Abstract
Background
Fracture healing is known to be delayed in postmenopausal, osteoporotic females under oestrogen-deficient conditions. Confirming this, experimental studies demonstrated impaired callus formation in ovariectomised animals. Oestrogen-deficiency is known to affect the immune system and the inflammatory response during wound healing. Because a balanced immune response is required for proper bone healing, we were interested to ascertain whether the early immune response after facture is affected by oestrogen depletion.
Methods
To address the above question, female mice received either a bilateral ovariectomy (OVX) or were sham-operated, and femur osteotomy was performed 8 weeks after OVX/sham operation. The effects of OVX on the presence of immune cells and pro-inflammatory cytokines were evaluated by flow cytometry and immunohistochemistry of the fracture calli on days 1 and 3 after fracture.
Results
One day after fracture, immune cell numbers and populations in the fracture haematoma did not differ between OVX- and sham-mice. However, on day 3 after fracture, OVX-mice displayed significantly greater numbers of neutrophils. Local expression of the oestrogen-responsive and pro-inflammatory cytokine midkine (Mdk) and interleukin-6 (IL-6) expression in the fracture callus were increased in OVX-mice on day 3 after fracture compared with sham-mice, indicating that both factors might be involved in the increased presence of neutrophils. Confirming this, Mdk-antibody treatment decreased the number of neutrophils in the fracture callus and reduced local IL-6 expression in OVX-mice.
Conclusions
These data indicate that oestrogen-deficiency influences the early inflammatory phase after fracture. This may contribute to delayed fracture healing after oestrogen depletion.
Keywords: Fracture healing, Oestrogen, Inflammation, Midkine, Neutrophils
Background
There is clinical evidence for a prolonged fracture healing time in postmenopausal, osteoporotic females [1, 2]; however, the pathomechanisms are currently not fully understood. Confirming clinical data, experimental studies demonstrated that ovariectomised rats and mice display impaired cartilaginous callus formation and reduced vascularisation during fracture healing [3–5]. In the late phase of healing, oestrogen-deficiency decreased the amount of the newly formed bone and the mechanical competence of the fracture callus [6–9]. On a cellular level, both osteoblast and osteoclast numbers were significantly increased, indicating high bone turnover with a shift towards bone resorption [10]. These studies indicate that osteoporotic bone healing is delayed due to impaired angiogenesis and cartilage formation, and an imbalance of osteoblast and osteoclast activities.
Oestrogen-deficiency also affects the immune system. Postmenopausal females display a pro-inflammatory phenotype with increased numbers of activated T-cells and B-lymphocytes [11] and higher levels of circulating pro-inflammatory cytokines, including interleukin-1 (IL-1), IL-6, IL-31 and tumour necrosis factor α (TNFα) [12–14]. Furthermore, the immune response is altered when the system is challenged. For example, the inflammatory response was increased in oestrogen-deficient mice after induction of paw inflammation or rheumatoid arthritis [15–17]. During wound healing, pro-inflammatory cytokines were up-regulated in rodents subjected to ovariectomy (OVX), resulting in delayed skin repair [18, 19].
A balanced immune response is regarded to be crucial also for successful bone healing, because it was shown that bone regeneration is disturbed under local and systemic inflammatory conditions [20–22]. However, the influence of oestrogen-deficiency on the inflammatory response in fracture healing has not yet been investigated, despite the high clinical relevance of delayed bone regeneration in postmenopausal osteoporosis. Our own previous work provided evidence that oestrogen-deficiency may affect the inflammatory response to fracture. We found that after bone fracture, OVX-mice displayed increased serum levels of midkine (Mdk) [23], a pro-inflammatory cytokine and a negative regulator of bone remodelling [24–26]. Mdk is an oestrogen-responsive gene and its expression is known to be up-regulated in the absence of oestrogen [27, 28] as well as during inflammatory diseases and tissue injury and regeneration [29–33]. The absence of Mdk reduced leukocyte recruitment to the sites of inflammation during nephritis, arthritis and other inflammatory diseases [33].
Therefore, we hypothesised that oestrogen-deficiency alters the early inflammatory response after fracture and that inflammatory mediators, including Mdk, may be involved in this effect. To test these hypotheses, we analysed the presence of immune cells and inflammatory cytokines in the fracture haematoma of OVX-mice as well as the impact of treatment with an antibody targeting Mdk.
Methods
Animal experiments
All animal experiments were in compliance with international regulations for the care and use of laboratory animals with the approval of the local ethical committee (No. 1079 and 1184, Regierungspräsidium Tübingen, Germany). Female C57BL/6J mice were maintained in groups of two to four animals per cage (370 cm2) on a 14-h light and 10-h dark circadian rhythm with water and food ad libitum. Mice aged 3–4 months underwent bilateral sham operation or OVX as described previously [34]. Osteotomy was performed 8 weeks after sham/OVX. The surgery was conducted according to the published protocol [35]. Briefly, the M. biceps femoralis and the M. vastus lateralis at the right femur were separated bluntly to minimise additional soft tissue trauma. A semi-rigid external fixator (axial stiffness of 3 N/mm) was mounted parallel to the femur shaft with four screws. The osteotomy was created in the middle of the femur diaphysis using a 0.4-mm gigli wire saw, and muscles and skin were adapted.
All mice were fed a phytoestrogen-free diet for the entire period of the experiments. Mice were euthanised on day 1, 3 or 23 after fracture using carbon dioxide. The uteri and fractured and intact femurs were removed for further analysis.
