Abstract
Dominance hierarchies are ubiquitous in social species that require social cognition to maintain. Status may be established initially through physical conflict but is maintained by social signals between individuals that depend critically on the relative social status of those interacting. How do individuals collect information they need to modulate their behavior? Using a particularly suitable fish model system living in a complex social environment, we describe how the social context of behavior shapes the brain and, in turn, alters the behavior of animals as they interact. These fish observe social interactions carefully to gather information vicariously that guides future behavior. Social opportunities produce rapid changes in gene expression in key brain nuclei, and both social success and failure produce changes in neuronal cell size and connectivity in reproductive centers of the brain. It remains unknown how social information is transduced into cellular and molecular changes. Understanding the cellular and molecular changes underlying animal cognition will yield unique insights into how the brain works.
It seems likely that cognition evolved in the service of social behavior because living in groups requires social awareness among individuals. A ubiquitous feature of such social systems are dominance hierarchies through which both social and sexual status are communicated directly among animals during social intercourse via chemical, visual, auditory, and postural signs in various combinations. Although many animal signals convey information about external events such as food, danger, nest sites, etc., more commonly animals signal information about themselves, their species and individual identity, and their social or sexual status. Dominance hierarchies, typically organized around male status, are most common where males will compete vigorously for high rank. This is because the perks of high status include increased access to food, reproductive opportunity, and improved health outcomes. This is not so for low-ranking animals who have limited access to food, a suppressed reproductive system as well as limited reproductive opportunities, and adverse health effects (e.g., Sapolsky 2005). The ubiquity of social status can be seen in its representation in brain structures including in humans (Zink et al. 2008), and social status in macaques has been suggested to covary with particular neural circuits (Noonan et al. 2014). Because psychopathology and social disorders like autism typically reflect a failure to act in socially acceptable ways, understanding the mechanisms responsible for normal social cognition may ultimately suggest methods for remediation.
To study cognition, we need a model system that can be assessed under controlled laboratory conditions mimicking the natural habitat but allowing careful experimentation without compromising behavioral interactions. Importantly, to study cognitive abilities in a social setting, we need to have animals living in natural social groups. Fishes, which comprise ~50% of all extant species, are a natural choice for research on social behavior for several reasons. Fish were the common ancestor of tetrapods so that all vertebrates share genetic features, including the brain. This means that similarities in brain structures are due to common ancestry, and, indeed, fish brain regions have well-described homologs with other vertebrate brains. Second, because fish species split from tetrapods into their own independent radiation now occupying a vast range of ecological niches, they have also evolved sensory systems exquisitely tuned to their particular environments. These include the usual senses—vision, olfaction, taste, and hearing—but also mechanosensory detection (e.g., lateral line organ), external taste buds, and electroreceptor systems that have driven evolution of specialized brain structures. Importantly, among fish species, virtually every known kind of social system has evolved from monogamy to harems to sex-changing animals.
Cognitive skills in various fish species have been shown in several domains including acquisition of foraging skills (Brännäs and Eriksson 1999), tool use (Timms and Keenleyside 1975; Paśko 2010), spatial memory, and manipulation of the environment (Hughes and Blight 1999). Examples of social intelligence in fish have been measured by how they interact in group-living environments (Balshine-Earn et al. 1998), enhance offspring survival with biparental care (Gross and Sargent 1985; Hourigan 1989; Alonzo et al. 2001; Van den Berghe and McKaye 2001), cooperate in hunting (Diamant and Shpigel 1985; Vail et al. 2013), and share information about predator inspection (Pitcher et al. 1986).
