Abstract
Purpose
Retinoblastoma is a rare malignancy in developing retina tissue in children with limited therapeutic options. Here we sought to investigate the potential clinical value of genistein, the phytoestrogen derived from the soybean with antioxidant activity, in this disease.
Methods
Retinoblastoma cells were treated with genistein. Colony formation capacity was measured with soft agar assay. MiRNA was identified with microarray. Post-transcriptional regulation of gene expression was determined with dual-luciferase reporter assay. Cell proliferation and apoptosis were measured with the Cell Counting Kit-8 (CCK-8) method and annexin V-propidium iodide (PI) staining. The xenograft model was administered with genistein, and tumor growth was monitored.
Results
The results showed that genistein treatment significantly suppressed proliferation and anchorage-independent growth of the human retinoblastoma cell line Y79 in vitro, which partially attributed to apoptosis induction. MicroRNA array screening identified that miR-145 was upregulated by genistein. Through post-transcriptional regulation of ABCE1, miR-145 functioned as a key downstream effector in genistein-mediated tumor suppression in retinoblastoma. Moreover, the in vivo data consolidated the inhibitory effect of genistein against retinoblastoma xenograft via upregulation of miR-145.
Conclusions
The data highlighted the therapeutic potency of genistein in this disease and showed that further clinical investigation is warranted.
Introduction
Retinoblastoma is a rare pediatric malignant tumor in the developing retina in children usually under 5 years old [1]. Occurrence of hereditary retinoblastoma is intimately associated with biallelic genetic or epigenetic inactivation of the retinoblastoma tumor susceptibility gene (RB1; Gene ID: 5925; OMIM: 614041) [2]. Somatic amplification of the MYCN oncogene (Gene ID: 4613; OMIM: 164840) accounts for some cases of sporadic, early-onset, aggressive, unilateral retinoblastoma [3]. Although the mortality associated with retinoblastoma is relatively low, most children who survive may lose their vision. Clinically, retinoblastoma is diagnosed with indirect ophthalmoscopy examination, and risk is evaluated by family history of retinoblastoma and detection of pathogenic aberrance in RB1 [4].
Early diagnosis and intervention can significantly reduce mortality and increase longevity, which is greatly limited by local medical conditions. Treatment options vary and largely depend on tumor stage, pathology, and genetic background [5]. Cryotherapy, chemotherapy, radiotherapy, and surgery are well-established standard treatments for retinoblastoma. Enucleation is considered only for late stage and recurrent tumors with little or no likelihood of restoration of vision. Cryotherapy induces secondary thrombosis and infarction in the vasculature of the tumor tissue by quick freezing, which is appropriate for the treatment of small primary and recurrent tumors [6]. Although retinoblastoma is sensitive to radiotherapy, the clinical practice is greatly limited by the associated risk in cosmetic deformity and subsequent neoplasms in patients with heritable retinoblastoma [7]. Chemotherapy, especially systematic and intravitreal modalities, has become the forefront of treatment within the past decade, which has shown promising and favorable clinical outcomes in several retrospective studies [8]. However, the intrinsic cytotoxicity and ensuing resistance undermine the therapeutic value of chemotherapy. Moreover, clinically successful target drugs widely used in other human cancers are still missing in retinoblastomas despite the well-acknowledged etiology of this disease. Therefore, novel and safer medicine is still urgently needed.
Genistein is a phytoestrogen derived from soy with diverse biologic activities [9]. In addition to antioxidant and anti-inflammatory effects, genistein acting as a preventative and therapeutic agent has received increasing attention [10]. In comparison with chemical agents, the soy-extracted genistein shows less toxicity, and antitumor potential has been investigated in various human cancers in vitro and in vivo. However, the therapeutic value of genistein in retinoblastomas has not been explored thus far. Therefore, we sought to evaluate the clinical potential of genistein in a retinoblastoma in vitro cell culture and in vivo mouse xenograft model.
Methods
Cell culture
The human embryonic kidney (HEK) 293T and retinoblastoma cell line Y79 were obtained from and authenticated by American Type Culture Collection (ATCC). Identification of the cell lines was performed for a gender-associated marker, amelogenin, and different short tandem repeats were amplified by using a commercial kit (EX20, AGCU ScienTech, Wuxi, China). Data were analyzed on an ABI Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA). The results for STR analysis are shown in Appendix 1. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) and 1% penicillin and streptomycin and cultured in 37 °C humidified incubator with 5% CO2. For treatment, equal volume of either dimethyl sulfoxide (DMSO) or genistein (purity ≥98%, a final concentration of 50 µM dissolved in DMSO, Sigma, St. Louis, MO) was added and incubated for 24 h.
CCK-8 assay
Cell proliferation was determined with a commercial Cell Counting Kit-8 (CCK-8) kit following the manufacturer’s instructions. Briefly, the appropriate number of cells was seeded in a 96-well plate and subjected to the indicated treatment. Ten microliters of CCK-8 solution were added to each well, and the chromogenic reaction was allowed for 1–4 h at 37 °C. Absorbance at 450 nm was measured with a microplate reader, and the relative cell viability was calculated.
