Abstract
In May 2016, the Division of Cancer Prevention and the Division of Cancer Control and Population Sciences, National Cancer Institute, convened a workshop to discuss a conceptual framework for identifying and genetically testing previously diagnosed but unreferred patients with ovarian cancer and other unrecognized BRCA1 or BRCA2 mutation carriers to improve the detection of families at risk for breast or ovarian cancer. The concept, designated Traceback, was prompted by the recognition that although BRCA1 and BRCA2 mutations are frequent in women with ovarian cancer, many such women have not been tested, especially if their diagnosis predated changes in testing guidelines. The failure to identify mutation carriers among probands represents a lost opportunity to prevent cancer in unsuspecting relatives through risk-reduction intervention in mutation carriers and to provide appropriate reassurances to noncarriers. The Traceback program could provide an important opportunity to reach families from racial, ethnic, and socioeconomic groups who historically have not sought or been offered genetic counseling and testing and thereby contribute to a reduction in health disparities in women with germline BRCA mutations. To achieve an interdisciplinary perspective, the workshop assembled international experts in genetics, medical and gynecologic oncology, clinical psychology, epidemiology, genomics, cost-effectiveness modeling, pathology, bioethics, and patient advocacy to identify factors to consider when undertaking a Traceback program. This report highlights the workshop deliberations with the goal of stimulating research and providing a framework for pilot studies to assess the feasibility and ethical and logistical considerations related to the development of best practices for implementation of Traceback studies.
CHALLENGES AND OPPORTUNITIES
Since 2007, the National Comprehensive Cancer Network guidelines have recommended the offering of genetic counseling for BRCA1 and BRCA2 mutation testing to women with ovarian cancer.1 This guideline offers a critical opportunity to identify BRCA1/2 mutation carriers who lack a family history of breast or ovarian cancer and would not have been offered genetic testing previously. The potential impact of this approach is demonstrated by data that show that 44% of 141 women with nonmucinous ovarian cancer positive for BRCA1/2 mutations did not report a family history of breast or ovarian cancer.2 Anecdotally, clinicians describe newly diagnosed BRCA1/2-related cancers in families in which relatives with breast and/or ovarian cancer were never tested for mutations. These findings highlight missed opportunities for cancer prevention and risk management through breast cancer screening, prophylactic mastectomy, and risk-reducing salpingo-oophorectomy.3-6
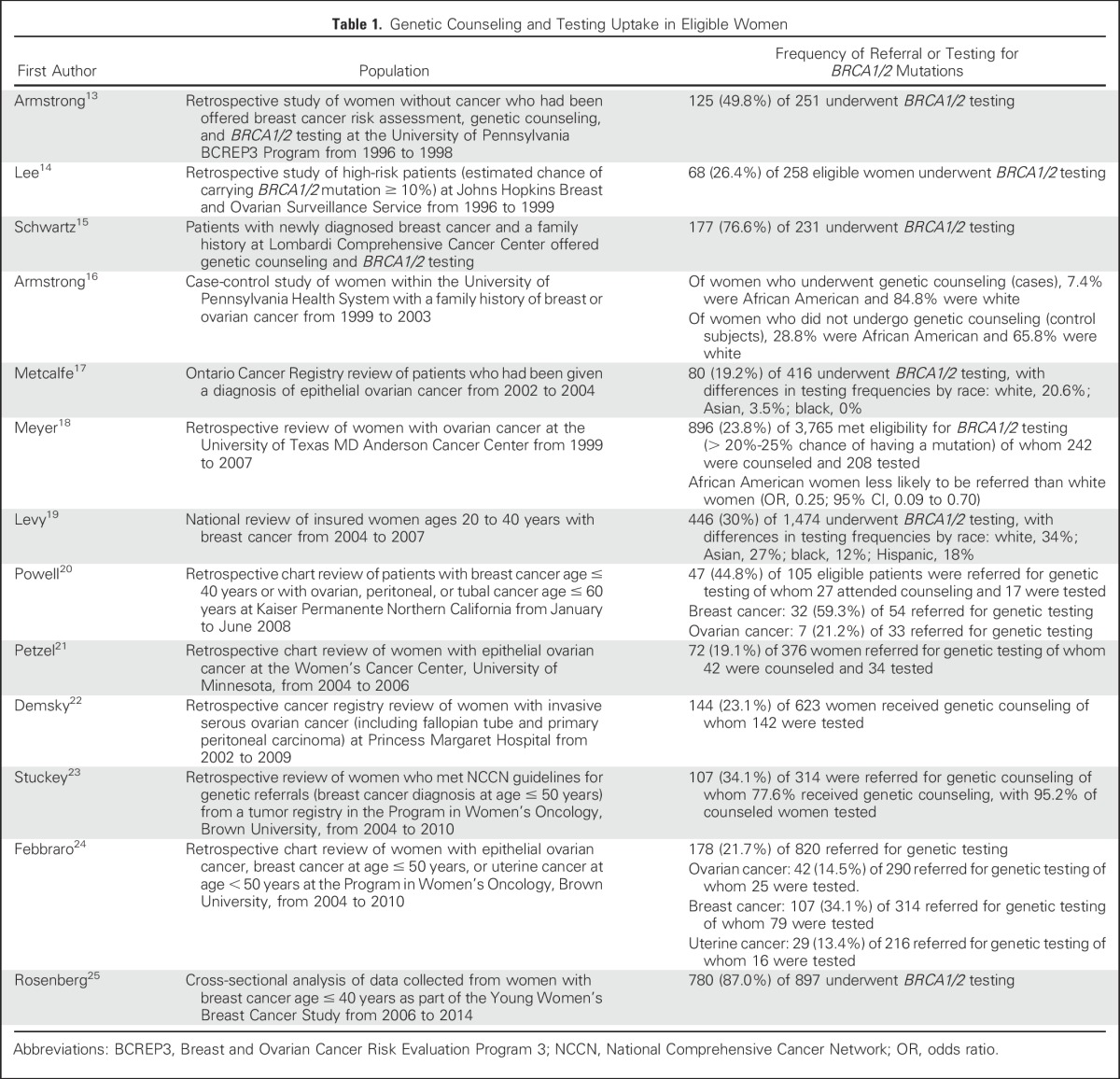
Women unselected for family history of breast or ovarian cancer with pathogenic BRCA1/2 mutations have a 45% to 65% risk for breast cancer and an 11% to 59% risk for ovarian cancer by age 70 years.7-9 High-grade ovarian, fallopian tube, and peritoneal cancers are important sentinel cancers for BRCA1/2 carriers, with germline mutations found in 15% of unselected women.10 Less information exists about the prevalence of BRCA1/2 mutations in nonwhite populations; in a study of the data repository of Myriad Genetics, BRCA1/2 pathogenic mutations were found in 14.8%, 15.6%, 12.7%, and 13.2% of tested Latin American, African, Asian, and Native American women, respectively.11 However, opportunities to counsel and test individuals at high risk of carrying BRCA1/2 mutations often are missed, especially among minority groups.12 On average, only approximately 20% to 30% of patients with cancer at high risk for BRCA1/2 mutations undergo genetic testing, with lower percentages among women with ovarian cancer (Table 1). This modest uptake of testing likely reflects a lack of referral, access, and follow-through by patients. Although referral rates for genetic testing have increased in the United States since 2004,18,19,24 it is estimated that only 48,700 of > 348,000 women who are BRCA1/2 mutation carriers have been identified.26 Approximately 220,000 of these BRCA1/2 carriers have not been given a diagnosis of cancer, which indicates challenges with regard to awareness of genetic risk and cascade testing of unaffected relatives. Given that reliance on self-referral, physician referral, and communication within families is not sufficient, a more active approach is needed to identify at-risk individuals.27
Table 1.
Genetic Counseling and Testing Uptake in Eligible Women
