Abstract
Autophagy is a vital process controlling the lysosomal degradation of cellular organelles and thereby regulating tissue homeostasis in an environment-dependent fashion. Recent studies have unveiled the critical role of tumor cell-derived autophagy in regulating pro-tumor and anti-tumor processes depending on different stages and tumor microenvironments. However, the precise mechanism whereby autophagy regulates tumor progression remains largely unclear. Since myeloid cells contribute to tumor progression and metastasis, we evaluated the role of myeloid cell-specific autophagy in the regulation of tumor progression. We found that the number and size of metastatic lesions were smaller in myeloid cell-specific autophagy-deficient mice. Furthermore, autophagy-mediated regulation of TGF-β in myeloid cells was associated with the induction of epithelial-mesenchymal transition (EMT), which increases the invasive and metastatic potentials of tumor cells. Myeloid-derived autophagy also plays a critical role in impairing antitumor immune responses and promoting the survival and accumulation of M2 macrophages in tumor tissues in a CSF-1 and TGF-β-dependent manner. Taken together, our findings elucidate previously unrecognized mechanisms by which myeloid cells promote tumor progression through autophagy-mediated regulation of malignancy and immune tolerance.
Introduction
Tumor microenvironments (TME) regulate the tumorigenic activities of transformed cells in coordination with multiple tumor-infiltrating normal cells such as endothelial cells, fibroblasts, mesenchymal stem cells and inflammatory cells [1,2]. In particular, recent studies have revealed the importance of tumor-associated myeloid cells (TAM) in tumor progression. TAM support tumor progression through various mechanisms including tumor angiogenesis, immune suppression, matrix remodeling and the epithelial-mesenchymal transition (EMT) of malignant cells [3,4]. Thus, the detailed evaluation of molecular mechanisms that govern the complex interplay between TAM and transformed cells must be defined in order to control the dismal clinical course of malignancy and improve patient responsiveness to anticancer therapeutics.
Autophagy is a vital physiological pathway that maintains metabolic homeostasis and controls stress responses by capturing intra- and extra-cellular components in autophagic vesicles and processing them in the lysosomal degradation system [5,6]. While accumulating evidence has clarified the contribution of autophagy to tumor initiation and progression, it has been proposed that autophagic signals in tumor cells either promote or suppress tumor growth in a context-dependent manner [7–10]. Deficiency of autophagy-essential genes, such as Beclin-1 and Atg8, increased tumorigenicity, and autophagy protects cells from transformation through protection excess oxidative stress in p62-dependent manner [7–9]. On the other hands, Ras utilize autophagy to facilitate lung tumorigenicity by modulating several metabolic pathway [10,11]. Thus, the mechanism by which autophagic pathways in tumor-infiltrating non-transformed cells regulate tumorigenicity in a TME-dependent manner remains elusive.
In this study, we demonstrate the unique role of myeloid cell autophagic pathways in the regulation of the malignant properties of tumor cells. Although myeloid cell-derived autophagy is dispensable for subcutaneous tumor growth, it facilitates the invasive and metastatic properties of tumor cells through TGF-β1-mediated induction of EMT and immune tolerance. Moreover, myeloid cell-derived autophagy contributes to the enhanced survival in stressed TME and the differentiation of M2-like macrophages induced by tumor-derived colony-stimulating factor-1 (CSF-1). Our findings reveal a new biological aspect of myeloid cell-derived autophagy in supporting tumor metastasis and progression.
Materials and methods
Mice
C57BL/6 and BALB/c mice were purchased from SCL. MMTV-PyMT mice were purchased from Jackson Laboratory. Atg5flox/flox, Lysozome M (LysM)-Cre/Atg5flox/flox (LysM-Atg5-/-) and OT-I mice were used as described previously [12,13]. All experiments were conducted under a protocol approved by the animal care committees of Hokkaido University, and all mice were cared ethically and strictly followed the declaration of Helsinki with proper Housing and husbandry environment. We were monitored at least once a week of all animal health conditions, and there were no case that severely ill or died at anytime prior to the experimental endpoint. We followed the protocol recommended by our institute for early euthanasia/humane endpoints for animals. CD11b-positive myeloid cells were purified by anti-CD11b-labelled microbeads (Miltenyi Biotech) from protease-digested tumor tissues.
Patient samples
The clinical protocols for this study were approved by the committees in the Institutional Review Board of Hokkaido University Hospital (Approval number: 10–0114). CD11b+ cells were obtained from the tumors or peripheral blood of patients with stage IV non-small cell lung carcinomas after written informed consent had been obtained. The cells were isolated by Ficoll-Hypaque density centrifugation, and further purified by anti-CD11b-labelled microbeads (Miltenyi Biotech).
Tumor cells
Tumor cells (B16-F10 melanoma & MC38 colon carcinoma) were obtained from the American Type Culture Collection (ATCC). All cell lines were obtained one year before being used in experiments and authenticated by the Central Institute for Experimental Animals (Kawasaki, Japan) for interspecies and mycoplasma contamination by PCR within 3 months of the experiments.
In vivo tumorigenic assays
C57BL/6 wild-type, LysM-Atg5-/- or Atg5flox/flox mice were injected subcutaneously in the flank with 1x105 B16-F10 or MC38 cells. Tumor growth was measured on the indicated days. For metastatic assays, B16-F10 or MC38 tumor cells were injected intravenously and intraperitoneally into LysM-Atg5-/- or Atg5flox/flox mice. The average areas, numbers and weight of lung and liver metastatic lesions were analyzed 3 weeks after the procedures. The average tumor areas were quantified by Image-J software. In some instances, anti-TGF-β1 mAb (1D11; 1 mg/kg per mouse) or CSF-1 receptor kinase inhibitor GW2580 (50 mg/kg) were administered intraperitoneally to the mice twice per week during the experimental period; in other experiments, anti-CD8 mAb (53–6.72, 1 mg/kg) was administered intravenously two days prior to tumor challenge. We confirmed the specificity of GW2580 since it inhibited expression of CSF-1 receptor but not CSF-2 receptor on monocytes.
In vitro cell viability assay
Atg5flox/flox or LysM-Atg5-/- CD11bhighTAM were isolated from MC38 or B16-deried tumors and briefly cultured for 16 h. MC38 cells were cultured with 50% of the TAM supernatants for 48 h, and then treated with cisplatin (CDDP) (10 μM) for 48 h. BMDM generated with recombinant CSF-1 (10 μg/mL) for 7 days were cultured under serum-starved conditions or low oxygen concentrations (1%) for 16 h, or exposed to ultraviolet light (300 J/m2) for 10 sec and cultured for 16 h. Cell viability was assessed with FITC-labelled annexin V and propidium iodide by flow cytometry. In some experiments, cells were treated with TGF-β receptor kinase inhibitor (SB431542) or blocking antibodies against murine TGF-β1 (Clone 1D11). The specificity of SB431542 was previously confirmed as reported [14].
In vitro invasion assay
Invasive activities of tumor cells were determined using the BD Bio-Coat Tumor Invasion Assay System (BD Bioscience) according to the manufacturer’s instructions. In brief, the upper chamber was coated with matrigel (100 μl), and serum-free media was used for the bottom chamber. MC38 murine colon cancer cells or A549 human lung cancer cells pretreated with or without myeloid cell supernatants were seeded into the upper chamber. The cells that invaded the bottom chamber were stained with 4 μg/mL calcein AM for 1 h, and then counted using a fluorescence microscope.
Immunofluorescence microscopy
MC38 cells (1 x 104) stimulated with Atg5flox/flox or LysM-Atg5-/- TAM or recombinant murine TGF-β1 (100 ng/mL) were cultured on a glass chamber plate overnight. The plate was washed three times to remove floating cells and adherent cells were fixed with 20% methanol at –20°C for 5 minutes. The cells were stained with antibodies for E-cadherin, N-cadherin, or vimentin and then Alexa-Flow 488- or Cy3-labeled secondary Ab and visualized using a TE2000-U inverted fluorescence microscope (Olympus).
