Abstract
Objective
ADAMTS13 (A Disintegrin And Metalloprotease with Thrombospondin type I repeats-13) prevents microvascular thrombosis by cleaving prothrombogenic ultra-large von Willebrand factor (ULVWF) multimers. Clinical studies have found association between reduced ADAMTS13 specific activity, ULVWF multimers and thrombotic angiopathy in patients with diabetic nephropathy. It remains unknown, however, whether ADAMTS13 deficiency or ULVWF multimers have a causative effect in diabetic nephropathy.
Approach and Results
The extent of renal injury was evaluated in wild-type (WT), Adamts13−/− and Adamts13−/−Vwf−/− mice after 26 weeks of streptozotocin-induced diabetic nephropathy. We found that WT diabetic mice exhibited low plasma ADAMTS13 specific activity and increased VWF levels (P<0.05 vs. WT-non diabetic mice). Adamts13−/− diabetic mice exhibited deterioration of kidney function (increased albuminuria, plasma creatinine and urea; P<0.05 versus WT diabetic mice), independent of hyperglycemia and hypertension. Deterioration of kidney function in Adamts13−/− diabetic mice was concomitant with aggravated intrarenal thrombosis (assessed by plasminogen activator inhibitor (PAI-1), VWF, fibrin(ogen) and CD41 positive microthrombi), increased mesangial cell expansion and extracellular matrix deposition (P<0.05 versus WT diabetic mice). Genetic deletion of VWF in Adamts13−/− diabetic mice improved kidney function, inhibited intrarenal thrombosis and alleviated histological changes in glomeruli, suggesting that exacerbation of diabetic nephropathy in the setting of ADAMTS13 deficiency is VWF-dependent.
Conclusions
ADAMTS13 retards progression of diabetic nephropathy, most likely by inhibiting VWF-dependent intrarenal thrombosis. Alteration in ADAMTS13-VWF balance may be one of the key pathophysiological mechanisms of thrombotic angiopathy in diabetes.
Keywords: ADAMTS13, Von Willebrand factor, intrarenal thrombosis, diabetic nephropathy
Subject code list: Basic Science Research
Introduction
Diabetic nephropathy is the major cause of end stage renal disease, contributing to approximately 40% of new cases, and is an independent risk factor for cardiovascular diseases.1 Current strategies to treat diabetic nephropathy include glycemic control with anti-diabetic drugs and anti-hypertensive medications such as angiotensin converting enzyme inhibitors (ACEi). Despite therapy, several patients remain at high risk because of adverse events.
Chronic hyperglycemia and oxidative stress-induced endothelial dysfunction and platelet activation are key features of progressive diabetic nephropathy.2 Oxidative stress was shown to be associated with accumulation of ultra-large von willebrand factor (ULVWF) multimers and thrombotic angiopathy in patients with diabetic nephropathy.3 Notably, ULVWF multimers, which are stored in platelet α-granules and endothelial Weibel-Palade bodies, are extremely large (up to 20,000 kDa) and are considered to be prothrombogenic. ULVWF multimers are not present in the plasma of healthy individuals because, upon release from platelets or endothelial cells, they are rapidly cleaved by the plasma protease ADAMTS13 (A Disintegrin And Metalloprotease with Thrombospondin type I repeats-13) into less active, smaller VWF multimers. ADAMTS13 is synthesized primarily by hepatic stellate cells4, 5 and to a lesser extent by endothelial cells6, 7 megakaryocytes,8 and podocytes.9 Deficiency of ADAMTS13 or very low levels of ADAMTS13 (<10%) increases plasma levels of ULVWF and causes thrombotic thrombocytopenic purpura (TTP), a disorder of thrombotic microangiopathy (TMA). Recent clinical studies suggest that chronic hyperglycemia can imbalance ADAMTS13-VWF axis, and, thereby may potentially contribute to thrombotic angiopathy in the setting of diabetes.10, 11 Despite these epidemiological findings, which predict that ADAMTS13-VWF axis imbalance may be causally linked to adverse renal and cardiovascular events in patients with diabetes, experimental evidence for causality is lacking.