Genetic counseling and testing for BRCA1/2 mutation carriers have emerged as a health disparity issue.28 Genetic testing varies across generations, ethnic groups, socioeconomic classes, and geographic regions with varying access to health services.29,30 This disparity is partly driven by lack of awareness of the value of testing, poor understanding of risk in relation to family history, lack of referral, and lack of capacity for genetic counseling and testing.31 Reaching out to disadvantaged populations to offer BRCA1/2 mutation testing represents an important goal particularly because these women have worse outcomes.30
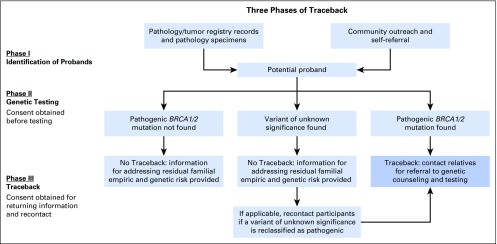
A Traceback approach of retrospective identification of mutation carriers provides an opportunity to offer informative genetic counseling, testing, and cancer risk assessments to probands and their family members (Appendix Table A1, online only). Furthermore, the approaches developed for retrospective identification of BRCA1/2 mutations among patients with ovarian cancer could be applied to other actionable high-penetrance mutations. However, substantial logistical, ethical, legal, social, and clinical challenges are associated with genetic testing of previously diagnosed and unreferred patients and communicating results to family members (Appendix Table A2, online only). Accordingly, the Traceback workshop and this report were organized around three interconnected themes that are summarized in Figure 1: (1) strategies to ascertain probands who carry pathogenic BRCA1/2 mutations; (2) approaches for molecular testing, including the scope of genetic testing and reporting; and (3) ethical considerations related to obtaining permission to perform genetic testing and communication of risk information to relatives.
Fig 1.
Three phases of Traceback. Phase I: potential previously diagnosed, unreferred probands are identified through searches of pathology or tumor registry records or through self-referral. Phase II: consent is obtained for BRCA1/2 genetic testing according to method used to identify potential probands. If proband is living and contactable, direct consent is obtained, and blood is tested in a clinically and molecularly certified laboratory. If archived pathology specimen is used to test potential proband because individual is deceased or cannot be contacted, consent is sought from next of kin (which also allows investigators to determine whether family members have already been tested). Phase III: variants of unknown significance are by definition not clinically actionable and, thus, should not be considered with respect to decision making. As such, a Traceback approach to genetic testing should only return pathogenic or likely pathogenic variants.32 If the potential proband is confirmed to have a BRCA1/2 pathogenic mutation, cooperation of the proband or next of kin is enlisted to reach relatives to offer education, counseling, and testing. If potential proband is not found to carry a BRCA1/2 pathogenic mutation or is found to have a variant of unknown significance, participants are informed about residual familial empirical and genetic risk. If a variant of unknown significance is later reclassified as a pathogenic mutation, relatives are contacted to offer education, counseling, and testing.
Identification of Probands to Reach Untested Individuals and Their Families
Germline BRCA1/2 mutations are particularly prevalent in epithelial ovarian cancers, which are efficient sentinel cancers to detect carriers among family members. Once identified, a carrier provides an entry point for reaching other family members who could potentially benefit from genetic counseling, genetic testing and interpretation of results, and appropriate risk-management strategies.
Several factors influence the feasibility, acceptability, and potential utility of genetic testing a proband’s blood or pathology specimen to determine BRCA1/2 mutation status: the value of identifying or ruling out a pathogenic mutation; access to genetic testing, counseling services, and future risk-reduction strategies, particularly for underserved populations; the ability to obtain consent for genetic testing; and the ability and willingness of probands to facilitate contact with relatives. Although a Traceback approach could be applicable to a number of BRCA1/2-associated cancers, workshop participants suggested the piloting of approaches with women with previous high-grade ovarian, fallopian tube, or peritoneal cancer because 15% of cases carry BRCA1/2 mutations in unselected populations.10 With feasibility and best-practice data derived from such a study, the approach could be extended to other predisposition genes and tumor types.
