ABSTRACT
Mediator is a conserved and essential coactivator complex broadly required for RNA polymerase II (RNAPII) transcription. Recent genome-wide studies of Mediator binding in budding yeast have revealed new insights into the functions of this critical complex and raised new questions about its role in the regulation of gene expression.
KEYWORDS: ChEC-seq, Mediator, Pre-initiation complex, RNA polymerase II, TFIID
Introduction
Mediator is a critical integrator of transcriptional regulatory signals that plays a central role in RNAPII transcription by communicating regulatory information directly to the RNAPII holoenzyme. Upon activation of transcription, Mediator is thought to be recruited to distal regulatory sequences, such as upstream activating sequences (UASs) in yeast and enhancers in metazoans, by DNA-bound transcription factors and then loop to target promoters to convey regulatory input. Mediator comprises 25 subunits in yeast and 30 subunits in human that are organized into four modules: head, middle, tail, and kinase. In yeast, the head and middle modules are essential and generally required for RNAPII transcription.1 In contrast, the tail and kinase modules are not required for viability and their loss only affects subsets of genes. The tail module is important for promoting transcription of stress response genes, which are generally characterized by the presence of a consensus TATA box promoter element and their requirement for SAGA, another coactivator complex.2 The dissociable kinase module appears to act as a context-dependent regulator through the phosphorylation of transcription factors and other targets.3 In this point of view, we summarize recent progress in understanding Mediator function and its role in the regulation of gene expression from recent genome-wide and structural studies and outline important directions for future studies of Mediator.
Genome-wide mapping of Mediator in budding yeast
Analysis of the genomic binding sites for Mediator in budding yeast has been performed for over a decade, often with ambiguous results. Early chromatin immunoprecipitation with tiled microarray analysis (ChIP-chip) experiments revealed the association of Mediator with over 1,200 sites both upstream of and within coding regions of genes.4 These observations were also supported by work in Schizosaccharomyces pombe.5 However, the view that Mediator binding is widespread was challenged by single-locus ChIP-PCR experiments that found little or no Mediator binding upstream of many highly transcribed genes.6 Further ChIP-qPCR analysis argued that the majority of sites detected in the early ChIP-chip studies, particularly those in gene bodies, were false positives.7 Such concerns with Mediator ChIP specificity may be related to the finding that irrelevant proteins, including nuclear-localized GFP, can be efficiently localized to highly transcribed genes via ChIP and high-throughput sequencing (ChIP-seq).8 Indeed, this “hyper-ChIPable artifact” can be seen in Mediator ChIP-seq profiles.9 Emphasizing the often ambiguous results obtained by ChIP analysis of Mediator binding, ChIP-seq for Med17 using a Med17 antibody or epitope-tagged Med17 gives notably different results.9,10 Most recently, ChIP-chip analysis normalized to a control ChIP from a strain without epitope-tagged Mediator has firmly placed Mediator upstream of ORFs,11,12 while ChIP-seq indicated little Mediator binding to any region under normal growth conditions.13
Given the longstanding ambiguity regarding the genome-wide localization of Mediator in budding yeast, we decided to apply a ChIP-orthogonal technique, chromatin endogenous cleavage, and high-throughput sequencing (ChEC-seq)14 to the problem. In ChEC-seq, a chromatin-associated protein of interest is tagged with micrococcal nuclease (MNase), enabling targeted, calcium-dependent cleavage of specific loci. Released fragments are then sequenced and mapped back to the genome, with peaks of fragment ends corresponding to genomic binding events. We mapped two Mediator head subunits, Med8 and Med17, by ChEC-seq under a variety of conditions.15 The high resolution of ChEC-seq unambiguously revealed that Mediator exclusively associated with UAS regions under all conditions tested, rather than core promoters or gene bodies. Our results indicated that Mediator was bound to the UAS regions of essentially all genes, and that, for the most part, the level of Mediator at the UAS was uncoupled from transcriptional output. Interestingly, we also noted that the distance from the average position of maximum Mediator enrichment to the TSS was different at SAGA- versus TFIID-dominated genes, perhaps indicating differences for Mediator function during the assembly of the transcription initiation machinery at these gene classes.