MicroRNA array analysis
The total RNA from cells with the indicated treatment was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA), and the quality and quantity were determined first. Affymetrix GeneChip miRNA 4.0 array (Thermo Fisher, Waltham, MA) processing was performed following the manufacturer’s instructions. Briefly, 1 µg RNA was labeled with the FlashTag Biotin HSR RNA Labeling kit (Affymetrix) and hybridized to the microarray in accordance with the provider’s standard protocol. In total, 2,578 mature human miRNAs were included along with miRNAs of other organisms. After washing and staining with the Affymetrix GeneChip Hybridization Wash and Stain Kit, the chips were scanned with the Affymetrix GeneChip Scanner 3000. The relative signal intensity was computed with Affymetrix GeneChip Command Console software (Affymetrix).
Establishment of stable cell lines
Either anti-miR-145 or scramble sequence (anti-miR-NC) was constructed to a pGCSIL-green fluorescent protein lentivirus vector (GeneChem, Shanghai, China). The HEK cell line 293T was used for virus packaging and production after transfection with the expression vectors and package vectors by Lipofectamine 2000 (Invitrogen). After continuous culture for 48 h, the supernatants were collected with brief centrifugation. Concentration was performed by ultracentrifugation (40,000 ×g at 4 °C for 30 min) if necessary, and the titer was determined by 293T infection. The exponentially growing Y79 cells were seeded into six-well plates the day before infection. The lentivirus with appropriate dilution was applied to each well for 24 h. The infected cells were sorted and pooled together as stable clones.
Soft agar
Y79 stable clones carrying either scramble or anti-miR-145 were prepared as a single cell suspension in complete DMEM and then mixed with an equal volume of 0.6% low-melting-point agarose (Sigma). The mixture was laid on top of a solidified layer of 0.6% agarose in growth medium. Cells were replaced with fresh growth medium every 3–4 days for up to 2 weeks. The colonies were stained with crystal violet and counted.
Cell apoptosis
Cell apoptosis was analyzed with annexin V-propidium iodide (PI) double staining assay with the fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ). Briefly, the Y79 cells (parental or transfectant with anti-miR-145) were treated with mock or 50 µM genistein for 24 h first. The single cell suspension was prepared in calcium-enriched HEPES buffer after washing with PBS (135 mM NaCl, 4.7 mM KCl, 10 mM Na2HPO4, 2 mM Na2HPO4, pH 7.4) and digested with trypsin. The cells were stained with annexin V-FITC and PI for 15 min and analyzed with flow cytometry (Beckman Coulter, Indianapolis, IN).
Dual-luciferase reporter assay
The Y79 cells were plated in 24-well plates the day before transfection. Either wild-type or seed-region mutated ABCE1 3′-untranslated region (UTR) sequences were constructed into pGL4 plasmids with double restriction digestion. The reporter plasmids were cotransfected with miR-145 or its mimic into Y79 cells with Lipofectamine 2000 (Invitrogen). Cells were harvested 48 h later, and relative luciferase activities were measured with the Dual-Luciferase Assay System (Promega, Madison, WI).
Real-time PCR
The relative expression of miR-145 was determined with qRT-PCR with the commercial TaqMan MicroRNA Assay (ID002278, Thermo Fisher Scientific, Waltham, MA). The human small nuclear RNA (snRNA) U6 was employed as the internal reference. The total RNA was extracted with TRIzol, and reverse transcription was performed with the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific). Initial denaturation: 94 °C for 4 min, followed by denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extention at 72 °C for 45 s (30 cycles), and a final extension at 72 °C for 5 min. The melting curve was plotted post-amplification, and the relative expression was calculated with the double delta comparative CT (2-△△Ct) method and normalized to U6. Each measurement was performed in triplicate independently.
Immunoblotting
The lysates were prepared from the indicated cells with regular RIPA lysis buffer. The protein content was quantified with the bicinchoninic acid (BCA) assay. A total of 15 µg of protein was separated with 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes in an ice bath. The membranes were then blocked with 5% skim milk for 1 h and incubated with primary antibodies against ABCE1 and GAPDH (#ab32270, #ab9483 from Abcam) overnight at 4 °C. The membranes were rigorously washed three times for 6 min with TBST (Tris-buffered saline plus 0.05% Tween-20) at room temperature and incubated with secondary anti-rabbit immunoglobulin G-horseradish peroxidase (IgG-HRP; 1:5,000, Sigma) for 2 h at room temperature. The membranes were subjected to a second wash with TBST at room temperature and visualized with enhanced chemiluminescence (ECL, Millipore, Billerica, MA) reagent according to the manufacturer’s instructions. The intensity of the individual bands was quantified with densitometry (Bio-Rad, Hercules, CA) and normalized to the corresponding input control (GAPDH).