Approaches for identifying these probands include a search of pathology records or tumor registry databases, community engagement campaigns, and self-referral on the basis of family (and/or personal) cancer history. Each approach poses specific ethical, legal, and logistical issues, including whether the individual is already a candidate for genetic testing according to current guidelines, procedures needed to obtain consent to perform genetic testing, and the proband’s vital status and availability for contact (as described in the section on Ethics and Privacy Considerations).
Identification of probands by using pathology records and tumor registry data.
Record searches may identify patients with cancer who have not undergone genetic testing and are unlikely to seek genetic counseling. For patients who can be contacted, informed consent and blood for germline genetic testing may be possible to obtain, or they could be directed to a high-risk clinic for genetic counseling. However, high-grade ovarian, fallopian tube, and peritoneal cancer have a high fatality rate, and many individuals may be deceased. DNA derived from normal tissues in archived pathology specimens should be suitable for assessing germline BRCA1/2 mutation status, although the sensitivity and specificity of this approach relative to more-conventional testing remain unclear.33,34
Evidence suggests that many women with high-grade ovarian, fallopian tube, or peritoneal cancers should be viewed as a single disease.35,36 Thus, patients with these cancers are appropriate candidates for genetic testing. In addition, this consideration may extend to individuals with serous tubal intraepithelial carcinoma, the presumptive precursor of ovarian, tubal, and peritoneal carcinomas.37,38 Histopathologic subtyping of these cancers is not entirely reproducible, and diagnostic practices have changed over time and differ among institutions39; in particular, cancers classified as endometrioid, serous, mixed, and undifferentiated have been inconsistently distinguished. Accordingly, consideration of inclusion of all nonmucinous high-grade ovarian, tubal, and peritoneal carcinomas would be appropriate. By contrast, genetic testing of nonepithelial ovarian cancers, low-grade carcinomas, or borderline (low malignant potential) neoplasms is unlikely to identify additional probands.
Diagnostic formalin-fixed paraffin-embedded surgical pathology blocks stored under adequate conditions and linked to medical records are the most appropriate biospecimens for genetic testing of tissues from previously diagnosed cases, with uninvolved lymph node or uterine tissues being the best source of normal DNA. Where normal tissue is not accessible, consideration could be given to the use of DNA from tumor material. Although the distinguishing of germline from somatic mutations would be difficult, a null result would be useful, and if pathogenic mutations are found, especially for established founder mutations, ascertainment of germline status among family members may be warranted. Consideration should also be given to the development of central repositories to store culled pathology specimens (and the associated issues around ownership, consent, cost, etc).
Community engagement and self-referral.
Traceback that is based on community engagement campaigns and that seeks to raise awareness about the genetic risk of breast or ovarian cancer provides a parallel strategy to recruit either affected individuals or relatives of women with ovarian cancer. Self-referral offers ease of consent and optimal collection of blood samples and may reflect an inclination to assist in identifying family members for referral. However, self-referral may not reach underserved groups unless appropriate media campaigns and community engagement are specifically tailored. Furthermore, in contrast to pathology-based proband identification in which a cancer diagnosis is relatively certain, self-referral could be based on incorrect recollections of cancer diagnoses among relatives, potentially leading to unnecessary genetic testing.
The Prevent Ovarian Cancer Program developed in Ontario, Canada, provides a model for a community Web-based campaign in which self-referred relatives of women with high-grade ovarian cancer participate in an online evaluation for genetic testing referral, if indicated. Media outreach has encompassed primary care practitioners, social media, and television and print news media. Since its launch in September 2015, the Prevent Ovarian Cancer Program has enrolled > 500 of the planned total recruitment of 1,000 women.
Scope of Molecular Testing and Reporting
Currently, detection of individuals with pathogenic mutations in BRCA1/2 provides the strongest argument for developing Traceback; however, numerous new variants in genes associated with BRCA-mediated DNA repair have been identified. Most of these variants are low risk, but some, such as RAD51C, RAD51D, and BRIP1, are associated with at least a moderate risk of ovarian cancer,10,40,41 and National Comprehensive Cancer Network guidelines recommend consideration of risk-reduction gynecologic surgery for women with pathogenic mutations in these genes.42 Multigene testing for mutations in many cancer susceptibility genes in parallel is increasingly being used as it becomes cost-effective, technically feasible, and increasingly accessible.
A prospective study of 1,046 individuals who were at risk for or given a diagnosis of breast or ovarian cancer and were not known BRCA1/2 mutation carriers found that 3.8% had positive test results for putative pathogenic mutations in moderate- or high-penetrance genes other than BRCA1/2.43 On the basis of existing guidelines, detection of these mutations was estimated to influence clinical management of approximately 52% of carriers and prompt genetic testing of additional first-degree family members in 72% of cases.43 These findings indicate that multigene testing for hereditary breast and ovarian cancer could provide clinical benefit beyond testing for BRCA1/2 alone; however, challenges persist related to the translation of a currently incomplete knowledge of risks and benefits into optimal clinical management.44,45
Multigene testing of DNA derived from fixed pathology samples has been demonstrated through either targeted capture– or multiplexed amplicon–based approaches followed by next-generation sequencing. Methodological limitations in using tissue blocks for genetic testing include variable quantity and preservation of tissue and DNA. Technical advances in sequencing methodology may enable more-affordable molecular testing and expand the ability to handle small or suboptimally preserved tissues with increasing specificity.33,34 Even under stringent conditions, some false-positive and -negative results that lead to misclassification of proband status may be unavoidable. False-positive results would likely raise concern and could result in harm if communicated to family members but may be resolved through further genetic testing. To minimize false-negative results in the proband, the offer of full gene sequencing for BRCA1/2 and/or a multigene germline hereditary cancer risk panel may be preferred. When a specific mutation is identified, targeted testing of relatives offers advantages. Full gene testing could be recommended for patients with breast cancer who have a relative with ovarian cancer to determine phenocopy or mutation carrier status. Participating individuals or communities in Traceback should be informed about the residual familial empirical and genetic risk caveats that apply when a mutation is not identified (uninformative testing). Conveying the scope of the mutation testing that was performed on the proband’s sample is critical to provide a clear future understanding of which genes were or were not evaluated. Informing family members that a relative has negative test results for pathogenic BRCA1/2 mutations may avoid unnecessary testing.
