. Peter Aplan, NUP98-PHF23 and NUP98-JARID1A in MSCV-IRES-puro by Dr. David Allis (Wang et al., 2009); and NUP98-TOP1 in MSCV-IRES-GFP and Meis1a in MSCV-IRES-YFP by Dr. Keith Humphries (Gurevich et al., 2004; Pineault et al., 2003). The MSCV-Hoxa9-Meis1-IRES-hygromycin, MSCV-Hoxa9-IRES-puro, MSCV-BirA-YFP, MSCV-MLL-AF9-IRES-GFP and NUP98-NSD1 plasmid have been previously described (Bernt et al., 2011; Deshpande et al., 2014).
Data Analysis and Statistical Methods
Microsoft Excel and GraphPad Prism software were used for statistical analysis. Statistical significance between 2 groups was determined by unpaired 2-tailed Student’s t- test. The Kaplan-Meier method was used to plot survival curves for murine leukemic transplant data, and the log-rank test was used to evaluate statistical differences. The hypergeometric test was performed in R to calculate the statistical significance of overlapped targets illustrated by Venn diagrams. A p value less than 0.05 was considered significant.
Supplementary Material
Significance.
To date, at least 28 different chromosomal rearrangements involving NUP98 have been identified. Understanding the molecular mechanisms of NUP98-fusion mediated leukemogenesis and elevated HOX gene expression may provide therapeutic options for treatment of NUP98-fusion driven leukemias. Here we show that the NUP98 fusions physically interact with mixed lineage leukemia 1 (MLL1) and the non-specific lethal (NSL) histone-modifying complexes. We also show the co-localization of NUP98 fusions and MLL1 at Hoxa and Hoxb cluster gene loci. We demonstrate an essential role for MLL1 in NUP98-HOXA9-induced gene expression as well as growth and survival of NUP98-HOXA9-transformed leukemia cells. These findings have profound therapeutic implications because they provide a strong rationale for targeting the MLL1 complex in NUP98 fusion-induced acute leukemia.
Highlights.
NUP98 fusions interact with MLL1 and NSL histone-modifying complexes
NUP98 fusions and MLL1 co-localize at Hoxa and Hoxb cluster gene loci
MLL1 is required for NUP98-HOXA9-induced leukemogenesis in vitro and in vivo
MLL1-dependent gene expression signatures resemble human NUP98-NSD1 AML
Acknowledgments
We acknowledge Alan Chramiec, Matthew Witkin and Li Yang for assistance with the preparation of ChIP-seq libraries, and Ke Xu for assistance with the confocal microscopy. This work was supported by NIH grants PO1 CA66996, R01 CA140575, the leukemia and lymphoma society and Gabrielle’s Angel Research Foundation (to S.A.A.); by a DoD Bone Marrow Failure Postdoctoral Fellowship Award (W81XWH-12-1-0568) (to H.X.); and by the Cure Childhood Cancer Foundation (to D.G.V.). S.A.A. is a consultant for Epizyme Inc and Imago Biosciences.
Footnotes
ACCESSION NUMBERS
RNA-seq and ChIP-Seq data have been deposited at the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov.ezp-prod1.hul.harvard.edu/geo/) with accession codes GSE75997 (Expression changes after loss of Mll1 in murine NHA9 transformed cells), GSE76001 (NUP98-HOXA9, NUP98-HOXD13, MLL1, H3K4me3 and H4K16ac ChIP-seq data), GSE83221(MLL1 ChIP-seq data with Hsieh lab antibody MO435, NUP98-TOP1 and N-NUP98 ChIP-seq data).
