Abstract
Background
While family history (FH) has been widely used to provide risk information, it captures only a small proportion of subjects with higher genetic susceptibility. Our objective is to assess whether a genetic risk score (GRS) calculated from prostate cancer (PCa) risk-associated single nucleotide polymorphisms (SNPs) can supplement FH for more effective risk stratification for PCa screening decision-making.
Methods
A GRS was calculated based on 29 PCa risk-associated SNPs for 4,528 men of European descent in the placebo arm of the Prostate Cancer Prevention Trial (PCPT). At study entry, participants were free of a PCa diagnosis. Performance of FH and GRS were measured by observed detection rate of PCa and high-grade PCa (Gleason score ≥7) during the 7-year study.
Results
GRS was a significant predictor of PCa in men with or without a positive FH (P=1.18×10−4 and P=4.50×10−16, respectively). Using FH alone, as expected, the 17% of men who were FH+ had a PCa detection rate that was significantly higher (29.02%) than FH− men (23.43%, P=0.001). When both FH+ or GRS>1.4 are considered, more than twice as many men (36%) can be classified as higher risk, as evidenced by a significantly higher PCa detection rate (30.98%) than in the remaining men (20.61%, P=5.30×10−15). If targeting only FH+ men, four of five PCa cases would go undetected, as would a similarly large fraction (~80%) of high-grade PCa cases. In comparison, if targeting FH+ or GRS>1.4 men, almost half of all PCa cases would be detected, including 45% of high-grade PCa cases.
Conclusions
GRS can supplement family history to better identify higher risk men for targeted intervention.
Keywords: Prostate cancer, single nucleotide polymorphisms, the Prostate Cancer Prevention Trial, risk, family history
Introduction
Screening for prostate cancer (PCa) and prostate biopsies have become controversial. Central among these concerns is that the use of prostate-specific antigen (PSA) for PCa screening may lead to over-detection and overtreatment of indolent cases. While the European Randomized study of Screening for Prostate Cancer (ERSPC) found a 21% reduction in PCa death for men who underwent PSA screening, no difference in PCa mortality was found the U.S. Prostate, Lung, Colorectal and Ovarian (PLCO) trial.[1,2] Considering that risks of PSA screening may outweigh the benefits, the United States Preventive Services Task Force (USPSTF) recommended against PSA screening in 2012.[3,4] The American Urological Association (AUA), on the other hand, recommends risk-based targeted PSA screening.[5] More precisely defining risk of developing PCa is essential for targeted PSA screening.
Family history (FH) of PCa is a commonly used risk stratification tool for PCa, which generally captures both genetic and shared environmental risk factors. Approximately 7–17% of men in the general population have a FH of PCa.[2,6,7] The increased PCa risk among men with a positive FH (FH+) has been consistently documented, with a relative risk estimated to be 1.3–1.5 from prospective studies.[6,7] However, for the majority of men in the general population for which there are no known affected relatives, FH is less informative, as lack of known FH at the time of examination may not necessarily indicate that men are at lower risk for PCa. Thus, additional methods of risk assessment are needed to identify men at higher risk of PCa, particularly among those without a FH of PCa.
Approximately 100 independent PCa risk-associated single nucleotide polymorphisms (SNPs) that have been identified from genome-wide association studies (GWAS) may represent an objective and novel measurement for PCa risk.[8,9] A genetic risk score (GRS) derived from a combination of these risk-associated SNPs has been consistently demonstrated in various study populations as an objective and significant predictor for PCa that is independent from other clinical and demographic predictors such as FH.[9–11] Importantly, GRS has consistently been shown to have a better predictive performance of PCa risk than FH.[12] Nevertheless, whether GRS can supplement FH to better stratify PCa risk has not previously been explicitly tested in prospective studies.
The primary objective of this study is to assess the predictive performance of FH supplemented by GRS in stratifying PCa risk in men enrolled in the placebo arm of the Prostate Cancer Prevention Trial (PCPT). Because all men in the study were free of PCa diagnosis at study entry and were followed for seven years for detection of PCa through for-cause or end-of-study prostate biopsies, regardless of the status of FH, the PCPT offered a unique study population and design for an objective assessment of GRS and FH as risk stratification tools. An important clinical implication of developing a more predictive risk assessment tool is for targeted PSA screening.
Materials and Methods
Patients
The demographics of the PCPT study have been described elsewhere.[13] Briefly, men 55 years of age or older with a normal digital rectal examination (DRE), no clinically significant coexisting conditions, an AUA symptom score of less than 20, and a PSA level of 3.0 ng/mL or lower were randomly assigned to receive either finasteride or a placebo. Since finasteride was found to significantly reduce PCa prevalence, the current study was limited to men in the placebo arm. Furthermore, since the vast majority of the PCa risk-associated SNPs were discovered and confirmed in men of European descent, the study was limited to Caucasian men with DNA previously available from the PCPT. The analytic cohort consisted of 4,258 men who were screened annually using a PSA and a DRE over a seven-year follow up period. Men not diagnosed with PCa during study follow up were recommended to undergo an end-of-study prostate biopsy. The typical number of biopsy cores taken was 6–10.
