Abstract
Purpose
To explore published evidence on health care costs associated with adherence or persistence to antidiabetes medications in adults with type 2 diabetes mellitus (T2DM).
Methods
Primary research studies published between January 2006 and December 2015 on compliance, adherence, or persistence and treatment in patients with T2DM that document a link with health care costs were identified through literature searches in bibliographic databases and 2015 abstract books for relevant DM congresses. Results were assessed for relevance by two reviewers. The review was part of a larger overview evaluating the impact of adherence and persistence on a range of clinical and economic outcomes; only findings from the cost element are reported herein.
Results
A total of 4,662 de-duplicated abstracts were identified and 110 studies included in the wider review. Of these, 19 reported an association between adherence (n=13), persistence (n=5), or adherence and persistence (n=1), and health care costs. All studies were retrospective, with sample sizes ranging from 301 to 740,195. Medication possession ratio was the most commonly employed adherence measure (n=11). The majority of adherence studies (n=9) reported that medication adherence was associated with lower total health care costs. Pharmacy costs were often increased in adherent patients but this was offset by beneficial effects on other costs. Findings were more variable in persistence studies; three reported that higher pharmacy costs in persistent patients were not sufficiently offset by savings in other areas to result in a reduction in total health care costs.
Conclusions
Few studies have evaluated the relationship between adherence, persistence, and health care costs in T2DM. However, it has been consistently shown that medication nonadherence increases health care costs, suggesting that cost savings from better adherence could be substantial. Available data support the economic case for identification of strategies that facilitate improved medication adherence in patients with T2DM.
Keywords: adherence, persistence, type 2 diabetes mellitus, costs, review
Introduction
Type 2 diabetes mellitus (T2DM) is a highly prevalent, chronic metabolic disease with considerable public health and economic implications. Recent estimates suggest that ~415 million adults aged 20–79 years worldwide have diabetes mellitus (DM) and that the global health care expenditure for adults with DM in 2015 was US$673 billion.1
Glycemic control is crucial for prevention or minimization of disabling or even life-threatening DM-related complications. Lowering of glycated hemoglobin (HbA1c) to ≤7% has been consistently associated with a reduction in the risk of microvascular and macrovascular complications.2 In addition, improvements in glycemic control have a positive economic impact. Using the CORE Diabetes Model, it was shown that modest and achievable improvements in glycemic control generate significant reductions in the incidence and cost of microvascular complications.3 A cost avoidance of £340 million was estimated after 5 years of sustained glycemic control, increasing to £5.5 billion after 25 years for the UK.3 Despite the overwhelming evidence for the importance of glycemic control with respect to patient and economic outcomes, a review of factors influencing adherence and outcomes indicated that <50% of patients on T2DM therapies actually achieve HbA1c targets.4
Two patient behaviors play a particularly important role in the achievement of glycemic control: adherence (the extent to which a medication is taken at the prescribed doses, intervals, and frequency) and persistence (continuation of treatment for the prescribed duration).5 Nonadherence and nonpersistence to prescribed T2DM medications are, however, common and remain a barrier to optimal health outcomes. For example, a meta-analysis of 27 studies that evaluated adherence rates to T2DM medications found that only 22% of studies reported ≥80% adherence among patients.6 A systematic review of observational studies reporting persistence with oral antidiabetes drugs (OADs) in patients with T2DM revealed a mean rate of 56.2%, with discontinuation estimates of 31.4%.7 Similarly, using claims data, rates of insulin glargine persistence in the first year after initiation of ~55.0% were reported.8,9 The reasons for T2DM medication nonadherence and nonpersistence are multifactorial and include suboptimal communication between patients and providers, inadequate patient knowledge about medications, complex regimens and follow-up, and unique issues surrounding insulin use.10 Compelling evidence demonstrates that treatment adherence and persistence help to achieve glycemic control in patients with T2DM and may improve outcomes.11
Given the importance of adherence and persistence for T2DM and its outcomes, the aim of this literature review was to identify evidence on health care costs associated with adherence/persistence (or lack of) to antidiabetes medications in adults with T2DM. The review was undertaken as part of a larger overview evaluating the impact of adherence/persistence on outcomes such as glycemic control, blood glucose, mortality, quality of life, and health care resource utilization. Only the findings from the cost element of the review are reported.
Methods
A protocol was developed for the review that outlined the focus with respect to scope, patient population, appropriate study type, and outcomes of interest, and also provided details of the search strategy and data extraction methods. The protocol was developed to reduce the risk of introducing bias, and to promote transparency and accountability.
Study selection criteria
Included were English language primary research studies on compliance, adherence or persistence, and treatment in adult patients with T2DM documenting a link with health care costs that were published as journal articles from 2006 to December 2015 or presented at selected 2015 congresses. The following were excluded: those not specifically in T2DM, or in pediatric patients; those with a focus on monitoring or nondrug treatment; studies in patients with T2DM using insulin pumps; studies reporting compliance, adherence, or persistence rates, with no attempt to link these with cost outcomes. Review papers, discussion papers, letters, and editorials were also not included.
Information sources
The following databases were searched: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, and NHS Economic Evaluation Database. In addition, the following 2015 congresses were searched: International Society for Pharmacoeconomics and Outcomes Research European, International, and Latin America meetings, and annual meetings of the European Association for the Study of Diabetes and the American Diabetes Association.
Search strategy
A base-case search strategy was developed for MEDLINE (Box S1) and adapted for the other databases. The search syntax was developed to target a sample of records likely to be most relevant to the research questions and the strategy designed to retrieve records containing the key major terms for the concepts of interest. For example, the search was limited to records explicitly including T2DM terms; no searches were carried out for nonspecific DM terms. In addition, the range of terms (subject headings and text-word terms) for the concepts of adherence, persistence, and compliance was focused on these three key terms only. Finally, subject headings were searched as major descriptors only, such that the search identified just those records wherein the indexer judged the subject heading to be the major study focus.
Titles and abstracts of the search results were assessed for relevance to the research questions by two independent reviewers. Studies considered as meeting or possibly meeting the eligibility criteria were selected for further review using the full-text record. Any disagreements between reviewers were resolved by discussion until consensus was reached.
Results
Study characteristic and costs included
The wider search, which included all primary research studies documenting a link between medication compliance, adherence, or persistence with clinical, humanistic, or economic outcomes, identified 4,662 de-duplicated abstracts and 130 full-text records were reviewed for relevance (Figure 1). A total of 110 studies were finally included in the wider review and 19 studies were identified that linked adherence and/or persistence to cost (13 reported on the association between adherence and cost, 1 on adherence and persistence, and 5 on persistence alone).
Figure 1.
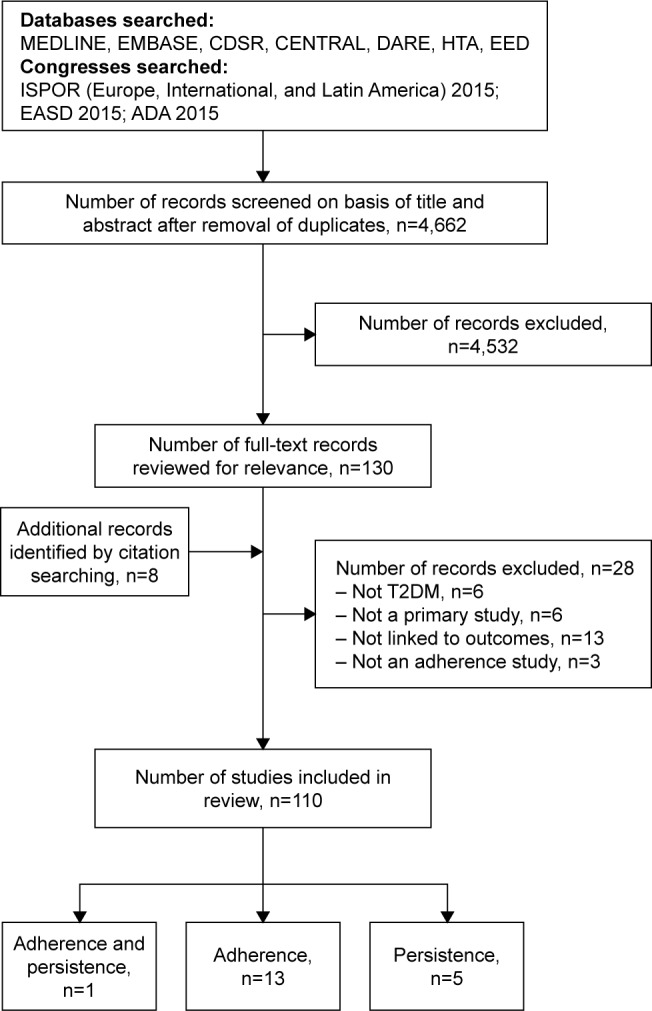
Flow chart of search results.