Author Contributions
Conceptualization, H.X. and S.A.A.; Methodology, H.X., D.G.V., S.H.C., G.L.B., and S.A.A.; Software, A.S. and R.P.K; Validation, H.X., D.G.V., and M.E.E.; Formal Analysis, H.X., D.G.V., A.S., R.P.K., W.H., and S.A.A.; Investigation, H.X., D.G.V., M.E.E., W.H., C.W.C., S.H.C., G.L.B., and C.Y.P.; Resources, J.J.H. and P.E.; Writing – Original Draft, H.X.; Writing – Review and Editing, H.X., D.G.V., M.E.E., and S.A.A.; Visualization, H.X., D.G.V., A.S., and R.P.K.; Supervision, S.A.A.; Funding Acquisition, H.X., D.G.V and S.A.A.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Nabi AM, Yassin ER, Varghese N, Deshmukh H, Yaseen NR. In vitro transformation of primary human CD34+ cells by AML fusion oncogenes: early gene expression profiling reveals possible drug target in AML. PloS one. 2010;5:e12464. doi: 10.1371/journal.pone.0012464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artinger EL, Mishra BP, Zaffuto KM, Li BE, Chung EK, Moore AW, Chen Y, Cheng C, Ernst P. An MLL-dependent network sustains hematopoiesis. Proc Natl Acad Sci U S A. 2013;110:12000–12005. doi: 10.1073/pnas.1301278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–2307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl J, Thompson A, Ramirez-Solis R, Krosl J, Grier DG, Lawrence HJ, Sauvageau G. Analysis of HSC activity and compensatory Hox gene expression profile in Hoxb cluster mutant fetal liver cells. Blood. 2006;108:116–122. doi: 10.1182/blood-2005-06-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisio V, Pigazzi M, Manara E, Masetti R, Togni M, Astolfi A, Mecucci C, Zappavigna V, Salsi V, Merli P, Rizzari C, Fagioli F, Locatelli F, Basso G. NUP98 Fusion Proteins Are Recurrent Aberrancies in Childhood Acute Myeloid Leukemia: A Report from the AIEOP AML-2001-02 Study Group. 56th ASH annual meeting; San Francisco. December 6–9, 2014. [Google Scholar]
- Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, Purohit T, Malik B, Zhao T, Wang J, et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer cell. 2015;27:589–602. doi: 10.1016/j.ccell.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Jin J, Swanson SK, Cole MD, Choi SH, Florens L, Washburn MP, Conaway JW, Conaway RC. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J Biol Chem. 2010;285:4268–4272. doi: 10.1074/jbc.C109.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao FTE, Karatas H, Xu J, Li L, Lee S, Liu L, Chen Y, Ouillette P, Zhu J, Hess JL, Atadja P, Lei M, Qin ZS, Malek S, Wang S, Dou Y. Targeting MLL1 H3K4 methyltransferase activity in mixed-lineage leukemia. Mol Cell. 2014;53:247–261. doi: 10.1016/j.molcel.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010;140:372–383. doi: 10.1016/j.cell.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Chen CY, Hou HA, Lin LI, Tang JL, Yao M, Tsay W, Ko BS, Wu SJ, Huang SY, Hsu SC, Chen YC, Huang YN, Tseng MH, Huang CF, Tien HF. Acute myeloid leukemia bearing t(7;11)(p15;p15) is a distinct cytogenetic entity with poor outcome and a distinct mutation profile: comparative analysis of 493 adult patients. Leukemia. 2009;23:1303–1310. doi: 10.1038/leu.2009.25. [DOI] [PubMed] [Google Scholar]
- D’Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij JD, Hollink I, Arentsen-Peters ST, van Galen JF, Berna Beverloo H, Baruchel A, Trka J, Reinhardt D, Sonneveld E, Zimmermann M, Alonzo TA, Pieters R, Meshinchi S, van den Heuvel-Eibrink MM, Zwaan CM. NUP98/JARID1A is a novel recurrent abnormality in pediatric acute megakaryoblastic leukemia with a distinct HOX gene expression pattern. Leukemia. 2013;27:2280–2288. doi: 10.1038/leu.2013.87. [DOI] [PubMed] [Google Scholar]