Laboratory methods
PCa risk-associated SNPs discovered from GWAS reported prior to December 2009 were included; each exceeded genome-wide significance levels in their initial reports (P<10−7) and has been replicated in independent populations. Genomic DNA was isolated from white blood cells or serum of peripheral blood. Genotyping was performed using either the Illumina GoldenGate 384-plex platform at the University of Texas Health Science Center at San Antonio or the Sequenom MassARRAY platform at Wake Forest School of Medicine. Twenty-nine SNPs were approved for genotyping by the PCPT committee (Supplementary Table 1). The average genotype call rate was 98%. None of the SNPs deviated from Hardy-Weinberg Equilibrium after being adjusted for multiple tests (P>0.05).
Measured outcomes
FH in this analysis was based on PCa information among first-degree relatives of the participants at study entry, since more distant FH is less associated with one’s risk and often unknown. Diagnoses of PCa were made at the participating PCPT sites and were confirmed by a Gleason sum that was made centrally at the Prostate Diagnostic Laboratory at the University of Colorado. High-grade PCa was defined as a Gleason score of 7 or higher.
Statistical methods
A GRS was calculated for each man based on his SNP genotype, which was weighted by the odds ratio (OR) and the allele frequency of each SNP.[14] Briefly, 1) the allelic OR of each SNP was obtained from an external study,[15] 2) the genotypic OR of each SNP was estimated from the allelic OR assuming a multiplicative model, 3) the risk relative to the average risk in the population was calculated for each genotype based on genotypic OR and genotype frequency in the HapMap CEU population, and 4) a GRS was obtained by multiplying the risks relative to the population of all SNPs. Therefore, a GRS of 1.0 indicates an average risk in the general population.
A univariate logistic regression model was used to test the association of PCa risk with each demographic and clinical variable.
Performance of FH and GRS for stratifying PCa risk were assessed by the detection rate of PCa and high-grade PCa during the seven years of follow up. Men with GRSs between 0.6 and 1.4 were classified as intermediate risk because they represent the middle ~50% of the analytical cohort, and various cutoff points were used to classify men at higher risk (>1.4, >1.6, >1.8, and >2.0) or men at lower risk (<0.6 and < 0.4). Performance of several potential risk stratification strategies based on FH and/or GRS for detection of PCa were assessed using positive predictive values (PPV), negative predictive values (NPV), proportion of cases detected, and proportion of cases missed.
Ethics
The Institutional Review Boards at the participating trial sites approved the PCPT. The Institutional Review Boards at the Wake Forest School of Medicine and the Johns Hopkins Bloomberg School of Public Health approved this study of genetics. All men enrolled in this study provided informed consent for participation.
Results
All PCa
During the seven years of follow-up, 1,104 of 4,528 men in the study (24.38%) were diagnosed with PCa. In the entire analytical cohort, the odds ratio (OR) of FH and GRS (as a continuous variable) for PCa risk was 1.34 (95% CI: 1.12–1.59, P=0.001) and 1.49 (95% CI:1.37–1.62, P=1.46×10−19), respectively. In FH+ men, GRS was significantly associated with PCa risk, with an OR of 1.57 (95% CI: 1.25–1.97-, P=1.18×10−4). In men with a negative FH (FH-), GRS was also significantly associated with PCa risk, with an OR of 1.47 (95% CI: 1.34–1.61-, P=4.50×10−16) (Table 1).
Table 1.