The development of multigene testing raises the important issue of genetic counseling and what findings to report. Some providers favor only the disclosure of pathogenic or likely pathogenic variants, whereas others may report variants of uncertain significance or likely benign or benign variants,46 and interpretations of variants can differ among clinical laboratories.47 Furthermore, risk estimates for many genes are imprecise48 and may be influenced by the presence or absence of other low-risk variants,49 which means that knowledge is likely to evolve over time. The complex nuances of interpreting and communicating risk related to the detection of wild-type BRCA1/2 or variants of unknown significance in the context of a family history of cancer must be considered, but the limiting of testing to specific mutations found in relatives with cancer can lessen the problem.
Research to clarify the implications of variants of unknown significance in a variety of clinical contexts is ongoing,50 and research associated with Traceback may enable the construction of research pedigrees that clarify the biologic importance of these findings. The responsibilities for recontacting Traceback participants when the pathogenicity of a variant of uncertain significance is reclassified should be defined at the outset and communicated to participants.
Ethics and Privacy Considerations
A Traceback protocol to identify and test previously diagnosed cases engenders ethical and legal concerns related to consent to perform genetic testing and to return results to probands and/or their relatives. These considerations may vary with the design of the protocol and in accordance with local, state, national, and institutional mandates and applicable laws.51
The most straightforward situation is proband self-referral because these patients have opted in for genetic testing. However, if probands are identified through medical records, ethical considerations may vary by vital status and ability to be contacted. If the patient is living and locatable, she can be approached to provide informed consent to undergo testing. Although a BRCA1/2 pathogenic mutation itself does not pose an imminent threat, the strong association between these mutations and potentially lethal, yet preventable cancers52 provides a strong justification for unsolicited contact or recontact.
If the potential proband cannot be reached, the ethical, legal, and social challenges of genetic testing of pathology blocks without antecedent consent from a representative of the family and returning results to family members are complex. One option is to test diagnostic blocks without consent and then contact next of kin if a pathogenic mutation is found; this approach was used for hereditary colorectal cancer in a health services research study in Australia. After approval by three human research ethics committees, the investigators successfully contacted 18 at-risk individuals or their next of kin, 17 of whom agreed to attend genetic counseling. The majority of the at-risk individuals were happy with the follow-up and considered it a valuable extension of their health care.53 The authors provided a detailed commentary on the reasons for and against proceeding without prior consent, which favored the opportunity to limit concern about a possible genetic risk to a minority of families, and advantages of cost, logistics, and speed of progress.53
Whether a nonconsented evaluation of ovarian cancer diagnostic specimens could be allowed will be dictated by local factors, including laws related to consent/authorization for testing, the receptivity of the community to genetic testing, laws related to genetic discrimination, and ability to access health and life insurance if a mutation is found. Accordingly, Traceback protocols should define ethical and legal requirements of the program, including the resolution of state and national privacy restrictions.
In the United States, the Health Insurance Portability and Accountability Act (HIPAA) speaks to obtaining consent before testing; returning genetic results to family members; and the logistics related to contacting family members, such as time frame, number of attempted contacts, and geographic limitations.51 In Australia, the National Health and Medical Research Council Guidelines for research that involves human subjects makes provisions for nonconsented access to diagnostic blocks under certain circumstances, although these are typically for research rather than for the clinical intent of Traceback.54 Similar guidelines exist in Japan concerning access to previously collected tissues. In Ontario, Canada, according to the Public Hospitals Act, all diagnostic tissue remains property of the hospital.55 Similar to Australia, this tissue could be used secondarily for research without informed consent but only if the risk of identification of the individual is considered low.56
Significant cultural differences exist about the trust and willingness of communities with regard to genetic research and clinical genetic testing,57-60 and genetic discrimination is a global concern.61,62 In some instances, the discovery of a high-risk gene can have an impact on the ability of unaffected carriers to obtain health or life insurance. In the United States, the Genetic Information Nondiscrimination Act and Affordable Care Act legally prohibit various forms of discrimination (including employment and health insurance) but still leave gaps in protection (eg, life insurance).63 In Canada, Bill S-201 (Genetic Non-Discrimination Act) was passed unanimously by the Senate in April 2016 and currently is being debated in the House of Commons. This bill would protect individuals from having to disclose genetic testing results to employers or insurance companies. Currently, Canada is the only Group of Seven country without such legislation to prevent genetic discrimination.
Once testing of a proband has occurred and a mutation is found, important issues related to disclosure remain. Studies have demonstrated that barriers to cascade testing within a family include the burden on a proband to inform, emotional and developental readiness, family culture, and genetic risk misinformation/misunderstanding.3 In addition, a shortage of adequately trained genetic specialists among health care providers and challenges with respect to reimbursement and insurance policies exist.64 Efforts to improve cascade testing are actively being pursued. Some investigators are examining improved technologies for sharing genetic results, including secure Web sites or the development of educational videos to send to relatives. Some specialists advocate for greater direct involvement of the clinician or genetic counselor to relieve probands of the pressure of communicating the results themselves.27 Greater involvement of a genetic specialist also streamlines testing by identifying the most appropriate family member to test first.64 Changes in health policy, such as the offer of tests at reduced costs or remote counseling, could also improve the uptake of cascade genetic testing.64
Under HIPAA, a designated personal representative may authorize disclosure of the genetic results of a deceased person under certain circumstances.65,66 If a deceased patient has not designated a personal representative, the law in most US states grants the responsibility to a default personal representative, such as a close relative, which potentially provides access to genetic test results by biologically related family members.67 HIPAA also permits disclosure of genetic information to health care providers who request it (for purposes of risk assessment and treatment of family members) provided that the individual has not previously restricted disclosure.