Mediator and PIC assembly
Recent advances in cryo-EM have provided spectacular insight into the PIC-Mediator complex architecture.1,16 Such studies of the budding yeast PIC-Mediator complex suggested that Mediator has surprisingly little effect on the structure of the core PIC, with Mediator-induced PIC rearrangements limited to TFIIH and TFIIS.1,16 Moreover, the addition of budding yeast Mediator to pre-formed PICs had no effect on transcription initiation.16 These findings are consistent with a role for Mediator in PIC assembly, e.g., by facilitating the recruitment of general transcription factors (GTFs) to the core promoter, as suggested by several in vitro studies. Mutations in Med17, Med18, and Med20 compromise PIC assembly in yeast nuclear extract,17 and Mediator-depleted HeLa cell nuclear extract is deficient in recruitment of several PIC components.18 However, the role of Mediator in PIC assembly in vivo is far less understood. A number of studies using temperature-sensitive mutants in various Mediator subunits have suggested roles of these subunits in the recruitment of GTFs.19,20 The nuclear depletion of the Mediator head module subunits Med17 and Med18 using anchor-away21 resulted in reduced, though not completely abrogated, TFIIB binding to core promoters.11 This finding is supported by recent structural work showing the association of Med18 and Med20 with TFIIB resulting in the stabilization of promoter bound TFIIB,1 probably through a change in TFIIB DNA binding kinetics.22
We investigated the potential role of Mediator in PIC formation in budding yeast by analyzing the effect of Mediator depletion on the recruitment of TFIID and vice versa.15 Cooperative assembly of Mediator and TFIID on chromatin was demonstrated in vitro,23,24 but this relationship was questioned by work in Drosophila cells indicating that RNAi-mediated depletion of the Mediator head subunit Med17 had no effect on TBP or Taf2 binding to a model promoter.25 To comprehensively address this relationship in vivo, we depleted Med14 via anchor-away, which is proposed to also remove all Mediator subunits saved for the Med2/3/15 tail triad from the nucleus,26 and mapped TFIID binding via ChEC-seq for Taf1, an essential subunit of TFIID that directly contacts promoter DNA.27 Depletion of Mediator reduced TFIID association with ∼70% of all promoters, indicating an important role for Mediator in TFIID recruitment. Depleting Taf1 and mapping Mediator via Med8 ChEC-seq revealed widespread reduction in Mediator association. Our data indicate that the cooperation in chromatin recruitment between Mediator and TFIID demonstrated in vitro occurs in vivo and supports a role for Mediator in PIC assembly in cells.