Characteriscs of study participants in the placebo arm of PCPT and results from univariate analyses
| Variables | Entire cohort | Men with a positive FH | Men with a negative FH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| PCa (N=1104) |
Non-PCa (N=3424) |
OR (95% CI) | P | PCa (N=224) |
Non-PCa (N=548) |
OR (95% CI) | P | PCa (N=880) |
Non-PCa (N=2876) |
OR (95% CI) | P | |
| Age, mean, year | 63.74 | 62.82 | 1.03(1.02–1.04) | 1.25E-06 | 62.9 | 62.32 | 1.02(0.99–1.05) | 1.89E-01 | 63.96 | 62.91 | 1.04(1.02–1.05) | 8.27E-07 |
| tPSA, median, ng/mL | 1.40 | 1.00 | 2.11(1.88–2.37) | 8.96E-36 | 1.40 | 1.10 | 1.85(1.41–2.42) | 8.86E-06 | 1.40 | 1.00 | 2.16(1.90–2.46) | 3.75E-31 |
| DRE, # (%) positive | 15.86 | 4.49 | 4.01(3.17–5.06) | 2.41E-31 | 15.98 | 4.44 | 4.10(2.34–7.17) | 7.86E-07 | 15.83 | 4.50 | 3.99(3.08–5.16) | 6.69E-26 |
| FH, # (%) positive | 20.29 | 16.00 | 1.34(1.12–1.59) | 1.02E-03 | ||||||||
| GRS, median | 1.08 | 0.87 | 1.49(1.37–1.62) | 1.46E-19 | 1.15 | 0.92 | 1.57(1.25–1.97) | 1.18E-04 | 1.05 | 0.85 | 1.47(1.34–1.61) | 4.50E-16 |
PCPT: Prostate Cancer Prevention Trial
PCa: Prostate cancer
tPSA: Total prostate-specific antigen
DRE: Digital rectal examination
FH: Family history
GRS: Genetic risk score
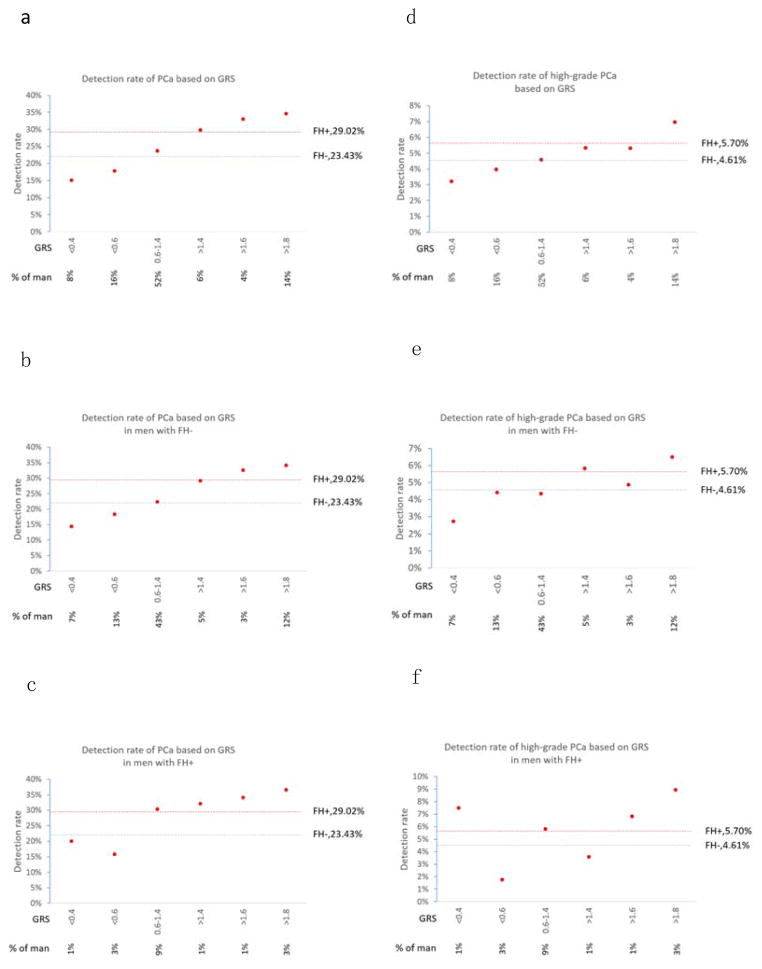
The performance of risk stratification using FH and GRS was assessed by the detection rate of PCa during the follow-up period. When FH was used to stratify risk, 17% of men were classified as higher risk based on positive FH. The observed PCa detection rates were significantly higher in men classified as higher risk (29.02%) than those classified as lower risk (23.43%), P=0.001 (Figure 1a). In comparison, when various GRS cutoff values were used to identify men at higher risk, considerably more men were implicated, and their higher estimated risks were confirmed by higher observed detection rates of PCa (see below). For example, when GRS >1.4 was used as a cutoff, 24% of men were classified as higher risk. The observed PCa detection rates were significantly higher in men classified as higher risk (33.18%) than those classified as lower risk (21.57%), P=6.30×10−15 (Figure 1b). When combining FH and GRS, 36% of men can be classified as higher risk (FH+ or GRS >1.4). The observed PCa detection rates were significantly higher in men classified as higher risk (30.98%) than those classified as lower risk (20.61%), P=5.30×10−15 (Figure 1c).
Figure 1.