Abbreviations: ADA, American Diabetes Association; CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; DARE, Database of Abstracts of Reviews of Effects; EASD, European Association for the Study of Diabetes; HTA, Health Technology Assessment Database; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; EED, Economic Evaluation Database; T2DM, type 2 diabetes mellitus.
An overview of study characteristics is provided in Table 1. The 19 studies were conducted across a range of geographies, although most (n=14) came from the USA. All of the studies were retrospective and utilized data from existing sources. Sample size varied across the 19 studies, although the studies were generally large as a consequence of the data sources utilized. Mean age of included patients was largely in the range of 50–60 years, and in most studies, the proportion of men was higher than women. A number of different T2DM treatment options were considered (eg, insulin therapy [n=9], OAD [n=8], liraglutide [n=1], and insulin or OADs12 [n=1]). All the persistence studies focused on insulin therapy.
Table 1.
Characteristics of studies reporting on the link between adherence and persistence and health care costs in adult patients with T2DM
| Study | Data source | Adherence measure used | Follow-up duration | Study sample | Treatments studied | Costs reported (cost year, if provided) |
|---|---|---|---|---|---|---|
| Adherence | ||||||
| Ayyagari et al22 (USA) | IMPACT Managed Care Database supplemented with laboratory assessments | Data-driven approach (insulin fills sufficient for entire quarter)a | 1 year | n=13,428; mean age: 54 years; 54% male | Initiation of basal insulin | Hospitalization (inpatient visits),b pharmacy (2010) |
| Chandran et al10 (USA) | Truven Health MarketScan® Commercial and Medicare Supplemental databases | Patients stratified by MPR quintile: least adherent (MPR, 0–0.20) to most adherent (MPR, 0.81–1.00) | 1 year | n=32,361; mean age: 59.1 years; 52.4% male; mean MPR (pen): 0.63; MPR ≥0.80: 33% | Initiating an insulin pen with use of any OAD | Inpatient, outpatient, ER, pharmacy (2011) Total and DM-related |
| Cheng et al18 (Taiwan) | Claims data from the National Health Research Institutes of Taiwan | MPR ≥0.80 | 7 years | n=11,580; mean age: 55.6 years; 53.0% male; mean MPR: 33% (year 1); MPR ≥0.80: 55.1% | OAD | Ambulatory visits, ER, hospitalization, laboratory tests, pharmacy, patient co-pay (2009) |
| Cobden et al25 (USA) | PharMetrics claims database | MPR ≥0.80 | 2 years | n=486; mean age: 45.1 years; 56.4% male; mean MPR: 68%; MPR ≥0.80: 56.2% | Insulin vial and syringe switching to pen use | ER, outpatient, hospitalization, physician visits, pharmacyc (assumed 2005) |
| Egede et al12 (USA) | VHA National Patient Care and Pharmacy Benefits Management databases | MPR ≥0.80 | 5 years | n=740,195; mean age: 65.6 years; 97.8% male | Insulin or OAD | Inpatient, outpatient, pharmacy (2006) |
| Encinosa et al26 (USA) | MarketScan database | Nonadherence = % of days on which the patient did not possess a DM medication; calculated as 1 minus MPR | 1 year | n=56,744; mean age: 54.0 years; 53.4% male | OAD | Hospitalization (admission and ER), hospital care, pharmacy (assumed 2002) |
| Gentil et al27 (Canada) | Longitudinal Quebec Survey on Seniorsd and administrative data from RAMQ | MPR ≥0.80 | 1 year | n=301; 42.9% aged 65–74 years; 35.2% male; MPR ≥0.80: 74.4% | OAD | Hospitalization, ambulatory visits (outpatient and ER), physician fees, outpatient medications (2009 and 2010) |
| Hagen et al19 (USA) | University of Michigan Health Management Research Center | PDC ≥0.80 | 1 year | n=4,978; mean age: 53.0 years; 85.2% male; mean PDC: 0.73; PDC ≥0.80: 57% | OAD | Medical (hospitalization, ER visits, outpatient services), pharmacy, disability costs paid by employer (NR) |
| Hansen et al28 (USA) | MEDSTAT MarketScan Research databases | MPR ≥0.80 | 2 years | n=108,592; mean age: 63.0 years; 50.2% male: MPR during 2 years: 61.3%–73.8% | Monotherapy metformin, pioglitazone, or sulfonylurea | Inpatient, outpatient, pharmacy, patient out-of-pocket expenses (2005) Total and DM-related |
| Hong and Kang14 (South Korea) | Korean National Health Insurance Program database | MPR ≥0.80 | 3 years | n=40,082; mean age: 55.3 years (adherent), 51.1 years (nonadherent); 61.4% male; MPR ≥0.80: 29.4% | OAD | Costs for procedures and therapies for all diseases (including T2DM) (NR) |
| Kleinman et al15 (USA) | Human Capital Management Services Research Reference database | MPR ≥0.80 | 1 year | n=1,588; mean age: 46.5 years; 52.1% male; mean MPR: 60.7%; MPR ≥0.80: 36.5% | Insulin | Medical, pharmacy (2006) |
| Shenolikar et al29 (USA) | North Carolina Medicaid database | MPR (costs determined per 10% change) | 3 years | n=1,073; mean age: 49.5 years; 26.1% male | Initiating pioglitazone | Medical, dental (including regular check-ups), office visits, home health care, inpatient and outpatient care, long-term care facility, prescription medications (NR) Total and DM-related |
| Stuart et al16 (USA) | Medicare Current Beneficiary Surveye | PDC ≥0.80 | 2 years | n=894; mean age: NR; 41.8% male; mean PDC: 74.3%; PDC ≥0.80: 58.2% | OAD | Medical, drug (2010) |
| Persistence | ||||||
| Anderten et al13 (Germany) | Disease Analyser (IMS Health) GP database (1,072 practices) | Early discontinuation: switching to a different basal insulin or another insulin regimen within 90 days of first basal insulin prescription (index date) | 1 year preindex, 1 year postindex | n=2,976;f mean age: 56.1 years/59.1 years (glargine/NPH); 53.2%/53.7% male (glargine/NPH) | Initiating glargine or NPH | Pharmacy, medical services (eg, visit costs based on frequency and complexity, therapeutic remedies and aids, diabetes education and training, and diagnostic procedures) (NR) |
| Ascher-Svanum et al23 (USA) | Truven Health Analytics MarketScan Commercial Claims and Encounters database | Early discontinuation: gap of ≥30 days between end of one prescription and subsequent fill date | 1 year | n=73,399 Mean age: 51.0 years; 54% male | Initiating basal or insulin mix | Hospitalization, ER, outpatient, pharmacy (2011) Total and DM-related costs |
| Hadjiyianni et al24 (Japan) | Claims data from the Japan Medical Center Database | Continuers: no gaps in insulin use; interrupters: ≥1 prescription after gap (≥30 days) in insulin use; discontinuers: no prescription after ≥30-day gap | 1 year | n=827 Mean age: 50.0 years; 71% male |
Initiating basal insulin (previously insulin naïve) | Inpatient, pharmacy (assumed 2013 and 2014) |
| Perez-Nieves et al17 (USA) | OptumHealth Reporting and Insights database | Continuers/persistent users: no therapy gaps ≥30 days; interrupters: ≥1 prescription after the first ≥30-day gap; discontinuers: no prescription claims after first ≥30-day gap | 2 years | n=19,110 Mean age (across groups): 59 years; ~60% male (across groups) |
Initiating basal insulin (previously insulin naïve) | Medical, pharmacy (NR) Total and DM-related costs |
| Wei et al11 (USA) | Pooled data from three retrospective claims database studies (IMPACT database) | Discontinuation: prescription not refilled within 90th percentile of the time (stratified by metric quantity supplied) between first and second fills among patients with ≥1 refill; treatment- persistent days: number of days on treatment without discontinuation or switching; nonpersistence: patients restarting initial study drug after a period without it during follow-up | 1 year | n=4,804 Mean age: 56.0 years; 57% male |
Initiating basal insulin (glargine or detemir) Previously insulin naïve | Pharmacy, total health care costs (NR) Total and DM-related costs (DM costs included medical claims, antidiabetes medications, glucose meters, and test strips) |
| Adherence and persistence | ||||||
| Busyman et al20 (USA) | Large US health plan affiliated with Optum | Adherence: PDC ≥0.80 and MPR ≥0.80; nonpersistence: gap in therapy >90 days | 1 year | n=1,321 Mean age: 53.0 years; 51% male; mean PDC: 0.59; 34% of patients adherent |
Initiating liraglutide | Ambulatory visits, ER, inpatient and other costs, pharmacy (NR) Total and DM-related costs |
Notes:
Effective days supply of insulin associated with each claim =90th percentile of all interfill times for same quantity and insulin type; dichotomous variable for insulin adherence defined in each quarter (consecutive, nonoverlapping 90-day intervals) for each insulin depending on whether patient had insulin supply for all days in that quarter; adherent patients in a quarter were those adherent to ≥1 insulin type.
