Abstract
The design and examination of 4,1,2-benzoxathiazin-3-one 1,1-dioxides as candidate serine hydrolase inhibitors are disclosed, and represent the synthesis and study of a previously unexplored heterocycle. This new class of activated cyclic carbamates provided selective irreversible inhibition of a small subset of serine hydrolases without release of a leaving group, do not covalently modify active site catalytic cysteine and lysine residues of other enzyme classes, and was found to be amenable to predictable structural modifications that modulate intrinsic reactivity or active site recognition. Even more remarkable and within the small pilot series of candidate inhibitors examined in an initial study, an exquisitely selective inhibitor for a poorly characterized serine hydrolase (PNPLA4, patatin-like phospholipase domain-containing protein 4) involved in adipocyte triglyceride homeostasis was discovered.
Graphical Abstract

Introduction
Serine hydrolases play key roles in human physiology and disease and are an important class of therapeutic targets. Serine hydrolases represent more than 1% of predicted proteins in humans, making it one of the largest and most diverse class of mammalian enzyme families.1 Not only do they make fundamental contributions to physiological and pathophysiological processes,2 but a large number of the serine hydrolases remain uncharacterized or unannotated, lacking a known role, endogenous substrate, or specific inhibitor. Selective chemical inhibitors for members of the serine hydrolase family have uniquely contributed to an understanding of the biological function of individual enzyme members. They have also led to new therapeutics, including new treatments for obesity, diabetes, microbial infections and Alzheimer’s disease.1 The active site catalytic triads that contain a serine nucleophile have inspired the design of many classes of small molecule inhibitors.3 The inhibitor classes include those that contain an electrophilic carbonyl such as trifluoromethyl ketones,4–6 α-ketoamides and esters,5,6 lactones,7,8 lactams,9 α-ketoheterocycles,10–12 carbamates,13,14 ureas,15–17 and other activated carbonyl-containing compounds,18 which act through covalent modification of the serine nucleophile.
The use of activity-based protein profiling (ABPP)19–21 paired with such selective inhibitor classes has allowed the rapid analysis of target serine hydrolases and monitoring of enzyme activity in complex biological systems. The use of ABPP probes developed for specific enzyme classes, including fluorophosphonate-rhodamine (FP-Rh)21,22 for selective serine hydrolase labeling, permits the rapid proteome-wide identification of inhibitor targets, assessment and optimization of inhibitor selectivity, and functional annotation of uncharacterized enzymes.23,24 This may be accomplished without recombinant enzyme expression, protein purification, knowledge of the endogenous substrate or the development of specific substrate assays as required by traditional methods.24–27
In efforts to interrogate serine hydrolases not yet successfully targeted by existing inhibitor classes, we have continued to explore new irreversible covalent inhibitor designs that might display a unique reaction selectivity among not only classes of enzymes, but also among a subset of the serine hydrolases.28 A large body of work on irreversible inhibitors of serine, cysteine and threonine proteases is available from which inspiration may be drawn.3 Tethered reactive moieties that acylate, phosphonylate, or sulfonylate active site nucleophiles were of particular interest, with the saccharin family of serine protease inhibitors serving as the inspiration for the new inhibitor class detailed herein.29 The saccharin family of 1,2-benzisothiazol-3-one 1,1-dioxides has been shown to inhibit serine proteases such as human leukocyte elastase30–32 and human mast cell tryptase33 through acylation of the nucleophilic serine active site residue (Figure 1). The mechanism of inhibition involves serine nucleophilic attack on the activated amide, collapse of the tetrahedral intermediate, and formation of an acyl enzyme intermediate in the form of an ester with release of the sulfonamide as the leaving group.30 The reactivity of such saccharin derivatives, their intrinsic stability and inhibitory potency can be modulated by core substitution. Activation of the leaving sulfonamide through N-acylation or N-arylation (R) or electronic modulation of the intrinsic carbonyl reactivity by C4/C6 aryl substitution have been detailed. However, the acyl bond formed with the active site residue is a serine ester that is susceptible to rapid deacylation and enzyme reactivation, limiting the utility of this inhibitor class for biological studies where sustained enzyme inhibition is required.
Figure 1.
The saccharin family of protease inhibitors. Proposed mechanism of inhibition and synthetic design for new inhibitor class.
The redesigned scaffold (1) detailed herein represents a modification of the saccharin ring system with insertion of a heteroatom (O, NH) adjacent to the carbonyl (Figure 1). Nucleophilic attack of an active site serine on the inhibitor carbonyl followed by collapse of the tetrahedral intermediate 2 provides a covalently-bound inhibitor 3 in the form of a more stable and potentially irreversible serine carbamate or carbonate, depending on the choice of heteroatom X (Figure 1). Remarkable in this day and age and while the urea version of the redesigned scaffold (4, X = NH) represents a well-established heterocycle class,34–38 the carbamate version (5, X = O) constitutes a class with only a single report of its inadvertent synthesis.39 The work detailed herein revealed that the former N-alkylated ureas may be insufficiently reactive to irreversibly inhibit serine hydrolases or other enzyme classes. However, the latter benzoxathiazin-3-one 1,1-dioxides (5, X = O) proved to be potent and remarkably selective irreversible serine hydrolase inhibitors. Moreover, these compounds constitute a class of serine hydrolase inhibitors that do not release a leaving group, are capable of predictable electronic modulation of the intrinsic reactivity and selectivity by C7 aryl substitution (R1), and can be tailored to bind individual enzyme targets through N-substitution (R2). Their use in the discovery of the first selective inhibitor of patatin-like phospholipase domain containing protein 4 (PNPLA4) is disclosed.
Results and Discussion
At the onset of our studies, we elected to probe the aryl substituent electronic effects (R1 = OMe, H, CN), utilizing the parent core heterocycles (R2 = H) and those that bear a small set of N-alkyl substituents that might impart representative selective enzyme active site targeting (R2 = CH2Ph, (CH2)5Ph vs H). In particular, the inclusion of the 5-phenylpentyl side chain might impart binding and inhibition of fatty acid amide hydrolase (FAAH) for which we were especially well equipped to examine as part of a pilot examination.10,25 Proteome-wide evaluation by gel-based ABPP was used to provide an initial assessment of the impact of expected reactivity trends (X = O > NH, R1 = CN > H > OMe), to define the utility and promiscuity of the parent core heterocycles (R2 = H) in fragment-based screening, and to probe their utility in the identification of more selective inhibitors for existing (e.g. FAAH) or uncharacterized serine hydrolase targets with the N-substituted heterocycles (R2 = CH2Ph, (CH2)5Ph).