For evaluation of the effects of Mdk on the fracture callus, sham- and OVX-mice received a subcutaneous injection with 25 mg/kg Mdk-antibody (Mdk-Ab) directly after the osteotomy surgery, as described previously [23]. The mouse-anti human Mdk IgG1 monoclonal antibody was shown to be cross-reactive to murine Mdk (ELISA EC50 to human Mdk: 31.7 ng/ml and ELISA EC50 to murine Mdk: 37.8 ng/ml) [36] and to block Mdk in the serum of fractured mice with an efficiency between 65 and 100% depending on the measurement time point [23, 36]. Mice were euthanised on day 3, and fractured femurs were subjected to decalcified histology.
Oestrogen ELISA
Sera of sham- and OVX-mice euthanised 1 day after fracture were tested for oestrogen concentrations using the commercially available oestrogen ELISA according to the manufacturer’s instructions (Calbiotech #ES180S-100, Spring Valley CA, USA).
Micro-computed tomography (µCT)
Femurs of mice sacrificed on day 23 were analysed using a µCT scanning device (Skyscan 1172, Bruker, Kontich, Belgium) operating at a voxel resolution of 8 µm (50 kV, 200 mA). Bone mineral density (BMD) was assessed using two phantoms each with a defined density of hydroxyapatite (250 and 750 mg/cm3) within each scan. Discrimination between non-mineralised and mineralised tissue was performed using a global threshold of 642 mg hydroxyapatite/cm3 according to Morgan et al. [37] and in accordance with the American Society for Bone and Mineral Research (ASBMR) guidelines for µCT analysis [38]. Three-dimensional (3D) reconstruction of the fracture callus between the two inner pin holes was performed using CTvol software (Bruker).
Fluorescent-activated cell sorting (FACS) analysis
To analyse inflammatory cells in the fracture haematoma, FACS analysis was performed. On day 1 after fracture, the fractured femur and the contralateral bone marrow were harvested. The fracture haematoma was collected using a surgical scissor. The contralateral bone marrow was flushed out using phosphate-buffered saline. The fracture haematoma was passed through a 70-µm cell strainer (Corning Inc., Durham, NC, USA) to obtain a single-cell suspension and the cells of the fracture haematoma and bone marrow were subjected to erythrolysis. For the identification of macrophages (Ly6G−, F4/80+, CD11b+), neutrophils (Ly-6G+, F4/80−, CD11b+), inflammatory monocytes (F4/80+, Ly-6G+, CD11b+), B-lymphocytes (CD19+), T-lymphocytes (CD3+), cytotoxic T-lymphocytes (CD3+, CD8+), and T-helper-lymphocytes (CD3+, CD4+), the antibodies listed in Table 1 were used. Specific isotype-matched immunoglobulin antibodies (Table 1) were used as negative controls. Cells (fracture haematoma: totality of cells isolated; bone marrow: 1 × 106 cells) were incubated with the antibodies for 30 min on ice. 7-aminoactinomycin (7-AAD, Sigma, Steinheim, Germany) was used for dead-cell discrimination. Cells were analysed using an LSR II flow cytometer (BD Bioscience) and FlowJo software v10 (FlowJo LLC, Ashland, OR).
Table 1.
Antibodies used for flow cytometry
| Antibody | Label | Product | Company | Dilution |
|---|---|---|---|---|
| Il-6 (rabbit anti-mouse) | – | bs-0782R | Bioss | 1:250 |
| Mdk (goat anti-mouse) | – | sc-1398 | SantaCruz | 1:100 |
| CCL2 (rabbit anti-mouse) | – | bs-1955R | Bioss | 1:150 |
| CXCL1 (rabbit anti-mouse) | – | ab86436 | Abcam | 1:200 |
| Ly6G (rat anti-mouse) | – | 127603 | BioLegend | 1:300 |
| CD45/B220 (rat anti-mouse) | – | RA3-6B2 | BioLegend | 1:100 |
| CD8 (rabbit anti-mouse) | – | bs-0648R | Bioss | 1:100 |
| F4/80 (rat anti-mouse) | – | ab6640 | AbD Serotec | 1:500 |
| Streptavidin | HRP | ZUC012 | Zytomed Sytems | 1:100 |
| IgG (donkey anti-goat) | Biotin | sc-3854 | SantaCruz | 1:100 |
| IgG (goat anti-rabbit) | Biotin | B2770 | Life Technologies | 1:100 |
| IgG (goat anti-rat) | Biotin | 31830 | Invitrogen | 1:100 |
Immunohistochemistry and immunofluorescence staining
Femurs of mice sacrificed 3 days post-surgery were fixed in 4% formalin, decalcified using 20% ethylenediaminetetraacetic acid (pH 7.2–7.4) for 10–12 days and embedded in paraffin after dehydration in an ascending ethanol series. Longitudinal cross-sections with a thickness of 7 μm were prepared. Immunohistochemical and immunofluorescence staining of IL-6, Mdk, CCL2, CXCL1, Ly6G (neutrophils), CD45R (B-lymphocytes), CD8 (cytotoxic T-lymphocytes) and F4/80 (macrophages) were performed using the antibodies specified in Table 2. Species-specific non-targeting immunoglobulins were used as isotype controls. 3-Amino-9-ethylcarbazol (Zytomed Systems) was used as the chromogen and the sections were counterstained using haematoxylin (Waldeck, Münster, Germany). FITC-streptavidin was used for immunofluorescence staining and the sections were counterstained using DAPI. The sections were examined by light or fluorescence microscopy (DMI6000 B, Leica, Heerbrugg, Switzerland). The amount of callus tissue and the number of positively stained cells were determined by image analysis software (Leica MMAF 1.4.0 Imaging System, Leica).
Table 2.