Medical costs were those paid by the health plan (no further details).
Analysis by adherence status only for all-cause health care costs; no breakdown detailed.
Survey conducted between 2005 and 2008 involving interviews with community-dwelling adults aged ≥65 years (n=2,811).
4,500 beneficiaries inducted into the survey each fall with 3 years’ follow-up; survey contains basic demographics, socioeconomic status, health insurance coverage, health status and functioning, and utilization of and payment for all medical services (reimbursed by Medicare or other payers).
Overall, 2,765 and 1,554 NPH patients identified; after propensity score matching for age, sex, DM duration, antidiabetes comedication, diabetologist care, and Charlson Comorbidity Index, 1,488 patients included in each group.
Abbreviations: DM, diabetes mellitus; ER, emergency room; GP, general practitioner; MPR, medication possession ratio; NPH, neutral protamine Hagedorn insulin; NR, not reported; OAD, oral antidiabetes drug; PDC, proportion of days covered; RAMQ, Régie de l’Assurance Maladie du Quebec (agency responsible for health plans in Quebec); T2DM, type 2 diabetes mellitus; VHA, Veterans Health Administration.
Most studies described the costs evaluated in their analyses, although the precise details varied somewhat across studies (Table 1). Pharmacy/drug/medication costs were specifically reported as being included in the analyses of all studies, 13 reported including inpatient, hospitalization, and/or emergency room (ER) costs, and outpatient or ambulatory costs were reported in 10 studies. Some studies were, however, vague about what expenses were actually considered, instead just referring to “medical” or “total” costs in their methodologies.11,13–17 Only two studies considered the costs of nonadherence outside the health care system: patient co-payments were included in one study and out-of-pocket expenses were included in another.18,19 One study included costs for short-term disability claims.19 These were based on employee salary, job type (hourly or salaried), and, if salaried, job classification (general or management). Employees on hourly contracts receiving short-term disability were paid 60% of their base-pay; general salaried employees received 100% of their salary for 3 months and 63% for up to an additional 9 months; employees in management received 100% of their salary for 6 months and 63% for up to an additional 6 months.19 It should be noted that despite describing the costs included in analyses, not all studies necessarily disaggregated individual cost elements in their results.
Measurement of adherence and persistence
Medication possession ratio (MPR) was employed in 10 studies, proportion of days covered (PDC) in two adherence studies, and both MPR and PDC were used in one study.20 MPR is generally calculated as the number of days for which the medication is supplied divided by the number of days in the study period.21 Calculation of PDC is by dividing the number of days medication available to the patient by the number of days in the follow-up period multiplied by 100 and capped at 1.21 Adherence was defined in most studies as an MPR or PDC of ≥0.80, although one study took a slightly different approach by reporting MPR as a continuous measure and stratifying patients by MPR quintile from least to most adherent.10 Using the stated days supply on a prescription fill claim for insulin as a measure of adherence can be challenging, since the dose is dependent on various factors such as body weight and disease progression; as such, one study adopted a data-driven approach and used a measure of adherence that estimated the number of days-worth of insulin in a prescription fill rather than using the reported days supply filed in medical claims.22
Persistence studies generally assessed whether there were gaps in therapy, but approaches to this varied (Table 1). Three studies defined a gap of 30 days as demonstrating nonpersistence,17,23,24 while one defined a longer gap of 90 days.13 Two studies provided a definition of “interrupters” as patients who received at least one prescription after a gap.17,24 A data-driven approach to the measurement of persistence was taken in one study, again due to the issues of using days supply encountered with injectable treatments.11
Relationship between adherence and persistence and health care costs
Studies were categorized as demonstrating that: 1) better adherence/persistence was associated with reduced health care costs; 2) there was no positive impact of greater adherence/persistence on costs; or 3) findings were variable, with some costs reduced in more adherent/persistent patients and others not affected or increased. Individual cost elements contributing to total health care costs varied from study to study (Table 1), although pharmacy costs were consistently documented as being included in the overall costs in all analyses and, where disaggregated, were found to be increased in adherent versus nonadherent patients (Table 2).
Table 2.