Their synthesis was anticipated to rely on a one-pot addition and cyclization of anilines (6) and phenols (7) with chlorosulfonyl isocyanate (8)40 to produce the unsubstituted benzothiadiazin-3-one 1,1-dioxides (4, X = NH) or benzoxathiazin-3-one 1,1-dioxides (5, X = O) poised for N-alkylation (Figure 1). Benzothiadiazin-3-one 1,1-dioxides (4, X = NH) were targeted first since a two-step synthesis was known for similar compounds, allowing access to the desired candidate inhibitor set.34 Interestingly, a number of compounds containing the substituted benzothiadiazin-3-one 1,1-dioxide core have been shown to exhibit effective biological activity,35,36,37,38,41 although none are reported to inhibit a hydrolytic enzyme target (Figure 2).
Figure 2.
Benzothiadiazin-3-one 1,1-dioxides that display biological activity.
The use of reported conditions for the synthesis of the benzothiadiazine-3-one 1,1-dioxides (known for R1 = OMe, Cl, Br),34,40,42,43 enlisting chlorosulfonyl isocyanate (CSI) and AlCl3 with the electron-rich anilines, provided 9 and 10 (Scheme 1), but failed to provide 12 with 4-aminobenzonitrile. Instead, after successful aniline addition to the isocyanate, attempts to promote electrophilic aromatic substitution led only to isolation of the 4-cyanophenylurea (13, R1 = CN) despite a screen of Lewis acids, reaction temperatures, and reaction solvents. However, the successful use of the method with p-bromoaniline provided 11 and permitted a subsequent cyanation reaction44 for accessing 12 (Scheme 1).
Scheme 1.
Synthesis of benzothiadiazin-3-one 1,1-dioxide cores
The four urea-containing core scaffolds were subjected to alkylation to incorporate the two R2 substituents (Figure 3). Initial studies with 9 (R1 = H), employing NaH and benzyl bromide, yielded a separable mixture of two products (14A and 14B). They were tentatively assigned to be the products of the desired N-benzylation (14A, 52%) and O-benzylation (14B, 22%) on the basis of the 1H/13C NMR chemical shifts and their structures were unambiguously established by single crystal X-ray structure determinations (Figure 3).45 Extension of this method with 10–12 proceeded smoothly to give 15A–17A in good yields (64–47%). Without optimization, the synthesis was also used to access the second R2 series, using NaH and 1-bromo-5-phenylpentane to provide 18A–21A (Figure 3).
Figure 3.
Alkylation of benzothiadiazin-3-one 1,1-dioxide cores and X-ray crystal structures of 14A and 14B.
Access to the targeted candidate inhibitors permitted their examination for inhibition of purified FAAH18 (Figure 4). The core scaffolds (R2 = H, 9A–12A) showed no activity (Ki >100 μM) and only a limited set of the benzyl substituted benzothiadiazin-3-one 1,1-dioxides (14A–17A) showed modest activity (16A and 17A). In the phenylpentyl series (18A–21A), all compounds exhibited modest activity. The most potent of the inhibitors (20A) was examined in detail. Compound 20A was found to exhibit simple competitive inhibition kinetics and it did not show a time-dependent increase in the inhibition potency, both of which are indicative of simple reversible competitive enzyme inhibition. Additionally, the full set of candidate inhibitors (9A–21A) was incubated with mouse brain membrane and soluble proteome and labeled with FP-rhodamine or iodoacetamide (IA)-rhodamine to examine serine and cysteine hydrolase inhibition (Supporting Information Figures S1 and S2). The reactivity with active site lysine-containing enzymes was also examined through use of a Kinativ™ kit. Ultimately, none of the gel-based ABPP studies showed evidence of significant target enzyme inhibition, and only weak reversible inhibition was detected at very high concentrations for a few compounds. The compounds also displayed a lack of hydrolytic reactivity of the potentially activated urea, being stable in methanol, DMSO and organic solvents as well as in weakly acidic and basic aqueous media (>24 h). Thus, initial studies with the benzothiadiazin-3-one 1,1-dioxides, unlike those with the carbamate variant that followed, suggest they lack the structure and reactivity to generally act as irreversible serine hydrolase inhibitors.
Figure 4.
FAAH inhibition by benzothiadiazin-3-one 1,1-dioxides.
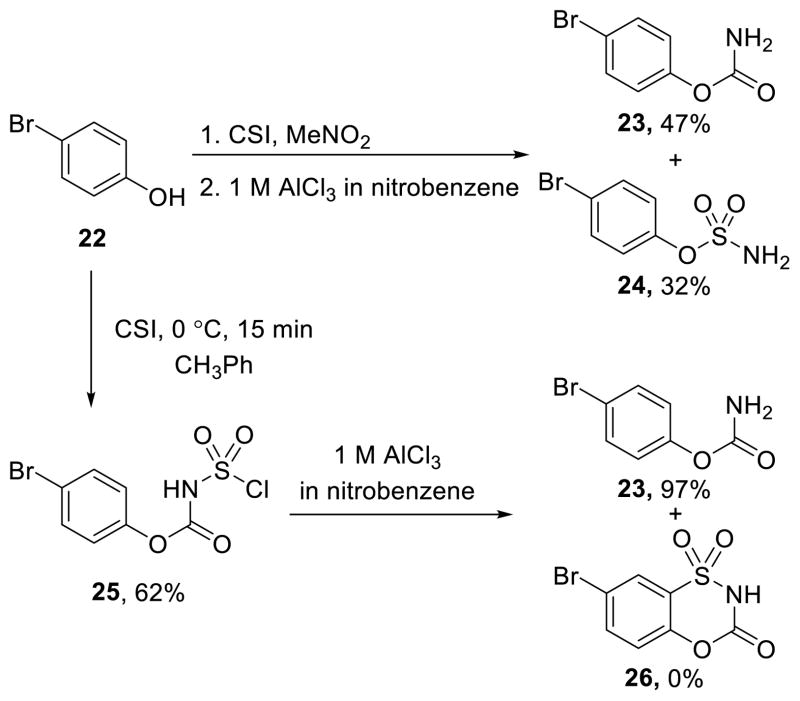
Remarkably, there is only a single report of the preparation of a 4,1,2-benzoxathiazin-3-one 1,1-dioxide (5, X = O) with CSI and it was isolated as a byproduct in the reaction with p-cresol.39 This, along with the single report of one N-substituted derivative (R2 ≠ H),46 made this unexplored carbamate a compelling class of compounds to prepare, characterize, and evaluate. p-Bromophenol was chosen as an initial substrate for examination of its reactivity towards CSI as a route to the expectedly more reactive carbamate versus urea series. In the reported synthesis, the cyclized product of p-cresol was isolated as a byproduct during sulfonylisocyanate formation.39 Attempts to extend this synthesis to 22 were unsuccessful (Scheme 2), even upon isolation and resubjection of intermediate 25 to the Lewis-acid catalyzed cyclization conditions.