Animal xenograft model
Female BALB/c nude mice were obtained from the SLAC Laboratory Animal Center (Shanghai, China) and housed in a pathogen-free environment. The experimental protocols were approved by the Institute Committee of Animal Care and Use. The indicated log phase cells were prepared in single-cell suspension in sterile PBS. Equal numbers (2 × 106) of each cell were inoculated subcutaneously into the right flanks of the immunodeficient mice. Tumor growth was monitored twice a week. Genistein was administrated via a tail vein injection. The experimental mice were euthanized by sodium pentobarbital (Sigma) 35 mg/kg, intraperitoneal injection at the indicated time points, and the actual size of the xenograft tumor was determined.
Statistical analysis
Data from three independent experiments were analyzed with SPSS 19.0 software, and all the results were shown as mean ± standard deviation (SD). The one-way ANOVA method was used for the multiple-group comparison analysis and the t test for the pairwise comparison group. The statistical significances between data sets were expressed as p values, and a p value of less than 0.05 was considered statistically different.
Results
Genistein inhibited proliferation and growth of retinoblastoma cells and induced apoptosis
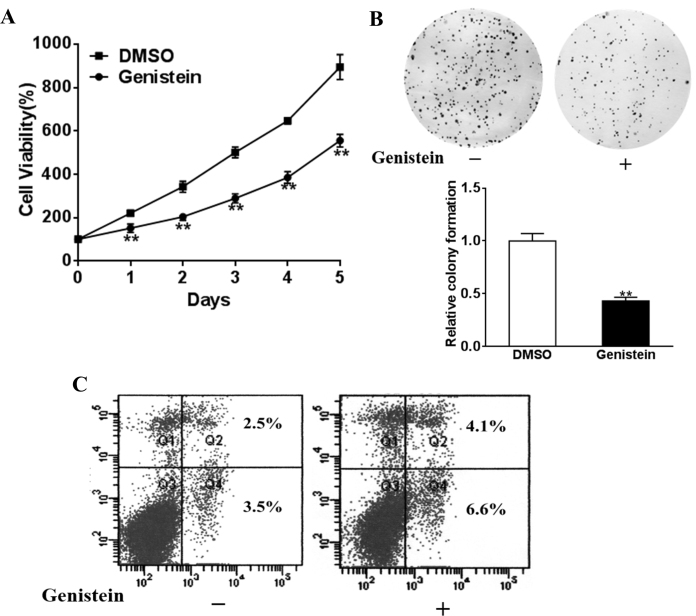
We employed the human retinoblastoma cell line Y79 to evaluate the therapeutic value of genistein in vitro. Cells were seeded in 96-well plates and treated with either mock or 50 μM genistein in triplicate for 5 consecutive days. No apparent cell toxicity was observed. As shown in Figure 1A, we examined cell viability with a commercial CCK-8 proliferation kit at the indicated time points. The cell proliferation was statistically significantly suppressed by genistein treatment in comparison with mock treatment with DMSO (Figure 1A). Consistent with decreased cell viability, the colony formation assay demonstrated a similar decline in colony formation capacity upon genistein treatment, which indicated the anchorage-independent growth of Y79 was also inhibited by genistein dosing (Figure 1B). To further define the inhibitory effect of genistein on retinoblastomas, cell apoptosis was determined after 24 h treatment with flow cytometry analysis following Annexin V-PI double staining. The apoptotic signal detected in the right section of the four-quadrant diagram increased in the genistein-treated cell population, which suggested that at least a partial inhibitory effect of genistein on retinoblastoma could contribute to induction of apoptosis (Figure 1C).
Figure 1.
Genistein inhibits retinoblastoma cell viability and growth and induces apoptosis. Y79 cells were treated with 50 μM genistein or dimethyl sulfoxide (DMSO). A: A Cell Counting Kit-8 assay was used to determine cell viability. B: Cloning efficiency was measured by the number of clones growing in soft agar. C: Cell apoptosis was analyzed with flow cytometry as described. Data were presented as mean ± standard deviation (SD) from three independent experiments with triple replicates per experiment. **p<0.01 indicates a statistically significant difference compared with the DMSO group.
Genistein induced expression of miR-145
Next, we attempted to understand the molecular events underlying cell growth suppression of Y79 by genistein. MicroRNA is a small non-coding RNA molecule with a length of about 22 nucleotides, widely expressed in plants, animals, and viruses. Through RNA silencing and post-transcriptional regulation of gene expression, microRNA is actively involved in every aspect of development and disease. Therefore, we sought to interrogate whether miRs participate in inhibition of genistein-mediated cell proliferation.
