Abstract
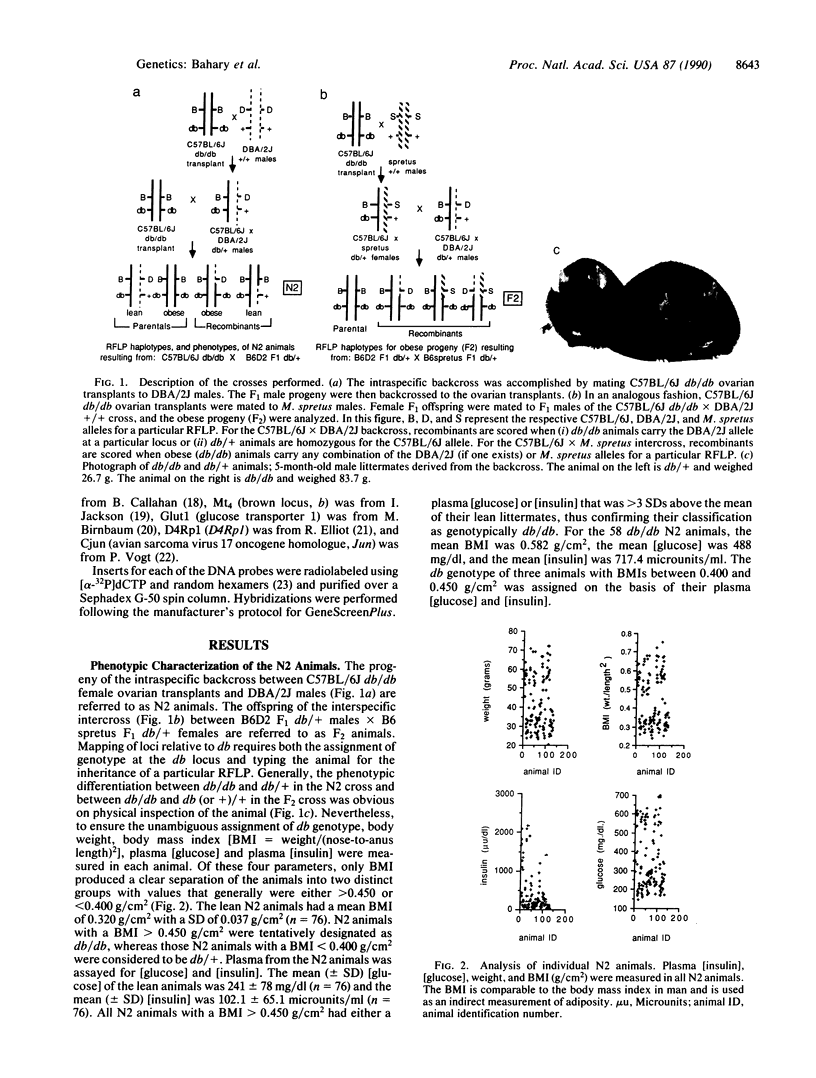
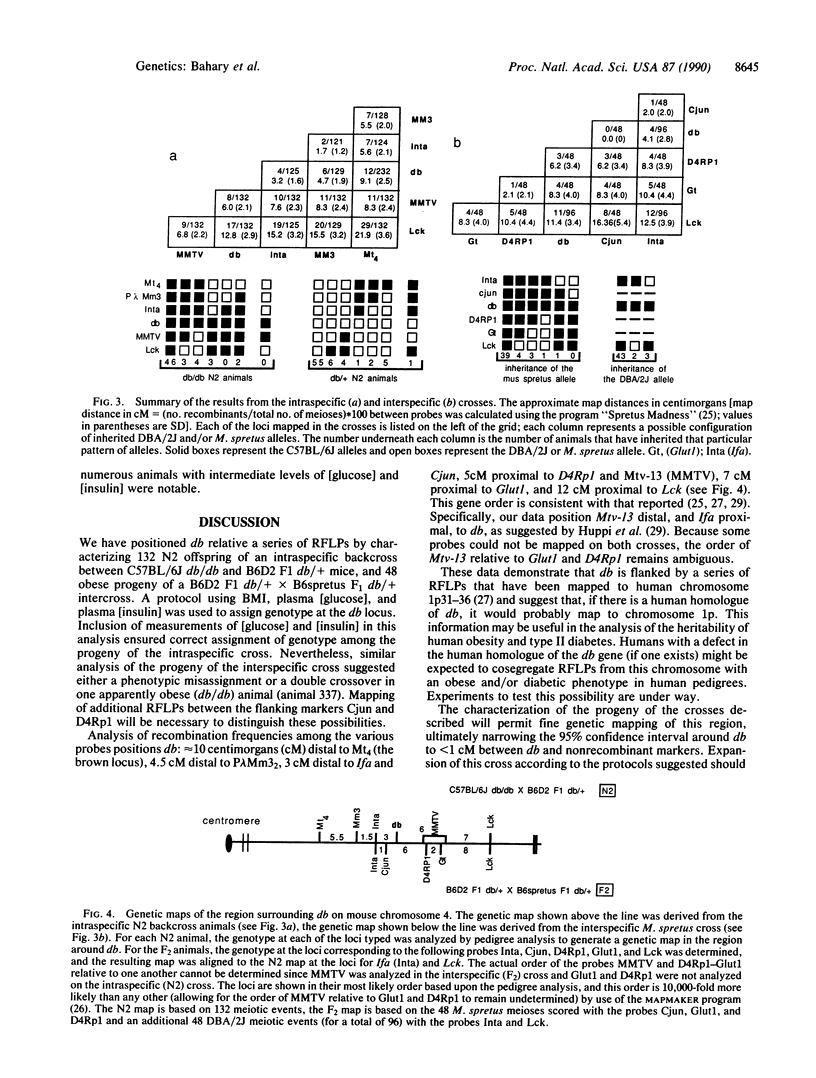
Diabetes (db) is an autosomal recessive mutation located in the midportion of mouse chromosome 4 that results in profound obesity with hyperphagia, increased metabolic efficiency, and insulin resistance. To clone this gene and generate a molecular map of the region around this mutation, two genetic crosses were established: an intraspecific backcross between C57BL/6J db/db females and C57BL/6J db/db x DBA/2J +/+ F1 (B6D2 db/+ F1) male mice and an interspecific intercross between B6D2 db/+ F1 males and C57BL/6J db/db x Mus spretus F1 (B6spretus db/+ F1) females. The progeny of both crosses were characterized for genotype at the db locus to map a series of restriction fragment length polymorphisms relative to the db locus. Measurements of body weight, body length, and plasma concentrations of glucose and insulin in the animals allowed the assignment of genotype (db/db vs. db/+ or +/+). A total of 132 progeny of the intraspecific cross and 48 db/db progeny of the interspecific cross were typed for individual restriction fragment length polymorphisms to generate a gene order of: centromere-brown (Mt4)-P lambda Mm3(2)-Ifa (Inta)-Cjun-db-D4Rp1-Glut1-Mtv-13-Lck. Several of the genes that are linked to db [Cjun, glucose transporter (Glut1) and Lck] map to human chromosome 1p, suggesting that db may be part of a syntenic group between human 1p and the distal portion of mouse chromosome 4. In addition, phenotyping of the progeny of these crosses revealed a wide range in plasma concentrations of glucose and insulin among the obese progeny, with some animals developing overt diabetes and other remaining euglycemic. Distributions of age-controlled plasma [glucose] and [insulin] among the intraspecific-cross obese progeny were not bimodal, suggesting a role for polygenic differences between the progenitor strains (C57BL/6J and DBA/2J) in the development of overt diabetes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L. C., Arnaud D., Cambrou J., Guenet J. L., Avner P. R. Mapping of the mouse X chromosome using random genomic probes and an interspecific mouse cross. EMBO J. 1985 Dec 30;4(13B):3695–3700. doi: 10.1002/j.1460-2075.1985.tb04137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avner P., Amar L., Dandolo L., Guénet J. L. Genetic analysis of the mouse using interspecific crosses. Trends Genet. 