Summary
Polyunsaturated fatty acids (PUFA) are essential for offspring development, but it is unclear whether pregnancy PUFA status affects growth and adiposity. In 985 mothers from the Singaporean GUSTO cohort, we measured plasma phosphatidylcholine PUFAs at 26-28 weeks’ gestation, including linoleic (LA) and docosahexaenoic (DHA) acid. We assessed the associations with fetal growth, neonatal body composition, abdominal adipose tissue volume, and postnatal growth and skinfold thicknesses. Regression coefficients were presented for 5% increase in PUFA levels. LA levels were positively associated with birthweight (β (95% CI): 0.04 (0.01, 0.08) kg), body mass index (0.13 (0.02, 0.25) kg/m2), and abdominal adipose tissue volume, but not with later outcomes. DHA levels, although not associated with birth outcomes, were related to higher length/height: 0.63 (0.09, 1.16) cm at 12 months and 1.29 (0.34, 2.24) at 5 years. LA was positively associated with neonatal body size, and DHA with child height. Pregnancy PUFA status may influence offspring growth and adiposity.
Keywords: Polyunsaturated fatty acids, fetal growth, infant growth, adiposity, body composition, growth modelling
1. Introduction
Polyunsaturated fatty acids (PUFAs) are classified into two series, omega-6 (n 6) and omega-3 (n 3). The most studied are the long-chain PUFAs (LCPUFAs) arachidonic (AA, 20:4 n 6), eicosapentaenoic (EPA, 20:5 n-3) and docosahexaenoic (DHA, 22:6 n 3) acids, because of their pleiotropic roles, especially in cell membrane structure, cell signaling and gene expression [1]. LCPUFAs can be ingested in the diet (mainly from animal sources) or synthetized from their essential precursors, linoleic (LA, 18:2 n-6) and α-linolenic (ALA, 18:3 n-3) acids, which can only be provided in the diet (mainly from plant sources). LCPUFAs are needed for the normal development of the fetus and the infant, including visual and cognitive development and the immune and cardiovascular systems, but less is known concerning growth and body composition [1, 2]. Yet, adipose tissue accretion begins in utero, and early-life growth plays a role in the life-course risk of obesity and its associated consequences [3, 4].
In randomized controlled trials (RCTs), the supplementation of LCPUFAs or of fish oil (rich in n-3 LCPUFAs) during pregnancy has shown inconclusive effects on offspring birth size, postnatal growth, and adiposity (body mass index (BMI) or body fat percentage) [5, 6]. The supplementation in n-3 LCPUFAs modestly increases birth weight (47 g) and length (0.48 cm), mainly owing to longer gestation [7]. Some RCTs reported that LCPUFA supplementation during pregnancy and/or lactation lowers adiposity later in childhood, but most showed no effect [6]. It remains difficult to draw any firm conclusions, however, because of differences in trial design, especially the amounts, balance and type of LCPUFA given (DHA, EPA+DHA or AA+DHA). Furthermore, the potential effects of their metabolic precursors LA and ALA have rarely been examined.
The results from observational studies are also mixed regarding the association of maternal n 3 or n-6 PUFA exposure with birth size [8, 9], or postnatal adiposity [6, 10, 11]. Again, differences in study design may explain this, particularly the type of outcomes and the age at which they were assessed. Longitudinal studies are scarce but repeated measures of growth and adiposity should help understand whether associations track from the prenatal period to childhood, or whether and when they increase or lessen with age.
Using data from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort, we assessed longitudinally the associations of maternal plasma phosphatidylcholine (PC) PUFA levels during pregnancy with fetal and postnatal growth, and body composition from the third trimester of gestation to 5 years.
2. Material and Methods
2.1. Study design
The GUSTO study is a Singaporean mother-offspring cohort focused on child health and development [12]. Between June 2009 and September 2010, 3751 pregnant women of Chinese, Malay or Indian ethnicity who attended their first antenatal ultrasound scan in two public maternity units (National University Hospital and KK Women’s and Children’s Hospital) were approached, of whom 2034 met eligibility criteria. The main exclusion criteria were intention to deliver out of the study centers, intention to move away from Singapore in the next 5 years, first visit after the first trimester and non-homogeneous ethnic background (including the unborn infant grandparents). The reasons for ineligibility of non-participants have been published [12]. Overall, 1247 pregnant women consented to participate, yielding 1171 singleton births. The study received ethical approval from the National Healthcare Group Domain Specific Review Board and the SingHealth Centralised Institutional Review Board.
2.2. Data collection
Maternal age, pre-pregnancy weight, highest education level (primary/secondary/post-secondary/university), parity (primiparous/multiparous) and monthly household income (<1000/1000-1999/2000-3999/4000-5999/≥6000 Singapore dollars) were obtained by self-administered questionnaire at enrolment. At 26-28 weeks’ gestation, maternal height and weight were measured and blood samples were collected. Fasting glucose and vitamin D levels were determined. Gestational age (determined by ultrasound) at delivery and child sex were obtained from birth records. Paternal weight and height were measured when fathers attended postnatal visits with their children, or were reported by mothers. BMI was calculated from weight (kg) divided by height squared (m2). Child fish consumption at 18 months and fish oil supplementation at 24 and 36 months were obtained by interviewer-administered questionnaires.
2.3. Fatty acid analysis
Plasma PC fatty acids were prepared from fasting blood samples collected at 26-28 week’ gestation and stored at -80°C, as previously described [13]. Briefly, lipid extraction was carried out with chloroform/methanol (Fisher Scientific). PC was separated by solid-phase extraction. PC fatty acid methyl esters were prepared by incubation with methanolic sulphuric acid. They were then separated by gas chromatography (BPX-70 column mounted on a Hewlett-Packard HP6890) and detected by flame ionization before quantification in µg/mL of plasma. For the eleven PUFAs identified, inter- and intra-assay variation coefficients were <6% and <3%, respectively. PUFAs were expressed in percentage of total fatty acids in plasma PC; we focused on five of interest (LA, ALA, AA, EPA and DHA) based on the literature [6]. The other PUFAs, mostly intermediates in the PUFA metabolic pathway, were nevertheless accounted for when summing the levels of PUFAs (≥18 carbons) and LCPUFAs (≥20 carbons) for each series (n-6 and n-3), as markers of the overall nutritional status. Ratios of n-6 to n-3 PUFAs were also analyzed.
2.4. Offspring outcomes
2.4.1. Fetal anthropometric measurements
Ultrasound scans were performed at 26-28 and 32-34 weeks’ gestation by trained ultrasonographers (n = 924 assessed at both visits and with PUFA data). Biparietal diameter, head (HC) and abdominal (AC) circumferences, and femoral length were measured. Fetal weight was estimated using the Hadlock’s four-parameter formula [14].