Herein, we determined the role of the ADAMTS13-VWF axis in the progression of diabetic nephropathy by testing the hypothesis that ADAMTS13 deficiency exacerbates diabetic nephropathy by aggravating intrarenal thrombosis mediated by ULVWF. We utilized Adamts13−/− (model of ULVWF multimers)12 and Adamts13−/−Vwf −/− mice13 in an experimental model of streptozotocin-induced diabetic nephropathy. We found that ADAMTS13 deficiency exacerbated diabetic nephropathy, whereas deletion of VWF alleviated the aggravated diabetic nephropathy in Adamts13−/− mice.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
WT diabetic mice exhibit reduced plasma ADAMTS13 specific activity and increased VWF levels
Reduced ADAMTS13 specific activity and elevated VWF levels have been reported in the plasma of patients with diabetic nephropathy.10, 11, 14 We determined whether progression of diabetic nephropathy in streptozotocin-induced diabetes model would cause an increase in VWF levels associated with decreased ADAMTS13 specific activity. In WT diabetic mice, we found that plasma ADAMTS13 specific activity was markedly reduced by ≅46%, whereas plasma VWF levels were increased by 3.8 fold (P<0.05 vs. WT-non diabetic mice, Figure 1A&B).
Figure 1. WT diabetic mice exhibits reduced ADAMTS13 specific activity and increased VWF levels.
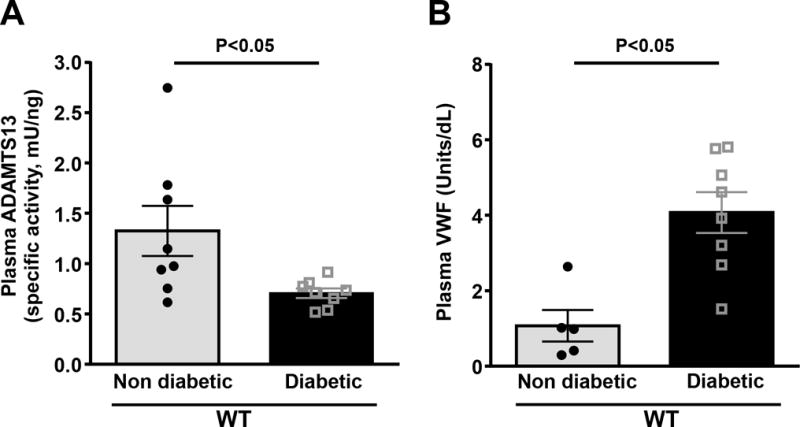
Plasma VWF and ADAMTS13 level was determined after 26 weeks of streptozotocin-induced diabetic nephropathy. A, Plasma ADAMTS13 specific activity. B, Plasma VWF levels. Data are presented as mean ± SEM. N=5–8 mice/group. Statistical analysis: unpaired t-test.
ADAMTS13 deficiency worsens kidney function during progression of diabetic nephropathy
Next, we determined whether ADAMTS13 deficiency exacerbates diabetic nephropathy. As expected, induction of diabetes in WT mice significantly deteriorated kidney function as assessed by increased urinary albumin secretion, plasma creatinine and urea levels concomitant with an increase in kidney weight (P<0.05 vs. WT-non diabetic mice, Figure 2). Adamts13−/− diabetic mice exhibited further deterioration in kidney function compared to WT diabetic mice (P<0.05, Figure 2), which was independent of hyperglycemia and hypertension (Figure SI–III).
Figure 2. Deterioration of renal function in the Adamts13−/− diabetic mice was VWF-dependent.