- Deshpande AJ, Deshpande A, Sinha AU, Chen L, Chang J, Cihan A, Fazio M, Chen CW, Zhu N, Koche R, et al. AF10 regulates progressive H3K79 methylation and HOX gene expression in diverse AML subtypes. Cancer cell. 2014;26:896–908. doi: 10.1016/j.ccell.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;121:873–885. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Ernst P, Mabon M, Davidson AJ, Zon LI, Korsmeyer SJ. An Mll-dependent Hox program drives hematopoietic progenitor expansion. Curr Biol. 2004;14:2063–2069. doi: 10.1016/j.cub.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Fontoura BM, Blobel G, Matunis MJ. A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J Cell Biol. 1999;144:1097–1112. doi: 10.1083/jcb.144.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam G, Takeda A, Camarata T, Moore MA, Viale A, Yaseen NR. The oncogene Nup98-HOXA9 induces gene transcription in myeloid cells. J Biol Chem. 2004;279:866–875. doi: 10.1074/jbc.M307280200. [DOI] [PubMed] [Google Scholar]
- Gough SM, Lee F, Yang F, Walker RL, Zhu YJ, Pineda M, Onozawa M, Chung YJ, Bilke S, Wagner EK, et al. NUP98-PHF23 is a chromatin-modifying oncoprotein that causes a wide array of leukemias sensitive to inhibition of PHD histone reader function. Cancer Discov. 2014;4:564–577. doi: 10.1158/2159-8290.CD-13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough SM, Slape CI, Aplan PD. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood. 2011;118:6247–6257. doi: 10.1182/blood-2011-07-328880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Altan N, Lippincott-Schwartz J, Powers MA. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol Biol Cell. 2002;13:1282–1297. doi: 10.1091/mbc.01-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich RMAP, Humphries RK. NUP98-topoisomerase I acute myeloid leukemia-associated fusion gene has potent leukemogenic activities independent of an engineered catalytic site mutation. Blood. 2004;104:1127–36. doi: 10.1182/blood-2003-10-3550. [DOI] [PubMed] [Google Scholar]
- Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, van Galen JF, Beverloo HB, Sonneveld E, Kaspers GJ, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118:3645–3656. doi: 10.1182/blood-2011-04-346643. [DOI] [PubMed] [Google Scholar]
- Hsieh JJ, Cheng EH, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell. 2003;115:293–303. doi: 10.1016/s0092-8674(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Asakawa H, Ohtsuki C, Osakada H, Koujin T, Hiraoka Y, Haraguchi T. Monoclonal antibodies recognize gly-leu-phe-gly repeat of nucleoporin nup98 of tetrahymena, yeasts, and humans. Monoclonal antibodies in immunodiagnosis and immunotherapy. 2013;32:81–90. doi: 10.1089/mab.2012.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–507. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Jude CD, Climer L, Xu D, Artinger E, Fisher JK, Ernst P. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell stem cell. 2007;1:324–337. doi: 10.1016/j.stem.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, Xia X, Jesneck J, Bracken AP, Silverman LB, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer cell. 2008;14:355–368. doi: 10.1016/j.ccr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Thorsteinsdottir U, Mayotte N, Nakamura T, Sauvageau G. NUP98-HOXA9 expression in hemopoietic stem cells induces chronic and acute myeloid leukemias in mice. EMBO J. 2001;20:350–361. doi: 10.1093/emboj/20.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HWT, Cashen A, Piwnica-Worms DR, Kunkle L, Vij R, Pham CG, DiPersio J, Cheng EH, Hsieh JJ. Proteasome inhibitors evoke latent tumor suppression programs in pro-B MLL leukemias through MLL-AF4. Cancer Cell. 2014;25:530–542. doi: 10.1016/j.ccr.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- Milne TA, Dou Y, Martin ME, Brock HW, Roeder RG, Hess JL. MLL associates specifically with a subset of transcriptionally active target genes. Proc Natl Acad Sci U S A. 2005;102:14765–14770. doi: 10.1073/pnas.0503630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne TA, Kim J, Wang GG, Stadler SC, Basrur V, Whitcomb SJ, Wang Z, Ruthenburg AJ, Elenitoba-Johnson KS, Roeder RG, Allis CD. Multiple interactions recruit MLL1 and MLL1 fusion proteins to the HOXA9 locus in leukemogenesis. Mol Cell. 2010;38:853–863. doi: 10.1016/j.molcel.