Antibodies used for immunohistochemistry
| Antibody | Label | Product | Company | Dilution |
|---|---|---|---|---|
| CD3e (Armenian hamster anti-mouse) | PE-Cyanine 7 | 25-0031 | Affymetrics eBioscience | 1:100 |
| CD4 (rat anti-mouse) | APC-eFlour® 780 | 47-0041 | Affymetrics eBioscience | 1:200 |
| CD8a (rat anti-mouse) | APC | 17-0081 | Affymetrics eBioscience | 1:800 |
| CD11b (rat anti-mouse) | Alexa Flour® 700 | 56-0112 | Affymetrics eBioscience | 1:400 |
| CD19b (rat anti-mouse) | PE | 12-0193 | Affymetrics eBioscience | 1:400 |
| F4/80 (rat anti-mouse) | FITC | 11-4701 | Affymetrics eBioscience | 1:50 |
| Ly-6G (rat anti-mouse) | V450 | 560603 | BD Bioscience | 1:400 |
| IgG Isotype (Armenian hamster) | PE-Cyanine 7 | 25-4888 | Affymetrics eBioscience | 1:100 |
| IgG2b K Isotype (rat) | APC-eFlour® 780 | 47-4031 | Affymetrics eBioscience | 1:200 |
| IgG2a K Isotype (rat) | APC | 17-4321 | Affymetrics eBioscience | 1:800 |
| IgG2b K Isotype (rat) | Alexa Flour® 700 | 56-4031 | Affymetrics eBioscience | 1:400 |
| IgG2a K Isotype (rat) | PE | 12-4321 | Affymetrics eBioscience | 1:400 |
| IgG2a K Isotype (rat) | FITC | 11-4321 | Affymetrics eBioscience | 1:50 |
| IgG2a K Isotype (rat) | V450 | 560377 | BD Bioscience | 1:400 |
Statistics
Statistical analysis was performed using Shapiro–Wilk test for normal distribution and ANOVA/LSD post hoc test with SPSS software (SPSS Inc., Chicago, IL, USA). All results are presented as the mean and standard deviation. Values of p < 0.05 were considered as statistically significant. Sample size was n = 5–6 per group and each time point.
Results
OVX-induced osteoporosis and delayed fracture healing
We first confirmed the known effects of OVX on the skeleton and on fracture healing [3, 39]. As expected, uteri of mice subjected to castration displayed severe atrophy (Fig. 1a) and a significantly decreased weight (Fig. 1b). Further, oestrogen serum levels were significantly reduced in OVX-mice (Fig. 1c). µCT analysis of the intact femurs demonstrated an osteoporotic phenotype in the trabecular compartment (Fig. 1d). Analysis of the fracture calli on day 23 showed considerably disturbed bony callus development and poor cortical bridging due to OVX (Fig. 1e).
Fig. 1.

Influences of ovariectomy (OVX) on uterus weight, intact bone and bony callus formation. a Uteri from sham-operated and OVX-mice 11 weeks after initial surgery. b Weights of the uteri. c Oestrogen serum levels at day 1 after osteotomy surgery. d Representative transversal µCT images of the metaphyseal region of the intact femurs. e Representative longitudinal 3D µCT images of the fracture calli of sham-operated and OVX-mice on day 23 after fracture
OVX increased the number of neutrophils in the fracture haematoma
We analysed the presence of immune cells in the fracture haematoma on day one after surgery by FACS analysis (Fig. 2a, b). The cell populations of the innate and adaptive immune systems did not differ significantly between sham- and OVX-mice at this time point at the fracture site. However, OVX significantly increased the number of B-lymphocytes and reduced the number of T-lymphocytes in the bone marrow (Fig. 2c). Within the T-lymphocyte population, cytotoxic T-cells were significantly reduced, whereas T-helper cells were significantly increased (Fig. 2d). Furthermore, the numbers of inflammatory monocytes and macrophages were not affected, whereas the number of neutrophils was significantly decreased. Three days after fracture, immune cells in the fracture callus were evaluated by immunohistochemistry. OVX-mice displayed significantly greater numbers of neutrophils in the periosteal callus (Fig. 3a). Neutrophils were predominantly found in the fracture gap surrounding fibrous tissue as well as in the early periosteal fracture callus (Fig. 4). The number of macrophages, which were mainly located in the bone marrow near the osteotomy gap, did not differ significantly between the groups (Fig. 3b). There were no differences in the number of B-lymphocytes or cytotoxic T-lymphocytes, which were located in the periosteal fracture callus (Fig. 3c, d).
Fig. 2.

Immune-cell populations in fracture haematoma and bone marrow on day 1 after fracture analysed by flow cytometry. a–d Percents of living B-lymphocytes (CD19+), inflammatory monocytes (Ly6G+, F4/80+, CD11b+), macrophages (Ly6G−, F4/80+, CD11b+), neutrophils (Ly6G+, F4/80−, CD11b+), T-lymphocytes (CD3+), cytotoxic T-lymphocytes (CD3+, CD8+) and T-helper lymphocytes (CD3+, CD4+) in the fracture haematoma and bone marrow of sham-operated (white bars) and OVX-mice (grey bars). *p < 0.05, **p < 0.01, ***p < 0.001 for comparison between sham- and OVX-mice
Fig. 3.