Scheme 2.
Direct reaction of phenol 22 with CSI
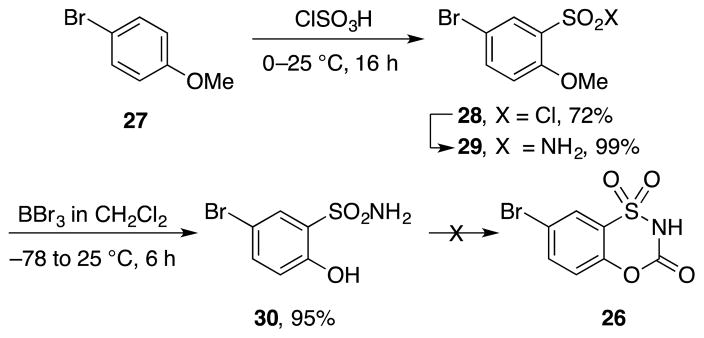
An alternative approach to 26 via the 2-hydroxybenzenesulfonamide 30 was pursued in which aryl chlorosulfonylation ortho to a protected phenol preceded cyclic carbamate formation (Scheme 3). Although successful in providing 29 and following methyl ether deprotection, attempts to form the final cyclized product from 30 with triphosgene, phosgene or 4-nitrobenzoyl chloride were unsuccessful. The highly polar, acidic and reactive nature of the product that made its isolation challenging and led to examination of a variation on this route, introducing the amine substituent prior to cyclization. This provides non acidic intermediates with increased organic solubility, avoiding the challenges of isolation of 26.
Scheme 3.
Attempted synthesis of benzoxathiazin-3-one 1,1-dioxide core
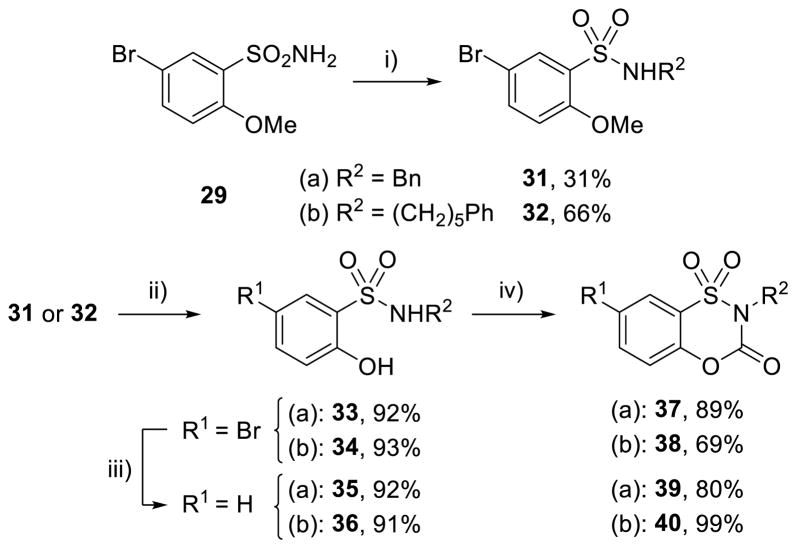
N-Alkylation of 29 (NaH, BnBr, DMF), with a methyl ether serving as a phenol protecting group to prevent O-alkylation, proceeded to give the 31 in 31% yield (Scheme 4), with recovered starting material and dialkylated product accounting for the remaining material. Methyl ether deprotection with boron tribromide provided 33 in 92% yield (Scheme 4). Formation of benzoxathiazin-3-one 1,1-dioxide was now successful when conducted with phosgene and NaH to yield 37 in 89% yield (Scheme 4). This provided a route to N-alkylated benzoxathiazin-3-one 1,1-dioxides and was applied to the additional targeted N-substitution product (R2 = (CH2)5Ph), which proceeded even more smoothly to form 38 (42% over three steps, Scheme 4). Hydrogenolysis of 33 and 34 (R1 = Br) proceeded smoothly to yield the C7-unsubstituted (R1 = H) precursors 35 and 36 in 92% and 91% yield, respectively, and subsequent carbamate formation yielded 39 and 40 (Scheme 4).
Scheme 4.
Stepwise synthesis of substituted benzoxathiazin-3-one 1,1-dioxides. Conditions: i) 1 equiv NaH, 2 equiv R2–Br, DMF; ii) BBr3, CH2Cl2, −78 to 25 °C; iii) Pd/C, H2, MeOH; iv) 5 equiv NaH, 2 equiv phosgene, CH2Cl2, 25 °C
Completion of the series with preparation of the C7-cyano and C7-methoxy derivatives is summarized in Scheme 5. Chlorosulfonylation of 4-methoxybenzamide (41) followed by subsequent dehydration of 42 and chlorosulfonylation of 1,4-dimethoxybenzene (44) were successfully utilized to access the sulfonyl chlorides 43 and 45 (Scheme 5). To further streamline the approach, a direct addition of the desired substituted amine to the sulfonyl chloride was used. Reaction of 43 and 45 with benzylamine and 5-phenylpentylamine produced the substituted sulfonamides 46–49 in high yield. Subjection of the methyl ethers 48 and 49 to the boron tribromide deprotection conditions selectively provided 52 and 53, whereas phenol deprotection in the p-cyano series required stronger reaction conditions (LiCl, 140 °C, DMF) to form hydroxysulfonamides 50 and 51. Preparation of the four additional benzoxathiazin-3-one 1,1-dioxides (54–57) proceeded upon treatment of 50–53 with phosgene and NaH. The structure of the candidate inhibitors was confirmed with a single crystal X-ray structure determination45 of 54 (Scheme 5).
Scheme 5.