Cytokine assays
TGF-β1, IL-6, IL-10, IL-12p40 and IFN-γ were measured by ELISA in culture supernatants from TAM and TAN generated from Atg5flox/flox or LysM-Atg5-/- mice, CD11b+ cells derived from tumors or peripheral bloods of non-small lung cancer patients according to the manufacturer’s directions (R&D Systems, BD Biosciences).
mRNA quantification by real-time PCR
The mRNA was isolated from Atg5flox/flox or LysM-Atg5-/- TAM. TGF-β1 forward and reverse primers were 5'-CACCGGAGAGCCCTGGATA-3' and 5'-TGTACAGCTGCCGCACACA—3', respectively. The mRNAs of TGF-β1 were quantified by real-time PCR (RT-PCR) using SYBR Green Gene Expression Assays (Applied Biosystems).
T cell differentiation assay
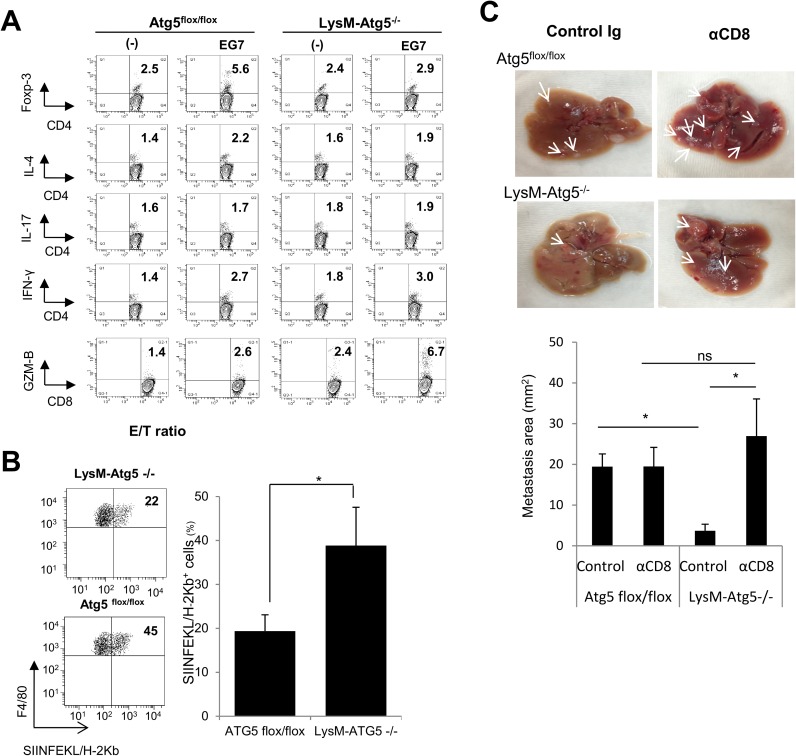
T cell differentiation assays were, respectively performed as previously described [15]. In brief, irradiated EG7 tumor cells (1 x 106) were loaded to Atg5flox/flox or LysM-Atg5-/- BMDM (1 x 105) to facilitate phagocytosis and antigen processing. Two hours after the EG7 cells were loaded, they were extensively washed, and then cultured with CD4+ or CD8+ T cells from wild-type mice for 96 h. Cells were stained with anti-CD3, -CD4, or -CD8 mAbs, fixed and permeabilized with Cytofix / Cytoperm (BD Biosciences). Cells were then further stained with anti-IFN-γ, anti-IL-4, anti-IL-17, anti-Foxp3, anti-granzyme-B and isotype control mAb (BD Biosciences), and analyzed by flow cytometry.
OVA-specific antitumor immune responses
OVA-specific CD8+ T cells isolated from OT-I mice were adoptively transferred (5 x 106 /mice) and two days later EG7 or B16-OVA cells (1 x 105 /mice) were inoculated subcutaneously into C57BL/6 wild-type, LysM-Atg5-/-, Atg5flox/flox or bone marrow chimeric mice as described below. The established tumors (~25 mm2) were then treated with CDDP (250 μg/mice) in the presence of control Ig or anti-TGF-β1 mAb on days 8, 10 and 12 after tumor inoculation. Four days after the final treatment, tumor-infiltrating myeloid cells were obtained from B16-OVA tumors using density gradient separation. The levels of SIINFEKL/H-2b complex on TAM were analyzed by flow cytometry.
Statistics
The differences between two groups were determined by the Student’s t test or the two-sample t test with Welch’s correction. The differences among three or more groups were determined by a one-way ANOVA. P values less than 0.05 are considered statistically significant. *: p<0.05, ns: not significant.
Results
Myeloid-specific autophagy promotes the metastatic potential of tumor cells
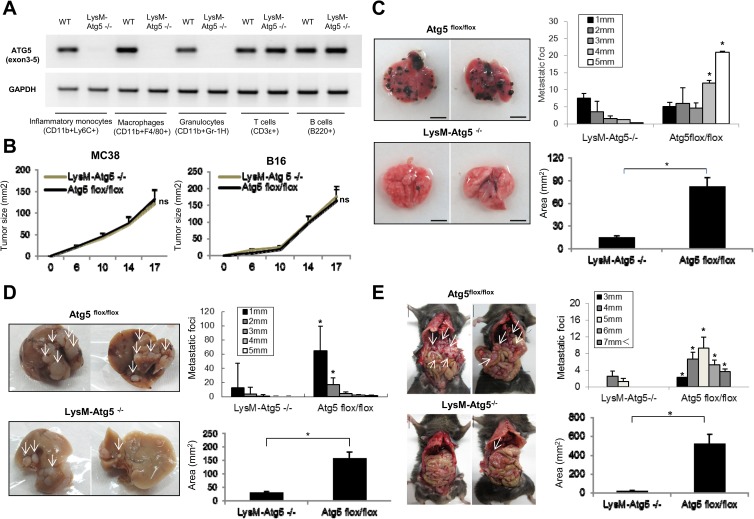
To characterize the role of myeloid cell autophagic pathways in tumor progression, we generated a myeloid cell-specific targeted deletion of the autophagic gene Atg5 by crossing mice carrying the lox-P flanked Atg5 allele to mice carrying lysozyme M (LysM) promoter-driven Cre recombinase [16]. In this myeloid cell-specific autophagy-deficient model (LysM-Atg5-/-), the Atg5 expression was specifically deleted in granulocytes, macrophages and Ly6Chigh inflammatory monocytes, and the defect of autophagy was confirmed in the LysM-Atg5-/- macrophages under amino acid starvation (Fig 1A). We select Atg5 as representative marker for autophagy function due to its essential role of autophagic processes [17]. We found that subcutaneous tumor growth was comparable in LysM-Atg5-/- and control Atg5flox/flox mice, suggesting that autophagy in myeloid cells made only a minor contribution to primary tumorigenesis (Fig 1B). In contrast, B16 melanomas yielded large and multiple pulmonary metastatic lesions in Atg5flox/flox mice by 28 days after tail vein injection, but the metastatic tumors were fewer and smaller in LysM-Atg5-/- mice (Fig 1C). Furthermore, splenic inoculation of MC38 colon carcinoma cells resulted in decreased numbers of liver metastatic tumors in LysM-Atg5-/- mice compared to their Atg5flox/flox counterparts (Fig 1D). In addition, myeloid cell-specific deletion of Atg5 resulted in the suppression of tumor dissemination to the peritoneal cavity upon splenic injection of MC38 tumor cells (Fig 1E). Splenomegaly by tumor infiltration was detected in Atg5flox/flox control, but not LysM-Atg5-/- mice. Quantitative analysis revealed that the metastasized tumor sizes in LysM-Atg5-/- mice were smaller than those in Atg5flox/flox mice (Fig 1C–1E).
Fig 1. Myeloid cell-specific deletion of autophagy attenuates tumor metastasis.