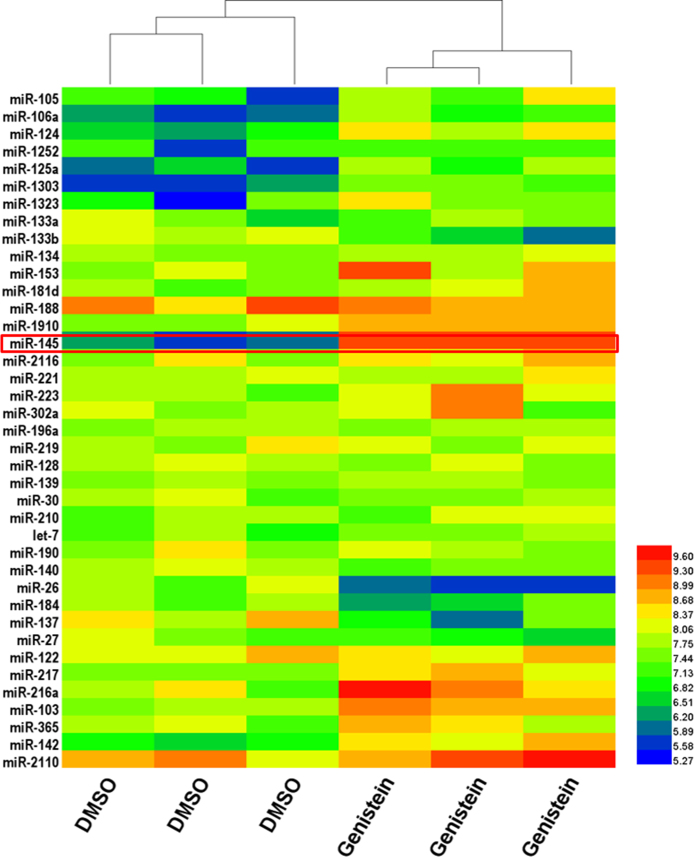
We employed a microRNA expression array from Affymetrix, which is capable of detecting 2,578 human mature miRs simultaneously for discovery purposes. The microRNA expression profiles were generated in mock and genistein treatment groups (Figure 2). Comparative analysis revealed 39 significant differentially expressed miRs, among which eight miRs were downregulated, and the rest were upregulated. We then picked miR-145 for further characterization because it was at the top of the list.
Figure 2.
Genistein induces miR-145 expression in retinoblastoma cells. Y79 cells were treated with 50 μM genistein or dimethyl sulfoxide (DMSO), and after 24 h, the expression levels of miRNAs were checked with a microarray.
Upregulation of miR-145-mediated inhibitory effect of genistein on retinoblastoma
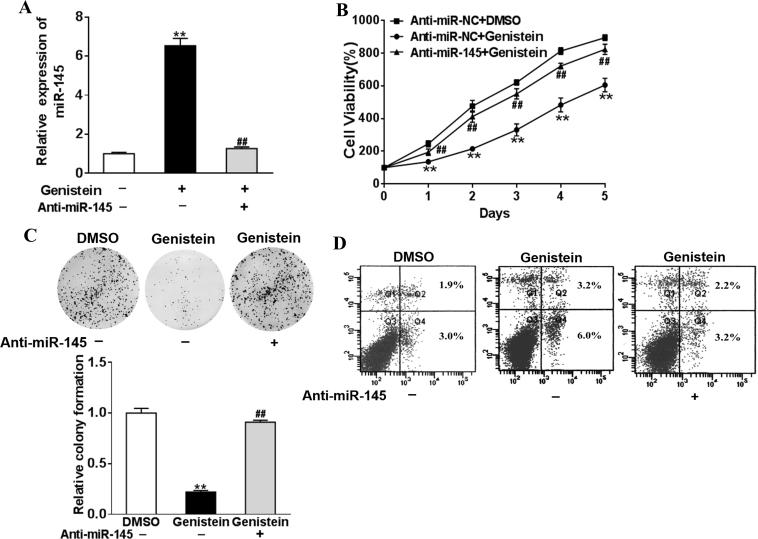
Based on the primary screening, we identified miR-145 which was dramatically upregulated in the genistein-treated Y79 cells. Next, we validated this regulatory effect with real-time PCR. As shown in Figure 3A, an almost sevenfold increase was detected in miR-145 expression after the genistein treatment (Figure 3A). The induction was readily suppressed by transfection with miR-145-specific siRNA. Therefore, we consolidated the upregulation of miR-145 by genistein in retinoblastomas.
Figure 3.
Silence of miR-145 reverses the suppression of genistein in the retinoblastoma. A: Y79 cells were transfected with the anti-miR-145 inhibitor (anti-miR-145) or control anti-sense RNA (anti-miR-NC) and were treated with 50 μM genistein or dimethyl sulfoxide (DMSO). After 48 h, the expression levels of miR-145 were analyzed with qRT-PCR. B: Cells were treated as above, and a Cell Counting Kit-8 assay was used to detected cell viability. C: Cloning efficiency was measured by the number of clones growing in soft agar. D: Cell apoptosis was analyzed with flow cytometry as described. Data are presented as mean + standard deviation (SD) from three independent experiments with triple replicates per experiment. **p<0.01 indicates a statistically significant difference compared with the anti-miR-NC + DMSO group. ##p<0.01 indicates a statistically significant difference compared with the anti-miR-NC + genistein group.