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BP, Zaffuto KM, Artinger EL, Org T, Mikkola HK, Cheng C, Djabali M, Ernst P. The histone methyltransferase activity of MLL1 is dispensable for hematopoiesis and leukemogenesis. Cell Rep. 2014;7:1239–1247. doi: 10.1016/j.celrep.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, MS, Yamada K, Sangel P, Hirata S, Maehara K, Kawakami K, Tachibana T, Ohkawa Y, Kimura H, Yoneda Y. Chromatin-prebound Crm1 recruits Nup98-HoxA9 fusion to induce aberrant expression of Hox cluster genes. Elife. 2016;5 doi: 10.7554/eLife.09540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist L, Pineault N, Wasslavik C, Humphries RK. Candidate genes for expansion and transformation of hematopoietic stem cells by NUP98-HOX fusion genes. PloS one. 2007;2:e768. doi: 10.1371/journal.pone.0000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Garcia P, Jeong J, Capelson M. Nucleoporin Nup98 associates with Trx/MLL and NSL histone-modifying complexes and regulates Hox gene expression. Cell Rep. 2014;9:433–442. doi: 10.1016/j.celrep.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Pineault N, Buske C, Feuring-Buske M, Abramovich C, Rosten P, Hogge DE, Aplan PD, Humphries RK. Induction of acute myeloid leukemia in mice by the human leukemia-specific fusion gene NUP98-HOXD13 in concert with Meis1. Blood. 2003;101:4529–4538. doi: 10.1182/blood-2002-08-2484. [DOI] [PubMed] [Google Scholar]
- Prestel M, Feller C, Straub T, Mitlohner H, Becker PB. The activation potential of MOF is constrained for dosage compensation. Mol Cell. 2010;38:815–826. doi: 10.1016/j.molcel.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Radu A, Blobel G, Moore MS. Identification of a protein complex that is required for nuclear protein import and mediates docking of importsubstrate to distinct nucleoporins. Proc Natl Acad Sci U S A. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja SJ, Charapitsa I, Conrad T, Vaquerizas JM, Gebhardt P, Holz H, Kadlec J, Fraterman S, Luscombe NM, Akhtar A. The nonspecific lethal complex is a transcriptional regulator in Drosophila. Mol Cell. 2010;38:827–841. doi: 10.1016/j.molcel.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, Humphries RK. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Saw JCD, Hussey DJ, Dobrovic A, Aplan PD, Slape CI. The fusion partner specifies the oncogenic potential of NUP98 fusion proteins. Leuk Res. 2013;37:1668–73. doi: 10.1016/j.leukres.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slape C, Hartung H, Lin YW, Bies J, Wolff L, Aplan PD. Retroviral insertional mutagenesis identifies genes that collaborate with NUP98-HOXD13 during leukemic transformation. Cancer Res. 2007;67:5148–5155. doi: 10.1158/0008-5472.CAN-07-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Goolsby C, Yaseen NR. NUP98-HOXA9 induces long-term proliferation and blocks differentiation of primary human CD34+ hematopoietic cells. Cancer Res. 2006;66:6628–6637. doi: 10.1158/0008-5472.CAN-06-0458. [DOI] [PubMed] [Google Scholar]
- Thiel AT, Blessington P, Zou T, Feather D, Wu X, Yan J, Zhang H, Liu Z, Ernst P, Koretzky GA, Hua X. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer cell. 2010;17:148–159. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, Luo JL, Patel DJ, Allis CD. Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature. 2009;459:847–851. doi: 10.1038/nature08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Powers MA. In vivo analysis of human nucleoporin repeat domain interactions. Mol Biol Cell. 2013;24:1222–31. doi: 10.1091/mbc.E12-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.