(A) The Atg5 gene was specifically deleted in myeloid cells of LysM-Atg5-/- mice. The cells isolated from in vivo growing tumors (MC38) were prepared from wild-type (WT) or LysM-Atg5-/- mice, and CD11b+Ly6C+ monocytes, CD11b+F4/80+ macrophages, CD11b+Gr-1+ granulocytes, CD3ε+ T cells and B220+ B cells were isolated by flow cytometry. Expression of Atg5 was evaluated by RT-PCR. (B) Atg5flox/flox or LysM-Atg5 -/- mice were inoculated subcutaneously with MC38 or B16 cells. Tumor growth was measured on the indicated days. (C) B16 cells were injected via tail veins of Atg5flox/flox or LysM-Atg5 -/- mice. 28 days later, the mice were sacrificed and subjected to macroscopic analysis for metastatic lesions in the lung. The experiments were performed at least ten times with similar results. (D) MC38 cells were injected into the spleen of Atg5flox/flox or LysM-Atg5 -/- mice. Twenty-one days later, the mice were sacrificed and subjected to macroscopic analysis for metastatic lesions in the liver. Representative data (left) and statistical analysis of average numbers and areas (mm2) of metastatic lesions are shown (right). The tumor metastatic lesions are shown by white arrows. The experiments were performed at least ten times with similar results. (E) MC38 cells were injected into the spleens of Atg5flox/flox or LysM-Atg5 -/- mice. Twenty-one days later, the mice were sacrificed and subjected to macroscopic analysis for disseminated lesions in the peritoneal cavity. Representative data (left) and statistical analysis of average numbers and areas (mm2) of metastatic lesions are shown (right). The experiments were performed five times with similar results. *: p<0.05, ns: not significant.
The myeloid cell-specific deletion of Atg7 also manifest the decreased lung metastasis of B16 tumors, implying that myeloid cell-specific autophagy promotes tumor metastasis in general. Taken together, these results demonstrate that the myeloid cell-specific autophagic pathway promotes the invasive and metastatic activities of tumor cells.
Myeloid-specific autophagy promotes pro-invasive tumor cell activity
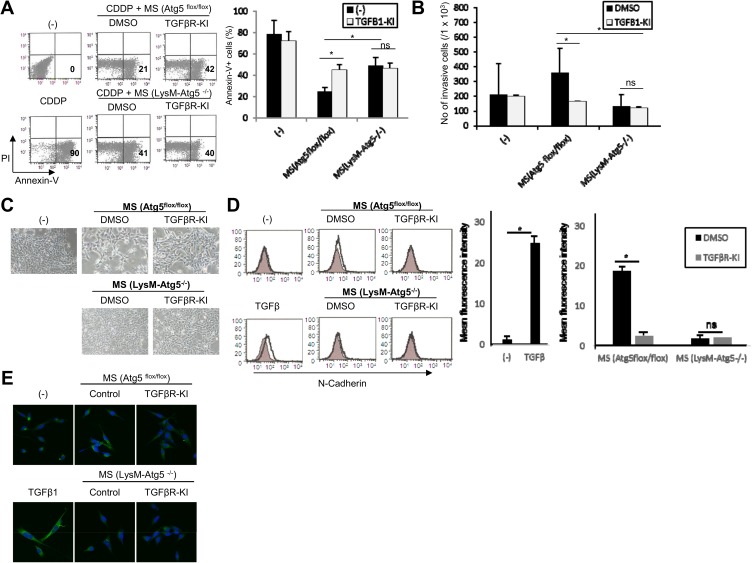
Autophagic pathways regulate either cellular survival or death in an environment-dependent fashion, but how autophagy in myeloid cells influences cellular homeostasis remains largely unknown. To investigate this, we examined whether myeloid cell-specific autophagy had an impact on tumor cell survival upon induction of stress such as exposure to cytotoxic agents. We isolated TAM from liver and lung metastatic lesions of MC38 and B16 tumors according to CD11bhigh expression, respectively. CD11bhigh population generally reflect either activation of inflammatory monocytes, macrophages and granulocytes, and we confirmed mixture of F4/80high/Gr-1low and F4/80low/Gr-1high subpopulation among CD11bhigh cells in tumor tissues. There was little difference between each mice on the composition of CD11bhighF4/80high/Gr-1low and CD11bhighF4/80low/Gr-1high in tumor tissues. and the frequencies of TAM populations were similar in both groups. Treatment of supernatants of TAM from wild-type or Atg5flox/flox mice triggered resistance of MC38 tumor cells to cisplatin, as revealed by decreased annexin V and propidium iodide (PI) staining compared to untreated MC38 cells. In contrast, these protective effects were significantly attenuated when supernatants of LysM-Atg5-/—derived TAMs were used (Fig 2A). These results underscore a vital contribution of myeloid cell-derived autophagy in supporting the resistance of tumor cells to cytotoxic therapy. We next examined whether myeloid cell autophagy regulates the invasive activities of MC38 tumor cells by in vitro matrigel assay. The treatment with supernatant from LysM-Atg5-/- TAM attenuated invasive activities of MC38 tumor cells compared to supernatants from control Atg5flox/flox cells (Fig 2B).
Fig 2. Myeloid cell-specific autophagy supports the chemoresistance and invasive properties of tumor cells through TGF-β1-dependent manner.
(A, B) MC38 colon cancer cells were cultured with supernatants from Atg5flox/flox or LysM-Atg5-/- TAM (MS) for 48 h in the presence of DMSO or the TGF-β receptor kinase inhibitor SB431542 (TGFβR-KI). (A) The indicated cells were then treated with Cisplatin (CDDP: 10 μM) for 24 h, and the cell viability was determined by flow cytometry with PI and Annexin V staining. (B) The indicated cells were evaluated for in vitro invasion by Matrigel assay. (C) MC38 tumor cells were cultured with supernatants from Atg5flox/flox or LysM-Atg5-/- TAM (MS) with or without SB431542 (TGFβR-KI) for 48 h. The cellular morphological appearance was analyzed by microscopy. (D, E) MC38 tumor cells were cultured with supernatants from Atg5flox/flox or LysM-Atg5-/- TAM (MS) with or without SB-431542 (TGFβR-KI) or recombinant TGF-β1 (100 ng/mL). The expression levels of N-cadherin (solid line) and vimentin (green) were analyzed by flow cytometry (D: left) and confocal microscopy (E), respectively. The statistical analysis was also performed for flow cytometry data (n = 3; D: right). *: p<0.05, ns: not significant.
Epithelial-mesenchymal transition (EMT) manifests as a critical step in conferring tumor cells with metastatic activities [18–20]. To investigate whether myeloid cell-specific autophagy regulates morphological and genetic profiles characterized as EMT in tumor cells, we performed confocal microscopy on MC38 tumor cells stimulated with supernatants from LysM-Atg5-/- or Atg5flox/flox TAM. Treatment with supernatants from Atg5flox/flox but not LysM-Atg5-/- TAM caused morphological changes with cobblestone-like epithelial layers of MC38 cells becoming scattered and presenting an elongated fibroblastic shape in appearance (Fig 2C). Furthermore, the tumor cells exhibited robust upregulation of various mesenchymal markers such as vimentin and N-cadherin when stimulated with supernatants from wild-type or Atg5flox/flox TAM (Fig 2D and 2E). In contrast, treatment with supernatant of LysM-Atg5-/- TAM did not induce the EMT-associated changes in MC38 tumor cells. These results demonstrate that autophagy in myeloid cells renders tumor cells with the ability to acquire chemo-resistance and invasive properties by inducing EMT.
Myeloid autophagy regulates the invasive activities of tumor cells through TGF-β
We found that TGF-β production was positively regulated in tumor-associated myeloid cells in an autophagy-dependent manner. Given critical role of TGF-β in regulating tumorigenicity by promoting anti-cancer drug resistance, metastatic potential and immune suppression [21–24], we next examine whether TGF-β blockade by TGF-β receptor kinase inhibitor (SB431542) modulated the anticancer drug sensitivity and invasive phenotypes of tumor cells stimulated with TAM supernatants. In agreement with our hypothesis, the sensitivity to cisplatin of tumor cells treated with Atg5flox/flox TAM were increased to the levels of those of LysM-Atg5-/- TAM supernatants in the presence of SB431542 (Fig 2A). On the other hands, the sensitivity to cisplatin and invasive activities of tumor cells treated with Atg5flox/flox TAM were reduced to the levels of those of LysM-Atg5-/- TAM supernatants in the presence of SB431542 (Fig 2B). Treatment with SB431542 partially reversed the epithelial morphology of MC38 tumor cells treated with supernatants of Atg5flox/flox TAM (Fig 2C). Moreover, treatment with SB431542 suppressed the induction of N-cadherin and vimentin, which are closely associated with EMT, in MC38 cells treated with supernatants from Atg5flox/flox TAMs, consistent with previous findings that TGF-β is critical for multiple tumorigenic processes including EMT [21, 22] (Fig 2D and 2E).