Next, we set out to evaluate the role of miR-145 plays in genistein-elicited growth suppression of Y79 cells. The inhibition of the proliferation of Y79 cells by genistein treatment was released while transfected with miR-145 specific siRNA (Figure 3B). miR-145 knockdown also restored the colony formation capacity of the genistein-treated Y79 cells (Figure 3C). Cell apoptosis induced by genistein treatment was apparently suppressed by siRNA-mediated downregulation of miR-145 (Figure 3D). The data showed the predominant role of miR-145 played in a suppressive effect of genistein on retinoblastoma.
miR-145 targeted ABCE1
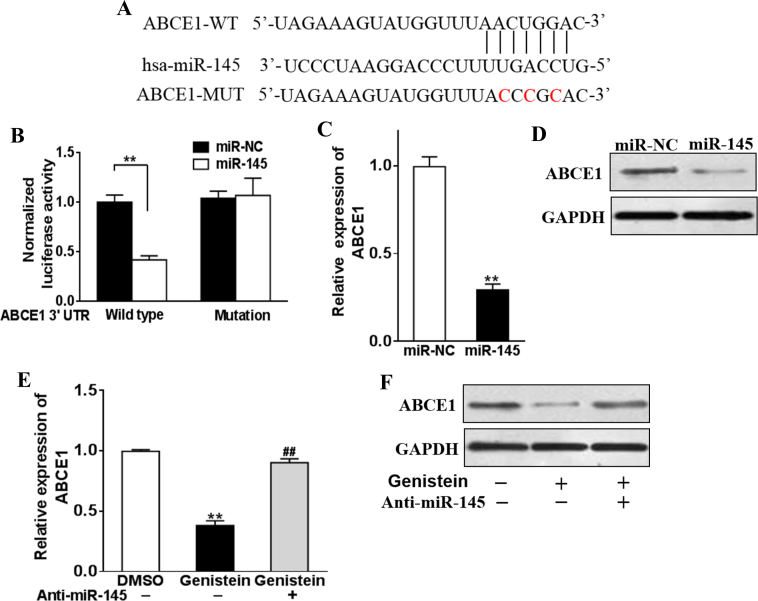
Cellular microRNAs function physiologically via post-transcriptional regulation of gene expression. Our previous results suggested an important role of miR-145 in genistein-mediated proliferation inhibition of retinoblastoma. However, the exact molecular target of miR-145 in this setting was undefined. We employed online bioinformatics tools, including Exiqon, miRDB, and microRNA, to predict the potential target of miR-145, and ABCE1 was highlighted by all three algorithms. Alignment of the seed region of miR-145 with the 3′-UTR of the ABCE1 transcript is shown in Figure 4A.
Figure 4.
Genistein-induced miR-145 directly targets ABCE1. A: Putative seed-matching sites or mutant sites (red) between miR-145 and the 3′-untranslated region (UTR) of ABCE1. B: Luciferase reporter assay was performed on Y79 cells to detect the relative luciferase activities of wild-type (WT) and mutant ABCE1 reporters. ** p<0.01. C–D: Y79 cells were transfected with miR-145 or control miR-NC mimics. After 48 h, the expression levels of ABCE1 were analyzed with quantative reverse transcription (qRT)-PCR and western blotting. ** p<0.01 indicates a statistically significant difference compared with the miR-NC group. E–F: Y79 cells were transfected with anti-miR-145 inhibitor (anti-miR-145), or control anti-sense RNA (anti-miR-NC), and were treated with 50 μM genistein or dimethyl sulfoxide (DMSO). After 48 h, the expression levels of ABCE1 were analyzed with qRT-PCR and western blotting. **p<0.01 indicates a statistically significant difference compared with the anti-miR-NC + DMSO group. ##p<0.01 indicates a statistically significant difference compared with the anti-miR-NC + genistein group.
Next, we validated the prediction experimentally. Either wild-type or mutant 3′-UTR sequences were cloned in a dual-luciferase reporter plasmid (Figure 4A). Exogenous expression of the miR-145 mimic statistically significantly decreased relative luciferase activity in the cotransfected Y79 cells, while scramble miR had no such effect on translational inhibition (Figure 4B). The expression of ABCE1 at the transcript (Figure 4C) and protein (Figure 4D) levels was inhibited by the introduction of the miR-145 mimic in comparison with the scramble control. Consistent with the induction of miR-145 by genistein treatment, expression of ABCE1 was remarkably suppressed in this setting, which was completely reversed by siRNA-mediated knockdown of miR-145 (Figure 4E). Alteration at the protein level measured with immunoblotting demonstrated a similar trend (Figure 4F). The data clearly showed that ABCE1 was per se the target of miR-145 and subjected to regulation by miR-145 in genistein-treated retinoblastoma.
Genistein inhibited retinoblastoma xenograft growth via upregulation of miR-145
Our previous in vitro data demonstrated genistein treatment efficiently suppressed cell proliferation and induced apoptosis, which was predominately mediated by upregulation of miR-145. Therefore, we further investigated the inhibitory effect of genistein on retinoblastoma in vivo.