Regulation of Mediator association with the genome
In vitro studies suggest that Mediator is recruited by activators, specific transcription factors that predominantly stimulate transcription of stress response genes, via interactions with the tail module of Mediator.28 In budding yeast, only 10% of genes are classified as stress response or SAGA-dominated genes.29 Thus, it has been a longstanding question how Mediator, if recruited to only a small fraction of genes, functions as a global regulator of gene expression. Recent genome-wide mapping by others and us revealed that Mediator is bound to UASs of essentially all genes.30,31 The mechanisms that are involved in Mediator recruitment to chromatin are still unclear. While the interaction between activators and the Mediator tail module has been studied in great detail in vitro,32-34 there is conflicting data on its role in vivo. We found that deletion of Med15/Gal11 (the main activator binding subunit in the tail module) does not abolish chromatin association of Mediator with UASs,30 while a pair of recent studies found that combined deletion of Med3 and Med15 results in decreased binding of Mediator to UASs and causes robust global decrease in TFIIB binding at both SAGA- and TFIID-dominated promoters.12,35 However, in agreement with its nonessential role in general transcription and structural work, the same studies reported that the tail is not required for Mediator association with the PIC,1,12,35 suggesting that association with a UAS before contacting the PIC is not necessarily required for Mediator function. It is, however, unclear how Mediator is recruited to the core promoter if it is not first recruited to the UAS. One possibility is that tailless Mediator remains capable of contacting the promoter-bound PIC, particularly through high-affinity interactions with the unphosphorylated RNAPII carboxy-terminal domain (CTD).16
New evidence suggests that, instead of the tail module, the kinase activity of Cdk8 in Mediator's kinase module may play an important role in global Mediator recruitment. The loss of the Mediator kinase module results in the increased binding of Mediator to UAS regions, indicative of a role for phosphorylation in modulating the association of Mediator with the genome.12,35 This could be achieved through kinase module phosphorylation of the Med3 tail subunit, which results in decreased Med3 protein levels via recruit of the Grr1 ubiquitin ligase.36 Consistent with this idea, co-deletion of Med3 and Med15 results in decreased binding of Mediator to UASs.12,35 Interestingly, these studies also showed that the kinase module associates with UASs but not core promoters, suggesting that the kinase module dissociates from core Mediator or is degraded before PIC association, though the mechanism underlying this compositional transition remains unknown.
It is also unclear if and how Mediator transits between its major binding site at the UASs and core promoters to facilitate PIC formation, especially since Mediator has not been detected at core promoters in genome-wide studies under normal growth conditions. Several Mediator subunits were originally discovered as suppressors of CTD truncations,37,38 and the association of Mediator with the CTD is disrupted by CTD phosphorylation in vitro.39 It was thus hypothesized that the CTD might be linked to the recruitment of Mediator to PICs in vivo. Indeed, the abrogation of CTD phosphorylation via chemical inhibition or anchor-away depletion of the CTD kinase Kin28 resulted in a dramatic increase in Mediator association with core promoters, but not UASs.11,13 It was therefore proposed that Mediator association with the PIC is transient, being quickly disrupted by CTD phosphorylation. We also investigated this phenomenon by performing Mediator ChEC-seq following chemical inhibition of Kin28. In contrast to the ChIP studies described above, we did not detect an increase in Mediator association with core promoters.15 These differing results may be due to technical differences in the experimental approaches. When fused to Med8, MNase could potentially be too far from promoter DNA to cleave, or DNA access could be sterically blocked by PIC components. It is also possible that, because Mediator contains no DNA binding domains, the interaction of Mediator with the PIC captured in ChIP experiments is mediated by protein–protein interactions trapped by formaldehyde crosslinking. Such interactions are proposed to occur via Mediator association with UASs and subsequent association with the PIC, with a single Mediator complex contacting both the UAS and PIC.12,15,35 We also note that the lack of robust Mediator association with core promoters observed in ChIP studies without Kin28 inhibition is consistent with work demonstrating a temporal threshold for formaldehyde crosslinking.40
Regulation of ribosomal protein genes by Mediator
Ribosomal protein genes (RPGs) are among the most highly transcribed genes in budding yeast. Despite the apparently global role of Mediator in RNAPII transcription,1 several studies have argued against a role for Mediator in the regulation of RPG transcription based on the poor enrichment of Mediator at RPG regulatory sequences.6,11,41 However, it has been argued that Mediator binding to RPG regulator sequences is highly dynamic, precluding its efficient detection by ChIP.11 Given that we detected Mediator at the UASs of essentially all genes, regardless of expression level, using ChEC-seq,15 we wondered if ChEC-seq might effectively capture Mediator enrichment at RPGs. We re-analyzed our Med8 ChEC-seq data from cells grown in both yeast extract-peptone-dextrose and glucose complete media and found robust enrichment upstream of the majority of RPGs (Fig. 1). In combination with the observation that Mediator is globally required for RNAPII transcription,1 these data indicate that Mediator regulates RPG transcription.