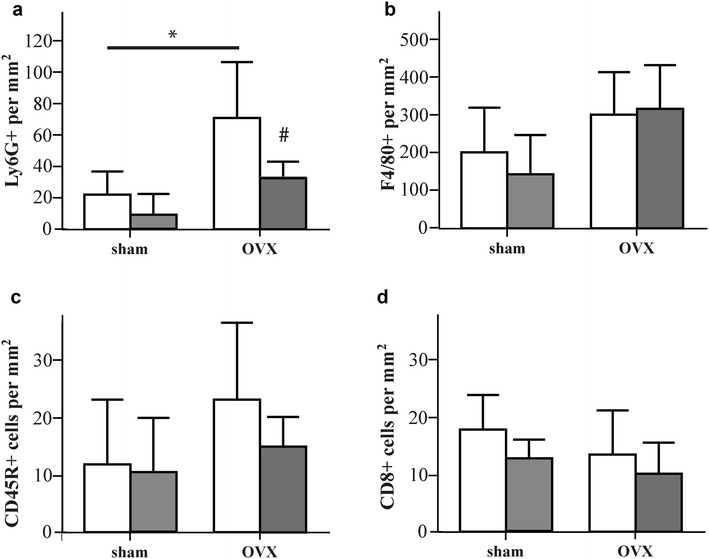
Number of immune cells in the fracture callus on day 3 after fracture analysed by immunohistochemistry. a Number of Ly6G-positive neutrophils per square mm of the periosteal callus. b Number of F4/80-positive macrophages per square mm of the marrow cavity. c Number of CD45R-positive B-lymphocytes per square mm of the periosteal callus. d Number of CD8-positive cytotoxic T-lymphocytes per square mm of the periosteal callus. *p < 0.05 for comparison between sham- and OVX-mice, # p < 0.05 for comparison between Mdk-Ab untreated (white bars) and treated mice (grey bars)
Fig. 4.
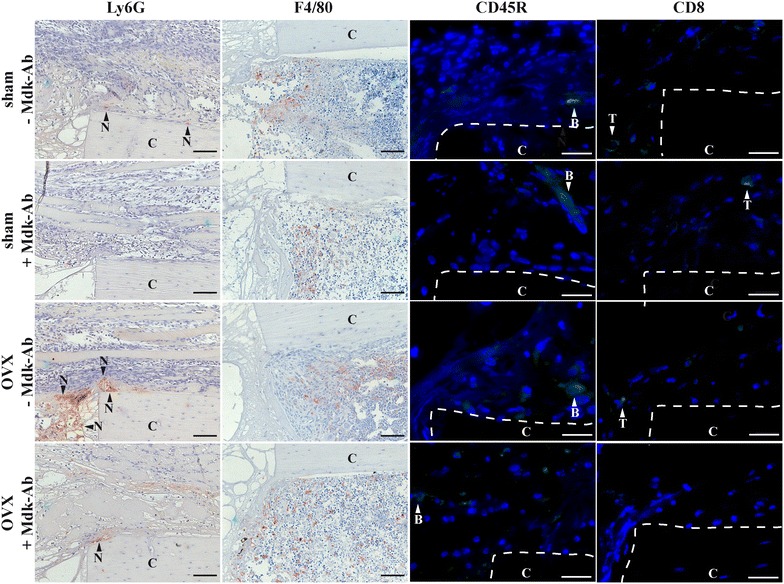
Immune-cell staining in the fracture callus on day 3 after fracture. Representative images of Ly6G−, F4/80−, CD45R− and CD8-positive cells in the fractured femurs from sham-operated and OVX-mice treated with and without Mdk-Ab. The periosteal callus proximal to the osteotomy gap is shown. N neutrophil, B B-lymphocyte, T T-lymphocyte, C bone cortex. Scale bar = 100 µm for immunohistochemical staining and 25 µm for immunofluorescence staining
OVX increased Mdk and IL-6 expressions in the fracture callus
We investigated the role of Mdk during the inflammatory phase of fracture healing. Therefore, we first analysed the local expression of this protein in the callus by immunostaining. On day 3, Mdk was expressed in the fracture callus of OVX-mice (Fig. 5). The protein was located at the periosteal transition zone from haematoma tissue to soft fracture callus proximal to the osteotomy gap. Mdk was expressed less in sham-operated animals. Because it is known that Mdk expression is associated with IL-6 expression [40] and because IL-6 is a potent inducer for neutrophil recruitment [41], IL-6 expression was evaluated by immunostaining. In sham-mice, IL-6 was expressed in muscle tissue and endosteal cells, but was expressed less in periosteal cells, whereas OVX-mice showed increased expression in periosteal cells adjacent to the fracture gap (Fig. 5). We hypothesised that the up-regulation of Mdk expression after OVX may be involved in increased neutrophil numbers in the periosteal callus. Therefore, we treated mice with an inhibitory Mdk-Ab directly after surgery. Mdk-Ab-treated mice displayed significantly lower numbers of neutrophils in the fracture callus compared with non-treated mice (Fig. 3a), whereas the numbers of macrophages, B-lymphocytes and cytotoxic T-lymphocytes were not significantly altered (Fig. 3b–d). Furthermore, Mdk-Ab-treated OVX-mice exhibited lower IL-6 expression in the periosteal cells next to the fracture gap (Fig. 5). Protein expressions of the proinflammatory cytokines, CCL2 and CXCL1, were similar between the groups (Fig. 5).
Fig. 5.