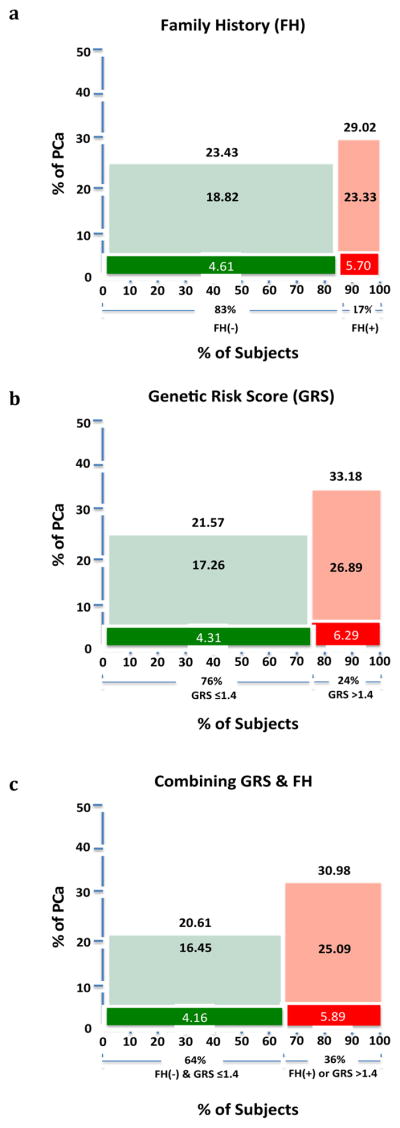
The detection rate of PCa and high-grade PCa among men defined as higher or lower risk based on family history alone (a), genetic risk score alone (b), and a combination of family history and genetic risk score. Green and red color represents men at lower or higher risk, respectively. Darker colors represent high-grade PCa.
The quantitative nature of GRS makes it feasible to further refine one’s risk for PCa (Figure 2a). Starting at GRS >1.4, the observed PCa detection rate became higher than that of FH (red dotted line), and highest at GRS >2.0. Conversely, men with GRS <0.6 had PCa detections rate lower than that of FH− men (blue dotted line). GRS was especially informative in men without FH, who represent the majority of men in the study and in general populations (Figure 2b). Although these men would typically be considered lower risk, many of them could be re-classified to higher risk based on their GRS, which confers a notably high potential PCa detection rate, even higher than that of men with a positive FH. For example, at a cutoff of GRS >1.4, 23% of FH− men were re-classified as higher risk and their observed detection rate of PCa was 32.72%, which is 13% higher than the rate of detection in FH+ men, P=0.10. GRS was also informative for further refining risk among FH+ men (Figure 2c). Approximately 22% of FH+ men could be reclassified to lower risk due to having a GRS <0.6, and their PCa detection rate was lower at 20%, which was 28% lower than the PCa detection rate of men with a FH-, P=0.06.
Figure 2.
Detection rate of prostate cancer (PCa) (a–c) and high-grade PCa (d–f) based on family history (FH) and/or genetic risk score (GRS). As a benchmark, detection rate of PCa for men with a positive FH (FH+) and a negative FH (FH-) is indicated in red dotted line and blue dotted line, respectively. Figures a and d were based on GRS for all participants in the study; Figures b and e were based on GRS in men with a negative FH; and Figures c and f were based on GRS in men with a FH+.
High-grade PCa
A total of 217 (4.79%) men were diagnosed with high-grade PCa. In univariate analysis, positive FH was not significantly associated with the detection of high-grade PCa (OR=1.21, 95% CI: 0.86–1.72, P=0.28). In contrast, GRS was significantly associated with high-grade PCa (OR=1.34, 95% CI: 1.16–1.54, P=5.86×10−5).
Men with a positive FH had a non-significantly (P=0.20) higher detection rate of high-grade PCa (5.70%) than those with a negative FH (4.61%) (Figure 1a and Figure 2d–f). In comparison, men with GRS >1.4 had a significantly higher detection rate of high-grade PCa (6.29%) than those men with lower risk (4.31%), P=0.008 (Figure 1b and Figure 2d). When combining FH and GRS, the observed high-grade PCa detection rate was significantly higher in men with positive FH or GRS >1.4 (5.89%) than the remaining (4.16%), P=0.009 (Figure 1c). GRS also performed better in predicting high-grade PCa among men with a negative FH (Figure 2e) or positive FH (Figure 2f).
Potential risk stratification strategies based on FH and/or GRS
The performances of several potential risk stratification strategies were compared for targeting men at higher risk of developing PCa (Table 2). The ‘FH+’ strategy, targeting FH+ men for detecting of PCa, would miss most PCa cases in the study. This method targeted only 17% of men and would detect only 20.29% of all PCa cases and 20.28% of all high-grade PCa cases in the study population. The ‘GRS’ strategy of selecting those with a higher GRS (>1.4) had a slightly better performance; it would target only 24% of men in the study and would detect 32.97% of all PCa cases and 31.80% of all high-grade PCa cases in the study. Finally, the ‘FH+ or GRS>1.4’ strategy performed best; it would target 36% of men in the study and detect 46.20% of all men who would develop PCa and 44.70% of all men who would develop high-grade PCa in the study population.
Table 2.