2.4.2. Postnatal anthropometric measurements
At each visit between birth and 5 years (birth, week 3, month 3, 6, 9, 12, 15, 18, 24, 36, 48, 54 and 60), offspring weight, length/height, HC and AC were measured in duplicate (and averaged) by trained clinic staff using Seca instruments (Seca GmBH & Co Kg, Hamburg, Germany) following standardized procedures [15]. Weight until 18 months was measured to the nearest 1 g using a calibrated mobile digital scale Seca 334, and after 18 months to the nearest 10 g using a digital scale Seca 813. Length (recumbent) and height (standing) were measured to the nearest 1 mm using a mobile infant mat Seca 210 and a stadiometer Seca 213, respectively. At 18 and 24 months, both length and height were measured.
2.4.3. Adiposity and body composition
Skinfold thicknesses were measured (in triplicate, the two closest were averaged) to the nearest 2 mm with skinfold calipers (Holtain Ltd, Crymych, UK): triceps and subscapular (at birth, 18, 24, 36, 48, 54 and 60 months), biceps (from 18 months onward), and suprailiac (from 48 months onward).
Whole body fat and fat-free mass at birth (n = 302 with PUFA data, age range 0-3 days) and age 10 days (n = 252 with PUFA data, age range 5-18 days) were measured with the PEA POD air displacement plethysmograph (COSMED USA Inc, Concord, CA), among the eligible participants (born at ≥34 weeks of gestation, birthweight ≥2000 g, no neonatal complications) who consented.
Neonatal abdominal adipose tissue volumes (n = 317 with PUFA data, age range 0-20 days) were assessed by magnetic resonance imaging (MRI) (Signa HDxt 1.5T, GE Healthcare, Wauwatosa, WI) among the eligible and consenting participants, as previously described [16]. Briefly, neonates were placed in an immobilization bag in supine position and their abdomen was scanned from the diaphragm to the pubic symphysis. Adipose tissue was categorized into three compartments (superficial subcutaneous, deep subcutaneous and internal) by a semi-automated algorithm using MATLAB (version 7.13; The MathWorks Inc., Natick, MA): it performed initial segmentation of subcutaneous and internal compartments which were further analyzed and optimized by two trained image analysts using anatomical judgement. Deep subcutaneous compartment was defined manually. Volumes of each compartment were generated by multiplying the number of voxels within each image slice by the voxel dimensions (except the voxels located at tissue boundaries tallied as half a voxel), and by summing the volumes in each slice. The unit of each compartment volume was expressed both in mL and % of total abdominal volume.
2.5. Postnatal growth modelling
Postnatal growth trajectories in length/height, weight, HC and AC were fitted using the Jenss-Bayley model, a parametric equation designed for child growth [17]. Using a mixed-effect approach, it accounts for heterogeneity in age at measurement, accommodates with missing data, and smooths measurement error. A study among US children showed that the Jenss-Bayley model provides better fit statistics and residual diagnostics than the Reed model, another commonly used parametric model for this period of age [18], a finding also observed with our data (not shown). As described elsewhere [19, 20], the Jenss-Bayley model fits growth from birth to age 6-8 years by assuming that growth increases monotonically following a four-parameter mathematical equation (a, b, c and d). It combines a straight line (a + b ⋅ t) corresponding to growth from 2-3 to 6-8 years, and a negatively accelerated exponential (−ec+d⋅t) allowing fitting growth from birth to 2-3 years. The full equation is interpreted as the jth growth measure of the ith subject; where y is the predicted measure and t is the child age (in days):
Unlike non-structural models, the Jenss-Bayley model provides four interpretable parameters: a is the predicted measure at birth; b is the growth velocity beyond 2-3 years; and c and d are the infant growth spurt magnitude and the decelerating growth rate (the curvature) over the first 2-3 years, respectively. Random effects were added on each parameter, allowing to estimate for each child, the four parameters and the size at any age. Since this model assumes weight increases monotonically, it does not fit the neonatal weight loss; to improve its fit, we did not include the birthweight measure into the modelling, as suggested by Botton et al. [19]. For length/height modelling, we used both length and height measured at 18 and 24 months to smooth the gap caused by the change in measuring method. The equation being non-linear in parameters, the mixed-effect modelling was realized using the R package saemix (version 1.2) [21]. Finally, BMI was derived in two steps using weight (kg) and length/height squared (m2), as predicted by their respective models. Growth modelling was performed in 1107 children, of whom 978 had PUFA data. Supplemental Table 1 shows the number of anthropometric measures and subjects modelled between birth and 5 years. Supplemental Table 2 and 3 show the descriptive statistics of the four parameters, and the fit statistics of the modelling, respectively. Goodness of fit (i.e., residuals according to age) is displayed in Supplemental Fig. 1.
2.6. Statistical Analysis
Flow diagram of mother-offspring pairs is shown in Fig. 1. Associations between maternal plasma PC PUFA levels and standardized Jenss-Bayley parameters were estimated by multivariable linear regression, with a single PUFA per model. Based on the literature and associations observed on our dataset, models were adjusted for the following potential confounders: study center, ethnicity, child sex, familial income, maternal education and age, parity, maternal height and pre-pregnancy BMI, gestational weight gain at 26-28 weeks’ gestation, fasting glucose and vitamin D levels. Models were also adjusted for paternal height and BMI, both predictive of the outcomes, to improve precision of estimates. We did not adjust for gestational age at birth and breast feeding which may be on the causal pathway: LCPUFA supplementation may increase birth size through a longer gestation, and breast milk contains PUFA whose levels depend on maternal status [22]. We tested the interactions of each PUFA with ethnicity and child sex by introducing the appropriate multiplicative terms into our models.
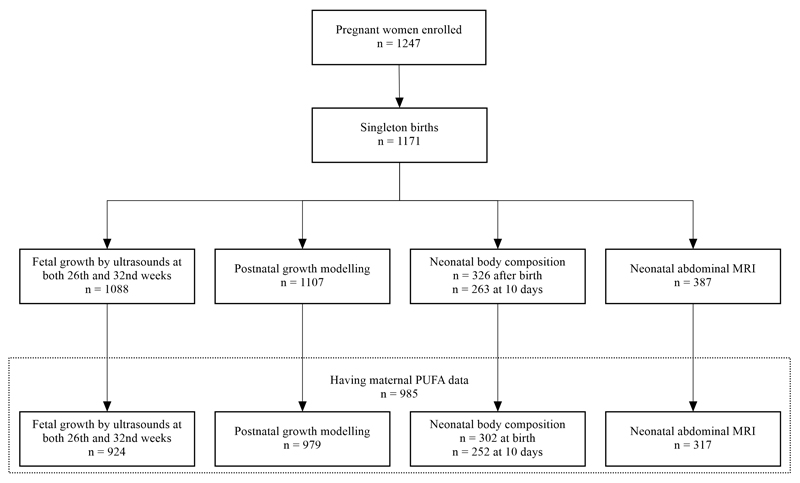
Fig. 1.