Cytokine expression in the fracture callus on day 3 after fracture. Representative images of Mdk-, IL-6-, CCL2- and CXCL1-immunostained sections of the fractured femurs from sham-operated and OVX-mice treated with and without Mdk-Ab. The periosteal callus proximal to the osteotomy gap is shown. C cortex. Scale bar = 100 µm
Discussion
It is well established that oestrogen affects the immune system and the severity of inflammatory disorders [42]. Because a balanced inflammatory response is considered to be crucial for proper bone healing [20], the question arises whether oestrogen-deficiency influences inflammation during early fracture healing. We analysed the immune cells in the bone marrow as the major source for migrating immune cells to the fracture haematoma [43]. Our results demonstrated that numbers of B-lymphocytes in the bone marrow were elevated in OVX-mice 1 day after fracture, whereas the number of bone marrow neutrophils and total T-lymphocytes were reduced but with an increased ratio of CD4+/CD8+ cells. It was previously reported that B-lymphocyte numbers increased in the bone marrow in response to OVX-induced oestrogen-deficiency [44–46]. Because B-lymphocytes are known to synthesise several inflammatory cytokines as well as recent finding suggesting that they are active regulators of the RANK/RANKL/OPG system, it was suggested that there is a strong association between increased numbers of B-lymphocytes and bone loss during menopause [47–49]. In general, the chronic inflammatory immune status in postmenopausal females is regarded to contribute to bone loss [50, 51]. It was also reported that oestrogen-deficiency considerably affects T-lymphocytes; however, data are conflicting, showing either an increase or decrease of T-lymphocytes in bone marrow and spleen [46, 52–54]. In agreement with our results, changes in the ratio of CD4+/CD8+ cells in the bone marrow were frequently observed in oestrogen-deficient animals [55–57]. Bone-marrow T-lymphocytes are suggested to contribute to the strong influence of the immune system on bone homeostasis and to modulate the bone-marrow environment in either an osteoclastogenic or an anti-osteoclastogenic manner, depending on the T cell subset [58]. Activated T-lymphocytes were shown to produce increased TNFα levels in response to oestrogen withdrawal, leading to increased bone resorption [52]. In particular, Th17 T-cells were demonstrated to link T-cell activation and osteoclast activation [59]. The roles of CD4+ and CD8+ T-cells during postmenopausal bone loss are strongly discussed. Depending on the manner in which these cells were activated, both subsets can either mediate osteoclastogenic or anti-osteoclastogenic effects [58]. In conclusion, the roles of T-lymphocytes in postmenopausal bone loss and chronic inflammation remain unclear. Even less is known about the contribution of bone-marrow neutrophils to osteoporotic bone loss. There are phenomenological studies showing increased [60], decreased [61] or unaltered [62] numbers of neutrophils after oestrogen withdrawal, but there are no mechanistic studies available.
Because it was shown that both B- and T-lymphocytes as well as neutrophils could affect fracture healing outcome [63–65], we evaluated the number of these cells in the early fracture haematoma. We did not find any differences between OVX- and sham-mice on day 1 after trauma, although the cell populations were different in the bone marrow. This finding indicates that the initial recruitment of inflammatory cells to the fracture callus was unaffected by oestrogen-deficiency. However, on day 3 after fracture, significantly more neutrophils were present in the periosteal callus of oestrogen-deficient mice, indicating a prolonged recruitment and/or an increased survival of neutrophils at the fracture site in the absence of oestrogen. In the literature, OVX is described to increase local activation of neutrophils after haemorrhagic shock [66], artery injury [67], lung damage [68] and during wound healing [69], although the mechanisms of interaction between oestrogen and neutrophils remain unclear. Our data suggest that the pro-inflammatory cytokine Mdk may be involved in the effects of oestrogen-deficiency on the inflammatory phase of fracture healing. It was shown previously that the promoter region of the Mdk gene contains oestrogen-responsive elements and that the expression of Mdk is enhanced in the kidney of diabetic, oestrogen-deficient mice [28]. In addition, oestrogen receptor α-deficient osteocytes displayed increased Mdk mRNA levels [27]. We demonstrated in a previous study, that Mdk serum levels were increased in oestrogen-deficient mice from day 3 to day 23 after fracture [23]. In the present study, we found increased local expression of Mdk in the fracture callus of OVX-mice on day 3 after fracture. Because Mdk is known to chemoattract both neutrophils and macrophages [33], we hypothesised that increased Mdk expression may be involved in the prolonged presence of neutrophils at the fracture callus in OVX-mice. Indeed, we found significantly decreased numbers of neutrophils after antagonising Mdk upon Mdk-Ab treatment. However, we did not detect significant changes in CXCL1 expression, one of the most important proteins for neutrophil recruitment. It is known from previous studies that Mdk-deficient mice displayed lower numbers of neutrophils and macrophages in the tubulointerstitium after ischaemic renal injury [32] and that Mdk-deficiency delayed the recruitment of macrophages to the fracture site during the regenerative phase of healing [70]. However, in the current study, we did not detect significant changes in the number of macrophages or the expression of monocyte chemoattractant protein 1 (CCL2). In addition, we did not detect changes in the numbers of B- or T-lymphocytes in the fracture callus, although it was demonstrated that Mdk regulated B-cell survival in vitro [71]. Therefore, the increased Mdk expression in the early fracture callus of oestrogen-deficient mice appears to predominantly affect the recruitment and survival of neutrophils.
In the literature, several pro-inflammatory cytokines are described to be involved in the increased severity of inflammatory disorders in oestrogen-deficient subjects. One of these cytokines is IL-6, which is known as a crucial factor for the recruitment of inflammatory cells [41, 72]. In addition, several studies demonstrated increased IL-6 expression after tissue injury in oestrogen-deficient mice [15, 16]. In the present study, we found higher IL-6 expression in the periosteal cells of OVX-mice. Indeed, it was shown previously that increased IL-6 expression was associated with greater numbers of neutrophils in the fracture callus after severe trauma [73]. Therefore, we suggest that increased IL-6 expression due to the lack of oestrogen might contribute to the increased number of neutrophils in the fracture callus of OVX-mice. In addition, there is some evidence in the literature that Mdk expression may be associated with IL-6 [40, 74].