Performance of strategies for defining higher PCa risk based on FH and GRS for targeted PCa detection in the placebo arm of PCPT
| Criteria for higher risk | #(%) of subjects | Any PCa | High-grade PCa | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| # of cases detected | Positive predictive value (PPV) | Proportion of all cases detected | Detect-to- targeted ratio | # of cases detected | Positive predictive value (PPV) | Proportion of all cases detected | Detect-to-targeted ratio | ||
| FH+ | 772(17%) | 224 | 29.02% | 20.29% | 1.19 | 44 | 5.70% | 20.28% | 1.19 |
| GRS>1.4 | 1,097(24%) | 364 | 33.18% | 32.97% | 1.37 | 69 | 6.29% | 31.80% | 1.31 |
| FH+ and GRS>1.4 | 223(5%) | 78 | 34.98% | 7.07% | 1.43 | 16 | 7.17% | 7.37% | 1.5 |
| FH+ or GRS>1.4 | 1,646(36%) | 510 | 30.98% | 46.20% | 1.27 | 97 | 5.89% | 44.70% | 1.23 |
PCPT: Prostate Cancer Prevention Trial
PCa: Prostate cancer
FH: Family history
GRS: Genetic risk score
High-grade PCa is defined as PCa with Gleason score ≥7
Conversely, we also compared the performance of several hypothetical strategies to define men at lower PCa risk in whom targeted PCa screening may be unnecessary (Table 3). Again, the strategy of ‘FH− only’ performed poorly. With this strategy, the vast majority of men in the study (83%) would not be targeted for PCa detection and, together with its lower NPV, it would miss 79.71% of men with PCa and 79.72% of men with high-grade PCa in the study population. The other two strategies, ‘GRS<0.6 only’ and ‘FH− and GRS<0.6’ performed better than that of ‘FH-’. For example, if men with negative FH and GRS<0.6 were considered low risk, 20% men in the study would not be targeted for PCa screening, and use of this strategy would miss only 14.13% of PCa and 16.13% of high-grade PCa.
Table 3.
Performance of strategies for defining lower PCa risk based on FH and GRS for targeted PCa detection in the placebo arm of PCPT
| Criteria for lower risk | # (%) of subjects | PCa | High-grade PCa | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| # of men negative | Negative predictive value (NPV) | False negative rate | # (%) of cases missed | # of men negative | Negative predictive value (NPV) | False negative rate | # (%) of cases missed | ||
| FH− | 3,756(83%) | 2876 | 76.57% | 23.43% | 880(79.71%) | 3583 | 95.39% | 4.61% | 173(79.72%) |
| GRS<0.6 | 1,076(24%) | 894 | 83.09% | 16.91% | 182(16.49%) | 1036 | 96.28% | 3.72% | 40(18.43%) |
| FH− and GRS<0.6 | 922(20%) | 766 | 83.08% | 16.92% | 156(14.13%) | 887 | 96.20% | 3.80% | 35(16.13%) |
PCPT: Prostate Cancer Prevention Trial
PCa: Prostate cancer
FH: Family history
GRS: Genetic risk score
High-grade PCa is defined as PCa with Gleason score ≥7
Discussion
Although FH is widely used for PCa risk stratification, its performance is modest, especially for the vast majority of men in the general population who do not have a FH of PCa. Thus, the primary goal of this study was to assess whether GRS can supplement FH to improve PCa risk stratification.
The association of GRS with PCa risk has been demonstrated consistently, including a large case-control study with 43,303 PCa cases and 43,737 controls [9], prospective biopsy cohorts [16–17], existing clinical trial populations [11,18], and others [10,19–24]. However, its association with PCa risk among men with a negative FH has not been previously evaluated. Utilizing the PCPT, we found that GRS was significantly associated with detection of both PCa and high-grade PCa during seven follow-up among men with and without a FH of PCa. More importantly, we found that GRS can identify a substantial proportion of men at higher risk for PCa among FH− men, and the observed risks for PCa and high-grade PCa in these men were even higher than FH+ men. When combining GRS with FH, we can identify twice as many higher risk men in the study than FH+ alone (36% vs 17%, respectively) and their estimated higher risk was supported by their higher observed detection rate of PCa and high-grade PCa during the seven-year study.
The validity of these findings is supported by the PCPT design. First, PCPT was a prospective study in which all men were free of a PCa diagnosis at study entry and were followed for seven years for the diagnosis of PCa. Second, because all men underwent prostate biopsies, either for-cause or end-of-study, the results are less likely to be influenced by detection bias related to PSA levels or FH. In contrast, the association of PCa risk with PSA and FH may be overestimated in many observational studies in which men with a higher PSA and/or FH+ may receive closer monitoring for PCa. Third, the PCa risk-associated SNPs selected in the study and ORs of these SNPs used in the calculation of GRS were all predetermined based on prior studies [15]. Therefore, the GRS results were not subjected to over-fitting.
