Abstract
Prednisone is typically co-administered with abiraterone in treatment of castrate resistant prostate cancer to prevent toxicities of secondary mineralocorticoid excess. However, many patients do not desire or cannot tolerate chronic glucocorticoid therapy. In this retrospective study, we report that eplerenone, a mineralocorticoid antagonist, can be safely used with abiraterone, and can obviate the need for concomitant prednisone in this patient population.
Background
Abiraterone acetate (hereafter abiraterone) is approved for metastatic castration refractory prostate cancer (mCRPC). Co-administration with prednisone is recommended to prevent toxicity from secondary mineralocorticoid excess such as hypertension, hypokalemia and edema. However, use of prednisone is often not desirable by patients because of potential for detrimental effects of long term therapy with corticosteroids, especially in those with comorbidities like diabetes or who have received prior immunotherapeutics. Eplerenone is a non-steroidal mineralocorticoid antagonist demonstrated to abrogate mineralocorticoid excess. In this retrospective study, we report real world experience of use of eplerenone with abiraterone in men with mCRPC who wished to avoid concomitant prednisone therapy.
Methods
Incidence and grade (CTCAE v4) of mineralocorticoid excess toxicities, along with baseline demographics, disease characteristics, and PFS in men with mCRPC treated with abiraterone, not willing to be treated with corticosteroids and thus received eplerenone were collected retrospectively, and compared with those treated with abiraterone and prednisone during the same time period. Continuous variables were assessed by Wilcoxon rank sum or student t-test, and categorical variables were assessed by Fischer's Exact test or chi-square as appropriate. PFS was compared by Kaplan-Meier method.
Results
Of 106 men treated with abiraterone, 40 received eplerenone and 66 received prednisone. Baseline and disease characteristics, incidence and grades of adverse events related to syndrome of mineralocorticoid excess, and median PFS were similar in both cohorts.
Conclusions
In real world population of men with mCRPC treated with abiraterone, corticosteroids may be avoided by concomitant treatment with eplerenone. Data need further validation.
Introduction
Abiraterone acetate (hereafter abiraterone) is an approved, and one of the most commonly used agents for men metastatic castration resistant prostate cancer (mCRPC). Abiraterone targets cytochrome P450 17A1 (CYP17A1), the rate-limiting hydroxylase in the androgen and steroid biosynthetic pathway.1
Abiraterone suppresses non-gonadal androgen synthesis (i.e. testosterone, cortisol) down-stream of CYP17A1. This leads to an an elevation in adrenocorticotrophic hormone (ACTH) leading to secondary excess in mineralocorticoids manifesting as hypertension, hypokalemia and fluid overload, often presenting as lower extremity edema.2 To suppress the hypothalamic-pituitary-adrenal (HPA) axis and diminish symptoms of mineralocorticoid excess, abiraterone was approved with concurrent prednisone administration.3 Currently, abiraterone is the most commonly prescribed drug in the first line setting of mCRPC, a population comprising predominantly the asymptomatic or minimally symptomatic men with mCRPC. The median overall survival in this patient population is approximately 3 years.4, 5 Long-term use of prednisone is often not desired by the relatively asymptomatic patients because of concerns of detrimental effects, especially in those with prior immunotherapy, or in the presence of comorbidities like diabetes.
Earlier phase I investigations of abiraterone employed peripheral inhibition of mineralocorticoid excess with the mineralocorticoid receptor antagonist eplerenone.2 Further clinical investigations employed central suppression with prednisone and other low-dose glucocorticoids. However, eplerenone remains a potentially viable option to mitigate secondary mineralocorticoid excess in clinical practice in men reluctant to be treated with long term prednisone.6-9
Herein we report our real world experience of use of eplerenone with abiraterone in men with mCRPC who wished to avoid concomitant therapy with prednisone. We also compare the patient and disease characteristics, clinical outcomes, and incidence and grade of mineralocorticoid excess in these men to those who were treated with abiraterone and prednisone during the same time period.
Methods
Patient Selection
We queried the University of Utah Huntsman Cancer Institute electronic medical record for men with mCRPC treated with abiraterone and either prednisone or eplerenone between 01/2013-08/2016. Inclusion criteria included those with prostate adenocarcinoma, documented castrate level of testosterone, disease progression per PCWG2 criteria prior to commencement of abiraterone, performance status of 0-2 and documented evidence of metastatic disease on imaging studies. Exclusion criteria consisted of absence of follow up data during treatment or discontinuation of treatment due to factors unrelated to mCRPC progression or toxicities. Prior to initiation of eplerenone therapy, patients agreed to transition from eplerenone to prednisone should they develop any drug-related toxicity or mineralocorticoid excess.