Key findings from studies reporting on the link between adherence and health care costs
| Study | Treatment | Key findings (adherent vs nonadherent patients) |
|---|---|---|
| Reduced overall health care costs | ||
| Chandran et al10 | Insulin pen | Significant decrease in total postindex health care costs in most vs least adherent:a US$23,839 vs US$26,310 (P=0.007); higher overall all-cause pharmacy costs in most vs least adherent: US$10,174 vs US$5,395 (P<0.001); least adherent patients had higher inpatient admissions cost vs most adherent: US$7,543 vs US$4,485 |
| Cobden et al25 | Vial/syringe switch to pen | MPR >0.80 associated with significant reductions in all-cause health care costs: OR, 0.55 (95% CI: 0.31–0.80); P<0.05 |
| Egede et al12 | OADs and insulin | Consistently higher (37% on average) pharmacy costs (US$1,762 vs US$1,132 in 2006) but lower (41% on average) inpatient costs (US$10,139 vs US$15,338 in 2006); estimated maximal incremental cost saving of US$204,530,778 if MPR increased from <0.80 to ≥0.80 |
| Encinosa et al26 | OADs | Increasing compliance from 50% to 100% increased DM drug costs by US$766 per patient but was associated with cost savings from averted hospitalizations and ER visits of US$886 (P=0.02); estimated cost offset of US$1.14 for every additional US$1.00 spent on DM drugs |
| Gentil et al27 | OAD | Lower total health care costs vs nonadherent regardless of comorbid anxiety and/or depression (adjusted cost differences:b US$11,124, with anxiety/depression and US$4,477, without; P<0.001); in patients with anxiety/depression lower total costs driven by reduced ambulatory and inpatient costs, physician fees, and medications; similar cost drivers in patients without anxiety and depression, but medication costs higher in adherent patients (US$444, P<0.001) |
| Hansen et al28 | Metformin, pioglitazone, sulfonylurea | All-cause total health care costs US$846 lower overall and in patients receiving metformin (US$336 lower), pioglitazone (US$1,140 lower), and sulfonylurea (US$1,509 lower);c significant difference in cost reductions between metformin and other drugs (P<0.05); lower costs primarily driven by reduced inpatient and outpatient care costs offsetting higher pharmacy costs |
| Hong and Kang14 | OADs | Lower health care costs in year 3 of follow-up in patients adherent for first 2 years (P<0.001);d costs decreased as MPR increased; year 3 costs lower in patients nonadherent (year 1) and adherent (year 2) vs patients nonadherent in both years 1 and 2 |
| Kleinman et al15 | Insulin | Lower medical and pharmacy costs (US$4,513 lower) and medical costs alone (US$5,110 lower) in patients with MPR =1.0 vs MPR =0.1 (P≤0.0005) who had higher prior medical spend;e estimated US$450 savings in total medical and pharmacy costs per 10% increase in MPR |
| Shenolikar et al29 | Pioglitazone | Reduction in total and DM-related health care costs with increasing adherence (2% and 4% decrease, respectively, with every 10% increase; P≤0.01) |
| No positive effect on health care costs | ||
| Cheng et al18 | OADs | Higher drug expenses (P<0.001) but lower hospitalization and ER costs (P<0.001); higher adherence associated with greater total health care costs (P<0.001); positive relationship between adherence and total costs declined at 5 years after diagnosis |
| Busyman et al20 | Liraglutide | Lower unadjusted DM-related medical costs (ambulatory, inpatient, ER, and other) (US$2,743 vs US$4,149; P=0.018) but higher mean pharmacy costs (US$6,338 vs US$3,568; P<0.001) and total health care costs (US$9,081 vs US$7,717; P=0.028) |
| Variable effect on health care costs | ||
| Ayyagari et al22 | Basal insulin | Increased pharmacy costs in adherent vs nonadherent pen or vial users (cost differences: US$2,074, vial and US$2,349, pen; P<0.001 both comparisons); no significant difference in total health care costs between adherent or nonadherent pen or vial users (cost differences: US$948, vial and US$766, pen); pharmacy costs higher for pen vs vial regardless of adherence status (P<0.001 adherent and nonadherent) |
| Hagen et al19 | OADs | Lower medical costs (US$4,627 vs US$5,974; P=0.0008) but higher pharmacy costs (US$3,155 vs US$1,668; P<0.0001) in year postindex;f total health care costs not significantly different (US$7,782 adherent vs US$7,642 nonadherent); lower short-term disability costs (P<0.0001) |
| Stuart et al16 | OADs | Significant reduction in medical costs (excluding drugs) vs nonadherence (US$3,464 and US$3,033 lower without and with adjustment for HAB, respectively; P<0.10)g and increase in drug costs (US$1,861 and US$1,374; P<0.05); total medical spend lower in adherent patients but not significant (US$1,667 and US$1,914 lower) |
Notes:
Most adherent = MPR >0.80; least adherent = MPR <0.20.
Adjusted for age, sex, marital status, education, CCI, and OAD exposure.
Adjusted for age, sex, geographic region, insurance type/origin, and major comorbidities.
Adjusted for age, sex, insurance type, medical institute, number of ambulatory care visits, comorbidities, and OAD (single or multiple).
Adjusted for employee versus spouse indicator, age, sex, CCI (with DM removed), prior medical costs, OAD use, number of non-DM medications, prior hospitalization/ER visit, employer, geographic region, index date month, co-pay per day insulin supply, glargine use indicator, and insulin MPR.
Adjusted for age, sex, CCI, and job type.
HAB occurs when other (unobserved) healthy behaviors influence adherence to treatment; two models were constructed, one that adjusted for HAB and another that did not.
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; DM, diabetes mellitus; ER, emergency room; HAB, healthy adherer bias; MPR, medication possession ratio; OAD, oral antidiabetes drug; OR, odds ratio.
Adherence studies
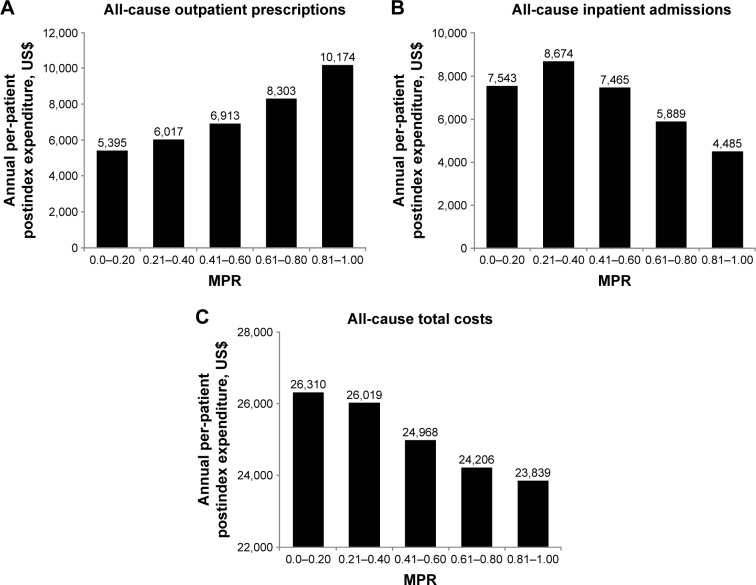
Table 2 provides an overview of adherence findings. Nine studies reported that an increase in medication adherence (insulin and/or OAD) was associated with a reduction in total health care costs.10,12,14,15,25–29 Four of these studies disaggregated cost elements and demonstrated that pharmacy costs were increased in more adherent patients, while other medical costs were reduced sufficiently to offset higher pharmacy expenses.10,12,26,28 This was clearly shown, for example, in one study that categorized patients using insulin pens into MPR quintiles from the least (MPR, 0–0.20) to the most adherent (MPR, 0.81–1.00): pharmacy costs were found to be highest in the most compared with the least adherent patients (P<0.001; Figure 2A).10 The higher pharmacy costs were, however, more than offset by substantial savings in other costs (mainly related to inpatient admissions, Figure 2B), resulting in lower total all-cause per-patient expenditure (inpatient, outpatient, ER, and pharmacy in the most versus least adherent individuals [P=0.007; Figure 2C]).10 Consistent with these findings were results from a very large (n=740,195) 5-year retrospective analysis of US veterans receiving insulin or OADs in which adherent patients had 37% higher pharmacy costs compared with nonadherent patients, but 41% lower inpatient costs (Table 2).12 Another US study also demonstrated that costs saved by averted hospitalizations and ER visits more than compensated for increases in drug spend, with a considerable cost offset realized if adherence was improved from 50% to 100% in patients with T2DM taking OADs (Table 2).26
Figure 2.
Mean postindex annual health care expenditures in insulin pen users with T2DM according to level of medication adherence.
Notes: Data from Chandran et al.10 Postindex pharmacy costs were higher in most versus least adherent patients (P<0.001), representing 43% of total costs versus 21%, respectively. Total all-cause per-patient expenditure (inpatient, outpatient, ER, and pharmacy) was 9.4% lower in most versus least adherent group (P=0.007).
Abbreviations: ER, emergency room; MPR, medication possession ratio; T2DM, type 2 diabetes mellitus.
Incremental increases in MPR were also shown to be associated with cost savings in another US study that evaluated insulin adherence.15 In this analysis, combined medical and pharmacy costs and medical costs alone were significantly lower in patients with 100% MPR and high prior medical costs than in patients with 10% MPR (P≤0.0005; Table 2); annual savings of US$450 in total medical and pharmacy costs for every 10% increase in MPR were also estimated (although a breakdown of individual cost elements included in medical expenditure was not provided).15
Reductions in health care costs associated with improved adherence appear to vary by type of OAD; all-cause total health care costs (inpatient, outpatient, pharmacy, and patient out-of-pocket expenses) were lower in patients adherent to metformin, pioglitazone, and sulfonylurea monotherapy compared with nonadherent individuals as estimated in a US retrospective cohort study.28 However, cost reductions were significantly higher in pioglitazone or sulfonylurea users versus metformin (P<0.05) (Table 2). Greater comorbidity and baseline costs were reported in patients receiving pioglitazone or sulfonylureas, suggesting a higher disease burden in these patients.28 As such, consistent therapy may have translated into greater clinical improvements. This study also found that the cost differences between adherent and nonadherent patients were not as pronounced with respect to DM-specific expenditures, indicating that the overall impact of adherence extends beyond DM care.28
Three studies reported that there was no difference in total health care costs (variously reported as including inpatient admissions, ER visits, outpatient services, and pharmacy) with better adherence.16,19,22 Pharmacy costs were significantly increased in more adherent patients across all these studies (P≤0.05; Table 2), suggesting a cost offset driven by reductions in other expenditures (individual elements not reported) (Table 2).