1988 Jan;4(1):18–23. doi: 10.1016/0168-9525(88)90123-0. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher D. W., Hayashi K., Rosenthal J., Notkins A. L. Virus-induced diabetes mellitus. III. Influence of the sex and strain of the host. J Infect Dis. 1975 Apr;131(4):462–466. doi: 10.1093/infdis/131.4.462. [DOI] [PubMed] [Google Scholar]
- Callahan R., Gallahan D., Kozak C. Two genetically transmitted BALB/c mouse mammary tumor virus genomes located on chromosomes 12 and 16. J Virol. 1984 Mar;49(3):1005–1008. doi: 10.1128/jvi.49.3.1005-1008.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci J. D., Siracusa L. D., Jenkins N. A., Copeland N. G. A molecular genetic linkage map of mouse chromosome 4 including the localization of several proto-oncogenes. Genomics. 1989 Nov;5(4):699–709. doi: 10.1016/0888-7543(89)90111-0. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Symposium IV: Diabetic syndrome in animals. Influence of genetic background on the expression of mutations at the diabetes locus in the mouse. II. Studies on background modifiers. Isr J Med Sci. 1975 Jul;11(7):708–713. [PubMed] [Google Scholar]
- Coleman D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 Mar;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher E. M., Cavanna J. S., Brown S. D. Microdissection and microcloning of the mouse X chromosome. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5846–5849. doi: 10.1073/pnas.82.17.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J. F. DNA polymorphism and human disease. Annu Rev Biochem. 1986;55:831–854. doi: 10.1146/annurev.bi.55.070186.004151. [DOI] [PubMed] [Google Scholar]
- Haluska F. G., Huebner K., Isobe M., Nishimura T., Croce C. M., Vogt P. K. Localization of the human JUN protooncogene to chromosome region 1p31-32. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2215–2218. doi: 10.1073/pnas.85.7.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hummel K. P., Coleman D. L., Lane P. W. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem Genet. 1972 Aug;7(1):1–13. doi: 10.1007/BF00487005. [DOI] [PubMed] [Google Scholar]