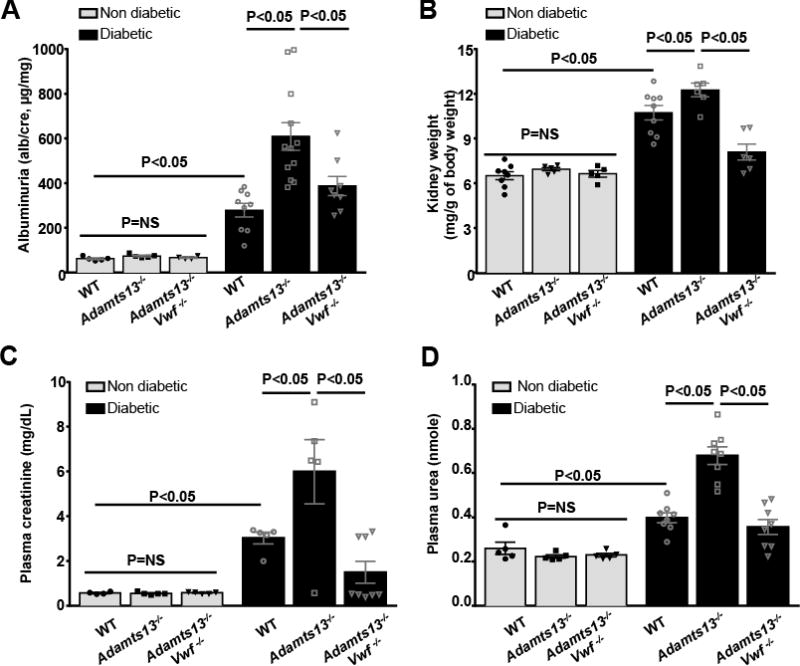
A, Urine albumin to creatinine ratio. B, Kidney weight/body weight. C, Plasma creatinine. D, Plasma urea. Data are presented as mean ± SEM. N=5–12 mice/group. Albuminuria, kidney weight, plasma creatinine and urea were significantly increased in diabetic mice when compared to non-diabetic mice in all the groups (P<0.05). Statistical analysis: one-way ANOVA followed by Holm-Sidak multiple comparison test.
ADAMTS13 deficiency aggravates intrarenal thrombosis and worsens key histological features of diabetic nephropathy
ADAMTS13 is known to prevent thrombus formation in the microvasculature by cleaving pro-thrombotic ULVWF multmers.12, 15 We hypothesized that ADAMTS13 deficiency aggravates intrarenal thrombosis and thereby exacerbates diabetic nephropathy. To evaluate intrarenal thrombosis, we quantified intrarenal fibrin(ogen) deposition, VWF, plasminogen activator inhibitor (PAI-1), and CD41 levels in kidney homogenates. We found that intrarenal fibrin(ogen) and CD41 levels (determined by Western blot), VWF and PAI-1 content (determined by ELISA) were significantly increased in Adamts13−/− diabetic mice compared to WT diabetic mice (P<0.05; Figure 3ABC). Consistent with these findings, PAI-1 mRNA expression levels were increased in kidney homogenates of Adamts13−/− diabetic mice (P<0.05 vs. WT diabetic mice; Figure SIV). Furthermore, we found an increase in intravascular platelet microthrombi (CD41-positive platelet deposits) in the glomeruli of Adamts13−/− diabetic mice (P<0.05 vs. WT diabetic mice, Figure 3D). Together, these results suggest that ADAMTS13 deficiency exacerbates intrarenal thrombosis. Next, we evaluated mesangial cell expansion and extracellular matrix (ECM) deposition (fibronectin and collagen) in the kidney glomeruli, two key histological features of diabetic nephropathy. Adamts13−/− diabetic mice exhibited significantly increased glomerular mesangial cell expansion as assessed by periodic acid Schiff (PAS) staining (P<0.05 vs. WT diabetic mice, Figure 4A). Concomitantly, Adamts13−/− diabetic mice showed increased ECM deposition and fibrosis as evident by increased fibronectin and collagen accumulation (P<0.05 vs. WT diabetic mice, Figure 4B&C). Non-diabetic WT and/or Adamts13−/− mice showed minimal changes in mesangial cell expansion and ECM deposition (Figure 4). Next, we determined inflammatory cytokines IL1β and TNFα levels in kidney extracts. Although, IL1β and TNFα levels were significantly increased in both WT and Adamts13−/− diabetic mice when compared to respective non-diabetic mice, they were comparable between WT diabetic and Adamts13−/− diabetic mice (Figure SV).
Figure 3. Increased intrarenal thrombosis in Adamts13−/− diabetic mice was VWF-dependent.