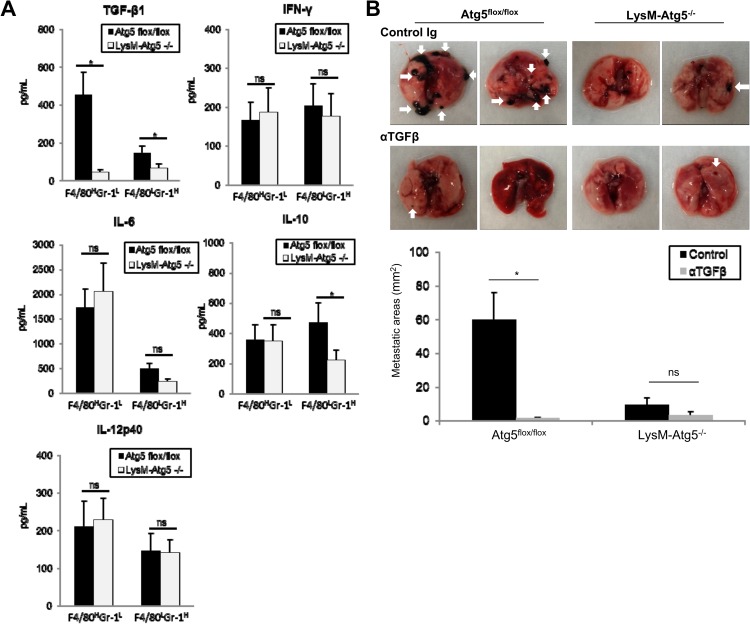
Myeloid cells regulate tumorigenic activities by releasing multiple sets of soluble factors. In addition, accumulating evidence reveals that autophagy contributes to the secretion of multiple cytokines and growth factors [25–27]. We therefore determined whether autophagic signals modulate cytokine profiles in myeloid cells. We found that Atg5flox/flox CD11b+F4/80highGr-1low and CD11b+F4/80lowGr-1high TAM produced TGF-β1 at greater amounts than their LysM-Atg5-/- counterparts (Fig 3A and 3B). In contrast, IL-10 was produced by Atg5flox/flox CD11b+F4/80lowGr-1high TAM at higher amounts than by their LysM-Atg5-/- counterparts, but was comparable in Atg5flox/flox and LysM-Atg5-/- CD11b+F4/80highGr-1lowTAM. The production of IFN-γ, IL-6 and IL-12p40 was similar in the Atg5flox/flox and LysM-Atg5-/- TAM (Fig 3A). Similarly, myeloid cells of normal splenic and liver macrophages and neutrophils produced comparable levels of these cytokines. We next evaluated whether TGF-β1 regulates the metastatic activities of tumors in vivo. Metastatic models of lung and liver cancer using tail vein or splenic administration of B16 or MC38 tumor cells revealed that treatment with anti-TGF-β1 neutralizing antibodies suppressed the formation of macroscopic metastatic foci in Atg5flox/flox mice, whereas it had little effect on metastatic lesions in LysM-Atg5-/- mice (Fig 3B). Overall, TGF-β1 released from myeloid cells in an autophagy-dependent fashion is a critical mediator that promotes tumor cell invasive and metastatic activities.
Fig 3. TGF-β upregulated by myeloid cell-specific autophagy is a major mediator in regulating the invasive properties of tumors.
(A)Production of TGF-β1, IL-6, IL-10, IL-12p40 and IFN-γ was assayed by ELISA in culture supernatants of CD11b+F4/80highGr-1low(F4/80HGr-1L) and CD11b+F4/80lowGr-1high (F4/80LGr-1H) TAM obtained from B16 lung metastasis lesions of Atg5flox/flox or LysM-Atg5-/- mice. (B) B16 melanoma cells were injected intravenously into Atg5flox/flox or LysM-Atg5-/- mice. Mice were then treated with anti-TGF-β1 mAb (1D11) or isotype control Ig twice per week. Twenty-one days later, the mice were sacrificed and subjected to macroscopic analysis for metastatic lesions in the lung. The tumor metastatic lesions are shown by white arrows. Representative data (top) and statistical analysis of average areas of metastatic lesions are shown (bottom). *: p<0.05, ns: not significant.
Myeloid cell autophagy supports TGF-β production by a translational level
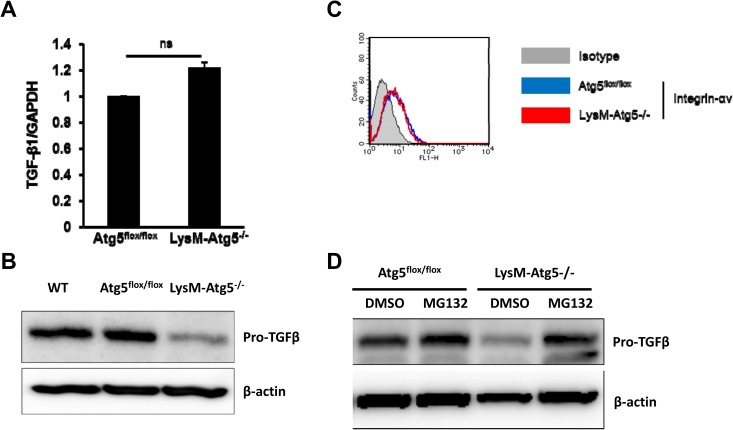
To examine mechanisms by which myeloid cells produce TGF-β1 through an autophagy-mediated mechanism, transcriptional and translational regulation of TGF-β1 expression was evaluated in Atg5flox/flox and LysM-Atg5-/- TAM. Although mRNA levels of TGF-β1 were comparable in both, pro-TGF-β1 expression was substantially decreased in LysM-Atg5-/- TAM compared to Atg5flox/flox TAM (Fig 4A and 4B). There was little difference between them on the expression of integrin-αv, which is important to process pro-TGF-β into the active form of TGF-β in myeloid cells (Fig 4A), indicating that autophagy may not be involved in the removal of latency-associated protein (LAP) to release the active form of TGF-β1 [28,29]. Interestingly, autophagy-mediated post-translational regulation of TGF-β might be operated through suppression of proteosomal degradation systems, because the treatment with a proteosomal inhibitor MG132 increased pro-TGF-β expression in LysM-Atg5-/- TAM at similar levels to those in Atg5flox/flox TAM (Fig 4D). Although these results suggest that autophagy supports TGF-β production by suppressing proteosomal degradation of pro-TGF-β in TAM, further study will be required for clarifying the precise mechanisms how myeloid cell-derived autophagy regulates TGF-β protein synthesis.
Fig 4. Mechanism of TGF-βinduction by myeloid cell-specific autophagy.
(A)The mRNA levels of TGF-β1 in Atg5flox/flox or LysM-Atg5-/- TAM were quantified by RT-PCR. (B) The protein levels of pro-TGF-β in wild-type (WT), Atg5flox/flox or LysM-Atg5-/- TAM were evaluated by western blot. (C) Integrin-αv expression on Atg5flox/flox or LysM-Atg5-/- TAM was evaluated by flow cytometry. (D) Atg5flox/flox or LysM-Atg5-/- TAMs were pretreated with MG132 or DMSO for 6h, and pro-TGF-β protein levels were measured by western blot.