Data Acquisition
We retrospectively analyzed charts to collect data on patient characteristics, such as age, prior therapies, Charlson Comorbidity Index (CCI), disease characteristics such as PSA at initiation of abiraterone therapy, Gleason score, presence of visceral metastasis, and data on the incidence and grading of mineralocorticoid excess related toxicities, such as hypertension, hypokalemia, and lower extremity edema while undergoing abiraterone therapy. Additionally, baseline body mass index (BMI), diabetes mellitus, and change of weight during therapy were recorded. Progression was determined based on PSA progression (per PCWG2 criteria) or clinical progression, or imaging progression (when imaging studies were done consistently). Grades of secondary mineralocorticoid excess were based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Data for men transitioning from therapy with eplerenone to prednisone due to grade 3 or 4 effects attributable to eplerenone were analyzed in the abiraterone with eplerenone group. Only toxicities secondary to syndrome of mineralocorticoid excess were recorded. Study was approved by the University of Utah Institutional Review Board.
Statistical Analysis
Data was analyzed using R statistical computing software.10 Fisher's Exact test was used for categorical variables with 2 or 3 categories (visceral metastasis, hypertension, edema, hypokalemia, any toxicity) and the Wilcoxon test was used for continuous variables and ordered categorical variables with 4 or more categories (age, PSA, Gleason score, previous lines of therapy, CCI). Median PFS was estimated using Kaplan-Meier methodology and a logrank test was used to compare PFS between groups. PFS was controlled for baseline prognostic factors by running a multivariate Cox proportional hazards model adjusting for previous lines of treatment, age, Gleason, PSA and CCI.
Results
Patient Characteristics
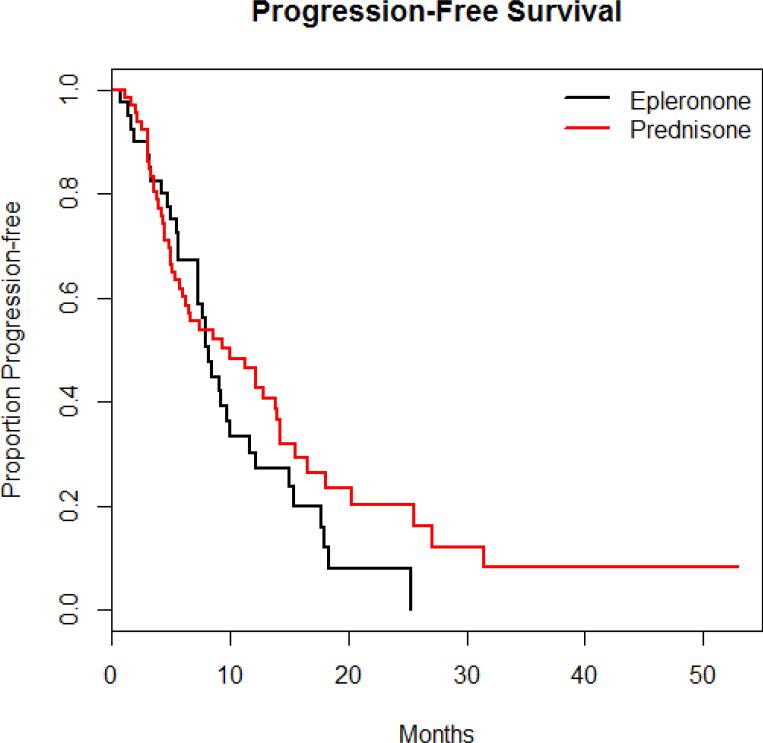
One hundred and six men met criteria for this study and were included in the analysis. Sixty-six were treated with abiraterone and prednisone (10 mg daily) and 40 were treated with abiraterone and eplerenone (50 mg daily). Age, comorbidities, Gleason, PSA, and baseline BMI on initiation of abiraterone were not statistically different between the two groups(Table 1). A greater number of lines of therapies preceded abiraterone in those treated with abiraterone plus eplerenone versus those treated with abiraterone plus prednisone. There was a greater number of lines of therapies preceded abiraterone in those treated with abiraterone plus eplerenone versus those treated with abiraterone plus prednisone. There was no difference in PFS in those who received eplerenone in place of prednisone (Figure 1).