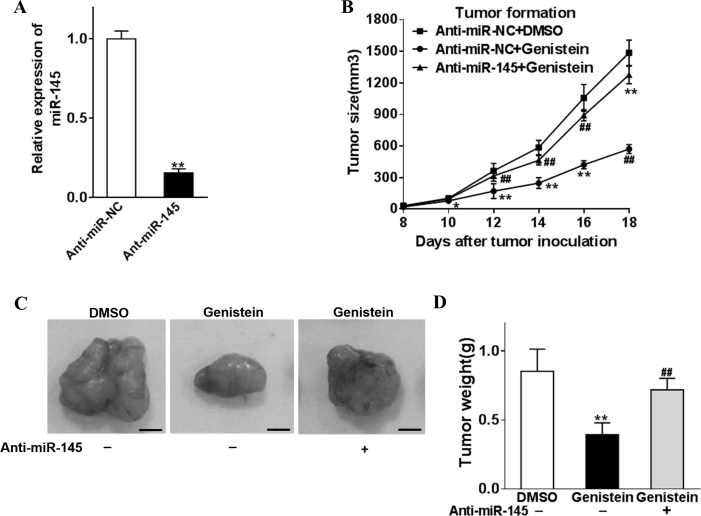
Stable clones derived from the Y79 cell line were established by lentivirus-mediated knockdown of endogenous miR-145, which were subsequently subcutaneously inoculated into nude mice for the in vivo study. The knockdown efficacy was validated with Q-PCR before transplantation (Figure 5A). Xenograft tumor progression was monitored every 2 days until the mice were euthanized. Tumor growth was apparently suppressed in the naïve Y79 retinoblastoma by administration of genistein via a tail vein injection, while only a slight inhibitory effect was observed in the miR-145 knockdown counterpart (Figure 5B). Macroscopic examination and weighing of the xenograft excised after the mice were euthanized showed similar results (Figure 5C,D). The data demonstrated that the administration of genistein efficiently inhibited tumor progression in vivo, which was heavily dependent on the expression of miR-145.
Figure 5.
Genistein inhibits retinoblastoma cell tumor formation in vitro by miR-145. Y79 was infected with anti-miR-145 or anti-miR-NC lentivirus to establish stable cell lines. A: The relative expression of the stable cells was analyzed with qRT-PCR. B–D: Anti-miR-145- or anti-miR-NC-overexpression cells (5 × 106) were dispersed in 100 μl of serum-free 1640 medium and were subcutaneously injected into each side of the posterior flank of the nude mice (n = 4), and then genistein (50 mg/kg) was added or not with a tail vein injection. Tumors were measured every 2 days after they were observed, and the volumes were calculated using the following formula: volume = 0.5 × Length × Width2. The tumor was excised and weighed after 18 days with representative pictures of the tumors shown (bar = 10 mm). Data were presented as mean + standard deviation (SD) from three independent experiments with triple replicates per experiment. *p<0.05 and **p<0.01 indicate a statistically significant difference compared with the anti-miR-NC+ dimethyl sulfoxide (DMSO) group. ##p<0.01 indicates a statistically significant difference compared with the anti-miR-NC + genistein group.
Discussion
Although retinoblastoma is considered a curable cancer in most cases, it still imposes a severe health burden on young patients and causes vision loss in a significant proportion [1]. Currently, the therapeutic modalities in the treatment of this disease mainly include chemotherapy, radiotherapy, and surgery [5]. The relatively conservative chemotherapy is applicable to primary tumor at early stage but is frequently associated with invoked drug resistance and cytotoxicity. Radiotherapy is highly efficient in progressive retinoblastoma, which displays great sensitivity and responsiveness, but entails a high risk of abnormality in cosmetic development and subsequent neoplasia. Although the etiology of retinoblastoma is relatively well understood, an efficient target therapy is far from available for this disease [2]. Moreover, immunotherapy with remarkable success in other solid tumors might be inapplicable to retinoblastoma due to the immaturity of the immune system in young patients [11]. Clinically available options for this disease are largely limited to early generation therapy. Therefore, the exploitation of highly efficient, specific, and safe drugs is still under intensive development.