Streamlined synthesis of candidate inhibitors. X-ray crystal structure of candidate inhibitor 54. Conditions: i) 2 equiv R2–NH2, 0.15 equiv Et3N, CH2Cl2; ii) 4 equiv LiCl, DMF, 140 °C; iii) BBr3, CH2Cl2, −78 to 25 °C; iv) 5 equiv NaH, 2 equiv phosgene, CH2Cl2
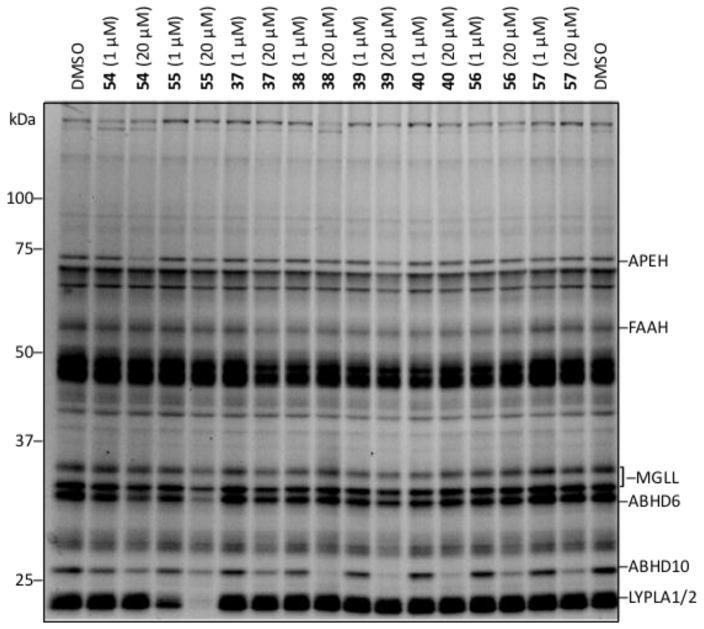
Access to the eight benzoxathiazin-3-one 1,1-dioxides allowed the examination of this carbamate series by gel-based ABPP. The use of mouse brain proteome (membrane fractions of proteome) and FP-rhodamine labeling showed clear inhibition of the serine hydrolase ABHD10 by nearly all compounds in the series, as well as low micromolar inhibitor activity against LYPLA1/2 by 55 (Figure 5). Interestingly, FAAH was not effectively inhibited by members of this class even with the candidate inhibitors that bear the 5-phenylpentyl side chain. Although not extensively profiled, IA-rhodamine and Alexa Fluor® labeling did not detect evidence of cysteine protease/hydrolase or lysine-containing enzyme inhibition (Supporting Information Figure S3 and S4).
Figure 5.
Screen of benzoxathiazin-3-one 1,1-dioxides by competitive gel-based ABPP. Mouse brain membrane proteome treated with compounds (at 1 μM and 20 μM, 30 min incubation) followed by FP-rhodamine (1 μM, 30 min). Fluorescent images shown in gray.
The results from this initial gel-based screen displayed interesting trends. First, the new inhibitor class displayed surprisingly selective inhibition among the serine hydrolases, displaying full inhibition of only three enzymes and partial inhibition of three more (Figure 5). Moreover, even within this small set of inhibitors, distinctive selectivity trends were observed toward these enzymes. Low micromolar inhibition against ABHD10 was observed for nearly all the inhibitors (R2 = (CH2)5Ph > CH2Ph) regardless of core substituent (R1 = CN, Br, H, OMe). Only the more reactive inhibitor 55 (R1 = CN) bearing only the phenylpentyl R2 substituent in the set of eight displayed LYPLA1/2 inhibition. Similarly, only inhibitors that contain the benzyl substituent (R2 = CH2Ph) inhibited APEH with core substituent trends reflecting the expected intrinsic reactivity of the inhibitors (R1 = CN > H > OMe). Finally, the inhibition was established to be irreversible against ABHD10 and LYPLA1/2 in studies analogous to those detailed latter herein (see Figure 12). As a result of these initial observations, a detailed examination of both the chemical and biochemical reactivity of these compounds was conducted to more comprehensively establish their behavior and the potential electronic effects of the varied substituents.
Figure 12.
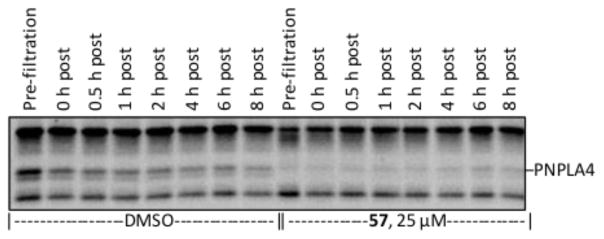
Irreversibility of the inhibition of PNPLA4 by 57. Incubation of HEK cell membrane proteome with 57 (25 μM, 30 min) and column filtration provided the covalent complex which was then subjected to FP-rhodamine labeling (1 μM, 30 min) at various time points post-filtration. Full gel shown in Supporting Information Figure S5.
The stability and reactivity of the benzoxathiazin-3-one 1,1-dioxides were briefly and initially surveyed with 37 (R1 = Br, R2 = Bn) and 54 (R1 = CN, R2 = Bn) and both were found to be stable in DPBS, H2O, THF and ethanol at 25 °C for over 24 h (Figure 6). Hydrolysis to provide 33 or 50 was observed in DMEM buffer (1–6 h at 25 or 37 °C, Figure 6) whereas reaction to produce unknown products was observed in Tris-HCl buffer (in less than 1 h at 25 °C). Finally, samples stored in DMSO showed slow conversion to the hydrolysis products 33 and 50 at both 25 °C (50% conversion in approximately 7 or 14 days, respectively) and at −78 °C (50% conversion in approximately 14 or 21 days, respectively).
Figure 6.
Stability and reactivity of 37 and 54.