In conclusion, our study demonstrated that oestrogen-deficiency significantly influenced the early inflammatory phase after fracture. Higher Mdk and IL-6 expression at the fracture site were associated with increased numbers of neutrophils in the callus.
Authors’ contributions
Study designed by MHL, VF and AI. Study conducted by MHL and VF. Data collection done by MHL, VF and KP. Data analysis done by MHL and VF. Data interpretation done by MHL, VF and AI. Drafting of the manuscript done by MHL, VF, KP, AL and AI. Revision of the manuscript content done by MHL, AL, AI, VF and KP. Approval of the final version of the manuscript done by MHL, AL, AI, VF and KP. MHL and VF take responsibility for the integrity of the data analysis. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Helga Bach, Marion Tomo, Sevil Essig and Uschi Maile for their excellent technical support. The authors are grateful to Cellmid Limited for providing the midkine monoclonal antibody.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal experiments were in compliance with international regulations for the care and use of laboratory animals with the approval of the local ethical committee (No. 1079 and 1184, Regierungspräsidium Tübingen, Germany).
Funding
This work was supported by the Elsbeth Bonhoff Foundation and was conducted within the framework of the Collaborative Research Centre CRC1149 (funded by the Germany Research Foundation).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Melanie Haffner-Luntzer and Verena Fischer contributed equally to this work
Contributor Information
Melanie Haffner-Luntzer, Email: melanie.haffner-luntzer@uni-ulm.de.
Verena Fischer, Email: verena.fischer@uni-ulm.de.
Katja Prystaz, Email: katja.prystaz@uni-ulm.de.
Astrid Liedert, Email: astrid.liedert@uni-ulm.de.
Anita Ignatius, Email: anita.ignatius@uni-ulm.de.
References
- 1.Nikolaou VS, Efstathopoulos N, Kontakis G, Kanakaris NK, Giannoudis PV. The influence of osteoporosis in femoral fracture healing time. Injury. 2009;40:663–668. doi: 10.1016/j.injury.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 2.Cheung WH, Miclau T, Chow SK, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury. 2016;47(Suppl 2):S21–S26. doi: 10.1016/S0020-1383(16)47004-X. [DOI] [PubMed] [Google Scholar]
- 3.Beil FT, Barvencik F, Gebauer M, Seitz S, Rueger JM, Ignatius A, Pogoda P, Schinke T, Amling M. Effects of estrogen on fracture healing in mice. J Trauma. 2010;69:1259–1265. doi: 10.1097/TA.0b013e3181c4544d. [DOI] [PubMed] [Google Scholar]
- 4.Hatano H, Siegel HJ, Yamagiwa H, Bronk JT, Turner RT, Bolander ME, Sarkar G. Identification of estrogen-regulated genes during fracture healing, using DNA microarray. J Bone Miner Metab. 2004;22:224–235. doi: 10.1007/s00774-003-0482-y. [DOI] [PubMed] [Google Scholar]
- 5.Xu SW, Yu R, Zhao GF, Wang JW. Early period of fracture healing in ovariectomized rats. Chin J Traumatol. 2003;6:160–166. [PubMed] [Google Scholar]
- 6.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–86. doi: 10.1016/S8756-3282(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 7.Meyer RA, Jr, Tsahakis PJ, Martin DF, Banks DM, Harrow ME, Kiebzak GM. Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res. 2001;19:428–435. doi: 10.1016/S0736-0266(00)90034-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang JW, Li W, Xu SW, Yang DS, Wang Y, Lin M, Zhao GF. Osteoporosis influences the middle and late periods of fracture healing in a rat osteoporotic model. Chin J Traumatol. 2005;8:111–116. [PubMed] [Google Scholar]
- 9.Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, Pei FX. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone. 2007;41:631–638. doi: 10.1016/j.bone.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Islam AA, Rasubala L, Yoshikawa H, Shiratsuchi Y, Ohishi M. Healing of fractures in osteoporotic rat mandible shown by the expression of bone morphogenetic protein-2 and tumour necrosis factor-alpha. Br J Oral Maxillofac Surg. 2005;43:383–391. doi: 10.1016/j.bjoms.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252:68–80. doi: 10.1016/j.cellimm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 13.Weitzmann MN, Pacifici R. Estrogen regulation of immune cell bone interactions. Ann NY Acad Sci. 2006;1068:256–274. doi: 10.1196/annals.1346.030. [DOI] [PubMed] [Google Scholar]
- 14.Ginaldi L, De Martinis M, Ciccarelli F, Saitta S, Imbesi S, Mannucci C, Gangemi S. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015;16:60. doi: 10.1186/s12865-015-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aydin A, Halici Z, Albayrak A, Polat B, Karakus E, Yildirim OS, Bayir Y, Cadirci E, Ayan AK, Aksakal AM. Treatment with carnitine enhances bone fracture healing under osteoporotic and/or inflammatory conditions. Basic Clin Pharmacol Toxicol. 2015;117:173–179. doi: 10.1111/bcpt.12384. [DOI] [PubMed] [Google Scholar]
- 16.Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quinones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine. 2015;72:121–129. doi: 10.1016/j.cyto.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue K, Inoue E, Imai Y. Female sex hormones ameliorate arthritis in SKG mice. Biochem Biophys Res Commun. 2013;434:740–745. doi: 10.1016/j.bbrc.2013.03.111. [DOI] [PubMed] [Google Scholar]