Table 1.
Baseline Patient Characteristics
| Baseline Characteristics | |||
|---|---|---|---|
| Abi + Eplerenone | Abi + Prednisone | P-value | |
| Number of patients | 40 | 66 | |
| Age (year, median) | 69 (IQR 62.8 – 75.3) | 72 (IQR 66.0 – 77.8) | 0.13 |
| Median PSA (ng/mL) | 34.4 (IQR 20.1 -71.0) | 24.5 (IQR 9.8 – 85.7) | 0.32 |
| Median Gleason score | 8 (IQR 7-9) | 8 (IQR 7-9) | 0.40 |
| Median lines of Tx for CRPC prior to Abi | 1 (IQR, 1-2) | 0 (IQR, 0-1) | < 0.001 |
| Visceral metastasis (%) | 1 (2.5%) | 7 (10.6%) | 0.25 |
| Median Charlson Comorbidity Index (CCI) | 6 (IQR, 6-7) | 7 (IQR, 6-8) | 0.18 |
| Baseline Diabetes | 4 (10.0%) | 13 (19.6%) | 0.28 |
| Baseline BMI | 30.12 | 29.54 | 0.84 |
Abbreviations: Abi, abiraterone; CRPC, castration resistant prostate cancer; IQR, interquartile range; BMI, body mass index; PFS, progression free survival
Figure 1.
Progression-free Survival Between the 2 Treatment Arms
Toxicities of Secondary Mineralocorticoid Excess
No significant differences between hypertension, hypokalemia or lower extremity edema were found comparing prednisone and eplerenone for management of secondary mineralocorticoid excess. Four men required transition from eplerenone to prednisone due to mineralocorticoid excess toxicity. All four men had grade 3 hypertension, and all four continued to have grade 2 hypertension after transitioning to prednisone. Each was successfully managed with optimization of antihypertensive medications on an outpatient basis without hospitalization. Overall, the incidence of grade 1/2 as well as grade 3/4 hypertension were similar in both groups. There were no grade 3 or 4 toxicities from hypokalemia or edema any patient regardless of treatment. Weight loss during therapy was significantly different between the arms with nearly a 5 kilogram loss in the eplerenone arm compared to no change in those treated with prednisone. A complete list of observed toxicities is provided in Table 2.
Table 2.
Toxicities Experienced in the 2 Treatment Arms
| Emergent Variables | |||
|---|---|---|---|
| Abi + Eplerenone | Abi + Prednisone | P-value | |
| Hypertension | 0.61 | ||
| None | 3 (7.5%) | 8 (12.1%) | |
| Grade 1/2 | 31 (77.5%) | 45 (68.2%) | |
| Grade 3/4 | 6 (15%) | 13 (19.7%) | |
| Edema | 0.30 | ||
| None | 31 (77.5%) | 57 (89.4%) | |
| Grade 1/2 | 9 (22.5%) | 9 (13.6%) | |
| Grade 3/4 | 0 (0%) | 0 (0%) | |
| Hypokalemia | 0.38 | ||
| None | 33 (82.5%) | 59 (89.4%) | |
| Grade 1/2 | 7 (17.5%) | 7 (10.6%) | |
| Grade 3/4 | 0 (0%) | 0 (0%) | |
| Any Toxicity | 39 (97.5%) | 61 (93.9%) | 0.41 |
| Median weight change during therapy (kg) | −4.80 (IQR −7.78 to −1.37) | −0.05 (IQR −3.40 to 3.00) | < 0.001 |
| Median PFS (months) | 8.17 (95% CI 7.30-11.6) | 9.97 (95% CI 6.03-14.2) | 0.23 |
Abbreviations: Abi, abiraterone; IQR, interquartile range; PFS, progression free survival
Discussion
Although currently an off-label treatment, in our patient population, eplerenone was well tolerated and provided non-inferior management of secondary mineralocorticoid excess as compared to prednisone.