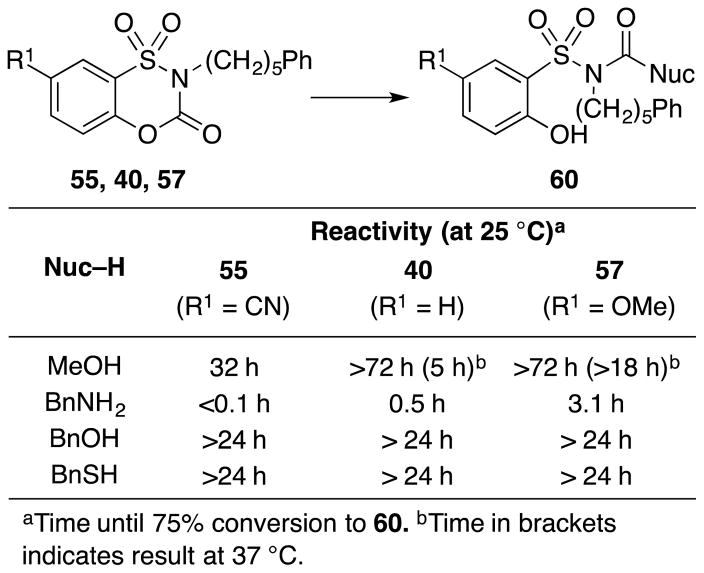
Their reactivity toward methanol as solvent provided the clearest indication of not only the relative reactivity (54 > 37), but also the reaction course. The reaction product derived from 37 and 54 in methanol was shown to be 58 or 59, and indicates that the phenol acts as the leaving group (vs –SO2NHR2). In this respect, the behavior of the benzoxathiazin-3-one 1,1-dioxides differs from the saccharin-based inhibitors where only the sulfonamide can serve as the leaving group. Subjection of the full set of phenylpentyl-substituted compounds (R1 = OMe, H and CN) to the reaction with methanol allowed a direct comparison of the magnitude of the electronic effect of the 7-substitution on the carbamate carbonyl reactivity (Figure 7). The compounds displayed predictable relative reaction rates (R1 = CN > H > OMe), where compound 55 with the electronic-withdrawing nitrile substituent exhibited the greatest reactivity observed even at room temperature. Compound 57 with the electron-donating methoxy substituent exhibited the lowest reactivity, requiring elevated temperatures to observe methanol addition, and the differences were of a magnitude indicative of a pronounced C7 substituent electronic effect (Figure 7).
Figure 7.
Reactivity of inhibitors.
The reactivity toward nucleophiles was also examined through subjection of the same series of compounds to reactions with benzylamine, benzyl alcohol and benzyl mercaptan (1 equiv, Figure 7). The reactions were performed in deuterated chloroform (0.1 M) with dibromomethane as an internal standard and analyzed by 1H NMR initially to determine the reaction time required for 75% conversion to products 60 (Figure 7). Neither benzyl alcohol nor benzyl mercaptan reacted with the compounds under these conditions. However, the reactivity profile with benzylamine exhibited a clear trend (Figure 7). Very rapid reaction was observed with the 7-cyano derivative 55 (t1/2 = 1.3 min, k = 5.3 M−1s−1) and a slower reaction was seen for the C7 unsubstituted compound 40 (t1/2 = 13 min, k = 1.8 M−1s−1). The 7-methoxy derivative 57 exhibited a much slower rate of reaction (t1/2 = 66 min, k = 0.24 M−1s−1) with full conversion observed only after 12 h. A Hammett plot (log k vs σp) of the results provided a slope (ρ value) of 1.2, indicating that the electronic effect of the substituent on this reactivity exceeds even that observed on benzoic acid acidity.
Because of their cyclic structure, the designed inhibitors are unique among previously explored carbamates in that there are not only two potential leaving groups (the sulfonamide or phenol), but no group is released from the enzyme upon initial covalent acylation of an active site serine due to the tethered nature of the carbonyl. Moreover, the compounds are also potentially capable of a subsequent cross-linking modification at the active site, where a second nucleophilic residue could attack the carbonyl of the covalently-bound inhibitor, leaving a carbonyl bound to two residues in the active site with release of the remainder of the molecule. To probe these two possibilities, compound 57 was incubated with purified ABHD10 (five-fold excess of inhibitor) for 30 min at 25 °C and examined by ESI-MS (Figure 8). Accordingly, the measured mass of ABHD10, established 1–4 h after the initial 30 min incubation, increased by 376 Da indicative of only initial acylation of the enzyme with no evidence of a subsequent cross-linking reaction (no increase of 26 Da). Thus, 57 serves as an irreversible inhibitor of ABHD10 that acts by mono-acylation of an active site serine without release of a leaving group under the conditions of our examinations. Although prolonged incubation conceivably could lead to cross-linking, these studies suggest it is unlikely to be observed with ABHD10. However, we would not want to rule out such behavior against other serine hydrolases with members of this inhibitor class.
Figure 8.
ESI-TOF-MS analysis of purified ABHD10 incubated with 57 (red), starting purified ABHD10 (black), and summary of results (top).
Additional proteome-wide screens of the compounds allowed the further comparison of their reactivity and serine hydrolase inhibition in vitro, by examining ABHD10 and LYPLA1/2 activity in cell lysate proteome samples from mouse neuroblastomas (N2A cells, Figure 9a) and human embryonic kidney cells (HEK 293T cells, Figure 9b). Treatment with the three related compounds 55, 40 and 57 (R2 = (CH2)5Ph), at 1, 50 and 100 μM, showed a clear trend of strongest inhibition observed with R1 = CN, and progressively less inhibition with R1 = H and R1 = OMe (Figure 9).
Figure 9.
In vitro analysis of compounds 55, 40, and 57 (at 1, 50, 100 μM, R2 = (CH2)5Ph) by gel-based ABPP with (a) processed N2A cells and (b) processed HEK cells showing electronic effect of aromatic core C7 substituents (R1 = CN, H, OMe) on inhibition of ABHD10 and LYPLA1/2.