Many plant-derived active ingredients demonstrate superior clinical potency due to low toxicity and few side effects [12]. Genistein is an isoflavone derived from the soybean and well recognized for antioxidative and hormonal activities [9]. Several pioneering epidemiology studies unambiguously indicated a preventative effect of soy food in human cancer, which was greatly attributed to the phytoestrogen composite, genistein [13,14]. Thereafter, the accumulation of evidence suggested the antitumor potency of genistein, in prophylaxis and treatment. For example, genistein was demonstrated to inhibit hepatocellular carcinoma cells via induction of apoptosis and regulation of cell cycle [13,15]. Tumor growth in human Belc7402 cell inoculated xenograft mice was significantly retarded when genistein was administered [16]. Genistein was also capable of suppressing primary gastric cancer cells in vitro via modulation of the expression of apoptotic-related factors such as Bcl-2 and Bax [17]. Another study from Yang’s laboratory demonstrated that genistein induced G2/M cell cycle arrest in SGC-7901 and BGC-823 cells through inhibition of the AKT signaling pathway [18]. The inhibitory effect during carcinogenesis of lung cancer was also investigated in several cell culture-based studies [19]. Genistein displayed a synergistic effect on lung cancer when in combination with either trichostatin A (TSA) or gefitinib [20,21]. Antitumor properties of genistein against colorectal cancer were also demonstrated by attenuation of the PI3K/AKT and Wnt pathways [22,23]. Remarkably, genistein manifested a paradoxical effect on estrogen receptor (ER)-positive breast cancer that a high dose significantly induced cell apoptosis while a low dose promoted cell proliferation via the ER signaling pathway [24,25]. However, the potential therapeutic role of genistein has not been exploited in retinoblastoma.
MicroRNAs are widely expressed small non-coding RNA molecules. Through RNA silencing or post-transcriptional regulation of gene expression, microRNAs are actively involved in critical physiologic processes. MicroRNAs also play crucial roles in tumor initiation, progression, resistance, etc. [26]. In the present study, we demonstrated that genistein significantly suppressed the proliferation and anchorage-independent growth of the human retinoblastoma cell line Y79 in vitro, which was partially mediated by induction of apoptosis. We elucidated the underlying molecular mechanism through identification of miR-145 as a key effector, which was subjected to regulation by genistein treatment and in turn modulated expression of ABCE1. ABCE1 is a member of the superfamily of ATP-binding cassette (ABC) transporters, which actively transport respective substrate molecules across the hydrophobic lipid bilayer membrane. Through direct interaction with and inhibition of RNase L, ABCE1 attenuates the interferon-regulated 2–5A/RNase pathway in response to virus infection. Several reports have suggested an oncogene-like property of ABCE1 in various human cancers. For instance, shRNA-mediated knockdown of ABCE1 elicited cell cycle arrest at the G1/S phase in oral cancer cells [27] while inhibited proliferation and invasion of breast cancer cells [28]. In addition, decreased expression of ABCE1 reversely correlated with sensitivity to chemotherapies, such as doxorubicin and 5-fluorouracil in lung cancer [29,30]. Our preliminary data suggested that ABCE1 might be involved in proliferative and malignant growth of retinoblastoma, which was a convincible target of miR-145 upregulated by genistein treatment. We further validated the therapeutic effect of genistein in the xenograft mice model and elucidated the predominant role of miR-145 in genistein-mediated tumor suppression [31].
In summary, we demonstrated the antitumor potency of genistein in retinoblastoma in vitro and in vivo. Mechanistically, genistein treatment significantly induced the intrinsic expression of miR-145, which subsequently inhibited the expression of oncogenic ABCE1 and eventually led to growth suppression of cancer cells. The data warrant further investigation of genistein in the management of retinoblastoma. Moreover, direct administration of exogenous miR-145 mimic might be an alternative therapeutic modality because of the predominant role of the miR-145 mimic in this setting. In view of the downstream position in our identified genistein-miR-145-ABCE1 signal axis and the nature of the membrane protein, we propose ABCE1 could be a molecular target for future drug exploitation.
Acknowledgments
This paper was supported by City Medical Support Funding (#JE072).
Appendix 1. STR analysis.
To access the data, click or select the words “Appendix 1.”