- 18.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17:42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 19.Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 20.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 21.Bastian OW, Kuijer A, Koenderman L, Stellato RK, van Solinge WW, Leenen LP, Blokhuis TJ. Impaired bone healing in multitrauma patients is associated with altered leukocyte kinetics after major trauma. J Inflamm Res. 2016;9:69–78. doi: 10.2147/JIR.S101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng Part B Rev. 2015;21:354–364. doi: 10.1089/ten.teb.2014.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffner-Luntzer M, Kemmler J, Heidler V, Prystaz K, Schinke T, Amling M, Kovtun A, Rapp AE, Ignatius A, Liedert A. Inhibition of midkine augments osteoporotic fracture healing. PLoS ONE. 2016;11:e0159278. doi: 10.1371/journal.pone.0159278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedert A, Schinke T, Ignatius A, Amling M. The role of midkine in skeletal remodelling. Br J Pharmacol. 2014;171:870–878. doi: 10.1111/bph.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liedert A, Mattausch L, Rontgen V, Blakytny R, Vogele D, Pahl M, Bindl R, Neunaber C, Schinke T, Harroch S, et al. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone. 2011;48:945–951. doi: 10.1016/j.bone.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Neunaber C, Catala-Lehnen P, Beil FT, Marshall RP, Kanbach V, Baranowsky A, Lehmann W, Streichert T, Ignatius A, Muramatsu T, et al. Increased trabecular bone formation in mice lacking the growth factor midkine. J Bone Miner Res. 2010;25:1724–1735. doi: 10.1002/jbmr.75. [DOI] [PubMed] [Google Scholar]
- 27.Kondoh S, Inoue K, Igarashi K, Sugizaki H, Shirode-Fukuda Y, Inoue E, Yu T, Takeuchi JK, Kanno J, Bonewald LF, Imai Y. Estrogen receptor alpha in osteocytes regulates trabecular bone formation in female mice. Bone. 2014;60:68–77. doi: 10.1016/j.bone.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond-Stanic MK, Romero-Aleshire MJ, Hoyer PB, Greer K, Hoying JB, Brooks HL. Midkine, a heparin-binding protein, is increased in the diabetic mouse kidney postmenopause. Am J Physiol Renal Physiol. 2011;300:F139–F146. doi: 10.1152/ajprenal.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohta S, Muramatsu H, Senda T, Zou K, Iwata H, Muramatsu T. Midkine is expressed during repair of bone fracture and promotes chondrogenesis. J Bone Miner Res. 1999;14:1132–1144. doi: 10.1359/jbmr.1999.14.7.1132. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu T, Kadomatsu K. Midkine: an emerging target of drug development for treatment of multiple diseases. Br J Pharmacol. 2014;171:811–813. doi: 10.1111/bph.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikutomo M, Sakakima H, Matsuda F, Yoshida Y. Midkine-deficient mice delayed degeneration and regeneration after skeletal muscle injury. Acta Histochem. 2014;116:319–326. doi: 10.1016/j.acthis.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol. 2001;167:3463–3469. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- 33.Weckbach LT, Muramatsu T, Walzog B. Midkine in inflammation. ScientificWorldJournal. 2011;11:2491–2505. doi: 10.1100/2011/517152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wehrle E, Liedert A, Heilmann A, Wehner T, Bindl R, Fischer L, Haffner-Luntzer M, Jakob F, Schinke T, Amling M, Ignatius A. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis Model Mech. 2015;8:93–104. doi: 10.1242/dmm.018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rontgen V, Blakytny R, Matthys R, Landauer M, Wehner T, Gockelmann M, Jermendy P, Amling M, Schinke T, Claes L, Ignatius A. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J Orthop Res. 2010;28:1456–1462. doi: 10.1002/jor.21148. [DOI] [PubMed] [Google Scholar]
- 36.Haffner-Luntzer M, Heilmann A, Rapp AE, Roessler R, Schinke T, Amling M, Ignatius A, Liedert A. Antagonizing midkine accelerates fracture healing in mice by enhanced bone formation in the fracture callus. Br J Pharmacol. 2016;173:2237–2249. doi: 10.1111/bph.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan EF, Mason ZD, Chien KB, Pfeiffer AJ, Barnes GL, Einhorn TA, Gerstenfeld LC. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 39.Wronski TJ, Dann LM, Scott KS, Cintron M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int. 1989;45:360–366. doi: 10.1007/BF02556007. [DOI] [PubMed] [Google Scholar]
- 40.Krzystek-Korpacka M, Neubauer K, Matusiewicz M. Clinical relevance of circulating midkine in ulcerative colitis. Clin Chem Lab Med. 2009;47:1085–1090. doi: 10.1515/CCLM.2009.248. [DOI] [PubMed] [Google Scholar]
- 41.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis. 2006;193:360–369. doi: 10.1086/499312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 43.Hoff P, Gaber T, Strehl C, Schmidt-Bleek K, Lang A, Huscher D, Burmester GR, Schmidmaier G, Perka C, Duda GN, Buttgereit F. Immunological characterization of the early human fracture hematoma. Immunol Res. 2016;64:1195–1206. doi: 10.1007/s12026-016-8868-9. [DOI] [PubMed] [Google Scholar]
- 44.Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onoe Y, Miyaura C, Ito M, Ohta H, Nozawa S, Suda T. Comparative effects of estrogen and raloxifene on B lymphopoiesis and bone loss induced by sex steroid deficiency in mice. J Bone Miner Res. 2000;15:541–549. doi: 10.1359/jbmr.2000.15.3.541. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Perez MA, Noguera I, Hermenegildo C, Martinez-Romero A, Tarin JJ, Cano A. Alterations in the phenotype and function of immune cells in ovariectomy-induced osteopenic mice. Hum Reprod. 2006;21:880–887. doi: 10.1093/humrep/dei413. [DOI] [PubMed] [Google Scholar]