Myeloid cell autophagy attenuates antitumor immune responses
Autophagy regulates multiple processes in immune responses, such as antigen processing and presentation, regulation of cytokine profiles and chemotherapy-mediated antitumor immune responses [6,30,31]. To explore the role of the autophagic pathway in myeloid cells in the regulation of effector activities, we co-cultured syngeneic wild-type CD4+ or CD8+ T cells with Atg5flox/flox or LysM-Atg5-/- bone marrow-derived macrophages (BMDM) loaded with dying OVA-expressing EG7 tumor cells and then analyzed the T helper cell differentiation and cytotoxic activities of T cells. LysM-Atg5-/- BMDM loaded with EG7 cells resulted in the preferential induction of granzyme B+ CD8+ T cells. In contrast, lower frequencies of Foxp3+ regulatory T (Treg) cells were detected when co-cultured with LysM-Atg5-/- compared to Atg5flox/flox BMDM (Fig 5A). However, LysM-Atg5-/- and Atg5flox/flox BMDM had comparable ability to promote T helper cell differentiation, as shown by similar frequencies of IFN-γ+, IL-4+ or IL-17+ CD4+ T cells (Fig 5B). To further analyze the mechanisms underlying antitumor immune responses, OVA-reactive OT-I cells were adoptively transferred into LysM-Atg5-/- and Atg5flox/flox mice. Two days later, mice were inoculated subcutaneously with B16-OVA cells and then treated with CDDP on days 8 and 10 after tumor inoculation. Lymphocytes were isolated from the established tumors 7 days after the final treatment. CDDP treatment elicited higher infiltration of TCR-Vβ5+ OT-I cells into tumor tissues in LysM-Atg5-/- mice compared to Atg5flox/flox mice (Fig 5B). Furthermore, depletion of CD8+ cells with anti-CD8 monoclonal antibody accelerated the formation of metastatic foci in LysM-Atg5-/- mice, whereas it had the little effect in Atg5flox/flox mice (Fig 5C). Together, these results demonstrate that myeloid cell-derived autophagy contributes to the impaired presentation of tumor antigens at local tumor microenvironments.
Fig 5. Intratumoral immunomodulation by myeloid cell-specific autophagy.
(A) Atg5flox/flox or LysM-Atg5 -/- BMDM were exposed to irradiated EG7 cells for 2 h and then co-cultured with wild-type CD4+ or CD8+ T cells for 96 h. CD4+ T cells expressing IFN-γ, IL-4, IL-17 or Foxp3, as well as CD8+ T cells expressing granzyme-B (GZM-B) were assayed by flow cytometry. (B) B16-OVA cells were inoculated subcutaneously into LysM-Atg5-/- or Atg5flox/flox mice that had been adoptively transferred with OT-I cells. After tumors were established (~25 mm2), tumor-infiltrating lymphocytes were isolated from the tumor tissues 4 days after the final treatment of CDDP and analyzed for the expression of SIINFEKL/H-2Kb complex on TAM by flow cytometry. Representative dot plots (left) and statistical analysis (n = 4; right) are shown. (C) MC38 cells were injected into the spleens of Atg5flox/flox or LysM-Atg5-/- mice two days after intravenous administration of anti-CD8 Ab. Twenty-one days later, the mice were sacrificed and subjected to macroscopic analysis for metastatic lesions in the liver. Representative data (upper) and statistical analysis of average areas of metastatic lesions are shown (bottom). White arrows indicate metastatic tumor areas. *: p<0.05, ns: not significant.
Impaired cross-priming of antitumor CTLs by myeloid cell-specific autophagy is dependent on TGF-β signals
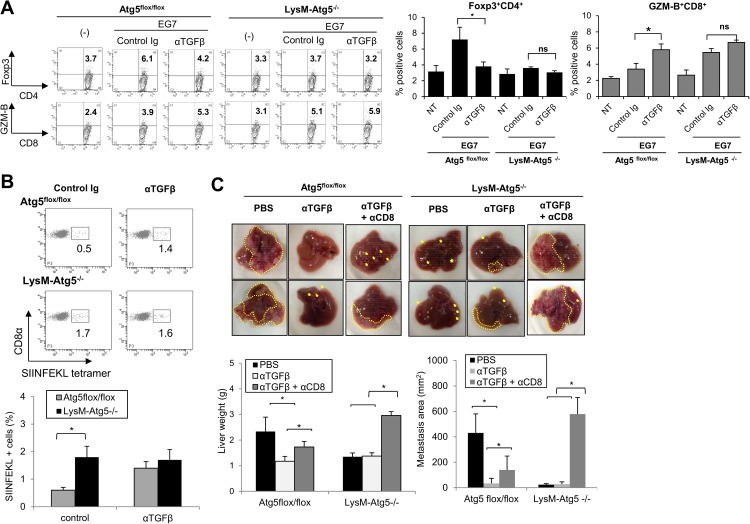
There are emerging evidences that Myeloid cell-derived TGF-β serves as critical mediator to promote tumor metastasis by suppressing IFN-ɤ and CD8+ T cell activation [23]. Moreover, TGF-β serves as a critical mediator for generating Treg cells and suppressing cytotoxic T lymphocytes (CTLs) [32–34], we next examined whether autophagy-mediated regulation of TGF-β was responsible for modulating antitumor immune responses. We found that treatment with anti-TGF-β1 neutralizing antibody largely reversed the Atg5flox/flox BMDM-mediated modulation of Foxp3 expression in CD4+ T cells and granzyme-B expression in CD8+ T cells (Fig 6A). To examine whether myeloid cell-derived TGF-β regulates cross-priming of tumor antigen-specific CD8+ CTL in an autophagy-dependent fashion, TAM were isolated from B16-OVA-tumor-bearing Atg5flox/flox or LysM-Atg5-/- mice, and then used for cross-priming of OVA-specific T cells. TAMs from LysM-Atg5-/- mice induced a higher percentage of CTLs reactive to an OVA-derived immunogenic peptide (SIINFEKL) compared to TAM from Atg5flox/flox mice (Fig 6B). Moreover, treatment with anti-TGF-β1 Abs augmented OVA-specific responses induced by TAM from Atg5flox/flox mice to levels similar to those from LysM-Atg5-/- mice, implying the importance of autophagy-dependent regulation of TGF-β1 in myeloid cells in the regulation of antigen-specific CTL activation (Fig 6B).
Fig 6. Myeloid cell autophagy suppresses antitumor immunity by TGF-β.
(A)Atg5flox/flox or LysM-Atg5-/- BMDM were exposed to irradiated EG7 thymoma cells for 2 h and then co-cultured with wild-type CD4+ or CD8+ T cells in the presence of anti-TGF-β1 neutralizing mAb (1D11) or control Ig for 96 h. CD4+ T cells expressing Foxp3 and CD8+ T cells expressing granzyme-B (GZM-B) were assayed by flow cytometry. (B) B16-OVA cells were inoculated intravenously into Atg5flox/flox or LysM-Atg5-/- mice, and TAM isolated from B16-OVA tumors in metastatic lungs were co-cultured with CD8+ T cells for 72 hr. The expression of OVA peptide (SIINFEKL) tetramer positive cells among the CD8+ T cells were analyzed by flow cytometry. Representative dot plots (top) and statistical analysis (n = 3; bottom) are shown. (C) MC38 cells were injected into the spleen of Atg5flox/flox or LysM-Atg5-/- mice two days after intravenous administration of anti-CD8 depleting mAb, anti-TGF-β1 neutralizing mAb, or both. Twenty-one days later, the mice were sacrificed and subjected to macroscopic analysis for metastatic lesions in the liver. Representative data (top) and statistical analysis of liver weight and average areas of metastatic lesions (mm2) are shown (bottom). The tumor metastatic lesions are shown by yellow arrows. *: p<0.05, ns: not significant.
Moreover, depletion of CD8+ T cells significantly attenuated the anti-metastatic effects of anti-TGF-β1 neutralizing Ab against MC38 liver tumors in the control Atg5flox/flox mice, whereas it afforded LysM-Atg5-/- mice with pro-metastatic ability irrespective of the treatment with anti-TGF-β1 Ab (Fig 6C). Together, these results demonstrate that myeloid autophagy negatively regulates CD8+ T cell-dependent antitumor immunity by promoting TGF-β production, which may be also linked with the metastatic potential of tumor cells.