References
- 1.Ortiz MV, Dunkel IJ. Retinoblastoma. J Child Neurol. 2016;31:227–36. doi: 10.1177/0883073815587943. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26023180&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 2.Mallipatna A, Marino M, Singh AD. Genetics of Retinoblastoma. Asia Pac J Ophthalmol (Phila) 2016;5:260–4. doi: 10.1097/APO.0000000000000219. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27488068&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Felsher DW. Role of MYCN in retinoblastoma. Lancet Oncol. 2013;14:270–1. doi: 10.1016/S1470-2045(13)70070-6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23498720&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson H. Retinoblastoma: diagnosis and management–the UK perspective. Arch Dis Child. 2015;100:1070–5. doi: 10.1136/archdischild-2014-306208. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25940424&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of Retinoblastoma in 2015: Agreement and Disagreement. JAMA Ophthalmol. 2015;133:1341–7. doi: 10.1001/jamaophthalmol.2015.3108. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26378747&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Shah PK, Narendran V, Kalpana N. Appearance and Spontaneous Resolution of Macular Pucker After Triple Freeze-Thaw Cryotherapy for Retinoblastoma. J Pediatr Ophthalmol Strabismus. 2009;48:E1–E2. doi: 10.3928/01913913-20090616-16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19645377&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Temming P, Arendt M, Viehmann A, Eisele L, Le Guin CH, Schundeln MM, Biewald E, Astrahantseff K, Wieland R, Bornfeld N, Sauerwein W, Eggert A, Jockel KH, Lohmann DR. Incidence of second cancers after radiotherapy and systemic chemotherapy in heritable retinoblastoma survivors: A report from the German reference center. Pediatr Blood Cancer. 2017;64:71–80. doi: 10.1002/pbc.26193. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27567086&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Shields JA. Intra-arterial Chemotherapy for Retinoblastoma. JAMA Ophthalmol. 2016;134:1201. doi: 10.1001/jamaophthalmol.2016.2712. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27533705&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Ganai AA, Farooqi H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed Pharmacother. 2015;76:30–8. doi: 10.1016/j.biopha.2015.10.026. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26653547&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–19. doi: 10.3945/an.114.008052. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26178025&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Wang Y, Wang H, Liu Y, Liu T, Kunda PE. Tandem therapy for retinoblastoma: immunotherapy and chemotherapy enhance cytotoxicity on retinoblastoma by increasing apoptosis. J Cancer Res Clin Oncol. 2013;139:1357–72. doi: 10.1007/s00432-013-1448-7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23689539&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 12.Normile D. Asian medicine. The new face of traditional Chinese medicine. Science. 2003;299:188–90. doi: 10.1126/science.299.5604.188. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12522228&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Zhu CF, Dai YL, Zhong Q, Sun B. Inhibitory effects of genistein on metastasis of human hepatocellular carcinoma. World J Gastroenterol. 2009;15:4952–7. doi: 10.3748/wjg.15.4952. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19842228&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, Yin P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288. doi: 10.1371/journal.pone.0089288. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24586662&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005;11:6512–7. doi: 10.3748/wjg.v11.i41.6512. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16425425&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chodon D, Banu SM, Padmavathi R, Sakthisekaran D. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Mol Cell Biochem. 2007;297:73–80. doi: 10.1007/s11010-006-9324-2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17006617&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Apoptosis of human primary gastric carcinoma cells induced by genistein. World J Gastroenterol. 2004;10:1822–5. doi: 10.3748/wjg.v10.i12.1822. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15188515&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YL, Zhang GQ, Yang Y, Zhang CY, Fu RX, Yang YM. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr Cancer. 2013;65:1034–41. doi: 10.1080/01635581.2013.810290. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24053672&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat Res. 2013;179:125–34. doi: 10.1667/RR2954.1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23237541&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu TC, Yang YC, Huang PR, Wen YD, Yeh SL. Genistein enhances the effect of trichostatin A on inhibition of A549 cell growth by increasing expression of TNF receptor-1. Toxicol Appl Pharmacol. 2012;262:247–54. doi: 10.1016/j.taap.2012.05.003. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22626855&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Cheng H, Ren Y, Liu ZG, Zhang YF, De Luo B. Synergistic inhibitory effects by the combination of gefitinib and genistein on NSCLC with acquired drug-resistance in vitro and in vivo. Mol Biol Rep. 2012;39:4971–9. doi: 10.1007/s11033-011-1293-1. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22160570&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Kim EJ, Shin HK, Park JH. Genistein inhibits insulin-like growth factor-I receptor signaling in HT-29 human colon cancer cells: a possible mechanism of the growth inhibitory effect of Genistein. J Med Food. 2005;8:431–8. doi: 10.1089/jmf.2005.8.431. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16379552&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Chen H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp Biol Med (Maywood) 2011;236:714–22. doi: 10.1258/ebm.2011.010347. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21571909&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Zhang YM, Song DF, Cui HB. Effect of apoptosis in human breast cancer cells and its probable mechanisms by genistein. Wei Sheng Yan Jiu. 2005;34:67–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15862028&dopt=Abstract [PubMed] [Google Scholar]
- 25.Seo HS, DeNardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR, Leclercq G, Brown PH. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99:121–34. doi: 10.1007/s10549-006-9191-2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16541309&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 26.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–9. doi: 10.1016/j.molmed.2014.06.005. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25027972&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Zhang M, Liu DX. Knock-down of ABCE1 gene induces G1/S arrest in human oral cancer cells. Int J Clin Exp Pathol. 2014;7:5495–504. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25337191&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 28.Huang B, Zhou H, Lang X, Liu Z. siRNAinduced ABCE1 silencing inhibits proliferation and invasion of breast cancer cells. Mol Med Rep. 2014;10:1685–90. doi: 10.3892/mmr.2014.2424. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25070080&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kara G, Tuncer S, Turk M, Denkbas EB. Downregulation of ABCE1 via siRNA affects the sensitivity of A549 cells against chemotherapeutic agents. Med Oncol. 2015;32:103. doi: 10.1007/s12032-015-0557-3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25744244&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 30.Zheng D, Dai Y, Wang S, Xing X. MicroRNA-299–3p promotes the sensibility of lung cancer to doxorubicin through directly targeting ABCE1. Int J Clin Exp Pathol. 2015;8:10072–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26617714&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2069852&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]