An ethical, legal, and social implications working group within a National Institutes of Health–funded program published a consensus paper that provides recommendations for the ethical and legal framework for returning a research participant’s genomic results to relatives, including communication after the participant’s death.51 Although a distinction exists between genomic results obtained for research versus clinical contexts, many of the recommendations and analyses may be applicable to Traceback. The consensus document stated that when research participants are found to bear pathogenic actionable genetic variants, the sharing of these results with relatives may be ethical if provision of this information can lead to a reduction in harm.51
A survey of institutional review board chair and vice chair perspectives on returning genetic research results to family members found that a majority of respondents favored disclosure of clinically actionable research results to family members if the proband is deceased and prior consent was given; only a minority agreed, however, to disclosure when consent was not expressly given.68 By contrast, a survey conducted by FORCE: Facing Our Risk of Cancer Empowered, an advocacy organization for patients with hereditary breast and ovarian cancer, to determine which factors influence decisions about communicating cancer risk to family members found that most respondents shared their genetic results with family members and were satisfied with the outcome, although decision making can be influenced by personal privacy, ease of contact, or the influence of other family members.69 Similarly, in a survey of individuals with pancreatic cancer and their family members, most respondents believed that genetic research results obtained after a patient’s death should be offered to his or her spouse and adult biologic children irrespective of whether the spouse wanted to know the information and even if the deceased’s wishes were unknown.70
Data from the aforementioned surveys and the experience in the Prevent Ovarian Cancer Program in Ontario suggest that Traceback might receive public acceptance; however, potential concerns exist related to risks and fear of genetic discrimination and costs for the individual as well as about important local community issues that should be recognized. As such, the workshop participants suggested a population-based survey to assess attitudes about seeking and testing potential probands and returning test results to relatives.
MARKERS OF SUCCESS
Important metrics for the success and cost-effectiveness of a Traceback program include the proportion of potentially eligible probands and relatives identified and tested, rates of mutation detection, and effectiveness of cascade testing. A recent study of the economic impact of screening in Singapore suggested that government subsidies for the testing of first-degree relatives are cost saving if ≥ 36% of relatives were tested, although these results were sensitive to assumptions about adherence to post-testing surveillance.71 Although formal cost-effectiveness analyses in the United States are needed, these results suggest that a target of 40% to 50% uptake would be reasonable for a pilot feasibility project. Other important parameters are related to the acceptability of the program to probands and relatives; the ability to reach underserved communities; and the impact on cancer incidence, mortality and overall quality of life. Information on program-specific resource utilization and downstream health interventions, such as testing facilities and genetic counseling services, also should be captured.
Pilot programs are warranted for a variety of reasons, including the opportunity to provide estimates of program-specific resource utilization and specific outcomes, which could be applied to the development of a large-scale Traceback program. Although a single approach may not prove equally effective in all communities, pilot studies will help to determine the relative weaknesses and strengths of different strategies as well as identify critical aspects germane to all designs.
CONCLUSION
Given that most BRCA1/2-related cancers can be potentially prevented by risk-reducing surgery or detected at early stages with screening (breast cancer), an increase in the identification of BRCA1/2 mutation carriers offers an important opportunity for cancer control. Efforts to increase physician referral of patients with ovarian cancer are under way,28 and population BRCA1/2 mutation testing has been proposed.72,73 Traceback seeks to leverage limited resources by using medical records and pathology specimens as well as community and individual education to identify probands and engage families with limited awareness of genetic risk. This approach is potentially cost-effective, more acceptable than population testing, and applicable to other hereditary cancers such as Lynch syndrome. In fact, the Cancer Moonshot Blue Ribbon Panel Report 2016 recommended a demonstration project to identify families who carry Lynch syndrome predisposition genetic mutations by initially germline sequencing patients diagnosed with a Lynch syndrome–related cancer and offering testing and counseling to relatives.74
Although considerable value is anticipated to arise from Traceback, the ethical, legal, and social implications of obtaining consent for genetic testing of a previously diagnosed case and communicating results to family members are complicated, are incompletely understood, and should not be underestimated. Although significant ethical and logistical challenges exist, the workshop participants formulated considerations related to the Traceback concept to stimulate interest and informative studies to evaluate the value of such programs in reaching the many untested individuals at elevated risk of carrying pathogenic mutations. The identification of such individuals would represent a major step toward providing genetic counseling, testing, and effective preventive interventions to lower cancer risk in mutation carriers.
Appendix
Table A1.
Elements of a Best-Practice Traceback Program
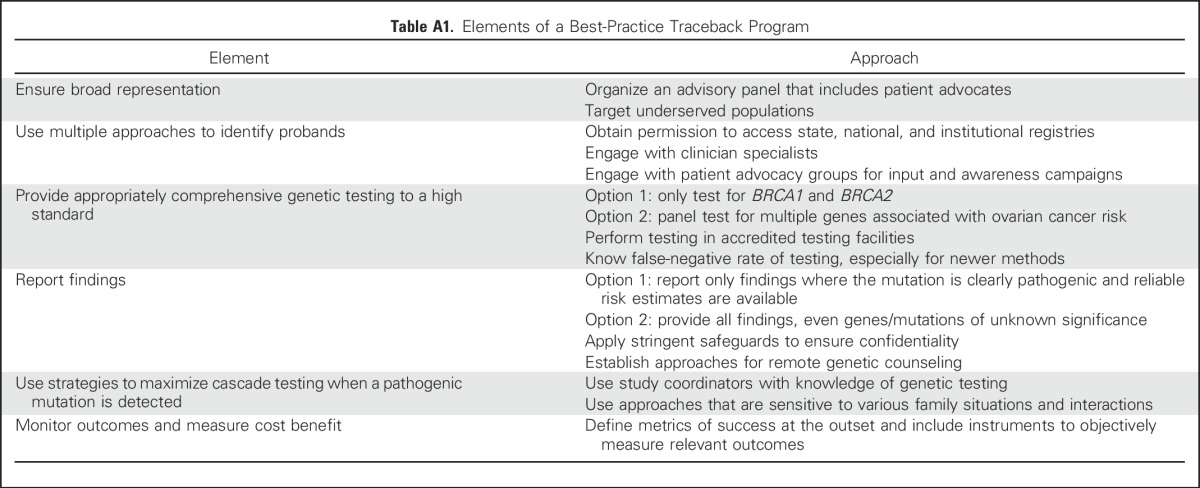
Table A2.