In order to more comprehensively define the relative selectivity of the inhibitors, the set comprised of 55, 40 and 57, bearing the same R2 group ((CH2)5Ph) and the varied R1 group (CN, H, OMe, respectively), were analyzed by ABPP-SILAC (Stable Isotopic Labeling with Amino Acids in Cell culture) (Figure 10).14,47 Whole cell proteome samples from isotopically-labeled HEK cell lysates were incubated with the compounds (at 25 μM) or DMSO control, then the activity-based probe FP-biotin. The FP-biotin-labeled proteins were enriched on streptavidin beads and then subjected to on-bead trypsin digestion. The resulting tryptic peptide mixtures were analyzed by multidimensional liquid chromatography–tandem mass spectrometry (LC/LC-MS/MS). The raw data was searched with the ProLuCID algorithm and SILAC ratios were quantified with CIMAGE48 generating the data graphically shown in Figure 10. The greatest spectrum of inhibition was seen with the 7-cyano substituted inhibitor 55, with ten enzymes inhibited at greater than 50% (Figure 10a), while only three enzymes were inhibited at this level by 40 (Figure 10b) and selective inhibition of only a single enzyme (PNPLA4) was observed for 57 (Figure 10c). These remarkable results beautifully display the C7 substituent electronic effect on the reactivity of the carbamate carbonyl of the benzoxathiazin-3-one 1,1-dioxides and its impact on the promiscuity of the inhibitor activity (Figure 10d). Additionally, the selectivity of the inhibition of LYPLA1/2 by 55, observed in the gel-based ABPP assay, was confirmed by the MudPIT analysis, as no inhibition of these two enzymes is observed for 40 and 57 (Figure 10). Like most but not all of the identified enzyme targets, ABHD10 inhibition followed the trends (CN > H > OMe) first observed by gel-based ABPP (Figure 10d). Most impressively, the analysis revealed the exquisite selectivity of 57 for a single human enzyme (not present in mouse): PNPLA4. Interestingly, both 57 and 40 inhibit PNPLA4 preferentially and completely, whereas the more reactive inhibitor 55 does so incompletely and with a greater preference for eight other enzymes. Clearly the more reactive inhibitor 55 identified a larger subset of serine hydrolases that can be targeted by the new inhibitor class, which most prominently also includes PNPLA8, AIG1, ABHD6, LIPE, and ABHD11, but the less reactive members 40 and 57 uniquely identified an enzyme (PNPLA4) that would have been overlooked by screening with 55 alone.
Figure 10.
ABPP-SILAC analysis of compounds (55 (a), 40 (b), 57 (c)) using HEK cell lysates showing effect of substituents on inhibitor potency and selectivity and (d) heat map showing trends for the three inhibitors and the selectivity for PNPLA4 inhibition.
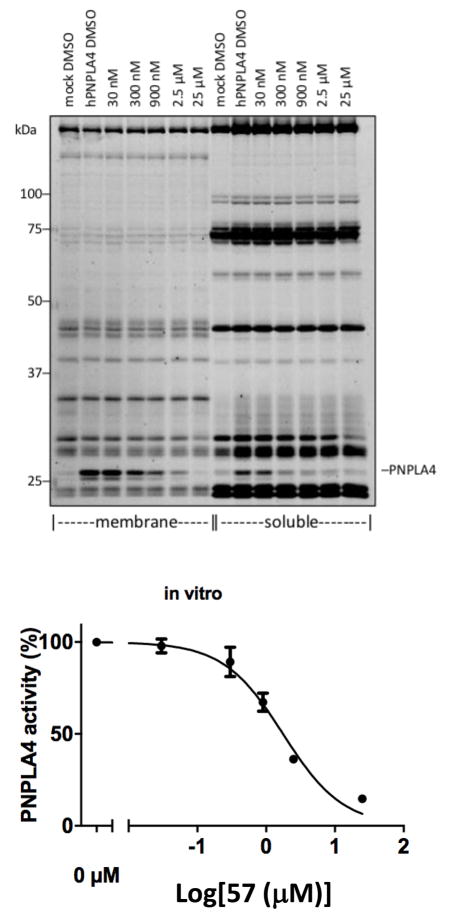
PNPLA4 (patatin-like phospholipase domain-containing protein 4), also known as GS2,49 is present in humans, but is absent in mouse.50 It constitutes a poorly characterized serine hydrolase that is known to hydrolyze triglycerides and retinol esters and is thought to be involved in adipocyte homeostasis. Thus, the discovery of a selective inhibitor of PNPLA4 constitutes a critical advance for characterizing the physiological role of PNPLA4. The use of overexpressed PNPLA4 in HEK cells allowed selective PNPLA4 inhibition by 57 to be observed in gel-based ABPP assays with whole cell lysates of the soluble and membrane-bound proteome fractions, with a calculated IC50 of 1.8 μM determined from the separated membrane proteome fraction (Figure 11).
Figure 11.
Selective PNPLA4 inhibition of cell lysates of transfected HEK cells by 57.
Subsequent studies were conducted that demonstrated the irreversibility of serine hydrolase inhibition through a gel-based ABPP study. After 30 min of incubation of compound 57 with the same HEK cell membrane proteome as Figure 11, filtration through a desalting column removed excess compound and allowed isolation of the proteome containing the enzyme-inhibitor complex. Aliquots of the complex in the full proteome were subjected to FP-rhodamine labeling at various time points to show the persistence of inhibition of PNPLA4, indicating both that binding is covalent, since the complex survives column filtration, and irreversible over the time scale since little enzyme reactivation was observed (<10% at 8 h post-filtration, Figure 12).
In preliminary studies, subjection of these same HEK cells to 57 in FBS-free media provided in-cell PNPLA4 inhibition, albeit at a 10-fold higher concentration (calculated IC50 = 11.7 μM), whereas treatment (50 μM 57) in the presence of fetal bovine serum (FBS) failed to display activity. At present, we do not know whether this loss of in-cell activity in the presence of FBS is due to serum protein binding or serum instability, although we suspect it is not due to serum instability (see below). These results are remarkable for a pilot study of a new serine hydrolase inhibitor chemotype and further optimization of 57 can be expected to provide a more potent, cellularly active inhibitor of PNPLA4 capable of use in defining its physiological role. Notably, 57 is among the most stable benzoxathiazin-3-one 1,1-dioxides examined, bearing an aryl electron-donating substituent that moderates its reactivity. Compound 57 is stable in DMSO at room temperature for at least 2 weeks, solid samples could be stored on the bench open to the air for greater than 6 months, and it was found to be stable to both DPBS (25 °C) and DMEM (25 and 37 °C) buffers over the time frames used in our assays (1–4 h, longer times not examined). Finally and although IA-rhodamine and Alexa Fluor® labeling did not detect evidence of competitive cysteine protease/hydrolase or lysine-containing enzyme inhibition by 57, it and members of this class have not been examined for activity against or reactivity toward other cellular targets.