The performances of FH, GRS, and even their combination in stratifying PCa risk are modest, which is not unexpected considering that PCa etiology is complex. While their performances are insufficient to justify their use as diagnostic markers, they can likely be useful for identifying men at higher risk for targeted PSA screening in primary care. This is especially relevant given the recent AUA recommendation of a risk-based PSA screening strategy [5]. The benefit of an FH-based targeted PSA screening strategy has already been demonstrated by a re-analysis of the PLCO study. In contrast to a lack of benefit in reducing PCa-specific mortality by non-targeted PSA screening [2], targeted PSA screening in the ~7% of men with a positive FH would reduce PCa-specific mortality by ~50% [25]. From a comparative effectiveness perspective and based on the current study, it is rational to suggest that GRS should be included in risk stratification methods. With better risk stratification by combining FH and GRS, it is expected that targeted PSA screening may further reduce PCa-specific mortality.
Several features and limitations of this study should be noted. First, because the PCPT was limited to men with PSA ≤3.0 ng/mL, caution should be exercised when attempting to apply our conclusions to men in the general population. Second, because many PCa cases were detected on an end-of-study biopsy, the detection rate of PCa during the seven-year follow-up (~24%) is higher than that in contemporary clinical settings. This is also a strength of the study as it minimizes the false negative PCa rate. Third, because this genetic study was approved in 2010, only 29 SNPs discovered by 2009 were analyzed. It is expected that adding additional risk-associated SNPs in the analysis would strengthen the current findings. Fourth, due to the PCPT design and inclusion criteria, most cases were less aggressive and few men died of PCa, and research is ongoing to link PCPT participants with the National Death Index [26]. As a result, we expect that risk stratification for lethal PCa will be possible in future analyses. Fifth, although we compared the performance of several hypothetical targeted strategies, the PCPT was not designed for PSA screening. Randomized trials for PSA screening such as PLCO and ERSPC would be more appropriate studies for such analyses. Lastly, because less than 8% of men enrolled in the PCPT were of other races and ethnicities, our current study was limited to Caucasian men. It would seem, however, reasonable to expect that the major findings from this study can be generalized to other ethnicities. Indeed, a significant association of PCa risk with race-specific GRS has been consistently reported in African American [23], Japanese [24], and Chinese populations [17], as well as multiethnic groups [27].
In summary, the current study demonstrates the novel finding that a GRS based on multiple PCa risk-associated SNPs can identify men at heightened risk for PCa, particularly among men with a negative FH. Combining FH with GRS may provide a better risk stratification tool for individualized decision making regarding PSA screening.
Supplementary Material
Acknowledgments
Funding
This work was partially supported by National Cancer Institute grants CA140262 to Xu and Platz, and CA148463 to Xu, National Key Basic Research Program Grant 973 of China (2012CB518301) to Xu, and the Key Project of the National Natural Science Foundation of China (81130047) to Xu, This study is partially supported by the Ellrodt-Schweighauser Family Chair of Cancer Genomic Research of NorthShore University HealthSystem to Xu. Support for the PCPT was from P01 CA108964 to Thompson, U10 CA37429 to C.D. Blanke, and UM1 CA182883 to Thompson and Tangen. Platz is also supported by P30 CA006973. Thompson is also supported by U01 CA086402 and P30 CA054174. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the participants in the PCPT and the clinicians and staff who contributed their expertise in recruiting participants and conducting this trial.
Footnotes
Conflict of interests
Jianfeng Xu, Karim Kader, Jielin Sun, S. Lilly Zheng, and William B Isaacs filed several patent applications related to the genetic risk score of prostate cancer risk-associated SNPs.