Ethical, Legal, and Social Issues Associated With the Testing of Potential Probands

Footnotes
This report reflects a summary of the discussion from the workshop and is not intended to represent an opinion or recommendation from the National Institutes of Health.
See accompanying Editorial on page 2226
AUTHOR CONTRIBUTIONS
Conception and design: Goli Samimi, Lawrence C. Brody, Charlisse F. Caga-anan, Georgia Chenevix-Trench, Fergus J. Couch, Ronny Drapkin, Michael Friedlander, Karen Hurley, Susan J. Ramus, Thomas P. Slavin, David D. Bowtell, Mark E. Sherman
Collection and assembly of data: Goli Samimi, Marcus Q. Bernardini, Lawrence C. Brody, Charlisse F. Caga-anan, Ian G. Campbell, Michael Dean, Joanne A. de Hullu, Heather Spencer Feigelson, Michael Friedlander, Mia M. Gaudet, Marline G. Harmsen, Paul A. James, Janice S. Kwon, Felicitas Lacbawan, Phuong L. Mai, Evan R. Myers, Mark E. Robson, Lisa F. Rezende, Patricia A. Shaw, Thomas P. Slavin, Elizabeth M. Swisher, Masataka Takenaka, David D. Bowtell, Mark E. Sherman
Data analysis and interpretation: Goli Samimi, Marcus Q. Bernardini, Charlisse F. Caga-anan, Ian G. Campbell, Michael Dean, Joanne A. de Hullu, Susan M. Domchek, Ronny Drapkin, Heather Spencer Feigelson, Michael Friedlander, Mia M. Gaudet, Marline G. Harmsen, Paul A. James, Janice S. Kwon, Felicitas Lacbawan, Stephanie Lheureux, Leah E. Mechanic, Lori M. Minasian, Evan R. Myers, Mark E. Robson, Lisa F. Rezende, Patricia A. Shaw, Elizabeth M. Swisher, David D. Bowtell, Mark E. Sherman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers Through Family-Based Outreach
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Goli Samimi
No relationship to disclose
Marcus Q. Bernardini
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Lawrence C. Brody
Consulting or Advisory Role: Boehringer Ingelheim (I), Roche (I), Global Blood Therapeutics (I)
Research Funding: Roche (I)
Travel, Accommodations, Expenses: Roche (I), Boehringer Ingelheim (I), Global Blood Therapeutics (I)
Charlisse F. Caga-anan
No relationship to disclose
Ian G. Campbell
No relationship to disclose
Georgia Chenevix-Trench
No relationship to disclose
Fergus J. Couch
Travel, Accommodations, Expenses: Ambry Genetics
Michael Dean
No relationship to disclose
Joanne A. de Hullu
No relationship to disclose
Susan M. Domchek
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), AbbVie (Inst), Pharmar (Inst)
Ronny Drapkin
No relationship to disclose
Heather Spencer Feigelson
No relationship to disclose
Michael Friedlander
Honoraria: Pfizer, AstraZeneca
Consulting or Advisory Role: AstraZeneca, Pfizer
Research Funding: AstraZeneca (Inst)
Mia M. Gaudet
No relationship to disclose
Marline G. Harmsen
No relationship to disclose
Karen Hurley
Honoraria: Sanofi, Genzyme, GeneDx, BioReference
Speakers’ Bureau: Sanofi, Genzyme, GeneDx
Paul A. James
No relationship to disclose
Janice S. Kwon
No relationship to disclose
Felicitas Lacbawan
Employment: Quest Diagnostics, Nichols Institute
Stock or Other Ownership: Quest Diagnostics
Stephanie Lheureux
Consulting or Advisory Role: AstraZeneca
Phuong L. Mai
No relationship to disclose
Leah E. Mechanic
No relationship to disclose
Lori M. Minasian
No relationship to disclose
Evan R. Myers
Consulting or Advisory Role: Merck, Ernst & Young
Mark E. Robson
Honoraria: AstraZeneca
Consulting or Advisory Role: McKesson, AstraZeneca
Research Funding: AstraZeneca (Inst), AbbVie (Inst), Myriad Genetics (Inst), Medivation (Inst)
Susan J. Ramus
No relationship to disclose
Lisa F. Rezende
Employment: Ventana Medical Systems (I)
Patricia A. Shaw
Honoraria: AstraZeneca
Thomas P. Slavin
No relationship to disclose
Elizabeth M. Swisher
No relationship to disclose
Masataka Takenaka
No relationship to disclose
David D. Bowtell
Research Funding: Roche (Inst), Genentech (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Genentech
Mark E. Sherman
No relationship to disclose
REFERENCES
- 1.Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: Breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–594. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 2.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakasis K, Burnier JV, Bowering V, et al. Ovarian cancer and BRCA1/2 testing: Opportunities to improve clinical care and disease prevention. Front Oncol. 2016;6:119. doi: 10.3389/fonc.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eccles DM, Balmaña J, Clune J, et al. Selecting patients with ovarian cancer for germline BRCA mutation testing: Findings from guidelines and a systematic literature review. Adv Ther. 2016;33:129–150. doi: 10.1007/s12325-016-0281-1. [DOI] [PubMed] [Google Scholar]
- 5.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 6.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall MJ, Reid JE, Burbidge LA, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean M, Boland J, Yeager M, et al. Addressing health disparities in Hispanic breast cancer: Accurate and inexpensive sequencing of BRCA1 and BRCA2. Gigascience. 2015;4:50. doi: 10.1186/s13742-015-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong K, Calzone K, Stopfer J, et al. Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. 2000;9:1251–1254. [PubMed] [Google Scholar]
- 14.Lee SC, Bernhardt BA, Helzlsouer KJ. Utilization of BRCA1/2 genetic testing in the clinical setting: Report from a single institution. Cancer. 2002;94:1876–1885. doi: 10.1002/cncr.10420. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MD, Lerman C, Brogan B, et al. Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14:1003–1007. doi: 10.1158/1055-9965.EPI-03-0545. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong K, Micco E, Carney A, et al. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 17.Metcalfe KA, Fan I, McLaughlin J, et al. Uptake of clinical genetic testing for ovarian cancer in Ontario: A population-based study. Gynecol Oncol. 2009;112:68–72. doi: 10.1016/j.ygyno.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer LA, Anderson ME, Lacour RA, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: Missed opportunities. Obstet Gynecol. 2010;115:945–952. doi: 10.1097/AOG.0b013e3181da08d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet Med. 2011;13:349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell CB, Littell R, Hoodfar E, et al. Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer. 2013;23:431–436. doi: 10.1097/IGC.0b013e318280f2b4. [DOI] [PubMed] [Google Scholar]