Figure 1.
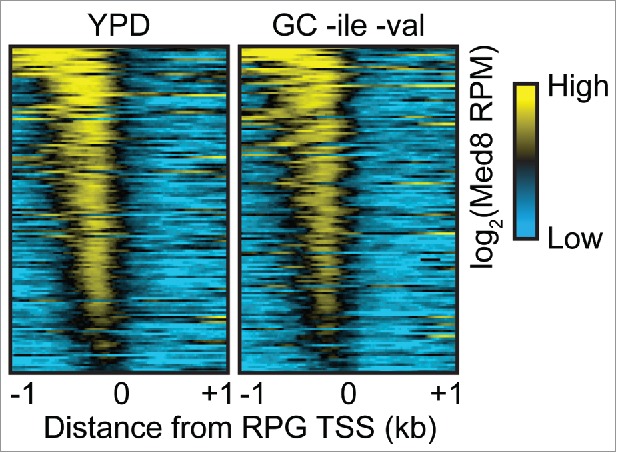
Mediator binds to ribosomal protein gene UASs, Heatmaps of Mediator ChEC-seq signal (1 min after calcium addition) at RPG TSSs from cells grown in yeast extract-peptone-dextrose (YPD) or glucose complete (GC) medium lacking isoleucine and valine. Heatmaps are normalized to reads per million, log2-transformed, and centered around a value of 10.
Conclusion and future perspectives
Genome-wide studies of Mediator binding and function in yeast have revealed valuable insights into the in vivo roles of this conserved, essential coactivator complex. These studies have also raised interesting questions about Mediator function, providing further avenues for investigation. For instance, what controls the dissociation of the kinase module from core Mediator before association with the PIC? How is tailless Mediator recruited to the PIC in the absence of the tail subunits that would tether it to DNA at UASs?
It will also be of interest to determine whether the mechanisms of Mediator function and regulation elucidated in yeast are conserved in metazoan systems. In this regard, previous work has suggested both similarities and discrepancies. In both yeast and metazoans, Mediator associates with distal regulatory elements (UASs in yeast, enhancers in metazoans), likely to facilitate looping to target promoters. However, Mediator association with promoters may be differentially regulated between yeast and metazoans. For instance, Mediator can be robustly detected at core promoters in metazoan cells under normal growth conditions,42 while it can only be detected at yeast core promoters following inhibition of CTD phosphorylation. While it remains to be tested if inhibition of CTD phosphorylation traps Mediator at core promoters in metazoan cells, the results of such experiments would likely be difficult to interpret due to promoter-proximal pausing of RNAPII in these organisms, which is itself regulated by Mediator and the Kin28 ortholog CDK7.3
Understanding how Mediator and other coactivators transmit regulatory signals directly to the transcription machinery is of great importance for understanding general gene regulation. We anticipate that further genome-wide studies that take into consideration structural analysis and high-resolution chromosome conformation capture technology will continue to yield new insights into the function of Mediator in transcriptional regulation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Moustafa Saleh for the critical reading of the manuscript.
Funding
S.G. was supported by NIH grants R01GM053451 and R01GM075114 and G.E.Z. was supported by Indiana University startup funds.