- Hummel K. P., Dickie M. M., Coleman D. L. Diabetes, a new mutation in the mouse. Science. 1966 Sep 2;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Kelly R., Taylor B. A., Bulfield G. Mouse DNA 'fingerprints': analysis of chromosome localization and germ-line stability of hypervariable loci in recombinant inbred strains. Nucleic Acids Res. 1987 Apr 10;15(7):2823–2836. doi: 10.1093/nar/15.7.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku K., Fiedorek F. T., Jr, Province M., Permutt M. A. Genetic analysis of glucose tolerance in inbred mouse strains. Evidence for polygenic control. Diabetes. 1988 Jun;37(6):707–713. doi: 10.2337/diab.37.6.707. [DOI] [PubMed] [Google Scholar]
- Kaku K., Province M., Permutt M. A. Genetic analysis of obesity-induced diabetes associated with a limited capacity to synthesize insulin in C57BL/KS mice: evidence for polygenic control. Diabetologia. 1989 Sep;32(9):636–643. doi: 10.1007/BF00274249. [DOI] [PubMed] [Google Scholar]
- Kelley K. A., Pitha P. M. Characterization of a mouse interferon gene locus I. Isolation of a cluster of four alpha interferon genes. Nucleic Acids Res. 1985 Feb 11;13(3):805–823. doi: 10.1093/nar/13.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Coleman D. L., Eisenstein A. B., Strack I. A new mutation (db3J) at the diabetes locus in strain 129/J mice. I. Physiological and histological characterization. Diabetologia. 1980 Jul;19(1):58–65. doi: 10.1007/BF00258313. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Coleman D. L., Hummel K. P. The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. III. Effect of H-2 haplotype and sex. Diabetes. 1981 Dec;30(12):1029–1034. doi: 10.2337/diab.30.12.1029. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Le P. H., Coleman D. L. Susceptibility to db gene and streptozotocin-induced diabetes in C57BL mice: control by gender-associated, MHC-unlinked traits. Immunogenetics. 1987;26(1-2):6–13. doi: 10.1007/BF00345448. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. The genetics of diabetes susceptibility in mice. FASEB J. 1989 Sep;3(11):2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Morrow P. L., Freedman A., Craighead J. E. Testosterone effect on experimental diabetes mellitus in encephalomyocarditis (EMC) virus infected mice. Diabetologia. 1980 Mar;18(3):247–249. doi: 10.1007/BF00251924. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]