Flow diagram of the participants assessed for growth and adiposity between 26th weeks’ gestation and age 5 years in the GUSTO cohort.
Based on our results for the period-specific Jenss-Bayley parameters, we further examined two consistent findings. First, we used multivariable linear regression to examine the associations of LA with outcomes near the birth period, namely fetal growth (ultrasound), neonatal adiposity (skinfold thicknesses), body composition (PEA POD and MRI), and growth till age 1 year. Second, we assessed the associations of DHA with postnatal growth (prediction by Jenss-Bayley modelling at the same ages for all the children) and adiposity (skinfold thicknesses) over the period from birth to 5 years, using generalized estimating equations (GEE) to account for within-subject repeated measurements. GEE covariance matrix structures were selected based on AIC and BIC criteria: first-order ante-dependence matrix for weight, length/height and BMI, and heterogeneous autoregressive matrix for HC and AC, and unstructured matrix for skinfold thicknesses. GEE models with skinfold thicknesses as outcomes were additionally adjusted for exact age. GEE models with weight, HC and AC, and skinfold thicknesses as outcomes were performed with and without adjustment for length/height.
Finally, we performed sensitivity analyses to evaluate whether i) excluding preterm infants, ii) excluding PUFA levels beyond ±4 SD from the mean, and iii) further adjusting for postnatal exposure to dietary n-3 LCPUFAs (fish consumption at 18 months, and fish oil supplementation at 24 and 36 months) had an influence on the estimates.
Statistical analyses were carried out using SAS 9.4 (SAS Institute Inc, Cary, NC) using PROC GLM for linear regressions and PROC MIXED with repeated statement for GEEs. Multiple imputation method (PROC MI) was used to deal with missing paternal height (n = 174) and BMI (n = 181). Regression coefficients (averaged across 10 imputed datasets) were reported for a 5% increase in PUFA levels to achieve a balance between biological plausibility and the number of decimals. We did no adjust for multiple comparisons [23]; the “significance” of our findings was evaluated based on the strength and the consistency of the associations we observed.
3. Results
Characteristics of participants with data both on fatty acids and at least one outcome are described in Supplemental Table 4. Average maternal levels (mean ± SD) of total n-6 and n-3 PUFA in plasma PC were 34.2 ± 3.3% and 6.2 ± 1.9%, respectively (Table 1). LA (21.8 ± 3.4%) and DHA (4.7 ± 1.4%) were the most prominent PUFAs within each series and were negatively correlated (r = -0.10) with each other. Main offspring outcomes from 26 weeks’ gestation to 5 years are summarized in Supplemental Table 5. Mothers who consented for body composition assessments were younger, less educated, and more likely to be Malay; their neonates had thicker skinfolds (not shown). PUFA levels, offspring birth size and parents’ height and BMI were however not differing between participants and non-participants.
Table 1.
Maternal plasma PC PUFA levels and ratios during pregnancy in the GUSTO cohort among participants with a least one outcome assessed (n = 985)1
| Fatty acids | Mean | SD | Range |
|---|---|---|---|
| 18:2 n-6 (LA) | 21.752 | 3.39 | 11.67, 41.32 |
| 20:4 n-6 (AA) | 7.91 | 1.67 | 2.66, 22.87 |
| Σ n-6 PUFA | 34.22 | 3.34 | 22.56, 51.29 |
| Σ n-6 LCPUFA | 12.41 | 2.25 | 6.52, 25.83 |
| 18:3 n-3 (ALA) | 0.21 | 0.14 | 0.01, 1.19 |
| 20:5 n-3 (EPA) | 0.69 | 0.59 | 0.06, 6.41 |
| 22:6 n-3 (DHA) | 4.69 | 1.42 | 1.30, 10.14 |
| Σ n-3 PUFAs | 6.36 | 1.85 | 2.22, 13.97 |
| Σ n-3 LCPUFAs | 6.15 | 1.83 | 2.03, 13.82 |
| LA/ALA | 157.26 | 109.43 | 15.39, 543.7 |
| AA/DHA | 1.84 | 0.67 | 0.63, 5.06 |
| Σ n-6 PUFAs/Σ n-3 PUFAs | 5.90 | 2.02 | 1.93, 16.56 |
| Σ n-6 LCPUFAs/Σ n-3 LCPUFAs | 2.21 | 0.83 | 0.80, 6.39 |
AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; LCPUFAs, long-chain polyunsaturated fatty acids; PUFAs, polyunsaturated fatty acids.
Values are expressed in % of total fatty acids.
Total plasma PC n-6 PUFA levels, driven by LA (the most abundant n-6 PUFA), were positively related to weight, length and HC at birth (Jenss-Bayley parameter a) and negatively with HC growth spurt in infancy (parameter c) (Table 2). DHA, but not the other n-3 PUFAs, was positively associated with length/height at birth (parameter a), growth velocity in weight, height and AC after 2-3 years (parameter b), and earlier weight growth deceleration in infancy (parameter d, more curved trajectory). Higher total n-6 LCPUFA levels were associated with a flatter AC growth rate (parameter d) between birth and 2-3 years. These results were overall consistent when considering ratios of n-6 to n-3 PUFAs (Supplemental Table 6).
Table 2.