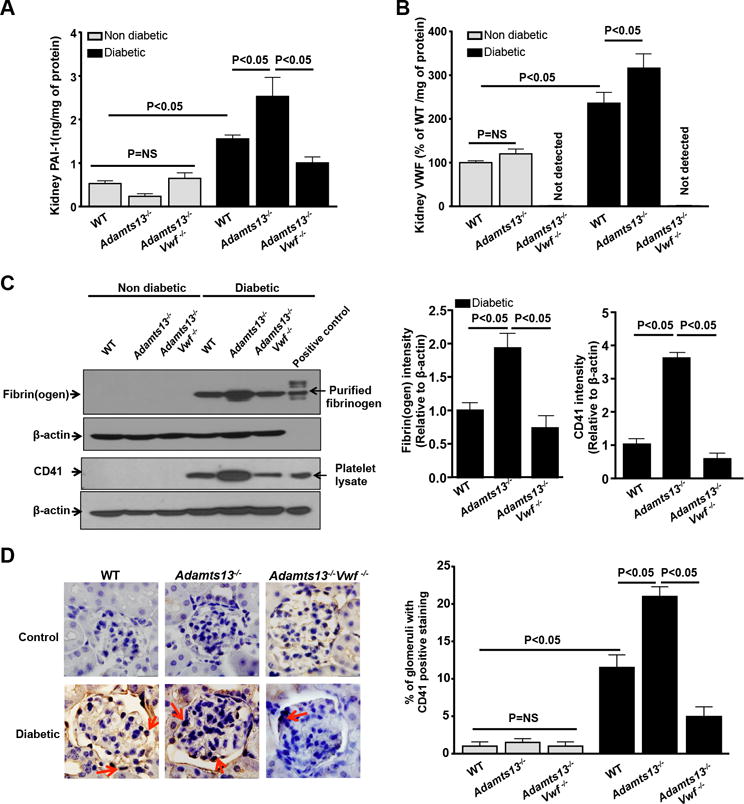
Kidney homogenates were prepared after 26 weeks of diabetes induction and as described in methods. A, Kidney PAI-1 levels. B, Kidney VWF levels. C, Left panels show representative images of immunoblots for fibrin(ogen) and CD41 positive platelets accumulation in the kidney homogenates of diabetic mice, but not in non-diabetic mice. Purified fibrin(ogen) and mouse platelet lysate was used as positive control. b-actin was used as loading control. Right panel shows densitometric quantification of the fibrin(ogen) and CD41 immunoblots (C) normalized to corresponding b-actin (loading control). D. Left panel shows representative images of CD41 positive stained microthrombi (red arrows) in the kidney section and right panel shows quantification. Data are presented as mean ± SEM. N=5 mice/group. P<0.05 for diabetic mice when compared to non-diabetic mice in all the groups. Statistical analysis: one-way ANOVA followed by Holm-Sidak multiple comparison test.
Figure 4. Increased mesangial cell expansion and extracellular matrix deposition in the glomeruli in Adamts13−/− diabetic mice was VWF-dependent.
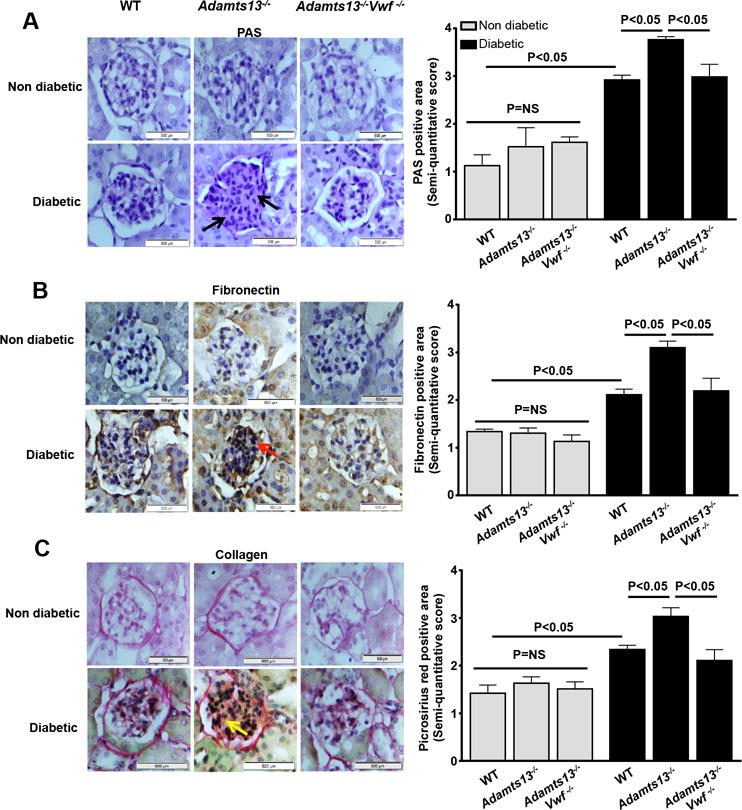
Left panel shows representative images of A, PAS stained (black arrow) kidney section, B, fibronectin stained (red arrow) kidney section and C, picrosirus red (yellow arrow) stained kidney section. Right panel shows semi-quantitative score for the respective staining. P<0.05 for diabetic mice when compared to non-diabetic mice in all the groups. Data are presented as mean ± SEM. Mean score of 5–20 glomeruli from each mouse were taken from 5–6 mice/group. Statistical analysis: one-way ANOVA followed by Holm-Sidak multiple comparison test.
