Abstract
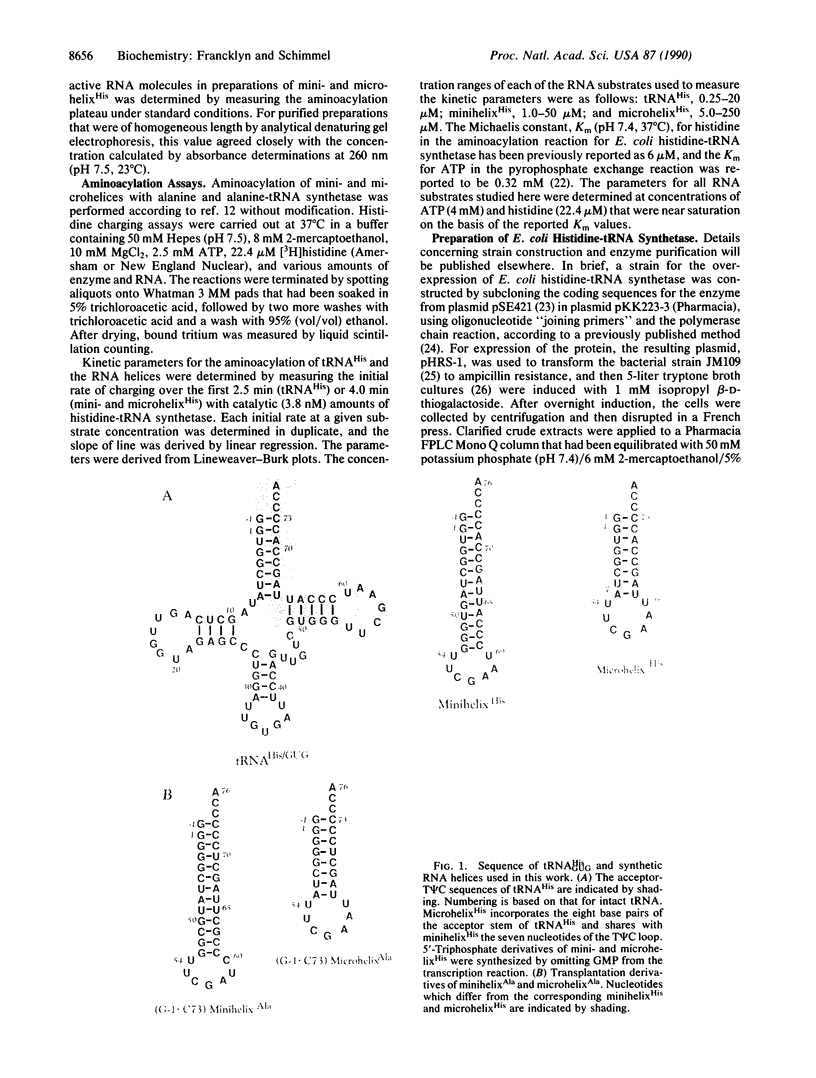
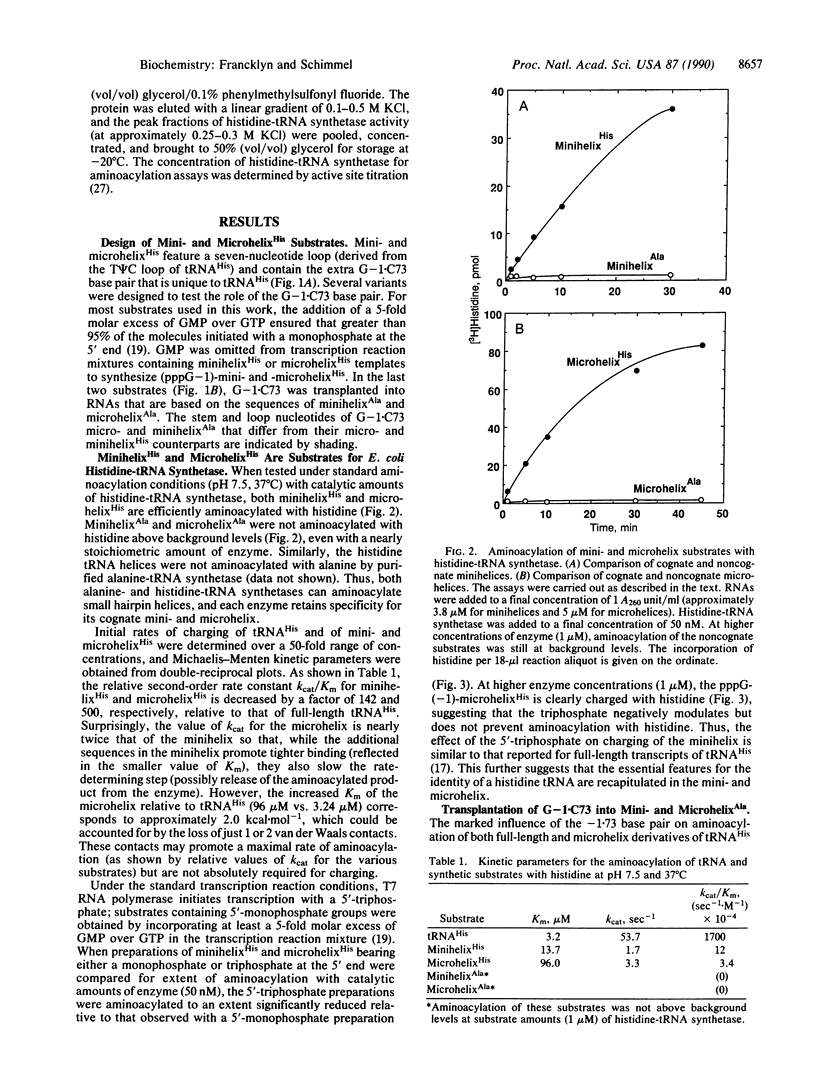
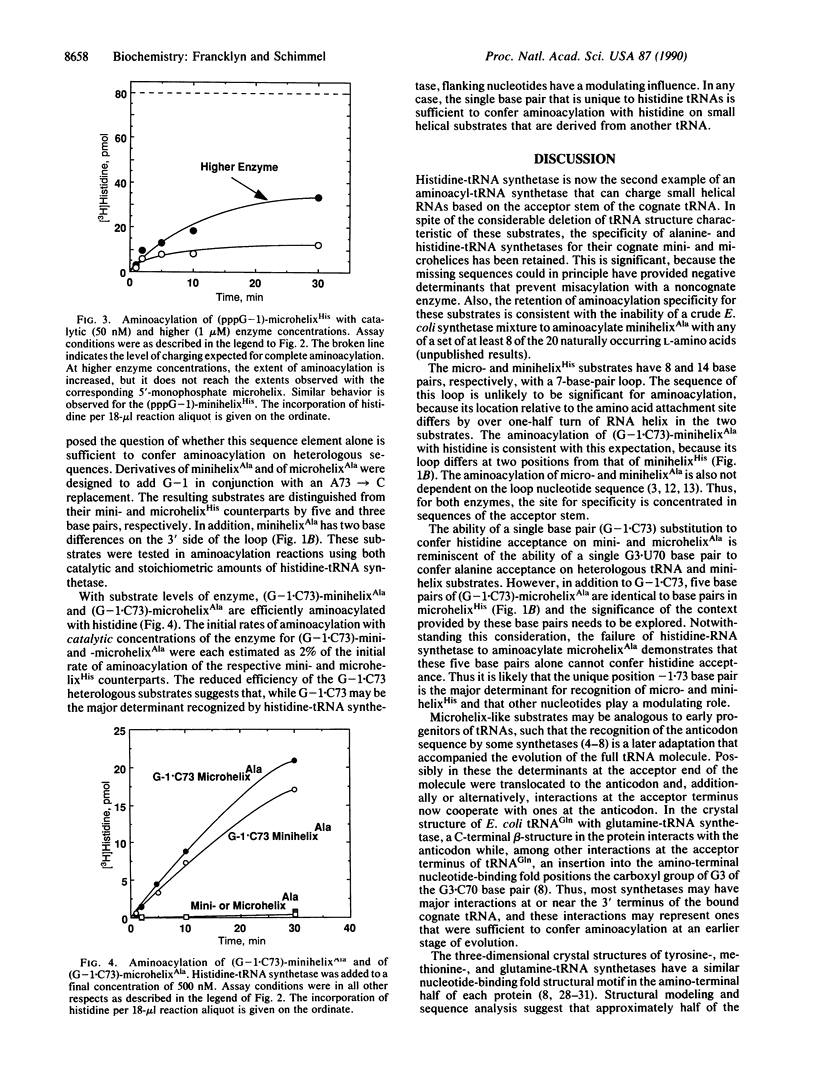
The major determinant for the identity of alanine tRNAs is a single base pair in the acceptor helix that is proximal to the site of amino acid attachment. A 7-base-pair microhelix that recreates the acceptor helix can be charged with alanine. No other examples of charging of small helices with specific amino acids have been reported, to our knowledge. We show here that a 13-base-pair and an 8-base-pair hairpin helix that reconstruct a domain and subdomain, respectively, of histidine tRNAs can be charged with histidine. We also show that transplantation of a base pair that is unique to histidine tRNAs is sufficient to consider histidine acceptance on a domain and subdomain of alanine tRNA. Both alanine and histidine aminoacyl-tRNA synthetases retain specificity for their cognate synthetic substrates. Alanine- and histidine-specific microhelices may resemble a system that arose early in the evolution of charging and coding.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat T. N., Blow D. M., Brick P., Nyborg J. Tyrosyl-tRNA synthetase forms a mononucleotide-binding fold. J Mol Biol. 1982 Jul 15;158(4):699–709. doi: 10.1016/0022-2836(82)90255-8. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Bhat T. N., Metcalfe A., Risler J. L., Brunie S., Zelwer C. Structural homology in the amino-terminal domains of two aminoacyl-tRNA synthetases. J Mol Biol. 1983 Dec 25;171(4):571–576. doi: 10.1016/0022-2836(83)90044-x. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Starzyk R. M., Schimmel P. Understanding structural relationships in proteins of unsolved three-dimensional structure. Proteins. 1990;7(2):99–111. doi: 10.1002/prot.340070202. [DOI] [PubMed] [Google Scholar]