References
- [1].Plaschka C, Lariviere L, Wenzeck L, Seizl M, Hemann M, Tegunov D, Petrotchenko EV, Borchers CH, Baumeister W, Herzog F et al.. Architecture of the RNA polymerase II-Mediator core initiation complex. Nature 2015; 518:376-380; PMID:25652824; https://doi.org/ 10.1038/nature14229 [DOI] [PubMed] [Google Scholar]
- [2].Ansari SA, Ganapathi M, Benschop JJ, Holstege FCP, Wade JT, Morse RH. Distinct role of Mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J 2012; 31:44-57; PMID:21971086; https://doi.org/ 10.1038/emboj.2011.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol 2015; 16:155-166; PMID:25693131; https://doi.org/ 10.1038/nrm3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andrau J-C, van de Pasch L, Lijnzaad P, Bijma T, Koerkamp MG, van de Peppel J, Werner M, Holstege FC. Genome-wide location of the coactivator mediator: binding without activation and transient Cdk8 interaction on DNA. Mol Cell 2006; 22:179-192; PMID:16630888; https://doi.org/ 10.1016/j.molcel.2006.03.023 [DOI] [PubMed] [Google Scholar]
- [5].Zhu X, Wirén M, Sinha I, Rasmussen NN, Linder T, Holmberg S, Ekwall K, Gustafsson CM. Genome-wide occupancy profile of mediator and the Srb8-11 module reveals interactions with coding regions. Mol Cell 2006; 22:169-178; PMID:16630887; https://doi.org/ 10.1016/j.molcel.2006.03.032 [DOI] [PubMed] [Google Scholar]
- [6].Fan X, Chou DM, Struhl K. Activator-specific recruitment of Mediator in vivo. Nat Struct Mol Biol 2006; 13:117-120; PMID:16429153; https://doi.org/ 10.1038/nsmb1049 [DOI] [PubMed] [Google Scholar]
- [7].Fan X, Struhl K. Where does mediator bind In Vivo? PLoS ONE 2009; 4:e5029; PMID:19343176; https://doi.org/ 10.1371/journal.pone.0005029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Teytelman L, Thurtle DM, Rine J, van Oudenaarden A. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. Proc Natl Acad Sci USA 2013; 110:18602-18607; PMID:24173036; https://doi.org/ 10.1073/pnas.1316064110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Paul E, Zhu ZI, Landsman D, Morse RH. Genome-wide association of mediator and RNA polymerase II in wild-type and mediator mutant yeast. Mol Cell Biol 2015; 35:331-342; PMID:25368384; https://doi.org/ 10.1128/MCB.00991-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Eyboulet F, Cibot C, Eychenne T, Neil H, Alibert O, Werner M, Soutourina J. Mediator links transcription and DNA repair by facilitating Rad2/XPG recruitment. Genes Dev 2013; 27:2549-2562; PMID:24298055; https://doi.org/ 10.1101/gad.225813.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jeronimo C, Robert F. Kin28 regulates the transient association of Mediator with core promoters. Nat Struct Mol Biol 2014; 21:449-455; PMID:24704787; https://doi.org/ 10.1038/nsmb.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jeronimo C, Langelier M-F, Bataille Alain R, Pascal John M, Pugh BF, Robert F. Tail and kinase modules differently regulate core mediator recruitment and function In Vivo. Mol Cell 2016; 64:455-466; PMID:27773677; https://doi.org/ 10.1016/j.molcel.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wong Koon H, Jin Y, Struhl K. TFIIH Phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol Cell 2014; 54:601-612; PMID:24746699; https://doi.org/ 10.1016/j.molcel.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zentner GE, Kasinathan S, Xin B, Rohs R, Henikoff S. ChEC-seq kinetics discriminates transcription factor binding sites by DNA sequence and shape in vivo. Nat Commun 2015; 6:8733; PMID:26490019; https://doi.org/ 10.1038/ncomms9733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grünberg S, Henikoff S, Hahn S, Zentner GE. Mediator binding to UASs is broadly uncoupled from transcription and cooperative with TFIID recruitment to promoters. EMBO J 2016; 35:2435-2446; PMID:27797823; https://doi.org/ 10.15252/embj.201695020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Robinson PJ, Trnka MJ, Bushnell DA, Davis RE, Mattei P-J, Burlingame AL, Kornberg RD. Structure of a complete mediator-RNA polymerase II pre-initiation complex. Cell 2016; 166:1411-1422.e16; PMID:27610567; https://doi.org/ 10.1016/j.cell.2016.