- 21.Petzel SV, Vogel RI, Bensend T, et al. Genetic risk assessment for women with epithelial ovarian cancer: Referral patterns and outcomes in a university gynecologic oncology clinic. J Genet Couns. 2013;22:662–673. doi: 10.1007/s10897-013-9598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demsky R, McCuaig J, Maganti M, et al. Keeping it simple: Genetics referrals for all invasive serous ovarian cancers. Gynecol Oncol. 2013;130:329–333. doi: 10.1016/j.ygyno.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23. doi: 10.1097/COC.0000000000000073. Stuckey A, Febbraro T, Laprise J, et al: Adherence patterns to National Comprehensive Cancer Network guidelines for referral of women with breast cancer to genetics professionals. Am J Clin Oncol 39:363-367, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Febbraro T, Robison K, Wilbur JS, et al. Adherence patterns to National Comprehensive Cancer Network (NCCN) guidelines for referral to cancer genetic professionals. Gynecol Oncol. 2015;138:109–114. doi: 10.1016/j.ygyno.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SM, Ruddy KJ, Tamimi RM, et al. BRCA1 and BRCA2 mutation testing in young women with breast cancer. JAMA Oncol. 2016;2:730–736. doi: 10.1001/jamaoncol.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drohan B, Roche CA, Cusack JC, Jr, et al. Hereditary breast and ovarian cancer and other hereditary syndromes: Using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–1737. doi: 10.1245/s10434-012-2257-y. [DOI] [PubMed] [Google Scholar]
- 27.Hampel H. Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer. 2016;15:423–427. doi: 10.1007/s10689-016-9893-5. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy AM, Bristol M, Domchek SM, et al. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol. 2016;34:2610–2618. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman AD, Hall MJ. Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J. 2009;15(suppl 1):S56–S62. doi: 10.1111/j.1524-4741.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 30.Simon MS, Petrucelli N. Hereditary breast and ovarian cancer syndrome: The impact of race on uptake of genetic counseling and testing. Methods Mol Biol. 2009;471:487–500. doi: 10.1007/978-1-59745-416-2_25. [DOI] [PubMed] [Google Scholar]
- 31.Randall TC, Armstrong K. Health care disparities in hereditary ovarian cancer: Are we reaching the underserved population? Curr Treat Options Oncol. 2016;17:39. doi: 10.1007/s11864-016-0417-1. [DOI] [PubMed] [Google Scholar]
- 32.Lindor NM, Goldgar DE, Tavtigian SV, et al. BRCA1/2 sequence variants of uncertain significance: A primer for providers to assist in discussions and in medical management. Oncologist. 2013;18:518–524. doi: 10.1634/theoncologist.2012-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mafficini A, Simbolo M, Parisi A, et al. BRCA somatic and germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing. Oncotarget. 2016;7:1076–1083. doi: 10.18632/oncotarget.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen AH, Aagaard MM, Nielsen HR, et al. Post-mortem testing; germline BRCA1/2 variant detection using archival FFPE non-tumor tissue. A new paradigm in genetic counseling. Eur J Hum Genet. 2016;24:1104–1111. doi: 10.1038/ejhg.2015.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crum CP, Drapkin R, Kindelberger D, et al. Lessons from BRCA: The tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurman RJ, Shih IeM. The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded. Am J Pathol. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 38.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 39.Madore J, Ren F, Filali-Mouhim A, et al. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. J Pathol. 2010;220:392–400. doi: 10.1002/path.2659. [DOI] [PubMed] [Google Scholar]
- 40.Song H, Dicks E, Ramus SJ, et al. Contribution of germline mutations in the RAD51B, RAD51C, and RAD51D genes to ovarian cancer in the population. J Clin Oncol. 2015;33:2901–2907. doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramus SJ, Song H, Dicks E, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015;107:djv214. doi: 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Comprehensive Cancer Network: NCCN clinical guidelines in oncology: Genetic/familial high-risk assessment: Breast and ovarian, version 1.2016. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 43.Desmond A, Kurian AW, Gabree M, et al. Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncol. 2015;1:943–951. doi: 10.1001/jamaoncol.2015.2690. [DOI] [PubMed] [Google Scholar]
- 44.Kurian AW, Kingham KE, Ford JM. Next-generation sequencing for hereditary breast and gynecologic cancer risk assessment. Curr Opin Obstet Gynecol. 2015;27:23–33. doi: 10.1097/GCO.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 45.Axilbund JE. Panel testing is not a panacea. J Clin Oncol. 2016;34:1433–1435. doi: 10.1200/JCO.2015.65.5522. [DOI] [PubMed] [Google Scholar]
- 46.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balmaña J, Digiovanni L, Gaddam P, et al. Conflicting interpretation of genetic variants and cancer risk by commercial laboratories as assessed by the prospective registry of multiplex testing. J Clin Oncol. 2016;34:4071–4078. doi: 10.1200/JCO.2016.68.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581–588. doi: 10.1038/nrclinonc.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mavaddat N, Pharoah PD, Michailidou K, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107:djv036. doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eccles DM, Mitchell G, Monteiro AN, et al. BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann Oncol. 2015;26:2057–2065. doi: 10.1093/annonc/mdv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf SM, Branum R, Koenig BA, et al. Returning a research participant’s genomic results to relatives: Analysis and recommendations. J Law Med Ethics. 2015;43:440–463. doi: 10.1111/jlme.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeps N, Iacopetta BJ, Schofield L, et al. Waiver of individual patient consent in research: When do potential benefits to the community outweigh private rights? Med J Aust. 2007;186:88–90. doi: 10.5694/j.1326-5377.2007.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 54. National Health and Medical Research Council: National statement on ethical conduct in human research (2007) - updated May 2015. https://www.nhmrc.gov.au/guidelines-publications/e72.