- Cooley L., Appel B., Söll D. Post-transcriptional nucleotide addition is responsible for the formation of the 5' terminus of histidine tRNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6475–6479. doi: 10.1073/pnas.79.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbeis S. J., Parker J. The nucleotide sequence of the promoter region of hisS, the structural gene for histidyl-tRNA synthetase. Gene. 1982 May;18(2):107–114. doi: 10.1016/0378-1119(82)90108-1. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Aminoacylation of RNA minihelices with alanine. Nature. 1989 Feb 2;337(6206):478–481. doi: 10.1038/337478a0. [DOI] [PubMed] [Google Scholar]
- Freedman R., Gibson B., Donovan D., Biemann K., Eisenbeis S., Parker J., Schimmel P. Primary structure of histidine-tRNA synthetase and characterization of hisS transcripts. J Biol Chem. 1985 Aug 25;260(18):10063–10068. [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sato S., Nishimura S. Unusual CCA-stem structure of E. coli B tRNAH(His)(1). FEBS Lett. 1972 Jan 1;19(4):352–354. doi: 10.1016/0014-5793(72)80078-4. [DOI] [PubMed] [Google Scholar]
- Hill K., Schimmel P. Evidence that the 3' end of a tRNA binds to a site in the adenylate synthesis domain of an aminoacyl-tRNA synthetase. Biochemistry. 1989 Mar 21;28(6):2577–2586. doi: 10.1021/bi00432a035. [DOI] [PubMed] [Google Scholar]
- Himeno H., Hasegawa T., Ueda T., Watanabe K., Miura K., Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNA(His) in aminoacylation. Nucleic Acids Res. 1989 Oct 11;17(19):7855–7863. doi: 10.1093/nar/17.19.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature. 1988 May 12;333(6169):140–145. doi: 10.1038/333140a0. [DOI] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. Evidence that a major determinant for the identity of a transfer RNA is conserved in evolution. Biochemistry. 1989 Aug 22;28(17):6800–6804. doi: 10.1021/bi00443a003. [DOI] [PubMed] [Google Scholar]
- Kalousek F., Konigsberg W. H. Purification and characterization of histidyl transfer ribonucleic acid synthetase of Escherichia coli. Biochemistry. 1974 Feb 26;13(5):999–1006. doi: 10.1021/bi00702a026. [DOI] [PubMed] [Google Scholar]