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ranish JA, Yudkovsky N, Hahn S. Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev 1999; 13:49-63; PMID:9887099; https://doi.org/ 10.1101/gad.13.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen X-F, Lehmann L, Lin Justin J, Vashisht A, Schmidt R, Ferrari R, Huang C, McKee R, Mosley A, Plath K et al.. Mediator and SAGA have distinct roles in pol II preinitiation complex assembly and function. Cell Rep 2012; 2:1061-1067; PMID:23177621; https://doi.org/ 10.1016/j.celrep.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eyboulet F, Wydau-Dematteis S, Eychenne T, Alibert O, Neil H, Boschiero C, Nevers MC, Volland H, Cornu D, Redeker V et al.. Mediator independently orchestrates multiple steps of preinitiation complex assembly in vivo. Nucleic Acids Res 2015; 43:9214-9231; PMID:26240385; https://doi.org/ 10.1093/nar/gkv782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eychenne T, Novikova E, Barrault M-B, Alibert O, Boschiero C, Peixeiro N et al.. Functional interplay between Mediator and TFIIB in preinitiation complex assembly in relation to promoter architecture. Genes Dev 2016; 30(18):2119-2132; PMID:27688401; https://doi.org/ 10.1101/gad.285775.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haruki H, Nishikawa J, Laemmli UK. The Anchor-Away Technique: Rapid, Conditional Establishment of Yeast Mutant Phenotypes. Mol Cell 2008; 31:925-932; PMID:18922474; https://doi.org/ 10.1016/j.molcel.2008.07.020 [DOI] [PubMed] [Google Scholar]
- [22].Zhang Z, English BP, Grimm JB, Kazane SA, Hu W, Tsai A, Inouye C, You C, Piehler J, Schultz PG et al.. Rapid dynamics of general transcription factor TFIIB binding during preinitiation complex assembly revealed by single-molecule analysis. Genes Dev 2016; 30:2106-2118; PMID:27798851; https://doi.org/ 10.1101/gad.285395.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson KM, Wang J, Smallwood A, Arayata C, Carey M. TFIID and human mediator coactivator complexes assemble cooperatively on promoter DNA. Genes Dev 2002; 16:1852-1863; PMID:12130544; https://doi.org/ 10.1101/gad.995702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson KM, Carey M. Assembly of a Mediator/TFIID/TFIIA complex bypasses the need for an activator. Curr Biol 2003; 13:772-777; PMID:12725737; https://doi.org/ 10.1016/S0960-9822(03)00283-5 [DOI] [PubMed] [Google Scholar]
- [25].Marr MT, Isogai Y, Wright KJ, Tjian R. Coactivator cross-talk specifies transcriptional output. Genes Dev 2006; 20:1458-1469; PMID:16751183; https://doi.org/ 10.1101/gad.1418806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Anandhakumar J, Moustafa YW, Chowdhary S, Kainth AS, Gross DS. Evidence for multiple mediator complexes in yeast independently recruited by activated heat shock factor. Mol Cell Biol 2016; 36:1943-1960; PMID:27185874; https://doi.org/ 10.1128/MCB.00005-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Louder RK, He Y, López-Blanco JR, Fang J, Chacón P, Nogales E. Structure of promoter-bound TFIID and model of human pre-initiation complex assembly. Nature 2016; 531:604-609; PMID:27007846; https://doi.org/ 10.1038/nature17394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hahn S, Young ET. Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics 2011; 189:705-736; PMID:22084422; https://doi.org/ 10.1534/genetics.111.127019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Basehoar AD, Zanton SJ, Pugh BF. Identification and distinct regulation of yeast TATA box-containing genes. Cell 2004; 116:699-709; PMID:15006352; https://doi.org/ 10.1016/S0092-8674(04)00205-3 [DOI] [PubMed] [Google Scholar]