Total health care costs were found to be increased in adherent compared with nonadherent patients in two studies.18,20 The first of these, a 7-year longitudinal analysis of Taiwanese claims data, showed that greater adherence to OAD therapy was associated with higher total health care costs (including expenditure related to ambulatory visits, ER visits, hospitalizations, laboratory tests, pharmacy, and patient co-pay [P<0.001 vs nonadherent]).18 Higher overall costs were primarily driven by greater drug costs in adherent versus nonadherent patients (P<0.001), since lower expenses for hospitalizations and ER visits were observed in adherent individuals (P<0.001). Reasons specific to the Taiwanese health care system may account for the overall higher costs in adherent patients (eg, high accessibility, low cost sharing, and fee-for-service reimbursement).18 The relationship between adherence and overall costs was attenuated 5 years after initial T2DM diagnosis, suggesting that, given the chronicity of T2DM, long-term follow-up is required to fully understand the links between costs and medication adherence.18 In the study that measured both adherence and persistence, it was reported that patients adherent to liraglutide therapy had significantly higher total health care costs (medical and pharmacy) compared with nonadherent patients (P=0.028).20 This was a result of significantly higher pharmacy costs (P<0.001) failing to be offset by lower DM-related medical costs (consisting of ambulatory, inpatient, ER, and other costs; Table 2).
Persistence studies
Findings with respect to total health care costs were mixed in persistence studies, with four reporting variable results and one reporting no impact of persistence on costs (Table 3). In addition, the study by Busyman et al that evaluated adherence and persistence failed to demonstrate a reduction in total health care costs in association with T2DM medication persistence.20 This study estimated numerically, but not statistically significantly, higher mean total unadjusted health care costs in persistent compared with nonpersistent liraglutide patients (US$8,675 vs US$7,447; P=0.092). This result was mainly driven by a significant increase in pharmacy costs in persistent versus nonpersistent patients (US$5,571 vs US$2,931; P<0.001) that was not offset by a reduction in medical costs (ambulatory and ER visits, inpatient, and other costs) (US$3,103 vs US$4,516; P=0.047).20 Anderten et al found that, while the annual cost difference for DM-related prescriptions was lower in German patients with T2DM who persistently used insulin glargine compared with patients who switched insulin type, this failed to reach statistical significance.13 Similarly, total treatment costs (DM-related prescriptions and other medical services) were lower but not significantly different between persistent and nonpersistent insulin glargine patients. In addition, no relevant cost differences were observed between persistent and nonpersistent neutral protamine Hagedorn insulin patients.13
Table 3.
Key findings from studies reporting on the link between persistence and health care costs
| Study | Treatment | Key findings |
|---|---|---|
| No effect on health care costs | ||
| Anderten et al13 | Glargine, NPH | No significant cost differences between persistent vs nonpersistent insulin glargine patients (DM-related prescription costs, €−74; total treatment costs, €−67) or in persistent vs nonpersistent NPH insulin patients (DM-related prescription costs, €−14; total treatment costs, €21) |
| Busyman et al20 | Liraglutide | Lower unadjusted DM-related medical costs (ambulatory, inpatient, ER, and other) in persistent vs nonpersistent patients (US$3,103 vs US$4,516; P=0.047) but higher mean pharmacy costs (US$5,571 vs US$2,931; P<0.001) and total health care costs (US$8,675 vs US$7,447; P=0.092) |
| Variable effect on health care costs | ||
| Ascher-Svanum et al23 | Basal or insulin mix | Early discontinuation of basal or mixed insulin was associated with 9.6% higher acute care costs (hospitalization and ER; P<0.0001) but lower outpatient (−6.4%), DM-related medication (−42.9%), all-cause medication (−34.0%), and total health care costs (−10.9%) compared with patients who did not discontinue early (all P<0.05)a |
| Hadjiyianni et al24 | Basal insulin | Patients who continued with basal insulin treatment had lower inpatient costs vs interrupters or discontinuers (¥132,013 vs ¥225,745 [P=0.054] and ¥320,582 [P=0.036], respectively) but higher pharmacy costs (¥158,403 vs ¥134,301 [P=0.039] and ¥121,593 [P=0.002]). However, total health care costs were not different across groups |
| Perez-Nieves et al17 | Basal insulin | Lower all-cause medical costs in patients who continued basal insulin treatment (year 1) vs interrupters or discontinuers (US$10,893 vs US$13,674 and US$13,021, respectively; P≤0.022) but higher pharmacy costs (US$7,449 vs US$5,239 and US$4,857; P<0.001) and no difference in total health care costs; lower DM-related medical costs in continuers vs interrupters (US$3,207 vs US$4,547; P<0.001) but not discontinuers (US$3,779) and higher pharmacy costs vs both interrupters and discontinuers (US$3,571 vs US$2,245 and US$1,690; P<0.001); total DM-related health care costs were similar between continuers vs interrupters but significantly higher vs discontinuers (P<0.001) |
| Wei et al11 | Basal insulin (glargine or detemir) | Compared with nonpersistent patients, patients who persisted with basal insulin treatment had higher pharmacy costs (US$5,761 vs US$4,319; P<0.0001) but similar total health care costs (US$17,007 vs US$18,367); there was a significant correlation between the number of treatment persistent days and pharmacy costs (R2=0.11601; P<0.0001); noted that the time period of 1 year may be insufficient to capture the full economic benefits of treatment persistence |
Note:
After controlling for patient characteristics, index medication prescribed, general health, baseline comorbidities, resource utilization, and medication usage.
Abbreviations: DM, diabetes mellitus; ER, emergency room; NPH, neutral protamine Hagedorn insulin.
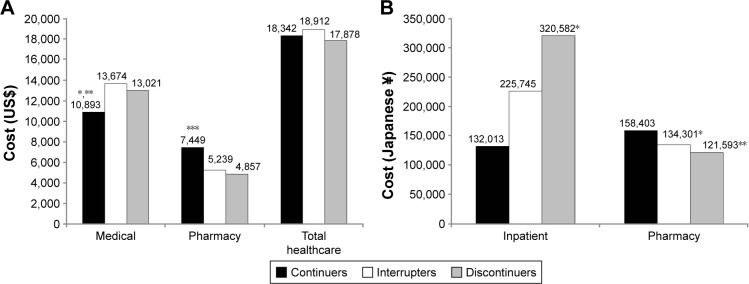
One retrospective US claims analysis demonstrated that acute care costs (hospitalization and ER visits) were lower in persistent patients compared with those who discontinued basal insulin therapy, although total health care costs (hospitalization, ER, outpatient, and pharmacy) were 10.9% higher (Table 3).23 Increased pharmacy costs were offset by savings in other areas in three of the other persistence studies, although not enough to result in a reduction in total health care costs in more persistent patients.11,17,24 One US claims analysis reported lower all-cause medical costs (costs not disaggregated) in the first year after treatment initiation in patients who continued basal insulin compared with interrupters or discontinuers (P≤0.022; Figure 3A), but the higher pharmacy costs also incurred by this group resulted in no difference in total health care costs (medical plus pharmacy) between patient cohorts.17 Using Japanese claims data, it was also shown that higher pharmacy expenditures in patients with T2DM who continued basal insulin therapy were not completely offset by significant reductions in inpatient costs reported in this group compared with interrupters or discontinuers (P≤0.039; Figure 3B).24 As such, total health care costs were not significantly different between persistent patients and those who interrupted or discontinued insulin. A pooled analysis of three previously published US retrospective studies also found that persistent users of basal insulin incurred significantly higher pharmacy costs (P<0.0001) but similar total health care costs (Table 3).11 In addition, this study reported a significant correlation between the number of treatment-persistent days and pharmacy costs (R2=0.116; P<0.0001).11
Figure 3.