Genetic deletion of VWF in Adamts13−/− mice improves kidney function by inhibiting intrarenal thrombosis and alleviating key hallmark features of diabetic nephropathy
To determine the molecular mechanism by which ADAMTS13 deficiency contributes to diabetic nephropathy exacerbation, we focused on VWF because it is the only known substrate for ADAMTS13 in multiple experimental models.13, 16–18 We hypothesized that genetic deletion of VWF in Adamts13−/− mice will alleviate diabetic nephropathy exacerbation. Blood glucose (Figure SIA), body weight (Figure SIB), diabetes incidence rate (Figure SII), systolic blood pressure (Figure SIII) and inflammatory cytokines (Figure SV) were comparable between diabetic WT, Adamts13−/− and Adamts13−/−Vwf −/− mice. Despite this, we found that genetic ablation of VWF in Adamts13−/− diabetic mice markedly improved kidney function, inhibited intrarenal thrombosis and alleviated histological changes in glomeruli (P<0.05 vs. Adamts13−/− diabetic mice, Figure 2, 3 & 4). Together these results strongly suggest that exacerbation of diabetic nephropathy observed in the setting of ADAMTS13 deficiency is VWF-dependent.
Discussion
The key novel findings of the current study are: 1) diabetes in mice results in increased VWF levels that are associated with decreased ADAMTS13 specific activity, 2) severe ADAMTS13 deficiency in mice exacerbates diabetic nephropathy, likely mediated in part due to increased intrarenal thrombosis, and 3) exacerbation of diabetic nephropathy observed in the setting of ADAMTS13 deficiency is VWF-dependent.
We found that WT diabetic mice have significantly low levels of ADAMTS13 specific activity and elevated levels of VWF antigen in the plasma compared to non-diabetic littermates. Our findings are consistent with several population-based studies, which have observed low ADAMTS13 activity and high VWF antigen levels in in type 1 and type 2 diabetic patients affected with diabetic nephropathy.10, 11, 14, 19, 20 The mechanisms by which ADAMTS13 specific activity is reduced in the setting of diabetes remain unclear. Possible mechanisms include: 1) reduced synthesis and secretion of ADAMTS13 by hepatic stellate cells in the liver; 2) increased ADAMTS13 consumption and/or incorporation into thrombi together with its substrate VWF, as observed in patients with acute MI;21 3) loss of function due to inhibitory autoantibodies to ADAMTS13;22 and 4) oxidative modification of ADAMTS13.23
Clinical studies have suggested that oxidative stress in type 2 diabetic patients affected with diabetic nephropathy is associated with increased ULVWF multimers.3 However, to date there is no experimental evidence to suggest a causal role for ULVWF multimers in the exacerbation of diabetic nephropathy. One can speculate that ULVWF multimers might be simply an associated marker of disease status, possibly secondary to endothelial cell dysfunction and/or oxidative stress. We utilized Adamts13−/− and Adamts13−/−Vwf −/− mice to determine the role of the ADAMTS13-VWF axis in experimental diabetic nephropathy. Herein, we provide evidence that Adamts13−/− diabetic mice are more susceptible to diabetic nephropathy and have deteriorated kidney function. Since VWF is the only known substrate for ADAMTS13,13, 16, 17 we determined whether exacerbated diabetic nephropathy in Adamts13−/− mice is VWF-dependent. We found that genetic deletion of VWF in Adamts13−/− mice alleviated exacerbated diabetic nephropathy. Together, these results strongly suggest a causal role for the ADAMTS13-VWF axis in progression of diabetic nephropathy.