Myeloid cell-derived autophagy and tumor-derived CSF-1 coordinate the survival and differentiation of M2 macrophages in stressful tumor microenvironments
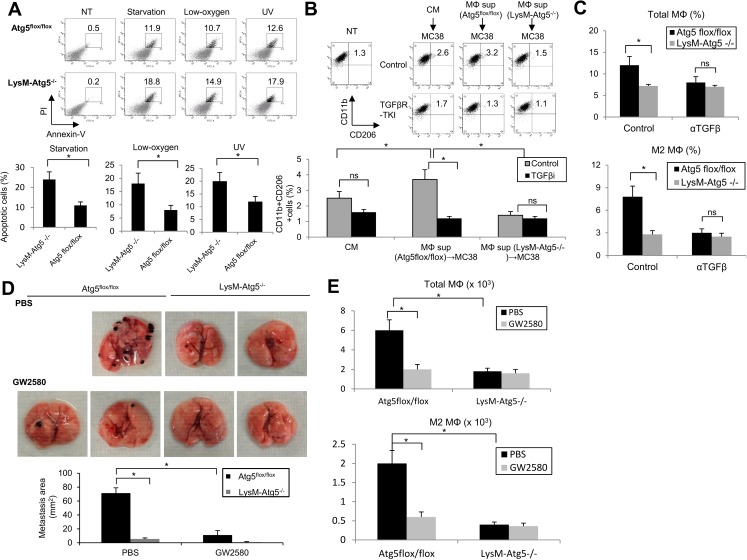
Accumulating evidence has revealed that autophagy serves as a defense system against excess cellular stress by eliminating unfolded proteins and damaged organelles [9,10,35]. Since TME are characterized by persistent replicative stresses of tumor cells, which must adopt a strategy for surviving under conditions of hypoxia and low nutrients, we hypothesized that myeloid cells may utilize autophagic systems to survive in TME. Indeed, LysM-Atg5-/- BMDM were more susceptible to various stresses, such as starvation, low oxygen or UV exposure compared to control Atg5flox/flox BMDM (Fig 7A). Interestingly, tumor cells primed by LysM-Atg5-/- BMDMs had a superior ability to induce the M2 markers CD206 and CD11b on wild-type BMDMs compared to tumor cells primed by Atg5flox/flox BMDM. Furthermore, treatment with TGFβR-TKI abrogated the M2-priming activities of tumor cells by LysM-Atg5-/- BMDM (Fig 7B). Moreover, myeloid cell-specific autophagy was responsible for the infiltration of F4/80highCD206+ M2-like macrophages in a TGF-β-dependent manner, while it had little impact on F4/80intCD206low M1-like macrophages (Fig 7C). These findings demonstrate that myeloid cell-intrinsic autophagy contributes to survival of tumor-infiltrating myeloid cells and promotes the differentiation of immunosuppressive M2 macrophages by cooperating with autophagy-mediated production of TGF-β in stressed tumor microenvironments.
Fig 7. CSF-1 supports the survival and differentiation of immunosuppressive macrophages in tumors.
(A) Atg5flox/flox or LysM-Atg5-/- BMDM were exposed to serum starvation for 16 h, low oxygen (1%) for 16 h, or UV light (300 J/m2) for 10 sec after 16h of culture. The cell viability was evaluated by quantifying annexin-V/PI-positive populations. Representative dot plots (upper) or statistical analysis (bottom) are shown. (B) MC38 cells were treated with culture medium (CM) or 50% supernatants of Atg5flox/flox or LysM-Atg5-/- BMDM for 72h. The supernatants of BMDM-primed MC38 cells were then used to treat wild-type BMDM in the presence of TGFβ receptor kinase inhibitor (TGFβR-KI: 10μM) or DMSO for 72 h. The expression of CD11b and CD206 was evaluated by flow cytometry. NT: untreated BMDM. (C) The percentages of F4/80+CD11b+macrophages, CD11b+CD206high M2 macrophages in MC38 tumors from control Ig or anti-TGF-β-treated Atg5flox/flox or LysM-Atg5-/- mice were quantified by flow cytometry. (D) B16 cells were injected intravenously to Atg5flox/flox or LysM-Atg5-/- mice two days after intraperitoneal administration of GW2580 (50 mg/kg) or PBS twice per week. Twenty-one days later, the mice were sacrificed and subjected to macroscopic analysis for metastatic lesions in the lung. Representative data (top) and statistical analysis of areas of metastatic lesions (mm2) are shown (bottom). (E) The numbers of total or M2 macrophages in B16 tumors from control Ig or anti-GW2580-treated Atg5flox/flox or LysM-Atg5-/- mice were quantified by flow cytometry. *: p<0.05, ns: not significant.
Recent studies have revealed that CSF-1 signals are critical for the survival, tumor infiltration and differentiation with immunosuppressive phenotypes of macrophages, and CSF-1 inhibitors have emerged as potent antitumor agents to prevent tumor growth and metastasis [36–38]. We therefore next examined whether pharmacological manipulation of CSF-1 impacts the tumorigenic potential of myeloid cell-derived autophagy. Interestingly, we found that CSF-1 was detected at much higher levels in tumors than in normal tissues (liver). Furthermore, treatment with CSF-1 receptor kinase inhibitor, GW2580, suppressed the lung and liver metastasis of B16 melanomas and liver metastasis of MC38 colon tumors in Atg5flox/flox mice, whereas it had little effect on their metastatic potentials in LysM-Atg5-/- mice (Fig 7D). Treatment with GW2580 substantially decreased the number of CD206+ macrophages infiltrating tumors in the control Atg5flox/flox mice (Fig 7E). Taken together, these findings demonstrate that myeloid cell-derived autophagy promotes the survival and infiltration of macrophages, which further differentiate into M2-like immunosuppressive cells via tumor-derived CSF-1. This suggests that myeloid cell-derived autophagy contributes to intrinsic survival signals by acting as a defensive system against stress-prone environments, thereby leading to an increased number of TGF-β-producing pro-tumorigenic macrophages in tumor microenvironments.
Patient-derived TAM confer tumor cells with invasive activity in an autophagy-dependent manner
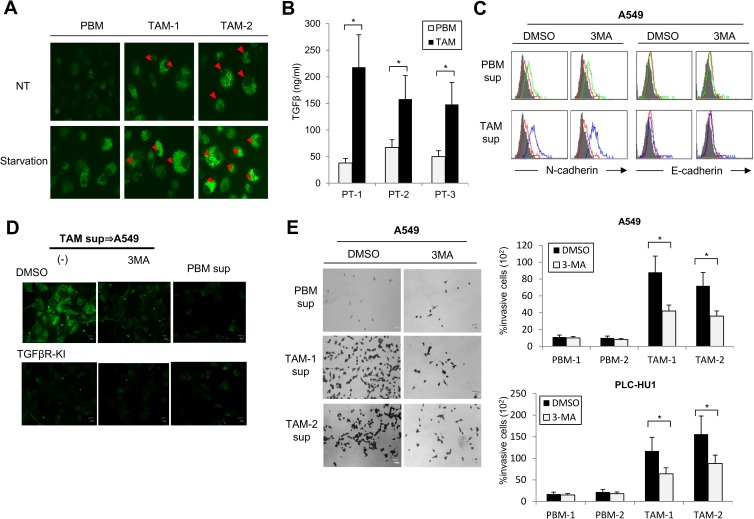
To determine whether the myeloid cell-specific autophagy-driven invasive properties of tumor cells were also operative in clinical specimens, we characterized five human CD11bhigh myeloid cells isolated from the tumors or peripheral blood of patients with non-small cell lung cancers. We found that autophagic activities, as revealed by punctate LC3 structures, were detected at higher levels in myeloid cells isolated from tumors than in the peripheral blood of patients or healthy donors (Fig 8A). In addition, the tumor-derived CD11b+ myeloid cells produced TGF-β at higher levels than their peripheral blood counterparts (Fig 8B). When co-cultured with A549 or primary NSCLC tumor cells, tumor-derived myeloid cells induced an EMT phenotype, with increased levels of the mesenchymal markers N-cadherin and vimentin, as well as augmented invasive activities. Treatment with the autophagy inhibitor 3MA suppressed the induction by tumor-derived myeloid cells of EMT-associated molecules (N-cadherin and vimentin) and invasion-related phenotypes of A549 and patient-derived primary NSCLC tumor cells (PLC-HU1) (Fig 8C–8E). In accord with the importance of TGF-β in the myeloid cell autophagic systems, the treatment with TGF-βR-KI suppressed the tumor invasive activities mediated by tumor-infiltrating myeloid cells to levels similar to those of cells undergoing autophagy inhibition (Fig 8D). Taken together, these findings highlight the broad impact of myeloid cell-specific autophagy on tumorigenic activities in cancer patients.