Impact of treatment persistence on health care costs in patients initiating basal insulin.
Notes: (A) Health care costs in previously insulin naïve patients with T2DM initiating basal insulin (retrospective cohort study using US claims data); continuers were patients having no gap between insulin prescriptions, interrupters had one or more prescriptions after a gap (≥30 days), and discontinuers had no prescription after a ≥30-day gap; *P=0.022 versus discontinuers, **P<0.001 versus interrupters, ***P<0.001 versus interrupters and discontinuers. Data from Perez-Nieves et al.17 (B) Health care costs in previously insulin-naïve patients with T2DM initiating basal insulin (retrospective longitudinal analysis of Japanese claims data);24 continuers were patients having no gap between insulin prescriptions, interrupters had one or more prescriptions after a gap (≥30 days), and discontinuers had no prescription after a ≥30-day gap; *P<0.04 versus continuers; **P=0.02 versus continuers. Data from Hadjiyianni et al.24
Abbreviation: T2DM, type 2 diabetes mellitus.
Disability costs
Employees with DM are more likely to be disabled and report lower productivity. Determination of the relationship between medication adherence and disability costs is, therefore, important for employers seeking to contain such expenditure.19 One study reported on disability costs borne by employers in patients with T2DM receiving OADs.19 Significantly fewer adherent patients (PDC ≥0.80) made a short-term disability claim compared with nonadherent individuals (16.0% vs 21.5%; P<0.0001), resulting in lower disability costs in the year following the first prescription (US$1,161 vs US$1,840 for adherent and nonadherent patients, respectively; P<0.0001).
Discussion
Determination of the economic burden of chronic diseases involves evaluation of more than just the patients’ total medical costs; it is suggested that a distinction should be made between expected and unexpected costs.23 Expected costs are those incurred in the support of comprehensive and continuous care such as outpatient care, medications, tests, and monitoring, which are a reflection of a patient’s engagement in their treatment. Unexpected costs, such as those associated with hospitalizations or ER visits, are, however, potentially modifiable and avoidable, and are often linked with poorer outcomes in the long term. The studies identified in this review clearly indicate that medication adherence and persistence have a considerable impact on health care costs in adult patients with T2DM. Generally, better adherence or persistence resulted in lower overall health care costs, which was mainly driven by beneficial effects on unexpected costs associated with hospitalization and ER visits. This was shown across different therapeutic approaches (insulin and/or OADs) and patient populations. Although an evaluation of the effects of adherence on glycemic control is outside the scope of this review, it is likely that the findings reflect improved disease control achieved through better medication adherence or persistence, which reduces the risk of complications and the consequent need for medical services. In contrast, patients who exhibited good adherence or persistence with medication were mostly reported to have increased pharmacy costs, which is not unexpected given that these are the individuals who, unlike nonadherent or nonpersistent patients, consistently take their medications as prescribed and continue to fill prescriptions as recommended. Several studies demonstrated that the beneficial effects of good adherence on medical costs such as hospitalization were sufficient to offset increases in drug expenditure such that total health care costs were either not significantly different between adherent and nonadherent patients11,16,17,19,22,24 or were significantly reduced.10,12,26,28 Rarely were total health care costs reported to be increased in adherent or persistent patients; and where this was the case, the significantly higher pharmacy costs were cited as the primary driver.18,20
The current review updates and expands upon the findings of previous reviews on this subject, which also concluded that better adherence is associated with lower health care costs.30–33 One study that was included in previous reviews but excluded from this on account of failure to meet search eligibility criteria does warrant mention. Clinical and economic outcomes were examined in a large national cohort of patients (n=135,639) identified from a US managed care company database.34 In patients whose adherence level increased (change in MPR from <0.80 to ≥0.80 during follow-up), the risk of hospitalization or ER visits declined by 13% to realize national annual cost savings of US$4.68 billion annually.34 Furthermore, it was estimated that eliminating loss of adherence (which occurred in 25% of the patient sample) would lead to an additional saving of US$3.61 billion.34
The evidence base in this area of research continues to grow and further studies relevant to the current review have been published after the search cut-off date. For example, Boye et al recently published an analysis of the associations between adherence to glucose-lowering agents and outcomes in older T2DM patients (≥65 years), an important population to study given that the rate of DM in this group is twice that in the overall adult population.35 A reduction in outpatient and acute care costs was determined with increasing medication adherence (US$10,788 and US$18,967, respectively, from least [PDC <20%] to most [PDC ≥80%] adherent patients; P<0.005).35 Consequently, a comparison of the least and most adherent patients was associated with total all-cause cost savings of US$28,824 over the 3-year study period. Furthermore, the study estimated savings of US$65,464 over 3 years for every 1% increase in adherence per 1,000 patients.35
Collectively, the studies included in this review and those in the broader literature highlight the importance of adherence and persistence with respect to clinical outcomes and economics. Indeed, medication adherence is now included in the Health care Effectiveness Data Information Set, one of the metrics used to assess US health plan quality and performance in the Centers for Medicare and Medicaid Services star ratings.21 It is, perhaps, then concerning that rates of medication adherence and persistence continue to be suboptimal. For example, some of the studies included herein found very low adherence rates among their patient samples: Hong and Kang reported that only 29.4% of patients receiving OADs had an MPR ≥0.80,14 and Chandran et al found that only 33% of insulin pen users were adherent (MPR ≥0.80).10 Given these observations, there is a clear and continued need for strategies that improve medication adherence in patients with T2DM. Numerous strategies, including telephone interventions by a health care provider, pharmacist-led interventions, health coaching and other educational interventions, integrated care managers, and HbA1c point-of-care testing, have been documented in the literature but with varying effectiveness.33,36 A recent systematic review evaluating interventions for DM medication adherence improvement concluded that telephone interventions performed by nurses, pharmacists, and other health care professionals did result in greater adherence, and that patients were also more likely to take medication when laboratory results were available through point-of-care HbA1c testing.33
With the increasing use of smartphones and the internet, the opportunity to use digital technology to self-manage DM has grown exponentially. As a consequence, numerous mobile applications are now available, many of which are focused on medication adherence.37 Adherence applications include MyMedSchedule, MyMeds, MedSimple, MedAgenda, PillManager, and RxmindMe Prescription,37 but there appear to be few analyses of their real-world effectiveness in improving adherence in patients with DM or other chronic diseases.38 Evidence for the effectiveness of medication reminders using a short message service (SMS, text messaging) in the improvement of DM adherence has, however, been published.38,39 This may represent a promising approach since it does not require any extensive investment of health care provider time and can be easily integrated into the daily lives of patients, although its long-term effectiveness is currently unknown.40
There is also evidence that nonadherence and nonpersistence are linked to regimen complexity in chronic diseases including DM.41 More frequent dosing of OADs or insulin has been correlated with poor adherence,42,43 and findings from the Global Attitudes of Patients and Physicians in Insulin Therapy study indicate that number of insulin injections and requirement for dosing at specific times are among the most commonly reported difficulties associated with insulin therapy.44
The current review highlighted several gaps in the evidence base regarding T2DM adherence and impact on health care costs. In particular, the lack of data from countries outside the USA makes it difficult to assess how the structure of local health care systems may impact adherence: more studies are needed in diverse geographies using consistent methodologies. Future studies also need to collect data over the longer term, as the impact of both adherence and persistence on clinical and economic outcomes may take time to manifest. Patients with T2DM are likely to be taking a range of different medications and it will be important for future studies to design methods for measuring adherence or persistence in such patients, as this is likely to vary between therapies. Little data appeared to be available regarding nonadherence costs that fall outside of health care budgets, such as social and community care, and patient out-of-pocket expenses. In addition, costs falling on employers were seldom reported and only one study assessed short-term disability costs.19 Thus, the severity and scope of the nonadherence problem may be underestimated; future studies should attempt to describe such costs.19
A number of limitations are associated with the evidence base explored in the current review. For example, identified studies varied widely with respect to methodologies, measurement of adherence or persistence, patient populations, and treatments, making direct comparisons between them impractical. Given the range of different treatment options evaluated, it was clear that patients are at different points in the disease continuum across studies and ranged from the newly diagnosed14 to those being initiated or established on insulin.17,25 This has important cost implications, since those with T2DM of longer duration are likely to experience more complications and thus exert a greater economic burden. Where reported, adjustment of analyses for confounding factors varied considerably, and it is possible that some studies may have failed to adjust the results for important measured and nonmeasured confounders.