It is intriguing to consider possible mechanisms by which the ADAMTS13-VWF axis may contribute to progression of diabetic nephropathy. Since podocytes and glomerular endothelial cells synthesize ADAMTS13, we hypothesized that ADAMTS13 deficiency may aggravate intrarenal thrombosis in the high shear environment of glomeruli due to accumulation of platelet rich microthrombi, and, thereby exacerbate diabetic nephropathy. Spontaneous thrombus formation has been demonstrated in activated microvessels of Adamst13−/− mice, but not in WT mice.15 Consistent with this hypothesis, we found that thrombosis markers such as PAI-1, VWF, fibrinogen, CD41 levels were significantly elevated in kidney homogenates of Adamts13−/− diabetic mice, suggesting aggravated intrarenal thrombosis. Consistent with these results, we found an increase in intravascular platelet microthrombi (CD41-positive platelet deposits) in the glomeruli of Adamts13−/− diabetic mice. On basis of these results, we suggest that ADAMTS13, by regulating ULVWF/VWF multimer size, may inhibit thrombotic angiopathy in the setting of diabetes. Indeed, a recent study has shown that recombinant ADAMTS13 inhibits thrombotic microangiopathy following systemic vascular endothelial growth factor inhibition.24 We and others have demonstrated that in addition to its role in thrombosis, the ADAMTS13-VWF axis also functions in the modulation of inflammatory responses in experimental models of stroke, myocardial infarction and atherosclerosis.16, 18, 25, 26 However, to our surprise, we found that the inflammatory cytokines IL1β and TNFα were comparable in kidney extracts of Adamts13−/− diabetic and WT diabetic mice, suggesting that most likely it is the aggravation of intrarenal thrombosis that mediates diabetic nephropathy exacerbation in Adamts13−/− mice in the setting of diabetes.
It is known that PAI-1 contributes to diabetic nephropathy exacerbation by regulating TGF-β and renal production of ECM proteins such as collagen and fibronectin.27 Accumulation of ECM proteins and mesangial cell expansion in the kidney glomerular and tubular compartments are hallmarks of diabetic nephropathy and worsening of renal function. We found that ADAMTS13 deficiency in mice promotes mesangial cell expansion and accumulation of collagen and fibronectin in the glomerular compartment, which was associated with increased PAI-1 protein levels. Although endothelial cells are the major source of PAI-1, platelets also contain significant amounts of PAI-1. We speculate that the source of increased PAI-1 levels in kidney homogenates of Adamts13−/− mice is both endothelial cells and activated platelets within thrombi. Based on our findings, we proposed a mechanistic model in which ADAMTS13 deficiency combined with hyperglycemia aggravates intrarenal thrombosis. Increased PAI-1 levels are released from activated endothelial cells and platelet-rich thrombi in the tubular micro-environment, which in turn potentiate TGF-β production to enhance production of ECM proteins including collagen and fibronectin and, thereby exacerbate diabetic nephropathy.
Our studies have some limitations. First, we have used a model of type 1 diabetes rather than more common type 2 diabetes. Second, type 2 diabetes patients with diabetic nephropathy often have only partially reduced ADAMTS13 activity rather than severe ADAMTS13 deficiency.10, 11, 14 The present study did not define whether partially reduced ADAMTS13 levels contribute to diabetic nephropathy exacerbation. Future studies will be required to determine the threshold of ADAMTS13 specific activity required to prevent exacerbated diabetic nephropathy. Despite this limitation, in our opinion, the results from this study may have clinical implications. First, our data suggest that ADAMTS13-VWF imbalance may causally contribute to thrombotic angiopathy in diabetic patients rather than simply serving as a biomarker of endothelial dysfunction and oxidative stress. Second, population-based studies have shown a lower efficacy for aspirin in preventing cardiovascular events in diabetes patients compared to non-diabetic patients.28, 29 It is possible that the lower efficacy could be in part related to the inability of aspirin to inhibit the formation of ULVWF-rich thrombi in diabetic patients. The use of recombinant ADAMTS13 or N-acetyl cysteine, which is known to reduce hyperactive ULVWF multimers to less active VWF multimers, combined with ACEi may be helpful in preventing cardiovascular events in diabetic patients.