- 47.Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Foger-Samwald U, Ellinger I. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62:128–137. doi: 10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K, Scheel-Toellner D. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. 2011;70:2022–2028. doi: 10.1136/ard.2011.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valenzona HO, Pointer R, Ceredig R, Osmond DG. Prelymphomatous B cell hyperplasia in the bone marrow of interleukin-7 transgenic mice: precursor B cell dynamics, microenvironmental organization and osteolysis. Exp Hematol. 1996;24:1521–1529. [PubMed] [Google Scholar]
- 50.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1301/nr.2007.dec.S147-S151. [DOI] [PubMed] [Google Scholar]
- 51.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7:134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 52.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, Kuvibidila S, Lucas EA, Smith BJ. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J Nutr Biochem. 2012;23:60–68. doi: 10.1016/j.jnutbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Safadi FF, Dissanayake IR, Goodman GG, Jago RA, Baker AE, Bowman AR, Sass DA, Popoff SN, Epstein S. Influence of estrogen deficiency and replacement on T-cell populations in rat lymphoid tissues and organs. Endocrine. 2000;12:81–88. doi: 10.1385/ENDO:12:1:81. [DOI] [PubMed] [Google Scholar]
- 56.Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS ONE. 2012;7:e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyagi AM, Srivastava K, Sharan K, Yadav D, Maurya R, Singh D. Daidzein prevents the increase in CD4 + CD28null T cells and B lymphopoesis in ovariectomized mice: a key mechanism for anti-osteoclastogenic effect. PLoS ONE. 2011;6:e21216. doi: 10.1371/journal.pone.0021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao W, Liu Y, Cahill CM, Yang W, Rogers JT, Huang X. The role of T cells in osteoporosis, an update. Int J Clin Exp Pathol. 2009;2:544–552. [PMC free article] [PubMed] [Google Scholar]
- 59.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jilka RL, Passeri G, Girasole G, Cooper S, Abrams J, Broxmeyer H, Manolagas SC. Estrogen loss upregulates hematopoiesis in the mouse: a mediating role of IL-6. Exp Hematol. 1995;23:500–506. [PubMed] [Google Scholar]
- 61.Figueroa-Vega N, Moreno-Frias C, Malacara JM. Alterations in adhesion molecules, pro-inflammatory cytokines and cell-derived microparticles contribute to intima-media thickness and symptoms in postmenopausal women. PLoS ONE. 2015;10:e0120990. doi: 10.1371/journal.pone.0120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szabo A, Hartmann P, Varga R, Janvari K, Lendvai Z, Szalai I, Gomez I, Varga G, Greksa F, Nemeth I, et al. Periosteal microcirculatory action of chronic estrogen supplementation in osteoporotic rats challenged with tourniquet ischemia. Life Sci. 2011;88:156–162. doi: 10.1016/j.lfs.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, Schell H, Lienau J, Serra A, Radbruch A, Duda GN. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res. 2011;26:113–124. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- 64.Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, Dahne M, Hartwig T, Akyuz L, Meisel C, et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5:177ra136. doi: 10.1126/scitranslmed.3004754. [DOI] [PubMed] [Google Scholar]
- 65.Kovtun A, Bergdolt S, Wiegner R, Radermacher P, Huber-Lang M, Ignatius A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur Cell Mater. 2016;32:152–162. doi: 10.22203/eCM.v032a10. [DOI] [PubMed] [Google Scholar]
- 66.Doucet DR, Bonitz RP, Feinman R, Colorado I, Ramanathan M, Feketeova E, Condon M, Machiedo GW, Hauser CJ, Xu DZ, Deitch EA. Estrogenic hormone modulation abrogates changes in red blood cell deformability and neutrophil activation in trauma hemorrhagic shock. J Trauma. 2010;68:35–41. doi: 10.1097/TA.0b013e3181bbbddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 68.Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L, Calabro G, Caputi AP, Maggi A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7:478–487. [PMC free article] [PubMed] [Google Scholar]
- 69.Hardman MJ, Ashcroft GS. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008;9:R80. doi: 10.1186/gb-2008-9-5-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haffner-Luntzer M, Heilmann A, Rapp AE, Beie S, Schinke T, Amling M, Ignatius A, Liedert A. Midkine-deficiency delays chondrogenesis during the early phase of fracture healing in mice. PLoS ONE. 2014;9:e116282. doi: 10.1371/journal.pone.0116282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen S, Shoshana OY, Zelman-Toister E, Maharshak N, Binsky-Ehrenreich I, Gordin M, Hazan-Halevy I, Herishanu Y, Shvidel L, Haran M, et al. The cytokine midkine and its receptor RPTPzeta regulate B cell survival in a pathway induced by CD74. J Immunol. 2012;188:259–269. doi: 10.4049/jimmunol.1101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rose-John S. The soluble interleukin-6 receptor and related proteins. Best Pract Res Clin Endocrinol Metab. 2015;29:787–797. doi: 10.1016/j.beem.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Recknagel S, Bindl R, Brochhausen C, Gockelmann M, Wehner T, Schoengraf P, Huber-Lang M, Claes L, Ignatius A. Systemic inflammation induced by a thoracic trauma alters the cellular composition of the early fracture callus. J Trauma Acute Care Surg. 2013;74:531–537. doi: 10.1097/TA.0b013e318278956d. [DOI] [PubMed] [Google Scholar]
- 74.Shindo E, Nanki T, Kusunoki N, Shikano K, Kawazoe M, Sato H, Kaneko K, Muraoka S, Kaburaki M, Akasaka Y, et al. The growth factor midkine may play a pathophysiological role in rheumatoid arthritis. Mod Rheumatol. 2016;27:1–6. doi: 10.1080/14397595.2016.1179860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