Notes
The study funders had no role in the design of this study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Paez A, Maattanen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, van der Kwast T, Kujala PM, Blijenberg BG, Stenman UH, Huber A, Taari K, Hakama M, Moss SM, de Koning HJ, Auvinen A, Investigators E. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC, Team PP. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, Gleitsmann K, Koenig HC, Lam C, Maltz A, Rugge JB, Lin K. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(11):762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 5.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad MH, Penson DF, Zietman AL. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA., Jr Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JA, 2nd, Gerber L, Moreira DM, Hamilton RJ, Banez LL, Castro-Santamaria R, Andriole GL, Isaacs WB, Xu J, Freedland SJ. Prostate cancer risk in men with prostate and breast cancer family history: results from the REDUCE study (R1) J Intern Med. 2012;272(1):85–92. doi: 10.1111/j.1365-2796.2011.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Sun J, Zheng SL. Prostate cancer risk-associated genetic markers and their potential clinical utility. Asian J Androl. 2013;15(3):314–322. doi: 10.1038/aja.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, Benlloch S, Hazelett DJ, Wang Z, Saunders E, Leongamornlert D, Lindstrom S, Jugurnauth-Little S, Dadaev T, Tymrakiewicz M, Stram DO, Rand K, Wan P, Stram A, Sheng X, Pooler LC, Park K, Xia L, Tyrer J, Kolonel LN, Le Marchand L, Hoover RN, Machiela MJ, Yeager M, Burdette L, Chung CC, Hutchinson A, Yu K, Goh C, Ahmed M, Govindasami K, Guy M, Tammela TL, Auvinen A, Wahlfors T, Schleutker J, Visakorpi T, Leinonen KA, Xu J, Aly M, Donovan J, Travis RC, Key TJ, Siddiq A, Canzian F, Khaw KT, Takahashi A, Kubo M, Pharoah P, Pashayan N, Weischer M, Nordestgaard BG, Nielsen SF, Klarskov P, Roder MA, Iversen P, Thibodeau SN, McDonnell SK, Schaid DJ, Stanford JL, Kolb S, Holt S, Knudsen B, Coll AH, Gapstur SM, Diver WR, Stevens VL, Maier C, Luedeke M, Herkommer K, Rinckleb AE, Strom SS, Pettaway C, Yeboah ED, Tettey Y, Biritwum RB, Adjei AA, Tay E, Truelove A, Niwa S, Chokkalingam AP, Cannon-Albright L, Cybulski C, Wokolorczyk D, Kluzniak W, Park J, Sellers T, Lin HY, Isaacs WB, Partin AW, Brenner H, Dieffenbach AK, Stegmaier C, Chen C, Giovannucci EL, Ma J, Stampfer M, Penney KL, Mucci L, John EM, Ingles SA, Kittles RA, Murphy AB, Pandha H, Michael A, Kierzek AM, Blot W, Signorello LB, Zheng W, Albanes D, Virtamo J, Weinstein S, Nemesure B, Carpten J, Leske C, Wu SY, Hennis A, Kibel AS, Rybicki BA, Neslund-Dudas C, Hsing AW, Chu L, Goodman PJ, Klein EA, Zheng SL, Batra J, Clements J, Spurdle A, Teixeira MR, Paulo P, Maia S, Slavov C, Kaneva R, Mitev V, Witte JS, Casey G, Gillanders EM, Seminara D, Riboli E, Hamdy FC, Coetzee GA, Li Q, Freedman ML, Hunter DJ, Muir K, Gronberg H, Neal DE, Southey M, Giles GG, Severi G, Cook MB, Nakagawa H, Wiklund F, Kraft P, Chanock SJ, Henderson BE, Easton DF, Eeles RA, Haiman CA Breast Prostate Cancer Cohort C, Consortium P, Consortium C, Consortium G-OE. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46(10):1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Balter K, Kader AK, Turner AR, Liu W, Bleecker ER, Meyers DA, Duggan D, Carpten JD, Chang BL, Isaacs WB, Xu J, Gronberg H. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358(9):910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 11.Kader AK, Sun J, Reck BH, Newcombe PJ, Kim ST, Hsu FC, D’Agostino RB, Jr, Tao S, Zhang Z, Turner AR, Platek GT, Spraggs CF, Whittaker JC, Lane BR, Isaacs WB, Meyers DA, Bleecker ER, Torti FM, Trent JM, McConnell JD, Zheng SL, Condreay LD, Rittmaster RS, Xu J. Potential impact of adding genetic markers to clinical parameters in predicting prostate biopsy outcomes in men following an initial negative biopsy: findings from the REDUCE trial. Eur Urol. 2012;62(6):953–961. doi: 10.1016/j.eururo.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Na R, Hsu FC, Zheng SL, Wiklund F, Condreay LD, Trent JM, Xu J. Genetic score is an objective and better measurement of inherited risk of prostate cancer than family history. Eur Urol. 2013;63(3):585–587. doi: 10.1016/j.eururo.2012.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 14.Pharoah PD, Antoniou AC, Easton DF, Ponder BA. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358(26):2796–2803. doi: 10.1056/NEJMsa0708739. [DOI] [PubMed] [Google Scholar]