In summary, for the first time to our knowledge, we show that severe ADAMTS13 deficiency exacerbates diabetic nephropathy, likely mediated by VWF-dependent increased intrarenal thrombosis. Alteration in ADAMTS13-VWF balance may be one of the key pathophysiological mechanisms of increased thrombotic angiopathies observed in diabetic patients.
Supplementary Material
Highlights.
ADAMTS13 is a plasma protease that cleaves prothrombotic ULVWF multimers, into smaller, less active VWF multimers.
Clinical studies have shown association between ULVWF multimers, and reduced ADAMTS13 specific activity with thrombotic angiopathies in patients with diabetic nephropathy.
We provide genetic evidence on the causal role of ADAMTS13-VWF axis in diabetic nephropathy exacerbation.
Alteration in ADAMTS13-VWF balance may be one of the key pathophysiological mechanisms of thrombotic angiopathy in diabetes.
Acknowledgments
Sources of funding
A.K.C lab is supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health (NHLBI/NIH) grants (R01 HL118246 and R01 HL118742) and by innovative grant 16IRG27490003 from American Heart Association. S.R.L lab is supported by NHLBI/NIH grant P01 HL062984.
Non-standard Abbreviations and Acronyms
- ULVWF
Ultra large von Willebrand factor
- ADAMTS13
A Disintegrin And Metalloprotease with Thrombospondin type I repeats-13
- PAS
Periodic acid Schiff
- PAI-1
Plasminogen activator inhibitor-1
Footnotes
Disclosures
None.
References
- 1.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 2.Domingueti CP, Dusse LM, Carvalho M, Gomes KB, Fernandes AP. Hypercoagulability and cardiovascular disease in diabetic nephropathy. Clin Chim Acta. 2013;415:279–285. doi: 10.1016/j.cca.2012.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Oggianu L, Lancellotti S, Pitocco D, Zaccardi F, Rizzo P, Martini F, Ghirlanda G, De Cristofaro R. The oxidative modification of von willebrand factor is associated with thrombotic angiopathies in diabetes mellitus. PLoS One. 2013;8:e55396. doi: 10.1371/journal.pone.0055396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of adamts13 to the stellate cells of human liver. Blood. 2005;106:922–924. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Inada M, Lee TP, Benten D, Lyubsky S, Bouhassira EE, Gupta S, Tsai HM. Adamts13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release adamts-13. J Thromb Haemost. 2006;4:1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 7.Tati R, Kristoffersson AC, Stahl AL, Morgelin M, Motto D, Satchell S, Mathieson P, Manea-Hedstrom M, Karpman D. Phenotypic expression of adamts13 in glomerular endothelial cells. PLoS One. 2011;6:e21587. doi: 10.1371/journal.pone.0021587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived vwf-cleaving metalloprotease adamts-13. J Thromb Haemost. 2005;3:2536–2544. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 9.Manea M, Kristoffersson A, Schneppenheim R, Saleem MA, Mathieson PW, Morgelin M, Bjork P, Holmberg L, Karpman D. Podocytes express adamts13 in normal renal cortex and in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2007;138:651–662. doi: 10.1111/j.1365-2141.2007.06694.x. [DOI] [PubMed] [Google Scholar]
- 10.Rurali E, Noris M, Chianca A, Donadelli R, Banterla F, Galbusera M, Gherardi G, Gastoldi S, Parvanova A, Iliev I, Bossi A, Haefliger C, Trevisan R, Remuzzi G, Ruggenenti P, Group BS Adamts13 predicts renal and cardiovascular events in type 2 diabetic patients and response to therapy. Diabetes. 2013;62:3599–3609. doi: 10.2337/db13-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi S, Hashiguchi T, Ono T, Takenouchi K, Nakayama K, Kawano T, Kato K, Matsushita R, Nagatomo M, Nakamura S, Nakashima T, Maruyama I. Association between reduced adamts13 and diabetic nephropathy. Thromb Res. 2010;125:e310–316. doi: 10.1016/j.thromres.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Motto DG, Chauhan AK, Zhu G, Homeister J, Lamb CB, Desch KC, Zhang W, Tsai HM, Wagner DD, Ginsburg D. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible adamts13-deficient mice. J Clin Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan AK, Walsh MT, Zhu G, Ginsburg D, Wagner DD, Motto DG. The combined roles of adamts13 and vwf in murine models of ttp, endotoxemia, and thrombosis. Blood. 