Adjusted associations of maternal plasma PC PUFA levels during pregnancy with the standardized parameters a, b, c and d resulting from the Jenss-Bayley growth modelling of weight, length/height, head circumference and abdominal circumference between birth and 5 years in children from the GUSTO cohort. (n = 979)1
| Fatty acids | Weight | Length/Height | HC | AC |
|---|---|---|---|---|
| Parameter a (model-predicted measure at birth2) | ||||
| 18:2 n-6 (LA) | 0.05 (0.01, 0.09) | 0.10 (0.02, 0.18) | 0.12 (0.03, 0.20) | 0.03 (-0.07, 0.12) |
| 20:4 n-6 (AA) | 0.02 (-0.07, 0.10) | 0.07 (-0.10, 0.25) | -0.04 (-0.22, 0.15) | 0.16 (-0.04, 0.37) |
| Σ n-6 PUFAs | 0.05 (0.01, 0.09) | 0.10 (0.01, 0.18) | 0.08 (-0.01, 0.17) | 0.08 (-0.02, 0.18) |
| Σ n-6 LCPUFAs | -0.02 (-0.08, 0.04) | -0.01 (-0.14, 0.12) | -0.10 (-0.23, 0.04) | 0.13 (-0.02, 0.28) |
| 18:3 n-3 (ALA) | 0.06 (-0.90, 1.01) | -0.75 (-2.73, 1.22) | -0.51 (-2.58, 1.56) | 0.98 (-1.32, 3.27) |
| 20:5 n-3 (EPA) | -0.21 (-0.44, 0.02) | -0.48 (-0.95, -0.01) | -0.09 (-0.59, 0.40) | 0.24 (-0.31, 0.79) |
| 22:6 n-3 (DHA) | 0.08 (-0.02, 0.17) | 0.22 (0.02, 0.42) | 0.09 (-0.11, 0.30) | -0.11 (-0.34, 0.12) |
| Σ n-3 PUFAs | 0.03 (-0.05, 0.10) | 0.09 (-0.06, 0.24) | 0.06 (-0.10, 0.22) | -0.02 (-0.20, 0.15) |
| Σ n-3 LCPUFAs | 0.03 (-0.05, 0.10) | 0.10 (-0.06, 0.25) | 0.06 (-0.10, 0.23) | -0.03 (-0.21, 0.15) |
| Parameter b (growth velocity beyond 2-3 years) | ||||
| 18:2 n-6 (LA) | -0.01 (-0.10, 0.08) | 0.00 (-0.09, 0.09) | 0.00 (-0.09, 0.10) | -0.09 (-0.18, 0.00) |
| 20:4 n-6 (AA) | -0.14 (-0.34, 0.05) | -0.01 (-0.20, 0.19) | -0.10 (-0.31, 0.11) | -0.09 (-0.29, 0.11) |
| Σ n-6 PUFAs | -0.07 (-0.16, 0.02) | 0.00 (-0.10, 0.09) | -0.04 (-0.14, 0.06) | -0.13 (-0.23, -0.04) |
| Σ n-6 LCPUFAs | -0.15 (-0.29, 0.00) | -0.04 (-0.18, 0.11) | -0.11 (-0.27, 0.04) | -0.10 (-0.25, 0.04) |
| 18:3 n-3 (ALA) | 0.23 (-1.95, 2.40) | 1.21 (-0.95, 3.37) | 0.14 (-2.21, 2.50) | -0.63 (-2.86, 1.60) |
| 20:5 n-3 (EPA) | -0.55 (-1.07, -0.03) | -0.21 (-0.72, 0.31) | -0.02 (-0.58, 0.55) | -0.08 (-0.62, 0.45) |
| 22:6 n-3 (DHA) | 0.28 (0.07, 0.50) | 0.27 (0.06, 0.49) | 0.07 (-0.17, 0.30) | 0.35 (0.12, 0.57) |
| Σ n-3 PUFAs | 0.13 (-0.04, 0.29) | 0.15 (-0.02, 0.32) | 0.05 (-0.14, 0.23) | 0.20 (0.03, 0.37) |
| Σ n-3 LCPUFAs | 0.13 (-0.04, 0.30) | 0.15 (-0.02, 0.32) | 0.05 (-0.14, 0.23) | 0.21 (0.03, 0.38) |
| Parameter c (growth spurt before 2-3 years) | ||||
| 18:2 n-6 (LA) | 0.03 (-0.06, 0.12) | -0.03 (-0.13, 0.06) | -0.11 (-0.21, -0.02) | 0.08 (-0.01, 0.17) |
| 20:4 n-6 (AA) | 0.04 (-0.16, 0.23) | 0.01 (-0.19, 0.22) | 0.03 (-0.17, 0.24) | -0.17 (-0.37, 0.02) |
| Σ n-6PUFAs | 0.04 (-0.05, 0.14) | -0.06 (-0.15, 0.04) | -0.10 (-0.20, 0.00) | 0.06 (-0.04, 0.15) |
| Σ n-6 LCPUFAs | 0.03 (-0.11, 0.18) | -0.03 (-0.18, 0.12) | 0.05 (-0.10, 0.20) | -0.09 (-0.23, 0.06) |
| 18:3 n-3 (ALA) | 0.57 (-1.65, 2.80) | -2.07 (-4.33, 0.20) | -0.65 (-2.94, 1.64) | 3.44 (1.24, 5.63) |
| 20:5 n-3 (EPA) | 0.52 (-0.01, 1.05) | 0.40 (-0.14, 0.95) | -0.09 (-0.64, 0.45) | 0.04 (-0.49, 0.56) |
| 22:6 n-3 (DHA) | -0.07 (-0.29, 0.16) | 0.01 (-0.22, 0.24) | -0.12 (-0.35, 0.11) | -0.01 (-0.23, 0.21) |
| Σ n-3 PUFAs | 0.05 (-0.13, 0.22) | 0.05 (-0.12, 0.23) | -0.09 (-0.27, 0.09) | 0.05 (-0.12, 0.22) |
| Σ n-3 LCPUFAs | 0.04 (-0.13, 0.22) | 0.07 (-0.11, 0.25) | -0.09 (-0.27, 0.09) | 0.03 (-0.14, 0.21) |
| Parameter d (deceleration rate before 2-3 years) | ||||
| 18:2 n-6 (LA) | 0.05 (-0.04, 0.14) | 0.06 (-0.03, 0.16) | 0.02 (-0.07, 0.11) | 0.04 (-0.05, 0.13) |
| 20:4 n-6 (AA) | -0.14 (-0.34, 0.06) | -0.02 (-0.22, 0.19) | -0.07 (-0.27, 0.13) | -0.19 (-0.39, 0.01) |
| Σ n-6 PUFAs | 0.00 (-0.10, 0.09) | 0.09 (0.00, 0.19) | 0.02 (-0.08, 0.11) | -0.04 (-0.14, 0.05) |
| Σ n-6 LCPUFAs | -0.14 (-0.29, 0.01) | 0.03 (-0.12, 0.18) | 0.00 (-0.15, 0.14) | -0.21 (-0.35, -0.06) |
| 18:3 n-3 (ALA) | 0.36 (-1.89, 2.62) | 2.43 (0.15, 4.71) | -0.76 (-3.00, 1.48) | -1.08 (-3.34, 1.19) |
| 20:5 n-3 (EPA) | -0.85 (-1.39, -0.31) | -0.16 (-0.71, 0.39) | -0.10 (-0.63, 0.44) | -0.39 (-0.93, 0.15) |
| 22:6 n-3 (DHA) | 0.23 (0.00, 0.45) | -0.07 (-0.30, 0.16) | -0.04 (-0.27, 0.18) | 0.20 (-0.03, 0.43) |
| Σ n-3 PUFAs | 0.03 (-0.14, 0.21) | -0.05 (-0.22, 0.13) | -0.03 (-0.21, 0.14) | 0.07 (-0.11, 0.25) |
| Σ n-3 LCPUFAs | 0.03 (-0.14, 0.21) | -0.06 (-0.24, 0.12) | -0.03 (-0.21, 0.15) | 0.08 (-0.10, 0.26) |
Models were adjusted for study center, ethnicity, child's sex, familial income, maternal education and age, parity, fasting glucose levels, vitamin D levels, gestational weight gain at 26-28 weeks’ gestation, maternal height and pre-pregnancy BMI, paternal height and BMI. AA, arachidonic acid; AC, abdominal circumference; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HC, head circumference; LA, linoleic acid; PUFAs, polyunsaturated fatty acids.