From inhibition of CYP17A1 and upregulation of ACTH, abiraterone can cause secondary mineralocorticoid excess. Hypertension, hypokalemia and lower extremity edema from secondary mineralocorticoid excess were initially documented in phase I analysis.2 In this study reported in 2008, these manifestations of secondary mineralocorticoid excess were managed with eplerenone. Only 3 of 38 patients required transition to dexamethasone.11 Phase II analyses used either prednisone alone or eplerenone with transition to low-dose glucocorticoids, reporting comparatively less hypokalemia, hypertension and lower extremity edema with prednisone.6, 7 Phase III analyses of abiraterone only used concomitant prednisone and not eplerenone, leading to the current FDA labeling requiring concomitant prednisone.12, 13
Although effective in diminishing secondary mineralocorticoid excess, prolonged administration of prednisone carries increased risk. Hyperglycemia, weight gain and decreased bone mineral density are major concerns. Of 66 men treated with prednisone, 1 patient developed dose-limiting hyperglycemia that improved after transitioning to eplerenone. Although eplerenone was generally well tolerated in phase I and II clinical trials, no evidence has yet been published regarding use of eplerenone for management of secondary mineralocorticoid excess with abiraterone in the real world setting which mostly consist of men with higher number of co-morbidities.
It should be noted that the objective of this study was to specifically compare the incidence and severity of syndrome of mineralocorticoid excess, and that the study was not powered to compare survival outcomes in these two cohorts. However, we did find the PFS intervals which are notably shorter in both arms than PFS durations reported in phase III trials with abiraterone and prednisone before chemotherapy. This likely represents the clinical heterogeneity of real-world analyses, but does provide further insight into the duration of responses expected for men with mCRPC treated with abiraterone. Furthermore, in majority of these patients, PFS was based on PSA progression which also reflects a real world practice where patients often do not wish to pursue imaging studies every three months. Lastly, based on in vitro data, concerns have been raised about androgen receptor (AR) agonist activities of both glucocorticoids as well as eplerenone.14 However, these in vitro experiences have not been tested in the clinical setting.
Our study is limited by its retrospective design, as well as the comparatively limited number of patients included in the study. Additionally, only toxicities secondary to syndrome of mineralocorticoid excess were recorded. Further prospective analyses can definitively determine the equivalency of eplerenone and prednisone for management of secondary mineralocorticoid excess following abiraterone therapy. However, this study provides important insight into the practical clinical experience of management of common side effects of abiraterone in men with mCRPC with abiraterone. Our data suggest prednisone and eplerenone are equally well tolerated and that eplerenone is a viable option in patients unable or unwilling to undergo therapy with prednisone.
Clinical Practice Points
Abiraterone is approved for treatment of CRPC and is commonly used by urologists and medical oncologists. As abiraterone blocks CYP17A1, there is upstream stimulation of ACTH leading to secondary mineralocorticoid excess and a syndrome of hypertension, hypokalemia and edema. Studied in early phase I trials, eplerenone reduced these symptoms through direct antagonism of mineralocorticoids but has never been studied in a real world clinical practice setting. Accordingly, only prednisone is approved for prevention of secondary mineralocorticoid toxicity. In this study of 106 patients treated with abiraterone and either eplerenone or prednisone for CRPC, we retrospectively analyzed rates of hypertension, hypokalemia and edema as well as baseline characteristics and PFS. In both arms, there were no significant differences between baseline characteristics or PFS. Additionally, patients treated with eplerenone has similar incidence and grades of toxicities of mineralocorticoid excess as those treated with prednisone. This study requires external validation as it retrospective and from a single institution, however, this data supports use of eplerenone in patients treated with abiraterone who either decline or cannot tolerate glucocorticoid therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grist E, Attard G. The development of abiraterone acetate for castration-resistant prostate cancer. Urol Oncol. 2015;33:289–294. doi: 10.1016/j.urolonc.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 3.Kluetz PG, Ning YM, Maher VE, et al. Abiraterone acetate in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2013;19:6650–6656. doi: 10.1158/1078-0432.CCR-13-2134. [DOI] [PubMed] [Google Scholar]
- 4.Malangone-Monaco E, Foley K, Varker H, Wilson KL, McKenzie S, Ellis L. Prescribing Patterns of Oral Antineoplastic Therapies Observed in the Treatment of Patients With Advanced Prostate Cancer Between 2012 and 2014: Results of an Oncology EMR Analysis. Clinical Therapeutics. 2016;38:1817–1824. doi: 10.1016/j.clinthera.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Rathkopf DE, Smith MR, De Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). European urology. 2014;66:815–825. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Shah S, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pia A, Vignani F, Attard G, et al. Strategies for managing ACTH dependent mineralocorticoid excess induced by abiraterone. Cancer Treat Rev. 2013;39:966–973. doi: 10.1016/j.ctrv.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 10.R Core Team . A language and environment of statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 11.Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 12.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer research. 2012;72:2176–2182. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]