Conclusions
A new class of serine hydrolase inhibitors was discovered, bearing a previously unexplored chemotype, based on a ring-expanded saccharin core obtained by introduction of a ring heteroatom that converted the saccharin amide to a carbamate. Although the analogous urea core displayed insufficient reactivity for use as candidate irreversible inhibitors of the serine hydrolases, the benzoxathiazin-3-one 1,1-dioxides, containing the carbamate core and a more reactive carbonyl, were found to be effective and selective irreversible serine hydrolase inhibitors. The benzoxathiazin-3-one 1,1-dioxides displayed tunable reactivity capable of C7-substituent electronic modulation of the inherent reactivity and proved amenable for active site targeting by variation of the N-substituent. Additionally and unique among the more traditional carbamate and urea based serine hydrolase inhibitors, they do not release a leaving group upon active site serine acylation.3 This latter feature avoids unknown or unanticipated pharmacology or toxicity derived from a released leaving group. In this regard, the inhibitors more closely parallel irreversible cysteine protease inhibition derived from halide displacement, conjugate addition, addition to epoxides or aziridines, or nucleophilic cleavage of weak bonds.3 Even more remarkable and within the limited series of candidate inhibitors examined, an exquisitely selective inhibitor was discovered for an uncharacterized hydrolase, PNPLA4. This enzyme bears a Ser-Asp catalytic dyad and is likely involved in adipocyte triglyceride homeostasis.51–53 Also known as protein GS2,49 it is present in a variety of human tissues, but is absent in mouse.50 PNPLA4 is known to catalyze the hydrolysis of triglycerides and participate in retinol ester hydrolytic metabolism.54,55 Further exploration of this unique PNPLA4-selective inhibition by 57 should allow the optimization and development of an even more potent and selective inhibitor suitable for detailed examination of this underexplored serine hydrolase. Just as significantly, the benzoxathiazin-3-one 1,1-dioxides represent a new and unexplored class of candidate enzyme inhibitors capable of selective irreversible inhibition of a subclass of serine hydrolases and amenable to structural modifications that substantially modulate intrinsic reactivity and/or active site recognition.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (DA015648, DLB and DA033760, BFC) and an NSF Graduate Research Fellowship (NSF/DGE-1346837, AFK) and the Skaggs-Oxford Scholarship (AFK). We thank Dr. M. Gembicky and A. Rheingold of the Crystallography Facility at the University of California, San Diego for the X-ray structure determinations of 14A, 14B, and 54.
Footnotes
The authors declare no competing financial interest.
The supporting information is available free of charge on the ACS Publication website at DOI: 10.1021/jacsXXXXXXX.
Full experimental details
Characterization data of all compounds
1H and 13C NMR spectra of all compounds
References
- 1.Bachovchin DA, Cravatt BF. Nat Rev Drug Discov. 2012;11:52–68. doi: 10.1038/nrd3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long JZ, Cravatt BF. Chem Rev. 2011;111:6022–6063. doi: 10.1021/cr200075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers JC, Asgian JL, Ekici OD, James KE. Chem Rev. 2002;102:4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- 4.Boger DL, Sato H, Lerner AE, Austin BJ, Patterson JE, Patricelli MP, Cravatt BF. Bioorg Med Chem Lett. 1999;9:265–270. doi: 10.1016/s0960-894x(98)00734-3. [DOI] [PubMed] [Google Scholar]
- 5.Koutek B, Prestwich GD, Howlett AC, Chin SA, Salehani D, Akhavan N, Deutsch DG. J Biol Chem. 1994;269:22937–22940. [PubMed] [Google Scholar]
- 6.(a) Patterson JE, Ollmann IR, Cravatt BF, Boger DL, Wong CH, Lerner RA. J Am Chem Soc. 1996;118:5938–5945. [Google Scholar]; (b) Cravatt BF, Lerner RA, Boger DL. J Am Chem Soc. 1996;118:580–590. [Google Scholar]
- 7.Bottcher T, Sieber SA. Angew Chem Int Ed. 2008;47:4600–4603. doi: 10.1002/anie.200705768. [DOI] [PubMed] [Google Scholar]
- 8.Hoover HS, Blankman JL, Niessen S, Cravatt BF. Bioorg Med Chem Lett. 2008;18:5838–5841. doi: 10.1016/j.bmcl.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tew DG, Boyd HF, Ashman S, Theobald C, Leach CA. Biochemistry. 1998;37:10087–10093. doi: 10.1021/bi9801412. [DOI] [PubMed] [Google Scholar]
- 10.Boger DL, Sato H, Lerner AE, Hedrick MP, Fecik RA, Miyauchi H, Wilkie GD, Austin BJ, Patricelli MP, Cravatt BF. Proc Natl Acad Sci USA. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boger DL, Miyauchi H, Hedrick MP. Bioorg Med Chem Lett. 2001;11:1517–1520. doi: 10.1016/s0960-894x(01)00211-6. [DOI] [PubMed] [Google Scholar]
- 12.Otrubova K, Boger DL. ACS Chem Neurosci. 2012;3:340–348. doi: 10.1021/cn2001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachovchin DA, Ji T, Li W, Simon GM, Blankman JL, Adibekian A, Hoover H, Niessen S, Cravatt BF. Proc Natl Acad Sci USA. 2010;107:20941–20946. doi: 10.1073/pnas.1011663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Blankman JL, Cravatt BF. J Am Chem Soc. 2007;129:9594–9595. doi: 10.1021/ja073650c. [DOI] [PubMed] [Google Scholar]
- 15.Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adibekian A, Martin BR, Wang C, Hsu KL, Bachovchin DA, Niessen S, Hoover H, Cravatt BF. Nat Chem Biol. 2011;7:469–478. doi: 10.1038/nchembio.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otrubova K, Ezzili C, Boger DL. Bioorg Med Chem Lett. 2011;21:4674–4685. doi: 10.1016/j.bmcl.2011.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patricelli MP, Patterson JE, Boger DL, Cravatt BF. Bioorg Med Chem Lett. 1998;8:613–618. doi: 10.1016/s0960-894x(98)00073-0. [DOI] [PubMed] [Google Scholar]