- 55. Public Hospitals Act, R.R.O. 1990, Reg. 965, ss.20(2)3, 31(1), 31(2)
- 56. Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada: Tri-Council policy statement: Ethical conduct for research involving humans, 2010. http://www.pre.ethics.gc.ca/archives/tcps-eptc/docs/TCPS%20October%202005_E.pdf.
- 57.Lagos VI, Perez MA, Ricker CN, et al. Social-cognitive aspects of underserved Latinas preparing to undergo genetic cancer risk assessment for hereditary breast and ovarian cancer. Psychooncology. 2008;17:774–782. doi: 10.1002/pon.1358. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez AG, Chalela P, Gallion KJ, et al. Attitudes toward breast cancer genetic testing in five special population groups. J Health Dispar Res Pract. 2015;8:124–135. [PMC free article] [PubMed] [Google Scholar]
- 59.Sheppard VB, Mays D, LaVeist T, et al. Medical mistrust influences black women’s level of engagement in BRCA 1/2 genetic counseling and testing. J Natl Med Assoc. 2013;105:17–22. doi: 10.1016/s0027-9684(15)30081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw JL, Robinson R, Starks H, et al. Risk, reward, and the double-edged sword: Perspectives on pharmacogenetic research and clinical testing among Alaska Native people. Am J Public Health. 2013;103:2220–2225. doi: 10.2105/AJPH.2013.301596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otlowski M, Taylor S, Bombard Y. Genetic discrimination: International perspectives. Annu Rev Genomics Hum Genet. 2012;13:433–454. doi: 10.1146/annurev-genom-090711-163800. [DOI] [PubMed] [Google Scholar]
- 62.Wauters A, Van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: A systematic literature review. J Hum Genet. 2016;61:275–282. doi: 10.1038/jhg.2015.151. [DOI] [PubMed] [Google Scholar]
- 63.Green RC, Lautenbach D, McGuire AL. GINA, genetic discrimination, and genomic medicine. N Engl J Med. 2015;372:397–399. doi: 10.1056/NEJMp1404776. [DOI] [PubMed] [Google Scholar]
- 64.George R, Kovak K, Cox SL. Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J Genet Couns. 2015;24:388–399. doi: 10.1007/s10897-014-9805-5. [DOI] [PubMed] [Google Scholar]
- 65. Department of Health and Human Services: 45 CFR 164.510 - Uses and disclosures requiring an opportunity for the individual to agree or to object. https://www.law.cornell.edu/cfr/text/45/164.510.
- 66. Department of Health and Human Services: Personal representatives: 45 CFR 164.502(g). http://www.hhs.gov/hipaa/for-professionals/privacy/guidance/personal- representatives.
- 67.Amendola LM, Horike-Pyne M, Trinidad SB, et al. Patients’ choices for return of exome sequencing results to relatives in the event of their death. J Law Med Ethics. 2015;43:476–485. doi: 10.1111/jlme.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beskow LM, O’Rourke PP. Return of genetic research results to participants and families: IRB perspectives and roles. J Law Med Ethics. 2015;43:502–513. doi: 10.1111/jlme.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. FORCE: Facing Our Risk of Cancer Empowered: Your experiences talking to family members about the inherited mutation in your family: Results from the ABOUT Network Family Communication Survey, http://www.facingourrisk.org/research-clinical-trials/research-findings/family-communication-survey.php.
- 70.Breitkopf CR, Petersen GM, Wolf SM, et al. Preferences regarding return of genomic results to relatives of research participants, including after participant death: Empirical results from a cancer biobank. J Law Med Ethics. 2015;43:464–475. doi: 10.1111/jlme.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. doi: 10.1136/jmedgenet-2016-104302. Li S-T, Yuen J, Zhou K, et al: Impact of subsidies on cancer genetic testing uptake in Singapore. J Med Genet, 10.1136/jmedgenet-2016-104302 [epub ahead of print on October 25, 2016] [DOI] [PubMed] [Google Scholar]
- 72.Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091–1092. doi: 10.1001/jama.2014.12483. [DOI] [PubMed] [Google Scholar]
- 74. Cancer Moonshot Blue Ribbon Panel Report 2016. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf.