SUMMARY
Object motion sensitive (OMS) W3-retinal ganglion cells (W3-RGCs) in mice respond to local movements in a visual scene, but remain silent during self-generated global image motion. The excitatory inputs that drive responses of W3-RGCs to local motion were recently characterized; but which inhibitory neurons suppress W3-RGCs’ responses to global motion, how these neurons encode motion information, and how their connections are organized along the excitatory circuit axis remains unknown. Here, we find that a genetically identified amacrine cell (AC) type, TH2-AC, exhibits fast responses to global motion and slow responses to local motion. Optogenetic stimulation shows that TH2-ACs provide strong GABAA receptor-mediated input to W3-RGCs, but only weak input to upstream excitatory neurons. Cell-type-specific silencing reveals that temporally coded inhibition from TH2-ACs cancels W3-RGC spike responses to global, but not to local motion stimuli and, thus, controls the feature selectivity of OMS signals sent to the brain.
EToc paragraph
Kim and Kerschensteiner report that a specific amacrine cell type (TH2-AC) distinguishes local and global motion in the kinetics of its responses. Optogenetic activation and cell-type-specific silencing show that TH2-ACs provide strong inhibitory input to object motion sensitive retinal ganglion cells (W3-RGCs), and that this input suppresses W3-RGC responses to global motion stimuli.
INTRODUCTION
In many parts of the nervous system, the diversity of inhibitory interneurons exceeds that of excitatory projection neurons (Freund and Buzsaki, 1996, Tasic et al., 2016). Due in part to this diversity, the function of many interneurons remains unknown and the organization of inhibitory circuits is not well understood (Isaacson and Scanziani, 2011). The diversity of interneurons may be greatest in the retina, which in mice contains approximately 50 types of ACs (Helmstaedter et al., 2013, MacNeil and Masland, 1998). Each AC type studied so far has a different task in vision; and as a population ACs support the encoding of diverse visual features in the spike trains of approximately 40 RGC types, which send retinal information to the brain (Euler et al., 2002, Grimes et al., 2010, Jacoby et al., 2015, Kim et al., 2015, Vlasits et al., 2014, Yoshida et al., 2001, Hoggarth et al., 2015, Dacheux and Raviola, 1986, Chen and Li, 2012, Baden et al., 2016). ACs can shape the output of RGCs directly through input to their dendrites (i.e. postsynaptic inhibition), or indirectly through synapses on bipolar cell axons or other ACs (i.e. presynaptic inhibition) (Demb and Singer, 2015, Franke et al., 2017). The contributions of most AC types to visual processing remain unknown. In addition, it is unclear whether individual AC types preferentially provide pre- or postsynaptic inhibition, or equally inhibit multiple components of the same circuit.
Detecting the movements of objects (e.g. an attacking predator) is an essential function of visual systems; and object motion strongly attracts attention (Abrams and Christ, 2003) and can elicit innate defensive behaviors in prey species (De Franceschi et al., 2016, Yilmaz and Meister, 2013). To reliably signal object motion, neural circuits need to distinguish it from self-generated motion (e.g. eye movements) (Olveczky et al., 2003). In the retinal image, object and self-generated motion cause local and global displacements, respectively. In mice, W3-RGCs receive excitatory input from bipolar cells and from VGluT3-expressing ACs (VG3-ACs) (Krishnaswamy et al., 2015, Kim et al., 2015, Lee et al., 2014). Local motion depolarizes bipolar cells and VG3-ACs, and elicits robust spiking in W3-RGCs; but during global motion, these neurons are silenced (Kim et al., 2015, Zhang et al., 2012). Global motion evokes inhibitory input to all three tiers of the excitatory circuit axis via the axons of bipolar cells, the neurites of VG3-ACs, and the dendrites of W3-RGCs (Kim et al., 2015, Krishnaswamy et al., 2015, Lee et al., 2014, Zhang et al., 2012). Which AC types provide this inhibitory input, how they distinguish local and global motion, and whether a single AC type preferentially targets a single tier of the excitatory circuit axis or provides similar input to all of them, is unknown.
Recent anatomical work showed that the neurites of a genetically identified AC type, TH2-AC, co-stratify with and appear to contact dendrites of W3-RGCs and neurites of VG3-ACs in the inner plexiform layer (IPL) (Bruggen et al., 2015, Knop et al., 2011). Here, using targeted patch clamp recordings, optogenetic circuit mapping, and cell-type-specific silencing, we identify a critical function of TH2-ACs in object motion encoding in the retinal output.
RESULTS
Temporal distinction of local vs. global motion by genetically identified TH2-ACs
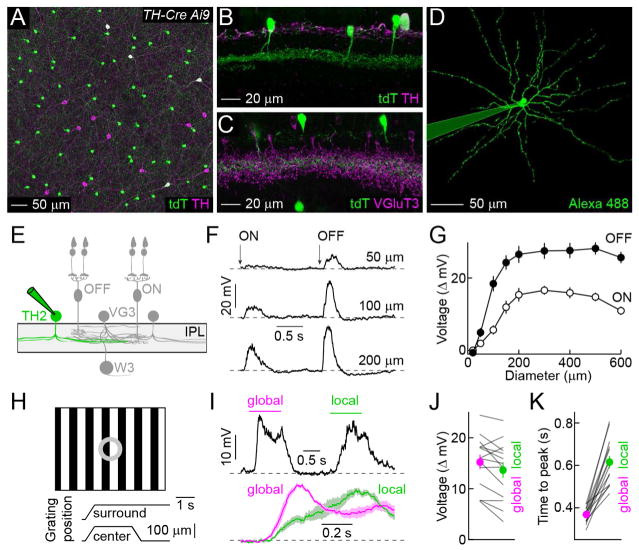
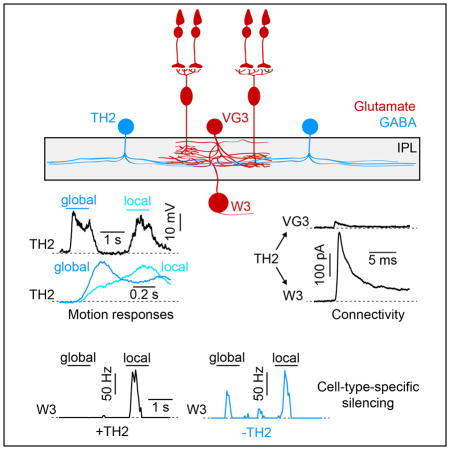
We crossed mice in which an 11-kb fragment of the tyrosine hydroxylase (TH) promoter drives expression of Cre recombinase (TH-Cre) to a fluorescent reporter strain (Ai9) (Savitt et al., 2005, Madisen et al., 2010). In the inner nuclear layer (INL) of TH-Cre Ai9 mice, a dense population of TH-negative cells expressed tdTomato (tdT), in addition to sparse subsets of dopaminergic ACs (i.e. TH-positive, DACs) and starburst ACs (Figures 1A and S1, SACs) (Vuong et al., 2015). The cell bodies of the dense TH-negative population were arranged in regular mosaics, indicating that they belonged to a single cell type (Figure S1). Neurites of this cell type stratified in the center of the IPL where they overlapped with neurites of VG3-ACs (Figures 1B, 1C, and S1). Under 2-photon guidance, we targeted the dense population in the INL for patch clamp recordings, and filled individual cells with an intracellular dye, revealing wide-field neurite arborizations (Figures 1D, 1E, and S1). Together with the genetic labeling and stratification patterns, this identified the dense TH-negative population as TH2-ACs (Knop et al., 2011, Vuong et al., 2015). Consistent with a previous study (Knop et al., 2011), we found that TH2-ACs depolarized to light increments (ON) and decrements (OFF) over a wide range of stimulus sizes (Figures 1F – 1G). Responses of TH2-ACs to visual motion had not yet been explored. Given co-stratification with OMS circuits in the middle of the IPL (Figures 1B, 1C and S1) (Bruggen et al., 2015, Jacoby and Schwartz, 2017, Knop et al., 2011), we tested responses of TH2-ACs to global and local motion stimuli previously used to characterize VG3-ACs and W3-RGCs (Figure 1H) (Zhang et al., 2012, Kim et al., 2015). TH2-ACs depolarized with slightly larger amplitudes to global than local motion (Figures 1I and 1J). More striking than differences in amplitudes, however, were differences in response kinetics to these motion stimuli. Whereas global motion elicited fast depolarizations, responses to local motion were much slower (Figures 1I and 1K). These differences in response kinetics persisted across a variety of stimulus contrasts (Figure S2). Thus, TH2-ACs appear to distinguish object and self-generated movements predominantly in their response timing. Intriguingly, similar differences in kinetics have been observed in the inhibitory inputs to VG3-ACs and W3-RGCs, with fast surround inhibition canceling excitatory center inputs during global motion (Kim et al., 2015, Zhang et al., 2012).
Figure 1. Genetically identified TH2-ACs distinguish local and global motion in their response timing.
(A) Representative image of the INL of a flat-mounted TH-Cre Ai9 retina stained for tdT and TH. (B and C) Vibratome sections of TH-Cre Ai9 retinas stained for tdT and TH (B) or VGluT3 (C). (D) An individual TH2-AC filled with Alexa 488 via a patch clamp electrode targeted under 2-photon guidance. (E) Schematic illustration of the W3-RGC circuit (ON and OFF bipolar cells, VG3-ACs, and W3-RGCs) and TH2-ACs. (F and G) Representative traces (F) and summary data (G, mean ± SEM, n = 12) of voltage responses of TH2-ACs to a stimulus in which intensity in spots of varying diameters was square-wave-modulated (1.5 s ON, 1.5 s OFF). (H) Schematic illustrating motion stimulus. Two square-wave gratings (bar width: 50 μm, contrast: 100 %) one covering the center and one the surround of the excitatory circuit axis, are separated by a gray annulus. During stimulus presentation, both gratings first move together (i.e. global motion) and then the center grating moves alone (i.e. local motion). (I) A representative voltage response of a TH2-ACs to the stimulus illustrated in (H) is presented in the top panel. Mean (± SEM) of responses of TH2-ACs (n = 14) to global (magenta) and local (green) stimuli aligned on the respective motion onsets are shown by lines (shaded areas) in the bottom panel. (J and K) Individual (gray lines) and population (mean ± SEM, circles with errorbars) data comparing response amplitudes (J, n = 14, p = 0.016) and timing (K, p = 1.2 × 10−4) to global (magenta) and local (green) motion stimuli. See also Figures S1 and S2.
Optogenetic exploration of inhibition from TH2-ACs to VG3-ACs and W3-RGCs
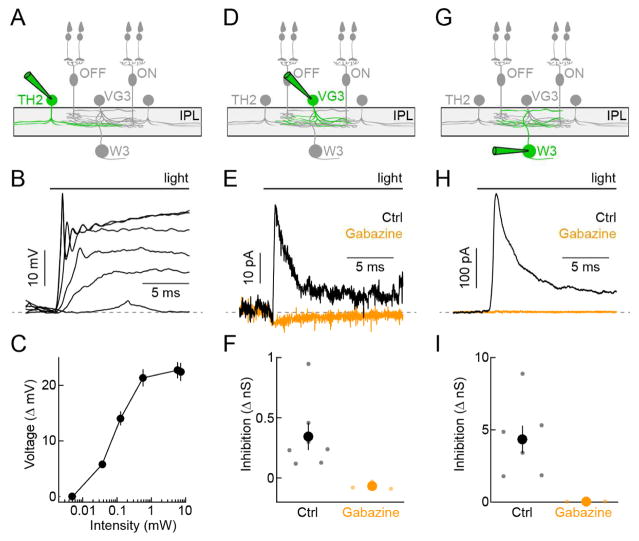
We used optogenetics to test if TH2-ACs provide input to VG3-ACs and/or W3-RGCs, and to analyze what transmitter(s) and receptor(s) mediate this putative communication. We first recorded TH2-ACs expressing channelrhodopsin 2 fused to yellow fluorescent protein (ChR2-YFP), to identify stimulus parameters that matched optogenetic depolarizations to those observed during photoreceptor-driven light responses (Figures 2A–2C; TH-Cre Ai32) (Park et al., 2015, Tien et al., 2016). In all optogenetic experiments, the transmission of photoreceptor signals to bipolar cells and excitatory synaptic transmission in the inner retina were blocked by inclusion of L-AP4 (20 μM), ACET (10 μM), D-AP5 (30 μM), and NBQX (40 μM) in the extracellular solution (Park et al., 2015, Tien et al., 2016). We obtained patch clamp recordings from VG3-ACs in mice in which both TH2-ACs and VG3-ACs (for targeting) express ChR2-YFP (Figure 2D; TH-Cre VG3-Cre Ai32). Holding VG3-ACs at 0 mV - the reversal potential of ChR2-mediated currents - allowed us to isolate inhibitory synaptic inputs. Optogenetic stimulation reliably elicited small inhibitory postsynaptic currents (IPSCs) in VG3-ACs (Figures 2E and 2F), which were not observed in mice in which only VG3-ACs express ChR2-YFP (data not shown; VG3-Cre Ai32). These IPSCs were blocked by application of the GABAA receptor antagonist gabazine (Figures 2E and 2F). In W3-RGCs, optogenetic stimulation elicited approximately 10-fold larger IPSCs than in VG3-ACs, which were equally blocked by gabazine (Figures 2G–2H; TH-Cre Ai32). Thus, TH2-ACs preferentially inhibit the final tier of the excitatory circuit axis, providing strong GABAA receptor-mediated input to W3-RGCs and only weak GABAA receptor-mediated input to VG3-ACs.
Figure 2. Optogenetic mapping of TH2-AC connectivity.
(A, D, G) Schematics illustrating the W3-RGC circuit with cell type recorded in the panels below highlighted (green). (B and C) Representative traces (B) and summary data (C, mean ± SEM, n = 6) of TH2-AC voltage responses to increasing optogenetic stimulus intensities presented in a 2-mm diameter spot (TH-Cre Ai32). To map connectivity stimuli of 0.57 mW were used. (E and F) Representative traces (E) and summary data (F) of IPSCs (Vhold = 0 mV) recorded in VG3-ACs during optogenetic stimulation of TH2-ACs (TH-Cre VG3-Cre Ai32) in the control conditions (black) and in the presence of gabazine (orange). In (F), each dot represents data from individual cells and the larger circles (errorbars) indicate the mean (± SEM) of the respective populations (control, n = 7, gabazine n = 4, p = 0.006). (H and I) Analogous to E (H) and F (I), but for IPSCs recorded from W3-RGCs (TH-Cre Ai32, control n = 7, gabazine n = 3, p = 0.016). TH2-AC-driven IPSCs are approximately 10-fold larger in W3-RGCs than in VG3-ACs (p = 5.8 × 10−4).
Cell-type-specific silencing of TH2-ACs selectively reduces inhibitory input to W3-RGCs
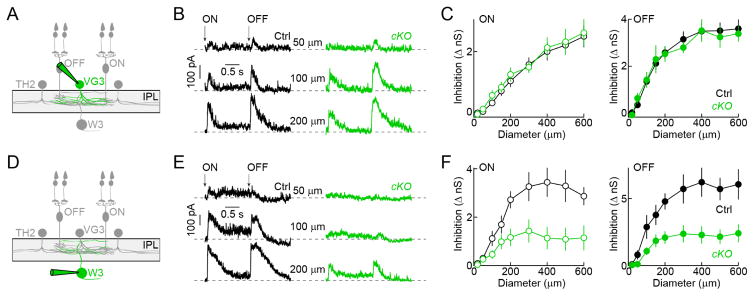
To reveal the contributions of TH2-ACs to visual processing, we endeavored to silence them in a cell-type-specific manner. We crossed TH-Cre mice to a conditional knockout strain of the vesicular GABA transporter (VGATcKO) (Tong et al., 2008). We used optogenetics to confirm that this manipulation suppressed inhibitory transmission from TH2-ACs. Indeed, VGAT deletion greatly reduced IPSCs in W3-RGCs without affecting the optogenetic activation of TH2-ACs (Figure S3; TH-Cre VGATcKO Ai32 vs. TH-Cre Ai32). The small residual IPSCs we observed in a subset of our recordings may be the result of incomplete Cre expression in the population of TH2-ACs and electrical coupling between TH2-ACs (Bruggen et al., 2015). We next analyzed how TH2-AC silencing affected photoreceptor-driven inhibition in VG3-ACs and W3-RGCs. Stationary ON and OFF stimuli of increasing size elicited increasing IPSCs in VG3-ACs. These IPSCs were unaffected by VGAT deletion in TH2-ACs (Figure 3A–3C; TH-Cre VG3-Cre VGATcKO Ai9 vs. TH-Cre VG3-Cre Ai9). By contrast, TH2-AC silencing reduced inhibitory inputs to W3-RGCs evoked by the same stimulus to less than half their normal amplitudes (Figures 3D–3F; TH-Cre VGATcKO vs. TH-Cre). Given the limited overlap of DAC and SAC neurites with OMS circuits in the middle of the IPL (Figure S1), and the fact that only sparse subsets of DACs and SACs are labeled in TH-Cre mice (Figure S1), it is unlikely that they contribute significantly to our findings. Interestingly, light responses of TH2-ACs themselves were largely unaffected by deletion of VGAT and by removal of VGluT3 (Figure S4, TH-Cre VGATcKO Ai9 and TH-Cre VGluT3KO Ai9 vs. TH-Cre Ai9); and no IPSCs were observed in TH2-ACs following optogenetic stimulation of TH2-ACs and VG3-ACs (data not shown; TH-Cre VG3-Cre Ai32). Together these results indicate that TH2-ACs neither inhibit each other, nor receive input from VG3-ACs, nor provide much output to them. Instead, TH2-ACs dominate surround inhibition to W3-RGCs.
Figure 3. Silencing of TH2-ACs reveals preferential inhibition to theW3-RGCs.
(A and D) Schematic illustrating the W3-RGC circuit with the cell type recorded in the panels to the right highlighted (green). (B and C) Representative traces (B) and summary data (C, mean ± SEM) of VG3-AC IPSCs elicited stationary ON and OFF stimuli of increasing size (i.e. spot diameter). Recordings from mice in which TH2-ACs lack VGAT (TH-Cre VG3-Cre VGATcKO Ai9, cKO, green, n = 10) and control mice (TH-Cre VG3-Cre Ai9, black, Ctrl, n = 24,) are compared (ON: p = 0.94, OFF: p = 0.902). (E and F) Analogous to B (E) and C (F), but for recordings of IPSCs in W3-RGCs, which are reduced when TH2-ACs are silenced (TH-Cre VGATcKO, cKO, green, n = 13) compared to when TH2-ACs are active (TH-Cre, Ctrl, black, n = 10, ON: p = 0.012, OFF: p = 0.006). See also Figures S3 and S4.
Cell-type-specific silencing of TH2-ACs reduces feature selectivity of OMS signals to the brain
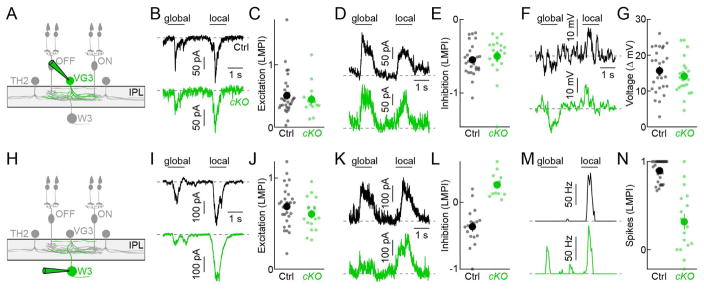
To analyze the impact of TH2-ACs on motion processing, we compared global and local motion responses along the excitatory circuit axis between mice in which TH2-ACs were silenced and control littermates. Due to surround inhibition onto bipolar cell axons, excitatory inputs to VG3-ACs are smaller during global than local motion (Kim et al., 2015). This preference for local motion, summarized by a local motion preference index (LMPI, s. Experimental Procedures), was unchanged when TH2-ACs were silenced, indicating that TH2-ACs do not inhibit the bipolar cell axons in this circuits (Figures 4A–4C; TH-Cre VG3-Cre VGATcKO Ai9 vs. TH-Cre VG3-Cre Ai9). Similarly, inhibitory inputs to VG3-ACs elicited by local and global motion were unchanged (Figures 4D and 4E), consistent with our results from stationary stimuli (Figures 3B and 3C); and voltage differences between VG3-ACs’ depolarizations to local motion and hyperpolarizations to global motion were preserved (Figures 4F and 4G). Because we observed no change in the bipolar cell input to VG3-ACs and in their voltage responses, we expected the excitatory input from bipolar cells and VG3-ACs to W3-RGCs to be unaltered as well. Indeed, excitatory synaptic conductances in W3-RGCs showed equal local motion preference in mice in which TH2-ACs were silenced and in control littermates (Figures 4H–4J; TH-Cre VGATcKO vs. TH-Cre). By contrast, inhibitory inputs to W3-RGCs elicited by global motion were drastically reduced by VGAT deletion in TH2-ACs (TH-Cre: 2.02 ± 0.2 nS, n = 17, TH-Cre VGATcKO: 0.8 ± 0.16 nS, n = 12, p = 1.8 × 10−4), resulting in a switch in motion preference (Figures 4K and 4L). As a consequence of this reduced surround inhibition, W3-RGCs spiked robustly during global motion when TH2-ACs were silenced (TH-Cre: 2 ± 0.2 Hz, n = 17, TH-Cre VGATcKO: 18.6 ± 4.1 Hz, n = 17, p = 0.0019), and the near-perfect object motion selectivity of output signals from W3-RGCs to the brain was lost (Figures 4M and 4N).
Figure 4. Silencing of TH2-ACs reduces feature selectivity of OMS signals to the brain.
(A and H) Schematic illustrating the W3-RGC circuit with the cell type recorded in the panels to the right highlighted (green). (B and C) Representative traces of excitatory input to VG3-ACs during global and local motion (B) and summary data of a local motion preference index (C, LMPI) compared between mice in which TH2-ACs lack VGAT (TH-Cre VG3-Cre VGATcKO Ai9, cKO, green, n = 13) and control mice (TH-Cre VG3-Cre Ai9, Ctrl, black, n = 30, p = 0.57). In (C), each dot represents data from individual cells and the larger circles (errorbars) indicate the mean (± SEM) of the respective populations. (D and E) Analogous to B (D) and C (E), but for inhibitory inputs to VG3-ACs (TH-Cre VG3-Cre VGATcKO Ai9, cKO, green, n = 19; TH-Cre VG3-Cre Ai9, Ctrl, black, n = 27, p = 0.14). (F and G) Representative traces of VG3-AC voltage responses during global and local motion (F) and summary data of the difference between both responses (G) compared between mice in which TH2-ACs lack VGAT (TH-Cre VG3-Cre VGATcKO Ai9, cKO, green, n = 22) and control mice (TH-Cre VG3-Cre Ai9, Ctrl, black, n = 27, p = 0.43). (I and J) Representative traces of excitatory input to W3-RGCs during global and local motion (I) and LMPI summary data (J, LMPI) compared between mice in which TH2-ACs lack VGAT (TH-Cre VGATcKO, cKO, green, n = 17) and control mice (TH-Cre, Ctrl, black, n = 28, p = 0.18). (K and L) Analogous to I (K) and J (L), but for inhibitory inputs to W3-RGCs (TH-Cre VGATcKO, cKO, green, n = 12; TH-Cre, Ctrl, black, n = 17, p = 3.5 × 10−5). (M and N) Analogous to I (M) and J (N), but for spike responses of W3-RGCs (TH-Cre VGATcKO, cKO, green, n = 17; TH-Cre, Ctrl, black, n = 30, p = 1.1 × 10−6).
DISCUSSION
We find that TH2-ACs, a genetically identified type of wide-field ACs (Vuong et al., 2015, Knop et al., 2011), distinguish local and global motion primarily in the speed of their responses (Figure 1). This temporal code of TH2-ACs was similarly observed in responses to stationary stimuli (Knop et al., 2011) and likely reflects the recruitment of voltage-dependent conductances that speed up depolarizations with increasing stimulus strengths. Response kinetics were unaffected by TTX (data not shown), arguing that TTX-sensitive voltage-gated sodium channels, which accelerate responses of other ACs (Tian et al., 2010), do not contribute to temporal coding in TH2-ACs. Previously observed effects of TTX on global motion suppression of VG3-ACs and W3-RGCs (Zhang et al., 2012, Kim et al., 2015), thus either reflect local effects on synaptic transmission from TH2-ACs not evident in recordings from their soma, and/or are accounted for by actions on other AC types contributing to surround inhibition in the W3-RGC circuit.
VGAT deletion in TH2-ACs drastically reduced inhibitory input to W3-RGCs during global but not during local motion (Figure 4), suggesting that the fast responses of TH2-ACs to global motion drive transmitter release more effectively than slow responses of similar amplitude during local motion. Several mechanisms including calcium channel inactivation, activity of calcium extrusion pumps, and vesicle pool dynamics may contribute to this differential transmission (Demb and Singer, 2015, Grant and Fuchs, 2008, Morgans et al., 1998). In addition to effective transmission, fast responses ensure that the inhibitory output of TH2-ACs coincides with and cancels excitatory inputs to W3-RGCs during global motion, whereas slower inhibition during local motion peaks after excitation (Zhang et al., 2012, Baccus et al., 2008, Kim et al., 2015). Thus, the temporal code of TH2-ACs is translated into feature-selective inhibition.
Inhibition occurs on three tiers of the excitatory axis via bipolar cell axons, VG3-AC neurites, and W3-RGC dendrites (Kim et al., 2015, Lee et al., 2014, Zhang et al., 2012). We find that TH2-ACs preferentially inhibit W3-RGCs, suppress their spike responses to global motion, and thus control the feature selectivity of OMS signals sent to the brain (Figures 2 and 4). Although optogenetic stimulation of TH2-ACs elicited small IPSCs in VG3-ACs (Figure 2), silencing of TH2-ACs showed no deficits at this or the preceding tier (Figures 3 and 4), arguing that presynaptic inhibition is dominated by other AC types. Interestingly, in salamander, a group of polyaxonal ACs was shown to provide presynaptic inhibition in an OMS circuit (Baccus et al., 2008). Whether homologous ACs serve a similar function in mammalian retinas; and to what extent the selectivity for pre- or postsynaptic inhibition is common among AC types remains to be determined.
We previously posited that if multiple AC types converge in the same circuit, they each make distinct and separable contributions to the overall computation (Tien et al., 2016). In the W3-RGC circuit, VG3-ACs selectively amplify responses to local motion (Kim et al., 2015), whereas TH2-ACs selectively suppress responses to global motion (Figure 4), highlighting this organizing principle. Other examples include the division of labor among AC types that inhibit Suppressed-by-Contrast RGCs during stimuli of different size and contrast (Tien et al., 2016, Tien et al., 2015, Jacoby et al., 2015, Lee et al., 2016), and between AC types that underlie the direction- and size-selectivity, respectively, of direction-selective RGCs (Hoggarth et al., 2015).
In addition to convergence, signals of AC types can diverge and may exert similar influences on multiple targets. It is tempting to speculate that in addition to W3-RGCs, TH2-ACs may contribute to surround inhibition of a recently identified group of RGCs that stratify in the center of the IPL and that like W3-RGCs track movements of small objects (Jacoby and Schwartz, 2017).
EXPERIMENTAL PROCEDURES
Animals
Throughout our study we used various combinations of the following mouse strains: VG3-Cre (Grimes et al., 2010) provided by Dr. R. H. Edwards; TH-Cre (Savitt et al., 2005) from Jackson Laboratories RRID:IMSR_JAX:008601; Ai9 (Madisen et al., 2010) from Jackson Laboratories stock #007909; Ai32 (Madisen et al., 2012) from Jackson Laboratories stock #012569; VGATcKO (Tong et al., 2008) from Jackson Laboratories stock #012897; VGluT3KO (Seal et al., 2008) from Jackson Laboratories stock #016931. Mice were housed in a 12 hr light/dark cycle and fed ad libidum. We isolated retinas from mice of both sexes aged between postnatal day 20 (P20) and P40, and compared littermates with appropriate genotypes in control and experimental groups. All experiments in this study were approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine and were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue preparation
Mice were dark adapted for > 2 hr, deeply anesthetized with CO2, killed by cervical dislocation, and enucleated. For electrophysiology, retinas were isolated in bicarbonate-buffered mACSF (mACSFNaHCO3) containing (in mM) 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, 26 NaHCO3 and 0.5 L-Glutamine equilibrated with 95% O2/5% CO2, and flat mounted on transparent membrane discs (Anodisc, Whatman). For anatomy, retinas were isolated in HEPES-buffered mACSF (mACSFHEPES) containing (in mM) 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 11 glucose and 20 HEPES (pH adjusted to 7.37 with NaOH) and flat mounted on black membrane discs (HABGO1300, Millipore). All procedures were carried out under infrared illumination.
Electrophysiology
Patch clamp recordings from TH2-ACs, VG3-ACs, and W3-RGCs were obtained in the dorsal halves of retinal flat mount preparations continually perfused (~8 mL/min) with warm (33 °C) mACSFNaHCO3 (Kim et al., 2015, Wang et al., 2011). TH2-ACs and VG3-ACs were targeted under 2-photon guidance in transgenic retinas in which these cells are fluorescently labeled. W3-RGCs were approached under conventional infrared illumination. Cell type identities were confirmed by characteristic neurite arbor morphologies visualized by 2-photon imaging of Alexa 488 (1 mM) included in the intracellular solution at the end of each recording. In addition, W3-RGCs were distinguished from other small RGCs by their longer response latencies to small spots of light (Jacoby and Schwartz, 2017). The intracellular solution for current clamp recordings contained (in mM): 125 K-gluconate, 10 NaCl, 1 MgCl2, 10 EGTA, 5 HEPES, 5 ATP-Na and 0.1 GTP-Na (pH adjusted to 7.2 with KOH). The intracellular solution for voltage clamp recordings contained (in mM): 120 Cs-gluconate, 1 CaCl2, 1 MgCl2, 10 Na-HEPES, 11 EGTA, 10 TEA-Cl and 2 Qx314 (pH adjusted to 7.2 with CsOH). Patch pipettes had resistances of 4–7 MΩ (borosilicate glass). Signals were amplified with a Multiclamp 700B amplifier (Molecular Devices), filtered at 3 kHz (8-pole Bessel low-pass) and sampled at 10 kHz (Digidata 1440A, Molecular Devices). In voltage-clamp recordings, series resistance (10–15 MΩ) was compensated electronically by ~75 %. Excitatory (EPSCs) and inhibitory postsynaptic currents (IPSCs) were isolated by holding cells at the reversal potential of inhibitory (−60 mV) and excitatory (0mV) conductances, respectively. In current clamp recordings, no bias current was injected. In recordings of optogenetic responses, the transmission of photoreceptor signals to bipolar cells and excitatory synaptic transmission in the inner retina were blocked by addition of L-AP4 (Tocris, 20 μM), ACET (Tocris, 10 μM), D-AP5 (Tocris, 30 μM), and NBQX (Tocris, 40 μM) to the mACSFNaHCO3. In a subset of recordings, gabazine (SR-95531, Sigma-Aldrich, 10 μM) was added to the mACSFNaHCO3 to block GABAA receptors.
Visual stimulation
Visual stimuli written in MATLAB (The Mathworks) were presented on an organic light-emitting display (OLED-XL, eMagin) using Cogent graphics extensions (John Romaya, Laboratory of Neurobiology at the Wellcome Department of Imaging Neuroscience, University College London), and were projected onto the photoreceptors of the retina via the substage condenser of an upright 2-photon microscope (FV1000 MPE, Olympus). All stimuli were centered on the soma of the recorded cells and their average intensity was kept constant at ~ 1500 rhodopsin isomerizations/rod/s. To measure the size preference of TH2-ACs, VG3-ACs and W3-RGCs, the intensity of spots of varying diameter was square-wave-modulated at 0.333 Hz (1.5 s ON, 1.5 s OFF) for 5 cycles (Michelson contrast: 96%). The order in which spots of different size were presented was randomly chosen for each cell, and the first stimulus in the sequence repeated at its end to confirm stability of the recording. To test responses to object vs. global motion stimuli, narrow square wave gratings (bar width: 50–75 μm) over the receptive field center and surround were moved separately or in unison (Kim et al., 2015, Zhang et al., 2012). A gray annulus was included in the spatial layout of the stimulus to reliably separate movement in the center and surround.
Optogenetics
To activate channelrhodopsin 2 (ChR2), light from a mercury bulb (Olympus) was bandpass filtered (426–446 nm, Chroma) and projected onto the RGC-side of the retina (spot diameter: 2 mm) through a 20 × 0.9 NA objective on an upright 2-photon microscope (FV1000 MPE, Olympus). Intensity of the optogenetic stimulus was varied by inserting different neutral density filters into its path; and a Uniblitz shutter (Vincent Associates) controlled stimulus timing.
Immunohistochemistry
Eye cups or flat-mounted retinas were fixed for 30 min in 4% paraformaldehyde (PFA) in mACSFHEPES at room temperature (RT) and washed three times for 10 min in PBS at RT. For vibratome sections (thickness: 60 μm), retinas were isolated from eye cups, embedded in agarose and cut (VT1000P, Leica) and then stained. Flat-mounted retinas were cryoprotected with a series of 10%, 20% and 30% sucrose in PBS for 1 hr (at RT), 1 hr (at RT) and overnight (at 4 °C), respectively. The tissue then underwent three cycles of freezing (held over liquid nitrogen) and thawing (in 30% sucrose in PBS), was washed three times in PBS for 10 min at RT, and subsequently stained. The following primary antibodies were in different combinations: rabbit anti-TH (EMD Millipore, 1:1000), rabbit anti-VGluT3 (Synaptic Sytems, 1:1000), goat anti-ChAT, (EMD Millipore, 1:500), and mouse anti-tdT (Abcam, 1:1000). Retinas were incubated with primary antibodies in PBS with 5% normal donkey serum and 0.5% Triton X-100 for 3–5 days (flat mounts) or overnight (sections) at 4 °C. Subsequently, they were washed three times for 10 min in PBS, stained with Alexa 488- Alexa 568-, and/or Alexa 633-conjugated secondary antibodies (Invitrogen, 1:1000) overnight at 4 °C (flat mounts) or 1 hr at RT (sections). The tissue was then washed three times in PBS for 10 min and mounted in Vectashield mounting medium (Vector Laboratories).
Confocal and 2-photon imaging
Confocal image stacks of fixed tissue were acquired through 20 × 0.85 NA or 60 × 1.35 NA oil immersion objectives (Olympus) on an upright microscope (FV1000, Olympus). The voxel sizes of confocal image stacks ranged from 0.62 μm – 1 μm (x/y – z) to 0.1 μm – 0.3 μm (x/y – z). Two-photon image stacks of neurons filled with Alexa 488 during patch clamp recordings were acquired through a 20 × 0.9 NA water immersion objective (Olympus) on an upright microscope (FV1000 MPE, Olympus) at a voxel size of 0.62 μm – 1 μm (x/y – z).
Analysis of electrophysiology data
Response amplitudes to visual and optogenetic stimuli were measured as baseline-subtracted averages during 100–200 ms time windows. To compare responses to local and global motion stimuli, we computed a local motion preference index (LMPI) according to:
where RL and RG indicate responses to local and global motion stimuli, respectively. For VG3-AC voltage recordings, we measured the difference between responses to local and global motion, rather than the LMPI, to avoid division by small denominators. Electrophysiology data were processed and analyzed using scripts written in MATLAB (The Mathworks).
Analysis of imaging data
To analyze the density and distribution of cells labeled in TH-Cre Ai9 retinas, flat mounts were co-stained for tdTomato (tdT), cholineacetyltransferase (ChAT), and tyrosine hydroxylase (TH). In agreement with previous studies (Knop et al., 2011, Vuong et al., 2015), we identified TH2-ACs as the cells that are positive for tdT, but negative for ChAT and TH. By contrast, starburst ACs are ChAT-positive and dopaminergic ACs are TH-positive. Soma positions of TH2-ACs were marked manually in projections of confocal image stacks through the INL and ganglion cell layer GCL and density recovery profiles calculated according to (Rodieck, 1991). Imaging data were processed and analyzed using Fiji (Schindelin et al., 2012) and scripts written in MATLAB (The Mathworks).
Statistical tests
To compare single value measurements between groups, we used Wilcoxon rank sum tests. To compare response amplitudes to increasing spot sizes between two groups (e.g. different genotypes) we used bootstrapping with 10,000 replicates. Differences in the actual average response curves were compared to differences of average response curves when data were randomly assigned to the compared groups.
Supplementary Material
Highlights.
TH2-ACs are a genetically identifiable wide-field amacrine cell type in mice
TH2-ACs distinguish global and local motion stimuli in their response timing
TH2-ACs provide strong GABAergic input to object motion sensitive W3-RGCs
Inhibitory input from TH2-ACs suppresses W3-RGC responses to global motion stimuli
Acknowledgments
We thank members of the Kerschensteiner lab for helpful comments and suggestions throughout this study. This work was supported by the National Institutes of Health (EY023341, EY026978, and EY027411 to DK and the Vision Core Grant EY0268) and by an unrestricted grant from the Research to Prevent Blindness Foundation to the Department of Ophthalmology and Visual Sciences at Washington University.
Footnotes
AUTHOR CONTRIBUTIONS
Experiments were designed by T.K. and D.K.; experiments were conducted by T.K.; data were analyzed by T.K. and D.K.; and the manuscript was written by T.K. and D.K..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABRAMS RA, CHRIST SE. Motion onset captures attention. Psychol Sci. 2003;14:427–32. doi: 10.1111/1467-9280.01458. [DOI] [PubMed] [Google Scholar]
- BACCUS SA, OLVECZKY BP, MANU M, MEISTER M. A retinal circuit that computes object motion. J Neurosci. 2008;28:6807–17. doi: 10.1523/JNEUROSCI.4206-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADEN T, BERENS P, FRANKE K, ROMAN ROSON M, BETHGE M, EULER T. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016;529:345–50. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUGGEN B, MEYER A, BOVEN F, WEILER R, DEDEK K. Type 2 wide-field amacrine cells in TH::GFP mice show a homogenous synapse distribution and contact small ganglion cells. Eur J Neurosci. 2015;41:734–47. doi: 10.1111/ejn.12813. [DOI] [PubMed] [Google Scholar]
- CHEN S, LI W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nat Neurosci. 2012;15:954–6. doi: 10.1038/nn.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DACHEUX RF, RAVIOLA E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–45. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE FRANCESCHI G, VIVATTANASARN T, SALEEM AB, SOLOMON SG. Vision Guides Selection of Freeze or Flight Defense Strategies in Mice. Curr Biol. 2016;26:2150–4. doi: 10.1016/j.cub.2016.06.006. [DOI] [PubMed] [Google Scholar]
- DEMB JB, SINGER JH. Functional Circuitry of the Retina. Annual Review of Vision Science. 2015;1:263–289. doi: 10.1146/annurev-vision-082114-035334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EULER T, DETWILER PB, DENK W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–52. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- FRANKE K, BERENS P, SCHUBERT T, BETHGE M, EULER T, BADEN T. Inhibition decorrelates visual feature representations in the inner retina. Nature. 2017;542:439–444. doi: 10.1038/nature21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUND TF, BUZSAKI G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- GRANT L, FUCHS P. Calcium- and calmodulin-dependent inactivation of calcium channels in inner hair cells of the rat cochlea. J Neurophysiol. 2008;99:2183–93. doi: 10.1152/jn.01174.2007. [DOI] [PubMed] [Google Scholar]
- GRIMES WN, ZHANG J, GRAYDON CW, KACHAR B, DIAMOND JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–85. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMSTAEDTER M, BRIGGMAN KL, TURAGA SC, JAIN V, SEUNG HS, DENK W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–74. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- HOGGARTH A, MCLAUGHLIN AJ, RONELLENFITCH K, TRENHOLM S, VASANDANI R, SETHURAMANUJAM S, SCHWAB D, BRIGGMAN KL, AWATRAMANI GB. Specific wiring of distinct amacrine cells in the directionally selective retinal circuit permits independent coding of direction and size. Neuron. 2015;86:276–91. doi: 10.1016/j.neuron.2015.02.035. [DOI] [PubMed] [Google Scholar]
- ISAACSON JS, SCANZIANI M. How inhibition shapes cortical activity. Neuron. 2011;72:231–43. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBY J, SCHWARTZ GW. Three Small-Receptive-Field Ganglion Cells in the Mouse Retina Are Distinctly Tuned to Size, Speed, and Object Motion. J Neurosci. 2017;37:610–625. doi: 10.1523/JNEUROSCI.2804-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBY J, ZHU Y, DEVRIES SH, SCHWARTZ GW. An Amacrine Cell Circuit for Signaling Steady Illumination in the Retina. Cell Rep. 2015;13:2663–70. doi: 10.1016/j.celrep.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM T, SOTO F, KERSCHENSTEINER D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. Elife. 2015:4. doi: 10.7554/eLife.08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOP GC, FEIGENSPAN A, WEILER R, DEDEK K. Inputs underlying the ON-OFF light responses of type 2 wide-field amacrine cells in TH::GFP mice. J Neurosci. 2011;31:4780–91. doi: 10.1523/JNEUROSCI.6235-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHNASWAMY A, YAMAGATA M, DUAN X, HONG YK, SANES JR. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature. 2015;524:466–70. doi: 10.1038/nature14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S, CHEN L, CHEN M, YE M, SEAL RP, ZHOU ZJ. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron. 2014;84:708–15. doi: 10.1016/j.neuron.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S, ZHANG Y, CHEN M, ZHOU ZJ. Segregated Glycine-Glutamate Co-transmission from vGluT3 Amacrine Cells to Contrast-Suppressed and Contrast-Enhanced Retinal Circuits. Neuron. 2016;90:27–34. doi: 10.1016/j.neuron.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACNEIL MA, MASLAND RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–82. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- MADISEN L, MAO T, KOCH H, ZHUO JM, BERENYI A, FUJISAWA S, HSU YW, GARCIA AJ, 3RD, GU X, ZANELLA S, KIDNEY J, GU H, MAO Y, HOOKS BM, BOYDEN ES, BUZSAKI G, RAMIREZ JM, JONES AR, SVOBODA K, HAN X, TURNER EE, ZENG H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADISEN L, ZWINGMAN TA, SUNKIN SM, OH SW, ZARIWALA HA, GU H, NG LL, PALMITER RD, HAWRYLYCZ MJ, JONES AR, LEIN ES, ZENG H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGANS CW, EL FAR O, BERNTSON A, WASSLE H, TAYLOR WR. Calcium extrusion from mammalian photoreceptor terminals. J Neurosci. 1998;18:2467–74. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLVECZKY BP, BACCUS SA, MEISTER M. Segregation of object and background motion in the retina. Nature. 2003;423:401–8. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- PARK SJ, BORGHUIS BG, RAHMANI P, ZENG Q, KIM IJ, DEMB JB. Function and Circuitry of VIP+ Interneurons in the Mouse Retina. J Neurosci. 2015;35:10685–700. doi: 10.1523/JNEUROSCI.0222-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODIECK RW. The density recovery profile: a method for the analysis of points in the plane applicable to retinal studies. Vis Neurosci. 1991;6:95–111. doi: 10.1017/s095252380001049x. [DOI] [PubMed] [Google Scholar]
- SAVITT JM, JANG SS, MU W, DAWSON VL, DAWSON TM. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci. 2005;25:6721–8. doi: 10.1523/JNEUROSCI.0760-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDELIN J, ARGANDA-CARRERAS I, FRISE E, KAYNIG V, LONGAIR M, PIETZSCH T, PREIBISCH S, RUEDEN C, SAALFELD S, SCHMID B, TINEVEZ JY, WHITE DJ, HARTENSTEIN V, ELICEIRI K, TOMANCAK P, CARDONA A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAL RP, AKIL O, YI E, WEBER CM, GRANT L, YOO J, CLAUSE A, KANDLER K, NOEBELS JL, GLOWATZKI E, LUSTIG LR, EDWARDS RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–75. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASIC B, MENON V, NGUYEN TN, KIM TK, JARSKY T, YAO Z, LEVI B, GRAY LT, SORENSEN SA, DOLBEARE T, BERTAGNOLLI D, GOLDY J, SHAPOVALOVA N, PARRY S, LEE C, SMITH K, BERNARD A, MADISEN L, SUNKIN SM, HAWRYLYCZ M, KOCH C, ZENG H. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–46. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIAN M, JARSKY T, MURPHY GJ, RIEKE F, SINGER JH. Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway. J Neurosci. 2010;30:4650–9. doi: 10.1523/JNEUROSCI.4212-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIEN NW, KIM T, KERSCHENSTEINER D. Target-Specific Glycinergic Transmission from VGluT3-Expressing Amacrine Cells Shapes Suppressive Contrast Responses in the Retina. Cell Rep. 2016;15:1369–75. doi: 10.1016/j.celrep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIEN NW, PEARSON JT, HELLER CR, DEMAS J, KERSCHENSTEINER D. Genetically Identified Suppressed-by-Contrast Retinal Ganglion Cells Reliably Signal Self-Generated Visual Stimuli. J Neurosci. 2015;35:10815–20. doi: 10.1523/JNEUROSCI.1521-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG Q, YE CP, JONES JE, ELMQUIST JK, LOWELL BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VLASITS AL, BOS R, MORRIE RD, FORTUNY C, FLANNERY JG, FELLER MB, RIVLIN-ETZION M. Visual stimulation switches the polarity of excitatory input to starburst amacrine cells. Neuron. 2014;83:1172–84. doi: 10.1016/j.neuron.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VUONG HE, PEREZ DE SEVILLA MULLER L, HARDI CN, MCMAHON DG, BRECHA NC. Heterogeneous transgene expression in the retinas of the TH-RFP, TH-Cre, TH-BAC-Cre and DAT-Cre mouse lines. Neuroscience. 2015;307:319–37. doi: 10.1016/j.neuroscience.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG YV, WEICK M, DEMB JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci. 2011;31:7670–81. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YILMAZ M, MEISTER M. Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol. 2013;23:2011–5. doi: 10.1016/j.cub.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA K, WATANABE D, ISHIKANE H, TACHIBANA M, PASTAN I, NAKANISHI S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–80. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- ZHANG Y, KIM IJ, SANES JR, MEISTER M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc Natl Acad Sci U S A. 2012;109:E2391–8. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.