- MacFerrin K. D., Terranova M. P., Schreiber S. L., Verdine G. L. Overproduction and dissection of proteins by the expression-cassette polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1937–1941. doi: 10.1073/pnas.87.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Changing the identity of a tRNA by introducing a G-U wobble pair near the 3' acceptor end. Science. 1988 May 6;240(4853):793–796. doi: 10.1126/science.2452483. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Uhlenbeck O. C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988 Nov 10;336(6195):179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- Normanly J., Abelson J. tRNA identity. Annu Rev Biochem. 1989;58:1029–1049. doi: 10.1146/annurev.bi.58.070189.005121. [DOI] [PubMed] [Google Scholar]
- Perret V., Garcia A., Grosjean H., Ebel J. P., Florentz C., Giegé R. Relaxation of a transfer RNA specificity by removal of modified nucleotides. Nature. 1990 Apr 19;344(6268):787–789. doi: 10.1038/344787a0. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Royal N. J., Neuman de Vegvar H., Herlihy W. C., Biemann K., Schimmel P. Primary structure of a large aminoacyl-tRNA synthetase. Science. 1981 Sep 25;213(4515):1497–1501. doi: 10.1126/science.7025207. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., DiRenzo A. B., Behlen L. S., Uhlenbeck O. C. Nucleotides in yeast tRNAPhe required for the specific recognition by its cognate synthetase. Science. 1989 Mar 10;243(4896):1363–1366. doi: 10.1126/science.2646717. [DOI] [PubMed] [Google Scholar]
- Schimmel P. Parameters for the molecular recognition of transfer RNAs. Biochemistry. 1989 Apr 4;28(7):2747–2759. doi: 10.1021/bi00433a001. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. The anticodon contains a major element of the identity of arginine transfer RNAs. Science. 1989 Dec 22;246(4937):1595–1597. doi: 10.1126/science.2688091. [DOI] [PubMed] [Google Scholar]
- Shi J. P., Francklyn C., Hill K., Schimmel P. A nucleotide that enhances the charging of RNA minihelix sequence variants with alanine. Biochemistry. 1990 Apr 17;29(15):3621–3626. doi: 10.1021/bi00467a005. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]