- 19.Cravatt BF, Wright AT, Kozarich JW. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 20.Evans MJ, Cravatt BF. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 21.Liu YS, Patricelli MP, Cravatt BF. Proc Natl Acad Sci USA. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Proteomics. 2001;1:1067–1071. doi: 10.1002/1615-9861(200109)1:9<1067::AID-PROT1067>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Leung D, Hardouin C, Boger DL, Cravatt BF. Nat Biotechnol. 2003;21:687–691. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]
- 24.Leung D, Du W, Hardouin C, Cheng H, Hwang I, Cravatt BF, Boger DL. Bioorg Med Chem Lett. 2005;15:1423–1428. doi: 10.1016/j.bmcl.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 25.Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, Hwang I, Hedrick MP, Leung D, Acevedo O, Guimarães CRW, Jorgensen WL, Cravatt BF. J Med Chem. 2005;48:1849–1856. doi: 10.1021/jm049614v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtman AH, Leung D, Shelton CC, Hardouin C, Boger DL, Cravatt BF, Saghatelian A. J Pharm Exp Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- 27.Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. J Pharm Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otrubova K, Srinivasan V, Boger DL. Bioorg Med Chem Lett. 2014;24:3807–3813. doi: 10.1016/j.bmcl.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martyn DC, Moore M, Abell AD. Curr Pharm Des. 1999;5:405–415. [PubMed] [Google Scholar]
- 30.Zimmerman M, Morman H, Mulvey D, Jones H, Frankshun R, Ashe BM. J Biol Chem. 1980;255:9848–9851. [PubMed] [Google Scholar]
- 31.Ashe BM, Clark RL, Jones H, Zimmerman M. J Biol Chem. 1981;256:11603–11606. [PubMed] [Google Scholar]
- 32.Hlasta DJ, Subramanyam C, Bell MR. J Med Chem. 1995;38:739–744. doi: 10.1021/jm00005a001. [DOI] [PubMed] [Google Scholar]
- 33.Combrink KD, Gülgeze HB, Meanwell NA, Pearce BC, Zulan P, Bisacchi GS, Roberts DG, Stanley P, Seiler SM. J Med Chem. 1998;41:4854–4860. doi: 10.1021/jm9804580. [DOI] [PubMed] [Google Scholar]
- 34.Boverie S, Antoine MH, Somers F, Becker B, Sebille S, Ouedraogo R, Counerotte S, Pirotte B, Lebrun P, de Tullio P. J Med Chem. 2005;48:3492–3503. doi: 10.1021/jm0311339. [DOI] [PubMed] [Google Scholar]
- 35.Majumdar KC, Ganai S. Beilstein J Org Chem. 2013;9:503–509. doi: 10.3762/bjoc.9.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayao S, Stryker W, Phillips B, Fujimori H, Vidrio H. J Med Chem. 1968;11:1246–1248. doi: 10.1021/jm00312a601. [DOI] [PubMed] [Google Scholar]
- 37.Buckheit RW, Jr, Fliakas-Boltz V, Decker WD, Roberson JL, Pyle CA, White EL, Bowdon BJ, McMahon JB, Boyd MR, Bader JP, Nickell DG, Barth H, Antonucci TK. Antiviral Res. 1994;25:43–56. doi: 10.1016/0166-3542(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 38.Arranz ME, Díaz JA, Ingate ST, Witvrouw M, Pannecouque C, Balzarini J, De Clercq E, Vega S. Bioorg Med Chem. 1999;7:2811–2822. doi: 10.1016/s0968-0896(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 39.Lohaus G. Chem Ber. 1972;105:2791–2799. [Google Scholar]
- 40.Girard Y, Atkinson JG, Rokach J. J Chem Soc, Perkin Trans 1. 1979:1043–1047. [Google Scholar]
- 41.Combrink KD, Gulgeze HB, Thuring JW, Yu KL, Civiello RL, Zhang Y, Pearce BC, Yin Z, Langley DR, Kadow KF, Cianci CW, Li Z, Clarke J, Genovesi EV, Medina I, Lamb L, Yang Z, Zadjura L, Krystal M, Meanwell NA. Bioorg Med Chem Lett. 2007;17:4784–4790. doi: 10.1016/j.bmcl.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 42.de Tullio P, Becker B, Boverie S, Dabrowski M, Wahl P, Antoine MH, Somers F, Sebille S, Ouedraogo R, Hansen JB, Lebrun P, Pirotte B. J Med Chem. 2003;46:3342–3353. doi: 10.1021/jm021117w. [DOI] [PubMed] [Google Scholar]
- 43.de Tullio P, Boverie S, Becker B, Antoine MH, Nguyen QA, Francotte P, Counerotte S, Sebille S, Pirotte B, Lebrun P. J Med Chem. 2005;48:4990–5000. doi: 10.1021/jm0580050. [DOI] [PubMed] [Google Scholar]
- 44.Schareina T, Zapf A, Mägerlein W, Müller N, Beller M. Chem Eur J. 2007;13:6249–6254. doi: 10.1002/chem.200700079. [DOI] [PubMed] [Google Scholar]
- 45.The structures 14A (CCDC 1501165), 14B (CCDC 1501166), and 55 (CCDC 1501167) were established by single crystal X-ray structure determinations.
- 46.Cremlyn RJ, Cronje T. Phosphorus, Sulfur Silicon Relat Elem. 1979;6:413–419. [Google Scholar]
- 47.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MBD, Bachovchin DA, Mowen K, Baker D, Cravatt BF. Nature. 2010;468:790–797. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao J, Simon M. J Invest Dermatol. 2005;124:1259–1266. doi: 10.1111/j.0022-202X.2005.23761.x. [DOI] [PubMed] [Google Scholar]
- 50.Kienesberger PC, Oberer M, Lass A, Zechner R. J Lipid Res. 2009;50:S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cognetta AB, Niphakis MJ, Lee HC, Martini ML, Hulce JJ, Cravatt BF. Chem Biol. 2015;22:1–10. doi: 10.1016/j.chembiol.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohda M, Tokuzawa Y, Kishita Y, Nyuzuki H, Moriyama Y, Mizuno Y, Hirata T, Yatsuka Y, Yamashita-Sugahara Y, Nakachi Y, Kato H, Okuda A, Tamaru S, Borna NN, Banshoya K, Aigaki T, Sato-Miyata Y, Ohnuma K, Suzuki T, Nagao A, Maehata H, Matsuda F, Higasa K, Nagasaki M, Yasuda J, Yamamoto M, Fushimi T, Shimura M, Kaiho-Ichimoto K, Harashima H, Yamazaki T, Mori M, Murayama K, Ohtake A, Okazaki Y. PLOS Genet. 2016;12:e1005679. doi: 10.1371/journal.pgen.1005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao JG, Simon M. J Invest Dermatol. 2006;126:2087–2095. doi: 10.1038/sj.jid.5700327. [DOI] [PubMed] [Google Scholar]
- 54.Gao JG, Shih A, Gruber R, Schmuth M, Simon M. Mol Gen Metab. 2009;96:253–260. doi: 10.1016/j.ymgme.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Holmes RS. 3 Biotech. 2012;2:277–286. doi: 10.1007/s13205-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.