- 15.Kim ST, Cheng Y, Hsu FC, Jin T, Kader AK, Zheng SL, Isaacs WB, Xu J, Sun J. Prostate cancer risk-associated variants reported from genome-wide association studies: meta-analysis and their contribution to genetic Variation. Prostate. 2010;70(16):1729–1738. doi: 10.1002/pros.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aly M, Wiklund F, Xu J, Isaacs WB, Eklund M, D’Amato M, Adolfsson J, Gronberg H. Polygenic risk score improves prostate cancer risk prediction: results from the Stockholm-1 cohort study. Eur Urol. 2011;60(1):21–28. doi: 10.1016/j.eururo.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Liu F, Wang Z, Na R, Zhang L, Wu Y, Zheng J, Lin X, Jiang D, Sun J, Zheng SL, Ding Q, Xu J. Prediction of prostate cancer from prostate biopsy in Chinese men using a genetic score derived from 24 prostate cancer risk-associated SNPs. Prostate. 2013;73(15):1651–1659. doi: 10.1002/pros.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindstrom S, Schumacher FR, Cox D, Travis RC, Albanes D, Allen NE, Andriole G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Crawford ED, Diver WR, Gaziano JM, Giles GG, Giovannucci E, Gonzalez CA, Henderson B, Hunter DJ, Johansson M, Kolonel LN, Ma J, Le Marchand L, Pala V, Stampfer M, Stram DO, Thun MJ, Tjonneland A, Trichopoulos D, Virtamo J, Weinstein SJ, Willett WC, Yeager M, Hayes RB, Severi G, Haiman CA, Chanock SJ, Kraft P. Common genetic variants in prostate cancer risk prediction--results from the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) Cancer Epidemiol Biomarkers Prev. 2012;21(3):437–444. doi: 10.1158/1055-9965.EPI-11-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kote-Jarai Z, Easton DF, Stanford JL, Ostrander EA, Schleutker J, Ingles SA, Schaid D, Thibodeau S, Dork T, Neal D, Donovan J, Hamdy F, Cox A, Maier C, Vogel W, Guy M, Muir K, Lophatananon A, Kedda MA, Spurdle A, Steginga S, John EM, Giles G, Hopper J, Chappuis PO, Hutter P, Foulkes WD, Hamel N, Salinas CA, Koopmeiners JS, Karyadi DM, Johanneson B, Wahlfors T, Tammela TL, Stern MC, Corral R, McDonnell SK, Schurmann P, Meyer A, Kuefer R, Leongamornlert DA, Tymrakiewicz M, Liu JF, O’Mara T, Gardiner RA, Aitken J, Joshi AD, Severi G, English DR, Southey M, Edwards SM, Al Olama AA, Consortium P, Eeles RA. Multiple novel prostate cancer predisposition loci confirmed by an international study: the PRACTICAL Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2052–2061. doi: 10.1158/1055-9965.EPI-08-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Sun J, Kader AK, Lindstrom S, Wiklund F, Hsu FC, Johansson JE, Zheng SL, Thomas G, Hayes RB, Kraft P, Hunter DJ, Chanock SJ, Isaacs WB, Gronberg H. Estimation of absolute risk for prostate cancer using genetic markers and family history. Prostate. 2009;69(14):1565–1572. doi: 10.1002/pros.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salinas CA, Koopmeiners JS, Kwon EM, FitzGerald L, Lin DW, Ostrander EA, Feng Z, Stanford JL. Clinical utility of five genetic variants for predicting prostate cancer risk and mortality. Prostate. 2009;69(4):363–372. doi: 10.1002/pros.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Kader AK, Hsu FC, Kim ST, Zhu Y, Turner AR, Jin T, Zhang Z, Adolfsson J, Wiklund F, Zheng SL, Isaacs WB, Gronberg H, Xu J. Inherited genetic markers discovered to date are able to identify a significant number of men at considerably elevated risk for prostate cancer. Prostate. 2011;71(4):421–430. doi: 10.1002/pros.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Bensen JT, Smith GJ, Mohler JL, Taylor JA. GWAS SNP Replication among African American and European American men in the North Carolina-Louisiana prostate cancer project (PCaP) Prostate. 2011;71(8):881–891. doi: 10.1002/pros.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akamatsu S, Takahashi A, Takata R, Kubo M, Inoue T, Morizono T, Tsunoda T, Kamatani N, Haiman CA, Wan P, Chen GK, Le Marchand L, Kolonel LN, Henderson BE, Fujioka T, Habuchi T, Nakamura Y, Ogawa O, Nakagawa H. Reproducibility, performance, and clinical utility of a genetic risk prediction model for prostate cancer in Japanese. PLoS One. 2012;7(10):e46454. doi: 10.1371/journal.pone.0046454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liss MA, Chen H, Hemal S, Krane S, Kane CJ, Xu J, Kader AK. Impact of family history on prostate cancer mortality in white men undergoing prostate specific antigen based screening. J Urol. 2015;193(1):75–79. doi: 10.1016/j.juro.2014.07.085. [DOI] [PubMed] [Google Scholar]
- 26.Thompson IM, Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, Lucia MS, Ford LG. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369(7):603–610. doi: 10.1056/NEJMoa1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann TJ, Van Den Eeden SK, Sakoda LC, Jorgenson E, Habel LA, Graff RE, Passarelli MN, Cario CL, Emami NC, Chao CR, Ghai NR, Shan J, Ranatunga DK, Quesenberry CP, Aaronson D, Presti J, Wang Z, Berndt SI, Chanock SJ, McDonnell SK, French AJ, Schaid DJ, Thibodeau SN, Li Q, Freedman ML, Penney KL, Mucci LA, Haiman CA, Henderson BE, Seminara D, Kvale MN, Kwok PY, Schaefer C, Risch N, Witte JS. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov. 2015 Aug;5(8):878–91. doi: 10.1158/2159-8290.CD-15-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.