Except for parameter a for weight which is not interpretable (see Methods), and was replaced by measured birthweight.
β coefficient (95% CI) are expressed in SD unit per 5% change in PUFA level.
LA levels were positively associated with all measures of fetal growth at 26 and 32 weeks’ gestation, and at birth, with the exception of birth length and AC (Table 3). However, LA was not associated with overall postnatal growth over the first year, since the associations observed at birth faded after 1 month. LA was positively associated with skinfold thicknesses among the infants who attended the 10-day visit, but not in the full sample assessed shortly after birth. LA was positively associated with neonates’ fat-free mass (PEA POD) and abdominal adipose tissue volumes (MRI), especially superficial subcutaneous and internal tissues (Table 3, and Supplemental Table 7 and 8).
Table 3.
Adjusted associations of maternal plasma PC LA levels during pregnancy with offspring fetal and postnatal growth, skinfold thicknesses, body composition and abdominal adipose tissue volumes in neonates from the GUSTO cohort1
| Outcomes | Fetal/infant age | GEE P-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 weeks | 32 weeks | Birth | 10 days | 1 month | 2 months | 3 months | 6 months | 9 months | 12 months | Global P | Pinteraction | |
| Anthropometrics | ||||||||||||
| n = | 924 | 979 | ||||||||||
| Weight2, kg | 0.013 (0.00, 0.02) |
0.03 (0.01, 0.05) |
0.04 (0.01, 0.08) |
0.01 (-0.01, 0.02) |
0.02 (-0.01, 0.05) |
0.03 (-0.02, 0.07) |
0.04 (-0.02, 0.09) |
0.04 (-0.03, 0.11) |
0.04 (-0.04, 0.12) |
0.03 (-0.06, 0.12) |
0.214 | 0.184 |
| Femur Length, cm | 0.02 (0.00, 0.04) |
0.03 (0.01, 0.05) |
- | - | - | - | - | - | - | - | ||
| Length, cm | - | - | 0.12 (-0.04, 0.27) |
0.12 (-0.04, 0.27) |
0.12 (-0.04, 0.28) |
0.12 (-0.05, 0.30) |
0.12 (-0.07, 0.31) |
0.10 (-0.12, 0.32) |
0.07 (-0.16, 0.31) |
0.05 (-0.20, 0.29) |
0.29 | 0.79 |
| BMI, kg/m2 | - | - | 0.13 (0.02, 0.25) |
-0.02 (-0.09, 0.05) |
0.01 (-0.08, 0.09) |
0.03 (-0.08, 0.14) |
0.04 (-0.08, 0.16) |
0.05 (-0.07, 0.17) |
0.04 (-0.08, 0.15) |
0.02 (-0.09, 0.14) |
0.40 | 0.23 |
| HC, cm | 0.11 (0.03, 0.19) |
0.12 (0.03, 0.21) |
0.11 (0.03, 0.19) |
0.10 (0.02, 0.18) |
0.09 (0.01, 0.17) |
0.08 (-0.01, 0.16) |
0.06 (-0.03, 0.15) |
0.04 (-0.05, 0.14) |
0.04 (-0.07, 0.14) |
0.03 (-0.07, 0.13) |
0.12 | 0.12 |
| AC, cm | 0.11 (0.01, 0.20) |
0.17 (0.04, 0.29) |
0.01 (-0.14, 0.15) |
0.10 (0.01, 0.18) |
0.16 (0.02, 0.30) |
0.15 (-0.02, 0.33) |
0.12 (-0.06, 0.30) |
0.07 (-0.11, 0.26) |
0.07 (-0.12, 0.25) |
0.06 (-0.13, 0.25) |
0.19 | 0.46 |
| Skinfold thicknesses | ||||||||||||
| n = | 940 | 369 | ||||||||||
| Triceps, mm | - | - | 0.06 (-0.05, 0.18) |
0.30 (0.09, 0.51) |
- | - | - | - | - | - | ||
| Subscapular, mm | - | - | 0.07 (-0.04, 0.17) |
0.27 (0.06, 0.48) |
- | - | - | - | - | - | ||
| Σ Skinfolds, mm | - | - | 0.13 (-0.07, 0.32) |
0.57 (0.18, 0.96) |
- | - | - | - | - | - | ||
| Body composition | ||||||||||||
| n = | 302 | 252 | ||||||||||
| Weight, kg | - | - | 0.07 (0.02, 0.13) |
0.09 (0.02, 0.16) |
- | - | - | - | - | - | ||
| Fat Free Mass, kg | - | - | 0.06 (0.02, 0.10) |
0.07 (0.02, 0.12) |
- | - | - | - | - | - | ||
| Fat Mass, kg | - | - | 0.01 (-0.01, 0.03) |
0.02 (-0.01, 0.05) |
- | - | - | - | - | - | ||
| Fat Mass, % | - | - | 0.14 (-0.40, 0.67) |
0.25 (-0.52, 1.02) |
- | - | - | - | - | - | ||
| Abdominal adipose tissue volumes | ||||||||||||
| n = | 317 | |||||||||||
| Total, mL | - | - | - | 6.4 (1.6, 11.2) |
- | - | - | - | - | - | ||
| Superficial Subcutaneous, mL | - | - | - | 4.6 (1.3, 7.8) |
- | - | - | - | - | - | ||
| Superficial Subcutaneous, % total | - | - | - | -0.01 (-0.7, 0.6) |
- | - | - | - | - | - | ||
| Deep Subcutaneous, mL | - | - | - | 0.6 (-0.2, 1.5) |
- | - | - | - | - | - | ||
| Deep Subcutaneous, % total | - | - | - | -0.1 (-0.5, 0.3) |
- | - | - | - | - | - | ||
| Internal, mL | - | - | - | 1.2 (0.1, 2.4) |
- | - | - | - | - | - | ||
| Internal, % total | - | - | - | 0.1 (-0.5, 0.7) |
||||||||
Models were adjusted for study center, ethnicity, child sex, familial income, maternal education and age, parity, fasting glucose levels, vitamin D levels, gestational weight gain at 26-28 weeks’ gestation, maternal height and pre-pregnancy BMI, paternal height and BMI. AC, abdominal circumference; GEE, generalized estimating equation; HC, head circumference; LA, linoleic acid.
Weight at 26 and 32 weeks’ gestation was estimated from ultrasound measures and Hadlock’s formula (n = 924), weight at birth is measured, and weight from 7 days to 12 months is predicted by the Jenss-Bayley model (n = 979).