The vast majority of included studies relied heavily on administrative claims data. While this kind of information has several advantages, the data are primarily collected for payment purposes and not for research, and may be subject to coding errors. In addition, the data cannot be used to determine causality and can only designate whether a prescription was filled, not if the medication was actually taken appropriately. Furthermore, the patient sample from which the data are derived may not be representative of the wider disease population. It has also been suggested that claims data may provide an unreliable basis for determining whether good medication adherence really does save payers money due to the issue of healthy adherer bias (HAB), which is generally not controlled for in published analyses.16 It is possible that drug adherence is correlated with other unobserved healthy behaviors and that some proportion of any apparent savings could be due to these behaviors. In this case, an overestimation of the cost savings associated with increased adherence would result. While this caveat should be acknowledged, one of the included studies demonstrated that even when HAB was controlled for, medical costs decreased and pharmacy costs increased in more adherent individuals.16 It is also important to recognize the difference between claims data and cost data. Cost of illness is calculated from charge data obtained from claims databases, but these data often do not accurately reflect the underlying costs or the rising costs of replacing and updating medical equipment. Most insurers negotiate reimbursement rates and receive substantial discounts on listed charges, with the implication that studies using charges can overestimate the direct cost of an illness.
Another issue inherent with administrative claims data is specifically related to the measurement of insulin adherence. Use of adherence or persistence measures that rely on the typical 30-day supply rule employed with oral medications fails to account for the wide variations in insulin dosing requirements across individual patients, and so can provide biased estimates of true adherence or persistence.11,22 Two studies reviewed herein used more data-driven approaches to measure insulin adherence and persistence, but it should be noted that even these may only provide an approximation of the true situation. As such, development of different methods for the analysis of insulin adherence and persistence in real-world clinical settings is required.
Limitations
The current review itself is also subject to certain limitations: searches were limited to the English language and to records that explicitly included T2DM terms, with searches not being undertaken for nonspecific DM terms; for the subject headings, only major descriptors were searched for (records where the subject heading is a major focus of the study as judged by the indexer). Given these limitations, it cannot be ruled out that other studies relevant to the research question have been published.
Conclusion
Despite considerable research into medication adherence in patients with T2DM, few studies have definitively evaluated the relationship between adherence, persistence, and health care costs. However, it has been consistently shown that nonadherence to T2DM medications increases health care costs and, while pharmacy costs are higher in more adherent patients, these are generally offset by savings in other areas such as inpatient expenses and costs of ER visits. The potential cost savings from increased T2DM medication adherence appear to be substantial and targeting poor adherence provides an opportunity to simultaneously improve health outcomes and reduce spending. Collectively, the findings from studies identified herein support the economic case for action from the medical community to identify strategies and technologies that can facilitate improved medication adherence in patients with T2DM, particularly in light of its growing global prevalence.
Supplementary material
MEDLINE search strategy
| S1 | MJMESH.EXACT.EXPLODE (“Diabetes Mellitus, Type 2”) | 75,500* |
| S2 | TI,AB,IF ((“maturity onset” or “mature onset” or “adult onset” or “non insulin dependent” or “non insulin responsive” or “noninsulin dependent” or “noninsulin responsive” or “insulin independent” or “type ii” or “type 2” or “type two” or stable or “ketosis resistant” or “keto resistant” or “slow onset” or “late onset” or lipoatrophic) near/5 (diabet* or dm)) | 109,113* |
| S3 | TI,AB,IF (dm2 or “dm-2” or t2dm or “t2-dm” or t2d or “t2-d” or niddm or iidm) | 22,156* |
| S4 | S1 or S2 or S3 | 128,259* |
| S5 | MJMESH.EXACT.EXPLODE (“Patient Compliance”) | 25,689* |
| S6 | TI,AB,IF (complian* or noncomplian* or comply* or noncomply* or complie*2 or noncomplie*2) | 143,820* |
| S7 | TI,AB,IF (adher* or nonadher*) | 160,970* |
| S8 | TI,AB,IF (persist* or nonpersist*) | 364,539* |
| S9 | S5 or S6 or S7 or S8 | 642,794* |
| S10 | S4 and S9 | 6,386* |
| S11 | MESH.EXACT.EXPLODE (“Animals”) not MESH.EXACT (“Humans”) | 4,076,479* |
| S12 | DTYPE (comment or editorial or letter or “case reports”) | 3,019,599* |
| S13 | TI (“case report”) | 177,148* |
| S14 | S10 not (S11 or S12 or S13) | 5,830* |
| S15 | (S10 not (S11 or S12 or S13)) AND la.exact (“ENG”) | 5,381* |
| S16 | PD (2006–2016) | 8,771,828* |
| S17 | S15 and S16 | 3,763° |
Notes:
Duplicates are removed from the search, but included in the result count.
Duplicates are removed from the search and from the result count.
Acknowledgments
The authors thank Mick Arber (York Health Economic Consortium [YHEC]) for assistance with the literature searches, and Susan Robinson, PhD, for assistance with writing and editing.
Footnotes
Author contributions
KSB participated in conception of the work and the critical revision of the manuscript. XP contributed to the design of literature review and super vised the work. TKM was commissioned to undertake the literature review. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosures
KSB is an employee and shareholder of Eli Lilly and Company. XP is an employee and shareholder of Eli Lilly and Company. The authors report no other conflicts of interest in this work.