2008;111:3452–3457. doi: 10.1182/blood-2007-08-108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domingueti CP, Dusse LM, Foscolo RB, Reis JS, Annichino-Bizzacchi JM, Orsi FL, Mazetto Bde M, Carvalho M, Gomes KB, Fernandes AP. Von willebrand factor, adamts13 and d-dimer are correlated with different levels of nephropathy in type 1 diabetes mellitus. PLoS One. 2015;10:e0132784. doi: 10.1371/journal.pone.0132784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan AK, Motto DG, Lamb CB, Bergmeier W, Dockal M, Plaimauer B, Scheiflinger F, Ginsburg D, Wagner DD. Systemic antithrombotic effects of adamts13. J Exp Med. 2006;203:767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. Adamts13 deficiency exacerbates vwf-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5224–5230. doi: 10.1182/blood-2012-06-440255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi C, Ahmad A, Wilson KM, Chauhan AK. Adamts13 modulates atherosclerotic plaque progression in mice via a vwf-dependent mechanism. J Thromb Haemost. 2014;12:255–260. doi: 10.1111/jth.12456. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan AK, Kisucka J, Brill A, Walsh MT, Scheiflinger F, Wagner DD. Adamts13: A new link between thrombosis and inflammation. J Exp Med. 2008;205:2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porta M, La Selva M, Molinatti PA. Von willebrand factor and endothelial abnormalities in diabetic microangiopathy. Diabetes Care. 1991;14:167–172. doi: 10.2337/diacare.14.2.167. [DOI] [PubMed] [Google Scholar]
- 20.Hirano T, Ookubo K, Kashiwazaki K, Tajima H, Yoshino G, Adachi M. Vascular endothelial markers, von willebrand factor and thrombomodulin index, are specifically elevated in type 2 diabetic patients with nephropathy: Comparison of primary renal disease. Clin Chim Acta. 2000;299:65–75. doi: 10.1016/s0009-8981(00)00274-6. [DOI] [PubMed] [Google Scholar]
- 21.Kaikita K, Soejima K, Matsukawa M, Nakagaki T, Ogawa H. Reduced von willebrand factor-cleaving protease (adamts13) activity in acute myocardial infarction. J Thromb Haemost. 2006;4:2490–2493. doi: 10.1111/j.1538-7836.2006.02161.x. [DOI] [PubMed] [Google Scholar]
- 22.Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, Konetschny C, Zimmermann K, Scharrer I, Peyvandi F, Galbusera M, Remuzzi G, Bohm M, Plaimauer B, Lammle B, Scheiflinger F. Adamts13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–1267. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Chen J, Ling M, Lopez JA, Chung DW, Fu X. Hypochlorous acid generated by neutrophils inactivates adamts13: An oxidative mechanism for regulating adamts13 proteolytic activity during inflammation. J Biol Chem. 2015;290:1422–1431. doi: 10.1074/jbc.M114.599084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erpenbeck L, Demers M, Zsengeller ZK, Gallant M, Cifuni SM, Stillman IE, Karumanchi SA, Wagner DD. Adamts13 endopeptidase protects against vascular endothelial growth factor inhibitor-induced thrombotic microangiopathy. J Am Soc Nephrol. 2016;27:120–131. doi: 10.1681/ASN.2014121165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von willebrand factor-cleaving protease adamts13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, Dietrich B, Rottensteiner H, Scheiflinger F, Wagner DD. Protective anti-inflammatory effect of adamts13 on myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5217–5223. doi: 10.1182/blood-2012-06-439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67:1297–1307. doi: 10.1111/j.1523-1755.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 28.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O’Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R, Prevention of Progression of Arterial D, Diabetes Study G, Diabetes Registry G, Royal College of Physicians E The prevention of progression of arterial disease and diabetes (popadad) trial: Factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y, Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes Trial I Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: A randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