β coefficients (95% confidence interval) are expressed in outcome unit per 5% increase in LA level.
GEEs P-values: global P represents overall effects of LA levels on postnatal growth outcomes, P for interaction is for interaction term between LA levels and infant age.
DHA was associated with postnatal length/height over the entire period from birth to 5 years (global P = 0.01) with no evidence of change of the effect over time (P for interaction with age = 0.29): for a 5% level increase, length/height was 0.63 (0.09, 1.16) cm and 1.29 (0.34, 2.24) cm higher at 1 and 5 years, respectively (Table 4). No associations were found with weight, BMI or HC. DHA levels were associated with larger AC and thicker triceps and biceps skinfolds (not shown), but no longer after adjusting for length/height (Table 4).
Table 4.
Adjusted associations of maternal plasma PC DHA levels during pregnancy with offspring’s postnatal growth and adiposity from birth to 5 years in children from the GUSTO cohort1
| Outcome | Age | GEE P-values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth | 12 months | 18 months | 24 months | 36 months | 48 months | 54 months | 60 months | Global P | Pinteraction | |
| Anthropometrics (model-predicted) | ||||||||||
| n = | 979 | |||||||||
| Weight, kg | -0.032 (-0.09, 0.04) |
-0.02 (-0.18, 0.13) |
-0.02 (-0.21, 0.17) |
0.00 (-0.23, 0.23) |
0.08 (-0.25, 0.41) |
0.17 (-0.28, 0.62) |
0.21 (-0.30, 0.73) |
0.26 (-0.32, 0.84) |
0.603 | 0.603 |
| Length/Height, cm | 0.20 (-0.17, 0.57) |
0.63 (0.09, 1.16) |
0.72 (0.11, 1.32) |
0.80 (0.14, 1.46) |
0.97 (0.21, 1.72) |
1.13 (0.28, 1.98) |
1.21 (0.31, 2.11) |
1.29 (0.34, 2.24) |
0.01 | 0.29 |
| BMI, kg/m2 | 0.20 (-0.09, 0.49) |
-0.04 (-0.31, 0.22) |
-0.04 (-0.32, 0.23) |
-0.02 (-0.32, 0.28) |
0.04 (-0.31, 0.40) |
0.10 (-0.30, 0.50) |
0.12 (-0.30, 0.54) |
0.14 (-0.30, 0.57) |
0.69 | 0.32 |
| HC, cm | 0.02 (-0.45, 0.49) |
-0.06 (-0.24, 0.13) |
-0.08 (-0.26, 0.11) |
-0.08 (-0.28, 0.11) |
-0.09 (-0.29, 0.11) |
-0.09 (-0.32, 0.14) |
-0.09 (-0.37, 0.19) |
-0.09 (-0.50, 0.31) |
0.59 | 0.80 |
| AC, cm | -0.32 (-1.38, 0.74) |
-0.15 (-0.50, 0.20) |
-0.13 (-0.49, 0.23) |
-0.08 (-0.47, 0.32) |
0.06 (-0.44, 0.57) |
0.21 (-0.47, 0.88) |
0.28 (-0.62, 1.18) |
0.35 (-1.24, 1.95) |
0.93 | 0.38 |
| Skinfold thicknesses | ||||||||||
| n = | 981 | |||||||||
| Triceps, mm | 0.03 (-0.23, 0.30) |
- | -0.20 (-0.65, 0.24) |
-0.30 (-0.76, 0.16) |
0.08 (-0.45, 0.61) |
0.14 (-0.51, 0.79) |
0.51 (-0.19, 1.21) |
0.33 (-0.43, 1.09) |
0.69 | 0.30 |
| Biceps, mm | - | - | -0.14 (-0.55, 0.27) |
0.37 (0.00, 0.74) |
-0.09 (-0.49, 0.31) |
0.04 (-0.44, 0.53) |
0.32 (-0.21, 0.86) |
0.36 (-0.20, 0.92) |
0.39 | 0.09 |
| Subscapular, mm | 0.04 (-0.20, 0.28) |
- | -0.21 (-0.56, 0.13) |
-0.19 (-0.56, 0.18) |
-0.20 (-0.66, 0.26) |
-0.04 (-0.66, 0.58) |
0.29 (-0.47, 1.04) |
0.21 (-0.56, 0.99) |
0.95 | 0.48 |
| Suprailiac, mm | - | - | - | - | - | 0.65 (0.02, 1.28) |
0.31 (-0.41, 1.04) |
0.74 (-0.03, 1.51) |
0.09 | 0.22 |
Models were adjusted for study center, ethnicity, child sex, familial income, maternal education and age, parity, fasting glucose levels, vitamin D levels, gestational weight gain at 26-28 weeks’ gestation, maternal height and pre-pregnancy BMI, paternal height and BMI. Models explaining weight, head circumference, abdominal circumference and skinfold thicknesses were additionally adjusted for length/height. AC, abdominal circumference; DHA, docosahexaenoic acid; GEE, generalized estimating equation; HC, head circumference.
β coefficients (95% confidence interval) are expressed in outcome unit per 5% increase in DHA level.
Global P represents overall effect of DHA levels on the outcome over the entire period of age. Pinteraction is for the interaction term between DHA levels and child age, i.e., the change of effect of DHA levels on the outcome over age.
AA, ALA, EPA and DHA levels were not associated with neonatal body composition (Supplemental Table 7 and 8). No interactions were found between PUFA levels and offspring sex or ethnicity. Our sensitivity analyses demonstrated a similar direction and strength of associations (not shown). In models further adjusted for postnatal n-3 LCPUFA exposure, this was not associated with offspring outcomes.
4. Discussion
We found positive associations of maternal plasma PC LA levels with fetal growth, birth size, fat-free mass and abdominal adipose tissue volumes. Higher maternal plasma PC DHA levels were associated with greater length/height on the overall period from birth to 5 years, without age-dependent effect modification. Higher DHA levels were also associated with larger AC and thicker skinfolds, but this was attributable to bigger body size. DHA status was not associated with other fetal or postnatal growth outcomes. The associations did not differ by sex or ethnicity and were robust to sensitivity analyses.
Our study has several strengths. First, we investigated a wide range of growth and body composition outcomes in mother-offspring dyads followed from early gestation to 5 years, with a relatively low attrition. Second, repeated growth and adiposity measures allowed us to analyze the data in a longitudinal way with the Jenss-Bayley model and GEEs. These methods account for multicollinearity and incomplete data and decrease measurement error, leading to more accurate estimates, an optimized sample size and improved statistical power. Lastly, the assessment of neonatal body composition by PEA POD and abdominal MRI is uncommon in birth cohorts and showed a novel in link with PUFA exposure.