- [30].Grunberg S, Henikoff S, Hahn S, Zentner GE. Mediator binding to UASs is broadly uncoupled from transcription and cooperative with TFIID recruitment to promoters. EMBO J 2016; 35:2435-2446; PMID:27797823; https://doi.org/ 10.15252/embj.201695020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jeronimo C, Robert F. Kin28 regulates the transient association of Mediator with core promoters. Nat Struct Mol Biol 2014; 21:449-455; PMID:24704787; https://doi.org/ 10.1038/nsmb.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brzovic PS, Heikaus CC, Kisselev L, Vernon R, Herbig E, Pacheco D, Warfield L, Littlefield P, Baker D, Klevit RE et al.. The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol Cell 2011; 44:942-953; PMID:22195967; https://doi.org/ 10.1016/j.molcel.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herbig E, Warfield L, Fish L, Fishburn J, Knutson BA, Moorefield B, Pacheco D, Hahn S. Mechanism of Mediator recruitment by tandem Gcn4 activation domains and three Gal11 activator-binding domains. Mol Cell Biol 2010; 30:2376-2390; PMID:20308326; https://doi.org/ 10.1128/MCB.01046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jedidi I, Zhang F, Qiu H, Stahl SJ, Palmer I, Kaufman JD, Nadaud PS, Mukherjee S, Wingfield PT, Jaroniec CP et al.. Activator Gcn4 employs multiple segments of Med15/Gal11, including the KIX domain, to recruit mediator to target genes in vivo. J Biol Chem 2010; 285:2438-2455; PMID:19940160; https://doi.org/ 10.1074/jbc.M109.071589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Petrenko N, Jin Y, Wong Koon H, Struhl K. Mediator Undergoes a Compositional Change during Transcriptional Activation. Mol Cell 2016; 64:443-454; PMID:27773675; https://doi.org/ 10.1016/j.molcel.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonzalez D, Hamidi N, Del Sol R, Benschop JJ, Nancy T, Li C, Francis L, Tzouros M, Krijgsveld J, Holstege FC et al.. Suppression of Mediator is regulated by Cdk8-dependent Grr1 turnover of the Med3 coactivator. Proc Natl Acad Sci U S A 2014; 111:2500-2505; PMID:24550274; https://doi.org/2693207 10.1073/pnas.1307525111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nonet ML, Young RA. Intragenic and Extragenic Suppressors of Mutations in the Heptapeptide Repeat Domain of Saccharomyces Cerevisiae RNA Polymerase II. Genetics 1989; 123:715-724; PMID:2693207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thompson CM, Koleske AJ, Chao DM, Young RA. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell 1993; 73:1361-1375; PMID:8324825; https://doi.org/ 10.1016/0092-8674(93)90362-T [DOI] [PubMed] [Google Scholar]
- [39].Max T, Søgaard M, Svejstrup JQ. Hyperphosphorylation of the C-terminal Repeat Domain of RNA Polymerase II Facilitates Dissociation of Its Complex with Mediator. J Biol Chem 2007; 282:14113-14120; PMID:17376774; https://doi.org/ 10.1074/jbc.M701345200 [DOI] [PubMed] [Google Scholar]
- [40].Schmiedeberg L, Skene P, Deaton A, Bird A. A Temporal Threshold for Formaldehyde Crosslinking and Fixation. PLoS ONE 2009; 4:e4636; PMID:19247482; https://doi.org/ 10.1371/journal.pone.0004636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ansari SA, He Q, Morse RH. Mediator complex association with constitutively transcribed genes in yeast. Proc Natl Acad Sci U S A 2009; 106:16734-16739; PMID:19805365; https://doi.org/20720539 10.1073/pnas.0905103106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS et al.. Mediator and cohesin connect gene expression and chromatin architecture. Nature 2010; 467:430-435; PMID:20720539; https://doi.org/ 10.1038/nature09380 [DOI] [PMC free article] [PubMed] [Google Scholar]