References
- 1.International Diabetes Federation (IDF) IDF Diabetes Atlas. 7th ed. 2015. [Accessed September 21, 2016]. Available from: http://www.diabetesatlas.org/
- 2.American Diabetes Association Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39(Suppl 1):S1–S119. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 3.Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with Type 1 and Type 2 diabetes on the number of clinical complications, and the associated financial benefit. Diabet Med. 2016;33(11):1575–1581. doi: 10.1111/dme.13062. [DOI] [PubMed] [Google Scholar]
- 4.García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 6.Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32(6):725–737. doi: 10.1111/dme.12651. [DOI] [PubMed] [Google Scholar]
- 7.Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283–1296. doi: 10.1185/03007995.2015.1053048. [DOI] [PubMed] [Google Scholar]
- 8.Baser O, Tangirala K, Wei W, Xie L. Real-world outcomes of initiating insulin glargine-based treatment versus premixed analog insulins among US patients with type 2 diabetes failing oral antidiabetic drugs. Clinicoecon Outcomes Res. 2013;5:497–505. doi: 10.2147/CEOR.S49279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Wei W, Miao R, Xie L, Baser O. Real-world outcomes of US employees with type 2 diabetes mellitus treated with insulin glargine or neutral protamine Hagedorn insulin: a comparative retrospective database study. BMJ Open. 2013;3(4):e002348. doi: 10.1136/bmjopen-2012-002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandran A, Bonafede MK, Nigam S, Saltiel-Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148–158. [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20(1):52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 12.Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35(12):2533–2539. doi: 10.2337/dc12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderten H, Dippel FW, Kostev K. Early discontinuation and related treatment costs after initiation of Basal insulin in type 2 diabetes patients: a German primary care database analysis. J Diabetes Sci Technol. 2015;9(3):644–650. doi: 10.1177/1932296814566232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong JS, Kang HC. Relationship between oral antihyperglycemic medication adherence and hospitalization, mortality, and healthcare costs in adult ambulatory care patients with type 2 diabetes in South Korea. Med Care. 2011;49(4):378–384. doi: 10.1097/MLR.0b013e31820292d1. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman NL, Schaneman JL, Lynch WD. The association of insulin medication possession ratio, use of insulin glargine, and health benefit costs in employees and spouses with type 2 diabetes. J Occup Environ Med. 2008;50(12):1386–1393. doi: 10.1097/JOM.0b013e3181875e9b. [DOI] [PubMed] [Google Scholar]
- 16.Stuart BC, Dai M, Xu J, Loh FH, S Dougherty J. Does good medication adherence really save payers money? Med Care. 2015;53(6):517–523. doi: 10.1097/MLR.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Nieves M, Kabul S, Desai U, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US. Curr Med Res Opin. 2016;32(4):669–680. doi: 10.1185/03007995.2015.1135789. [DOI] [PubMed] [Google Scholar]
- 18.Cheng SH, Chen CC, Tseng CH. Does medication adherence lead to lower healthcare expenses for patients with diabetes? Am J Manag Care. 2013;19(8):662–670. [PubMed] [Google Scholar]
- 19.Hagen SE, Wright DW, Finch R, Talamonti WJ, Edington DW. Impact of compliance to oral hypoglycemic agents on short-term disability costs in an employer population. Popul Health Manag. 2014;17(1):35–41. doi: 10.1089/pop.2013.0009. [DOI] [PubMed] [Google Scholar]
- 20.Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–355. doi: 10.1007/s12325-015-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46(11):1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]
- 22.Ayyagari R, Wei W, Cheng D, Pan C, Signorovitch J, Wu EQ. Effect of adherence and insulin delivery system on clinical and economic outcomes among patients with type 2 diabetes initiating insulin treatment. Value Health. 2015;18(2):198–205. doi: 10.1016/j.jval.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Ascher-Svanum H, Lage MJ, Perez-Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):225–242. doi: 10.1007/s13300-014-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjiyianni I, Desai U, Ivanova JI, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in Japan. Value Health. 2015;18(7):A611–A612. [Google Scholar]
- 25.Cobden D, Lee WC, Balu S, Joshi AV, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(7):948–962. doi: 10.1592/phco.27.7.948. [DOI] [PubMed] [Google Scholar]
- 26.Encinosa WE, Bernard D, Dor A. Does prescription drug adherence reduce hospitalizations and costs? The case of diabetes. Adv Health Econ Health Serv Res. 2010;22:151–173. doi: 10.1108/s0731-2199(2010)0000022010. [DOI] [PubMed] [Google Scholar]
- 27.Gentil L, Vasiliadis HM, Préville M, Berbiche D. Adherence to oral antihyperglycemic agents among older adults with mental disorders and its effect on health care costs, Quebec, Canada, 2005–2008. Prev Chronic Dis. 2015;12:E230. doi: 10.5888/pcd12.150412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen RA, Farley JF, Droege M, Maciejewski ML. A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clin Ther. 2010;32(7):1308–1319. doi: 10.1016/j.clinthera.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Shenolikar RA, Balkrishnan R, Camacho FT, Whitmire JT, Anderson RT. Comparison of medication adherence and associated healthcare costs after introduction of pioglitazone treatment in African Americans versus all other races in patients with type 2 diabetes mellitus: a retrospective data analysis. Clin Ther. 2006;28(8):1199–1207. doi: 10.1016/j.clinthera.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Breitscheidel L, Stamenitis S, Dippel FW, Schöffski O. Economic impact of compliance to treatment with antidiabetes medication in type 2 diabetes mellitus: a review paper. J Med Econ. 2010;13(1):8–15. doi: 10.3111/13696990903479199. [DOI] [PubMed] [Google Scholar]
- 31.Salas M, Hughes D, Zuluaga A, Vardeva K, Lebmeier M. Costs of medication nonadherence in patients with diabetes mellitus: a systematic review and critical analysis of the literature. Value Health. 2009;12(6):915–922. doi: 10.1111/j.1524-4733.2009.00539.x. [DOI] [PubMed] [Google Scholar]
- 32.Banerji MA, Dunn JD. Impact of glycemic control on healthcare resource utilization and costs of type 2 diabetes: current and future pharmacologic approaches to improving outcomes. Am Health Drug Benefits. 2013;6(7):382–392. [PMC free article] [PubMed] [Google Scholar]
- 33.Capoccia K, Odegard PS, Letassy N. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ. 2016;42(1):34–71. doi: 10.1177/0145721715619038. [DOI] [PubMed] [Google Scholar]
- 34.Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood) 2012;31(8):1836–1846. doi: 10.1377/hlthaff.2011.1198. [DOI] [PubMed] [Google Scholar]
- 35.Boye KS, Curtis S, Lage M, Garcia-Perez LE. Associations between adherence and outcomes among older, type 2 diabetes patients: evidence from a Medicare Supplemental database. Patient Prefer Adherence. 2016;16:1573–1581. doi: 10.2147/PPA.S107543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zullig LL, Gellad WF, Moaddeb J, et al. Improving diabetes medication adherence: successful, scalable interventions. Patient Prefer Adherence. 2015;9:139–149. doi: 10.2147/PPA.S69651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah VN, Garg SK. Managing diabetes in the digital age. Clin Diabetes Endocrinol. 2015;1:16. doi: 10.1186/s40842-015-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc. 2013;53(2):172–181. doi: 10.1331/JAPhA.2013.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81(9):594–604. doi: 10.1016/j.ijmedinf.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Vervloet M, Linn AJ, van Weert JCM, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31(3):213–224. doi: 10.1007/s10865-007-9147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19(4):279–284. doi: 10.1046/j.1464-5491.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly LA, Morris AD, Evans JM, DARTS/MEMO collaboration Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM. 2007;100(6):345–350. doi: 10.1093/qjmed/hcm031. [DOI] [PubMed] [Google Scholar]
- 44.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Factors associated with injection omission/non-adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab. 2012;14(12):1081–1087. doi: 10.1111/j.1463-1326.2012.01636.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
| S1 | MJMESH.EXACT.EXPLODE (“Diabetes Mellitus, Type 2”) | 75,500* |
| S2 | TI,AB,IF ((“maturity onset” or “mature onset” or “adult onset” or “non insulin dependent” or “non insulin responsive” or “noninsulin dependent” or “noninsulin responsive” or “insulin independent” or “type ii” or “type 2” or “type two” or stable or “ketosis resistant” or “keto resistant” or “slow onset” or “late onset” or lipoatrophic) near/5 (diabet* or dm)) | 109,113* |
| S3 | TI,AB,IF (dm2 or “dm-2” or t2dm or “t2-dm” or t2d or “t2-d” or niddm or iidm) | 22,156* |
| S4 | S1 or S2 or S3 | 128,259* |
| S5 | MJMESH.EXACT.EXPLODE (“Patient Compliance”) | 25,689* |
| S6 | TI,AB,IF (complian* or noncomplian* or comply* or noncomply* or complie*2 or noncomplie*2) | 143,820* |
| S7 | TI,AB,IF (adher* or nonadher*) | 160,970* |
| S8 | TI,AB,IF (persist* or nonpersist*) | 364,539* |
| S9 | S5 or S6 or S7 or S8 | 642,794* |
| S10 | S4 and S9 | 6,386* |
| S11 | MESH.EXACT.EXPLODE (“Animals”) not MESH.EXACT (“Humans”) | 4,076,479* |
| S12 | DTYPE (comment or editorial or letter or “case reports”) | 3,019,599* |
| S13 | TI (“case report”) | 177,148* |
| S14 | S10 not (S11 or S12 or S13) | 5,830* |
| S15 | (S10 not (S11 or S12 or S13)) AND la.exact (“ENG”) | 5,381* |
| S16 | PD (2006–2016) | 8,771,828* |
| S17 | S15 and S16 | 3,763° |
Notes:
Duplicates are removed from the search, but included in the result count.
Duplicates are removed from the search and from the result count.