Our main study limitation is that the neonatal body composition assessment was limited (for feasibility reasons) to a subsample which slightly differed from the non-participants; this might explain stronger effect sizes observed for the skinfold measures at 10 days (subsample) compared to birth (full sample). Another limitation concerns the goodness of fit for length/height, HC and AC modelling, which was not as great as for weight, since we observed a dependency in the residuals across time. In length/height modelling, residuals were wider at early ages than at older ones, and for AC, the overall systematic error was relatively high (1.9 cm). This may be due to a lower precision in measures. Nonetheless, this is unlikely to have affected our results to an important extent, since we obtained similar effect sizes (but on less subjects) using growth data as measured instead of model predictions.
The measurement of PUFA exposure from maternal plasma PC level at 26-28 weeks’ gestation may not fully capture fetal exposure throughout pregnancy, and PC fatty acid composition reflects only 75% of plasma phospholipids, which in turn reflect only a portion of overall fatty acids in plasma. To overcome this limitation, we expressed fatty acids in percentage instead of absolute concentration. Moreover, maternal PUFA status is moderately correlated with fetal levels, since the placenta transfers maternal PUFAs to the fetal circulation not only through passive diffusion, but also through selective mechanisms targeting LCPUFAs, especially DHA [24]. Using cord blood PUFA, not available in our study, could result in different findings, as shown in another study [10]. Nonetheless, our exposure variable remains an objective measure collected when the fetal need for LCPUFAs is high [25].
We found positive associations between LA and fetal growth, neonatal body composition and postnatal growth until 1-2 months, but not at later ages. Animal studies have shown that high LA intake during pregnancy and lactation promotes weight gain and adipose tissue accretion [26–28]. These studies also suggest that LA intake may increase the placental uptake of the LA metabolite AA, which then stimulates the pro-inflammatory prostacyclin pathway in the fetus, thereby increasing weight gain and adipose tissue accretion. The absence of association with postnatal growth in our study, however, suggests a short-term effect of prenatal LA exposure on fetal growth and birth outcomes, which diminishes postnatally owing to variation in postnatal LA intake. The relationship between DHA and offspring growth has been more investigated in the literature. RCTs suggest that prenatal DHA may have a marginal effect on birth size [29], and no effect on postnatal growth [30–32]. Nevertheless, RCTs have often been underpowered to detect modest effect sizes, and variability in their designs calls for cautious interpretation. For instance, the placebos used often contain vegetable oil with high LA concentrations (soybean and corn oil) and may have other effects than a real placebo [30, 32]. Baseline maternal PUFA status is also rarely accounted for, and the efficiency of the supplements is not always assessed. In line with the findings of the current study and those of another cohort study, a RCT observed a positive correlation of the increase in DHA status over gestation with postnatal growth [11, 33]. How DHA might promote growth remains unclear. A study in adolescents has observed that DHA supplementation increases the level of insulin-like growth factor-1, a mediator of growth hormone [34]. It could also involve bone growth mechanisms, since DHA inhibits osteoclastogenesis [35]. Adult height in Greenland and European populations has been related to genetic variants in the fatty acid desaturase genes [36], which encode for desaturases that catalyze DHA synthesis from ALA. Together with our findings, this suggests that enhanced endogenous synthesis of DHA, but not of other n-3 PUFAs, may stimulate statural growth. Whether prenatal DHA exposure can have a long-lasting effect on growth remains unknown. Maternal status may actually reflect shared dietary habits and genetic inheritance, and thus be a proxy for offspring status. In our study, this latter measure was not available, which constitutes a further limitation. Although in sensitivity analyses adjusting for postnatal exposure to dietary n-3 LCPUFA did not change our results, residual confounding may remain.
We measured fatty acids in maternal plasma PC. DHA in plasma PC may originate either from the diet, from pre-existing maternal stores of DHA, or from endogenous synthesis from ALA. It is well described that alterations in intake of DHA are reflected in changes in DHA in plasma PC [37]. LA is an essential fatty acid and must come from the diet. Thus, LA in plasma PC must originate from the diet or from pre-existing maternal stores of LA. The relationship between variation in dietary LA intake and plasma PC LA is, however, not well described in the literature.
5. Conclusions
To conclude, we found associations between plasma LA status during pregnancy and fetal growth, neonatal body size and body composition, and between DHA status and child height. No consistent associations were found with the other PUFAs studied. Although replication is needed, our findings suggest that maternal PUFA intake and/or metabolism during pregnancy may influence fetal and later child growth. Our study may help refine current dietary recommendations on LA and DHA intakes for pregnant women.
Supplementary Material
6. Acknowledgements
We thank the members of the GUSTO study group: Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Carolina Un Lam, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Xiu Ling Loo, Fabian Yap, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Izzuddin Bin Mohd Aris, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Peter D. Gluckman, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, S. Sendhil Velan, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan, Yung Seng Lee and Zhongwei Huang.
Sources of financial support: This research was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding of the present study was provided by the Singapore Institute for Clinical Sciences, A*STAR and Nestec. KMG and PCC are supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and KMG is supported by the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement number 289346. The sponsors were not involved in study design, data collection, analysis and interpretation, manuscript writing, and the decision to submit the article for publication.
Abbreviations
- AA
arachidonic acid
- AC
abdominal circumference
- ALA
α-linolenic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FADS
fatty acid desaturase
- GEE
generalised estimating equation
- GUSTO
growing up in Singapore towards healthy outcomes
- HC
head circumference
- LA
linoleic acid
- LCPUFA
long chain polyunsaturated fatty acids
- PC
phosphatidylcholine
- PUFA
polyunsaturated fatty acids
- RCT
randomized controlled trial
Footnotes
Conflict of interest Statement: LS, YSC, PDG, KMG and YSL have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. They are part of an academic consortium that has received research funding from Abbott Nutrition, Nestle, and Danone. All other authors have nothing to disclose.
Clinical Trial Registration Number: This study was registered at clinicaltrials.gov as NCT01174875.