Fig 8. TAM-derived autophagy contributes to the invasive activities of cancer patient-derived tumor cells.
(A) Detection of LC3 in CD11bhighmyeloid cells from tumors (TAM) or peripheral bloods (PBM) of NSCLC patients (n = 5). Red arrows indicate the cells with punctate LC3 structures. (B) Protein levels of TGF-β1 in culture supernatants of CD11bhigh myeloid cells from tumors (TAM) or peripheral bloods (PBM) derived from NSCLC patients as measured by ELISA (n = 10). (C-E) A549 or primary NSCLC cancer cells (PLC-HU1) were cultured with supernatants of myeloid cells from tumors (TAM) or peripheral bloods (PBM) of NSCLC patients (n = 3) for 48 h in the presence of 3MA or TGFβR-KI. The indicated cells were evaluated for the expression of E-cadherin and N-cadherin by flow cytometry (C), the expression of vimentin by fluorescence microscopy (D) and the in vitro invasion by Matrigel assay (E). *: p<0.05, ns: not significant.
Discussion
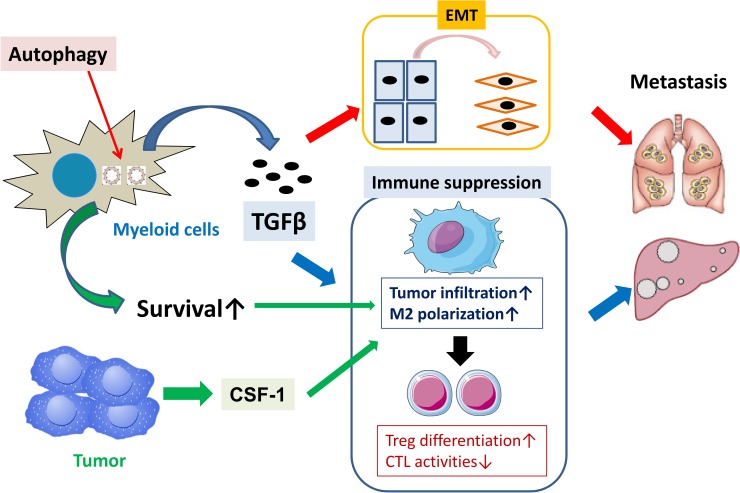
Here we demonstrate that autophagic responses specifically operating in myeloid cells are key events in supporting the invasive and metastatic properties of tumor cells. As a mechanism by which myeloid cell-specific autophagy mediates tumorigenic cascades, the autophagy triggers EMT by promoting TGF-β production by myeloid cells. In accord with the importance of myeloid cell-derived TGF-β, TGF-β blockade suppressed the invasive and metastatic activities of tumors in an autophagy-dependent manner. Moreover, autophagy provides pro-survival signals to protect myeloid cells from stressful tumor microenvironments, and bestows the surviving tumor-infiltrating myeloid cells with the ability to gain pro-tumorigenic properties through tumor-derived factors such as CSF-1. These findings provide new insights into the mechanisms regulating interactions between myeloid cells and tumors (Fig 9).
Fig 9. Schema of mechanism by which myeloid cell-specific autophagy promotes tumorigenesis.
Myeloid cell-derived autophagy promotes tumor metastasis through TGF-β-mediated EMT, as well as activates pro-survival signals to protect myeloid cells from stress-prone tumor microenvironments in support with tumor-derived CSF-1. These dual cascade bestow the surviving tumor-infiltrating myeloid cells to further amplify pro-metastatic cascade.
Tumorigenic cells manifest deregulation of intrinsic autophagic pathways through altered signaling networks that include multiple oncogenic and tumor suppressor signals [39,40]. According to previous studies, autophagy serves as a safeguard against pro-tumorigenic metabolic and oxidative stresses, thus preventing tumor initiation. In contrast, autophagy confers a survival advantage to established tumor cells by protecting them from excessive metabolic stresses [7,9]. Thus, autophagy plays a multifaceted role in tumor initiation and progression in a cell-autonomous manner. On the other hand, it remains largely obscure whether autophagy in non-transformed stromal cells has an impact on the tumorigenesis. Our findings that myeloid cell-derived autophagy regulates metastatic potential provide a new perspective that further clarifies the functional role of autophagic pathways in the regulation of tumor progression. In accord with our findings, recent studies have also revealed the role of tumor-associated fibroblasts in supporting tumor growth by modulating the metabolic and oxidative profiles of tumor cells [41–43]. These findings raise the possibility that autophagic pathways in non-transformed stromal cells, including inflammatory cells, endothelial cells, fibroblasts as well as extracellular matrices, regulate tumorigenesis through distinct but coordinated mechanisms. In addition, previous studies revealed that genetic disruption of autophagy genes in tumor cells increased their sensitivity to diverse chemotherapeutic agents [44,45]. Thus, it is crucial to address whether myeloid cell-derived autophagy regulates the responses of tumors to anticancer therapies.
TGF-β is an important mediator in the control of tumor progression through stromal and tumor cell interactions. Herein we have demonstrated that myeloid cell-derived TGF-β contributes to tumor progression by inducing EMT in tumor cells in a paracrine manner. In addition, TGF-β modulates pro- and anti-tumor activities in TME in various ways [21–25]. Recent studies demonstrated that TGF-β activated autophagy and mediated antitumor responses in hepatocellular carcinoma cells [45]. On the other hand, TGF-β-mediated activation of EMT increased the stemness and tumorigenic activities of basal-type breast carcinoma cells through loss of fructose-1, 6-biphosphatase (FBP1) and the consequent activation of pyruvate kinase M2 [46]. Our findings further provide a new framework of TGF-β-mediated regulation of tumorigenesis, which is dependent on myeloid cell-specific autophagic pathways.
It remains unclear how autophagy regulates TGF-β production by myeloid cells. In our system, the protein levels of pro-TGF-β were decreased in LysM-Atg5-/- macrophages compared to their Atg5flox/flox counterparts, whereas mRNA levels were comparable in LysM-Atg5-/- and Atg5flox/flox macrophages. This suggests that autophagy controls TGF-β expression at the translational level. Recent studies suggest that autophagy positively regulates unconventional Golgi-ER secretory phenotypes through formation of an mTOR-autophagy spatial coupling compartment [27]. Furthermore, proinflammatory cytokines induced by autophagy-dependent secretory pathways play a critical role in increasing the metastatic potential of rasV12-activated melanoma cells [26]. However, a subset of immune-regulatory cytokines, such as TGF-β and IL-10, were regulated by myeloid cell-derived autophagy in our experiments, making the involvement of unconventional secretory phenotypes unlikely. Alternatively, metabolic cascades rewired as consequence of the stressed tumor microenvironments might stimulate specific secretory phenotypes in myeloid cells through coordinated activation of ER stress and autophagy, as shown in the case of senescent tumor cells [47]. Further studies are required to clarify the molecular mechanisms linking autophagic signals with the translational regulation of TGF-β in myeloid cells.
Autophagy plays a pivotal role in the regulation of innate and adaptive immune responses through multiple mechanisms. We found that myeloid cell-derived autophagy negatively regulates Th1 differentiation and cross-priming of CTL. These differences may result mainly from the distinct properties of the antigenic sources, antigen-presenting cell (APC) subsets, or microenvironments at the site of virus infection or tumors. In addition, tumors may induce different molecules and pathways, compared to infectious diseases, which influence autophagic activities in coordination with unique environmental factors. Thus, autophagy in myeloid cells may have a distinct function in immunosurveillance in different pathologic microenvironments.