References
- [1].Uauy R, Mena P, Rojas C. Essential fatty acids in early life: structural and functional role. Proc Nutr Soc. 2000;59:3–15. doi: 10.1017/s0029665100000021. [DOI] [PubMed] [Google Scholar]
- [2].Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36:5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- [3].Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, Brug J. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11:695–708. doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- [5].Delgado-Noguera MF. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeedingmothers for improving child growth and development (Review) Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007901.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Voortman T, van den Hooven EH, Braun KV, van den Broek M, Bramer WM, Chowdhurry R, Franco OH. Effects of polyunsaturated fatty acid intake and status during pregnancy, lactation, and early childhood on cardiometabolic health: A systematic review. Prog Lipid Res. 2015 doi: 10.1016/j.plipres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- [7].Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006:CD003402. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- [8].Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Drouillet P, Forhan A, De Lauzon-Guillain B, Thiebaugeorges O, Goua V, Magnin G, Schweitzer M, Kaminski M, Ducimetiere P, Charles MA. Maternal fatty acid intake and fetal growth: evidence for an association in overweight women. The 'EDEN mother-child' cohort (study of pre- and early postnatal determinants of the child's development and health) Br J Nutr. 2009;101:583–591. doi: 10.1017/S0007114508025038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr. 2011;93:780–788. doi: 10.3945/ajcn.110.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moon RJ, Harvey NC, Robinson SM, Ntani G, Davies JH, Inskip HM, Godfrey KM, Dennison EM, Calder PC, Cooper C, S.W.S.S. Group Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab. 2013;98:299–307. doi: 10.1210/jc.2012-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stunkel W, Holbrook JD, Kwek K, Chong YS, Saw SM, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- [13].Lim WY, Chong M, Calder PC, Kwek K, Chong YS, Gluckman PD, Godfrey KM, Saw SM, Pan A, G.S. Group Relations of plasma polyunsaturated Fatty acids with blood pressures during the 26th and 28th week of gestation in women of Chinese, Malay, and Indian ethnicity. Medicine (Baltimore) 2015;94:e571. doi: 10.1097/MD.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- [15].Aris IM, Soh SE, Tint MT, Saw SM, Rajadurai VS, Godfrey KM, Gluckman PD, Yap F, Chong YS, Lee YS. Associations of gestational glycemia and prepregnancy adiposity with offspring growth and adiposity in an Asian population. Am J Clin Nutr. 2015;102:1104–1112. doi: 10.3945/ajcn.115.117614. [DOI] [PubMed] [Google Scholar]
- [16].Tint MT, Fortier MV, Godfrey KM, Shuter B, Kapur J, Rajadurai VS, Agarwal P, Chinnadurai A, Niduvaje K, Chan YH, Aris IB, et al. Abdominal adipose tissue compartments vary with ethnicity in Asian neonates: Growing Up in Singapore Toward Healthy Outcomes birth cohort study. Am J Clin Nutr. 2016;103:1311–1317. doi: 10.3945/ajcn.115.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jenss RM, Bayley N. A mathematical method for studying the growth of a child. Hum Biol. 1937;9:556–563. [Google Scholar]
- [18].Regnault N, Gillman MW, Kleinman K, Rifas-Shiman S, Botton J. Comparative study of four growth models applied to weight and height growth data in a cohort of US children from birth to 9 years. Ann Nutr Metab. 2014;65:167–174. doi: 10.1159/000365894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Botton J, Scherdel P, Regnault N, Heude B, Charles MA. Eden Mother-Child Cohort Study Group, Postnatal weight and height growth modeling and prediction of body mass index as a function of time for the study of growth determinants. Ann Nutr Metab. 2014;65:156–166. doi: 10.1159/000362203. [DOI] [PubMed] [Google Scholar]
- [20].Carles S, Charles MA, Forhan A, Slama R, Heude B, Botton J, Emcs group A Novel Method to Describe Early Offspring Body Mass Index (BMI) Trajectories and to Study Its Determinants. PLoS One. 2016;11:e0157766. doi: 10.1371/journal.pone.0157766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Comets E, Lavenu A, Lavielle M. SAEMIX, an R version of the SAEM algorithm. 20th meeting of the Population Approach Group in Europe; Athens, Greece. 2011. [Google Scholar]
- [22].Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- [23].Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- [24].Gil-Sanchez A, Koletzko B, Larque E. Current understanding of placental fatty acid transport. Curr Opin Clin Nutr Metab Care. 2012;15:265–272. doi: 10.1097/MCO.0b013e3283523b6e. [DOI] [PubMed] [Google Scholar]
- [25].Kuipers RS, Luxwolda MF, Offringa PJ, Boersma ER, Dijck-Brouwer DA, Muskiet FA. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot Essent Fatty Acids. 2012;86:13–20. doi: 10.1016/j.plefa.2011.10.012. [DOI] [PubMed] [Google Scholar]
- [26].Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, Guesnet P, Amri EZ, Negrel R, Ailhaud G. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res. 2003;44:271–279. doi: 10.1194/jlr.M200346-JLR200. [DOI] [PubMed] [Google Scholar]
- [27].Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, Mohsen-Kanson T, Amri EZ, Ailhaud G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res. 2010;51:2352–2361. doi: 10.1194/jlr.M006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alvheim AR, Torstensen BE, Lin YH, Lillefosse HH, Lock EJ, Madsen L, Froyland L, Hibbeln JR, Malde MK. Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids. 2014;49:59–69. doi: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Makrides M, Gibson RA, Udell T, Ried K, L.I. International Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am J Clin Nutr. 2005;81:1094–1101. doi: 10.1093/ajcn/81.5.1094. [DOI] [PubMed] [Google Scholar]
- [30].Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics. 2001;108:E82. doi: 10.1542/peds.108.5.e82. [DOI] [PubMed] [Google Scholar]
- [31].Lucia Bergmann R, Bergmann KE, Haschke-Becher E, Richter R, Dudenhausen JW, Barclay D, Haschke F. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J Perinat Med. 2007;35:295–300. doi: 10.1515/JPM.2007.085. [DOI] [PubMed] [Google Scholar]
- [32].Gonzalez-Casanova I, Stein AD, Hao W, Garcia-Feregrino R, Barraza-Villarreal A, Romieu I, Rivera JA, Martorell R, Ramakrishnan U. Prenatal Supplementation with Docosahexaenoic Acid Has No Effect on Growth through 60 Months of Age. J Nutr. 2015;145:1330–1334. doi: 10.3945/jn.114.203570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bergmann RL, Bergmann KE, Richter R, Haschke-Becher E, Henrich W, Dudenhausen JW. Does docosahexaenoic acid (DHA) status in pregnancy have any impact on postnatal growth? Six-year follow-up of a prospective randomized double-blind monocenter study on low-dose DHA supplements. J Perinat Med. 2012;40:677–684. doi: 10.1515/jpm-2012-0080. [DOI] [PubMed] [Google Scholar]
- [34].Damsgaard CT, Molgaard C, Matthiessen J, Gyldenlove SN, Lauritzen L. The effects of n-3 long-chain polyunsaturated fatty acids on bone formation and growth factors in adolescent boys. Pediatr Res. 2012;71:713–719. doi: 10.1038/pr.2012.28. [DOI] [PubMed] [Google Scholar]
- [35].Akiyama M, Nakahama K, Morita I. Impact of docosahexaenoic acid on gene expression during osteoclastogenesis in vitro--a comprehensive analysis. Nutrients. 2013;5:3151–3162. doi: 10.3390/nu5083151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jorgensen ME, Korneliussen TS, Gerbault P, Skotte L, Linneberg A, Christensen C, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–1347. doi: 10.1126/science.aab2319. [DOI] [PubMed] [Google Scholar]
- [37].Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.