Autophagy serves as a vital system to protect damaged cells from excess stress [31,35,48]. We demonstrated that myeloid cell-derived autophagy provides a safeguard for tumors by shielding them from metabolic and oxidative stresses in tumor microenvironments resulting from the reduction in oxygen and nutrients. In this sense, it is noteworthy that tumor cells have a unique intrinsic property that activates antioxidant signaling, which attenuates excess genotoxic insults, resulting in resistance to anticancer chemotherapy [49]. Moreover, CSF-1 produced at high levels from tumor tissues promotes the differentiation of CD206+ M2 macrophages from the surviving precursors. Even more interestingly, treatment with a CSF-1 receptor inhibitor exhibited remarkable anti-metastatic activities against tumors in control mice, whereas it showed little additive effect in autophagy-deficient mice. Thus, our present findings provide new insight into the mechanisms whereby myeloid cells support tumorigenicity through the coordinated actions of myeloid cell-intrinsic autophagy and tumor-derived myeloid-stimulating factors.
The pro-tumorigenic activities of myeloid cell-derived autophagy highlight the complex and dual roles of autophagic pathways in tumor microenvironments in the regulation of tumor progression. Although the recent development of autophagy modulators has produced significant benefits for various pathological conditions such as autoimmune and malignant disorders [50, 51], it is critical to further clarify the optimal conditions for which autophagy-targeting drugs should be used for the clinical development. Moreover, the identification of novel molecules that regulate autophagic pathways, particularly in a cell lineage-specific manner, would facilitate the development of new diagnostic and therapeutic tools useful for combating various diseases including cancer.
Acknowledgments
We thank Dr. N. Mizushima (University of Tokyo) for Atg-5flox/flox mice and LC3-GFP transgenic mice, and Mr. M. Baghdadi, Mr. A. Yoneda, Mr. S. Chiba and Ms. K. Kudo (Hokkaido University) for technical assistance. This study is partially supported by a Grant-in-Aid for Scientific Research and Scientific Research for Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Ministry of Health, Labor and Welfare (M. J.). The authors declare no conflicts of interest in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This study is partially supported by a Grant-in-Aid (26670183) for Scientific Research for Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT; http://www.mext.go.jp/en/) and the Ministry of Health, Labor and Welfare (Grant number: H24-B創-肝炎-一般-008 to MJ; http://www.mhlw.go.jp/english/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hanahan D and Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012; 21: 309–22. doi: 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140: 883–99. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A and Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010; 22: 231–7. doi: 10.1016/j.coi.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 4.Qian BZ. and Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010; 141: 39–51. doi: 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima N and Komatsu M. Autophagy: renovation of cells and tssues. Cell. 2011; 147: 728–41. doi: 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 6.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011; 469: 323–35. doi: 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011; 25: 795–800. doi: 10.1101/gad.2016211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest.2003; 112: 1809–20. doi: 10.1172/JCI20039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew R, Karp CM, Beaudoin M, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009; 137: 1062–75. doi: 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, et al. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 2013; 11:1272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–7. doi: 10.1073/pnas.1307237110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006; 441: 885–9. doi: 10.1038/nature04724 [DOI] [PubMed] [Google Scholar]
- 13.Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009; 206: 1317–26. doi: 10.1084/jem.20082614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogunjimi AA, Zeqiraj E, Ceccarelli DF, Sicheri F, Wrana JL, David L.Structural basis for specificity of TGFβ family receptor small molecule inhibitors. Cell Signal. 2012;24:476–83. doi: 10.1016/j.cellsig.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinushi M, Nakazaki Y, Dougan M, Carasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links pro- and anti-inflammatory activities of GM-CSF. J Clin Invest. 2007; 117: 1902–13. doi: 10.1172/JCI30966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo E.F., Dekonenko A., Arko-Mensah J., Mandell M.A., Dupont N., Jiang S., et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci USA. 2012; 109: 3168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004; 432:1032–6. doi: 10.1038/nature03029 [DOI] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133: 704–15. doi: 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J and Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. 2008; Dev Cell. 14: 818–29. doi: 10.1016/j.devcel.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 20.Chaffer CL and Weinberg RA. A perspective on cancer cell metastasis. Science. 2001; 331: 1559–64. [DOI] [PubMed] [Google Scholar]
- 21.Ikushima H and Miyazono K. TGF-beta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010; 10: 415–24. doi: 10.1038/nrc2853 [DOI] [PubMed] [Google Scholar]
- 22.Bierie B and Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006; 6: 506–20. doi: 10.1038/nrc1926 [DOI] [PubMed] [Google Scholar]
- 23.Pang Y, Gara SK, Achyut BR, Li Z, Yan HH, Day CP, et al. TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 2013;3:936–51. doi: 10.1158/2159-8290.CD-12-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan HH, Jiang J, Pang Y, Achyut BR, Lizardo M, Liang X, et al. CCL9 Induced by TGFβ Signaling in Myeloid Cells Enhances Tumor Cell Survival in the Premetastatic Organ. Cancer Res. 2015;75:5283–98. doi: 10.1158/0008-5472.CAN-15-2282-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009; 23: 798–803. doi: 10.1101/gad.519709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lock R, Kenific CM, Leidal AM, Salas E, Debnath J. Autophagy-dependent production of secreted factors facilitates oncogenic Ras-driven invasion. Cancer Discov. 2014; 4: 466–79. doi: 10.1158/2159-8290.CD-13-0841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011; 332: 966–70. doi: 10.1126/science.1205407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alphav beta6 binds and activates latent TGF beta1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999; 96: 319–28. [DOI] [PubMed] [Google Scholar]
- 29.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007; 449: 361–5. doi: 10.1038/nature06110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010; 32: 227–39. doi: 10.1016/j.immuni.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaud M, Mertins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011; 334: 1573–7. doi: 10.1126/science.1208347 [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003; 198: 1875–86. doi: 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorelik L and Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001; 7: 1118–22. doi: 10.1038/nm1001-1118 [DOI] [PubMed] [Google Scholar]
- 34.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity. 2001; 34: 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lum JJ, DeBerardinis RJ and Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005; 6: 439–48. doi: 10.1038/nrm1660 [DOI] [PubMed] [Google Scholar]
- 36.Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013; 19: 1264–72. doi: 10.1038/nm.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011; 1: 54–67. doi: 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ries CH, Cannarile MA, Hoves S, Benz J, Warsha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014; 25: 846–59. doi: 10.1016/j.ccr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 39.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012; 12: 401–10. doi: 10.1038/nrc3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi AM, Ryter SW, Levine, B. Autophagy in human health and disease. N Engl J Med. 2013; 368: 651–62. doi: 10.1056/NEJMra1205406 [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Outschoorn UE, Pavlides S, Chiavarina B, Bonuccelli G, Casey T, Tsirigos A, et al. The autophagic tumor stroma model of cancer or "battery-operated tumor growth": A simple solution to the autophagy paradox. Cell Cycle. 2010; 21: 4297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, et al. Metabolic reprogramming of stromal fibroblasts through p63-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014; 26: 121–35. doi: 10.1016/j.ccr.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao X, He Y and Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int J Cancer. 2013; 132: 1–8. doi: 10.1002/ijc.27664 [DOI] [PubMed] [Google Scholar]
- 44.Song J, Quo, Guo X, Zhao Q, Zhao X., Gao L, et al. Hypoxia-induced autophagy contributes to the chemoresistance of hepatocellular carcinoma cells. Autophagy. 2009; 5: 1131–44. [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, et al. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012; 72: 230–8. doi: 10.1158/0008-5472.CAN-11-2001 [DOI] [PubMed] [Google Scholar]
- 46.Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano MR, et al. Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer Res. 2009; 69: 8844–52. doi: 10.1158/0008-5472.CAN-08-4401 [DOI] [PubMed] [Google Scholar]
- 47.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013; 23: 316–31. doi: 10.1016/j.ccr.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dörr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013; 50: 421–5. [DOI] [PubMed] [Google Scholar]
- 49.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Gene Dev. 2007; 21: 1621–35. doi: 10.1101/gad.1565707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc (-) and thereby promotes tumor growth. Cancer Cell. 2011; 19: 387–400. doi: 10.1016/j.ccr.2011.01.038 [DOI] [PubMed] [Google Scholar]
- 51.Rubinsztein DC, Codogno P and Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012; 11: 709–30. doi: 10.1038/nrd3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.