MODEL SOCIAL SYSTEM
To understand the mechanisms underlying social cognition, my laboratory analyzes the social behavior of a cichlid fish species from Lake Tanganyika, East Africa, Astatotilapia burtoni (formerly Haplochromis burtoni). The A. burtoni male hierarchial social system requires particular social skills for success in maintaining a high status. Changes in social status cause changes in body color and behavior, as well as to neural connections related to reproduction. There are several reasons why A. burtoni is a valuable and unique species for understanding the role of social cognition: (a) The social system is based on resource guarding, and its natural state can be reliably and accurately replicated in the laboratory; (b) male status is signaled phenotypically via bright coloration, including a dark bar through the eye, making high-status animals easy to distinguish and their behavior readily quantifiable (see Fig. 1); (c) in this species, as in all vertebrates, gonadotropin-releasing hormone (GnRH1) neurons in the brain ultimately control reproduction (in A. burtoni these neurons are directly regulated by male social status and their size and connectivity changes with social status); (d) we can readily measure behavior in conjunction with levels of circulating hormones, tissue-specific peptides, and DNA expression; and (e) the A. burtoni genome has been sequenced (Brawand et al. 2014), so we can use transgenic and other genomic technologies.
Figure 1.
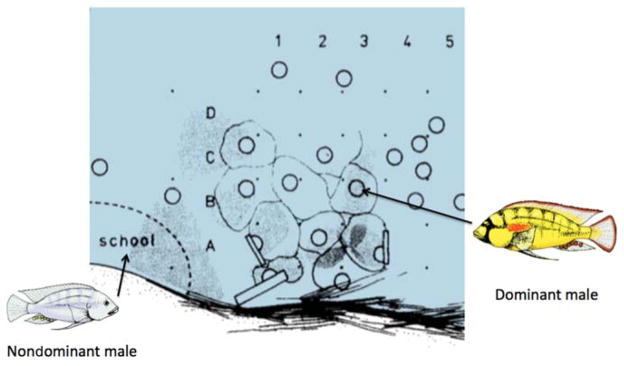
Sketch of an observation area in Lake Tanganyika, Burundi, Africa. Solid dots are grid stakes spaced ~50 cm apart that are labeled (1–5; A–D) for identification. Dark circles represent spawning pit locations of dominant males. Lighter colored outlines circumscribe the territories of individuals. Male territories are arrayed over the food source of detritus. Nondominant males and females school together near the territorial area. (Based on Fernald and Hirata 1977.)
SOCIAL SYSTEM OF A. BURTONI: CONSEQUENCES OF STATUS
Astatotilapia burtoni males live as one of two reversible, socially controlled phenotypes: reproductively competent dominant (D) males and reproductively incompetent nondominant (ND) males (see Fig. 1). D males are brightly colored, aggressively defend territories, and actively court females (Fernald and Hirata 1977). In striking contrast, ND males have a dull coloration, mimic female behavior, and school with females and other ND males, except when fleeing from an attacking D male.
These obvious external differences are reflected in major physiological and neural adaptations in response to differences in social status. As animals transition from one phenotype to the other, some changes, including expression of the black bar through the eye, brightening of the body color, and switching behavioral repertoires, are expressed in minutes (Fig. 1), whereas concomitant physiological and neural changes happen over days.
ND males attend closely to the unfolding social scene, assessing when they might be able to gain a territory by defeating a resident male. When this happens, there is typically a dramatic fight during which males engage in mouth-to-mouth biting, hitting each other with their bodies, and nipping at each other’s fins. If the ND male successfully defeats the resident, he rapidly turns on his bright body colors (Fernald and Hirata 1977; Burmeister et al. 2005) and will quickly begin performing the 17 distinct behaviors characteristic of dominant males upon his social ascent. Over a few days, the reproductive system of the ascending male is remodeled rendering the male reproductively competent, changes that are evident at several levels along the hypothalamic-pituitary-gonadal (HPG) axis (Maruska and Fernald 2014). In A. burtoni, as in all vertebrates, reproduction is controlled by gonadotropin-releasing hormone (GnRH1) containing neurons in the hypothalamus that deliver the eponymously named GnRH decapeptide to the pituitary. When a male ascends (ND→D), delivery of this molecule sets in motion a cascade of actions ultimately resulting in reproductive competence and release of sex steroids from the gonads. The GnRH neurons increase in volume by eightfold (Davis and Fernald 1990), extend their dendrites (Fernald 2012), and rapidly increase production of GnRH mRNA (Burmeister et al. 2005) and GnRH peptide (White et al. 2002). However, when a D male is moved into a social system with larger D males (>5% longer), it abruptly loses its color (<1 min) and joins other ND males and females in a school. Its GnRH-containing neurons in the preoptic area (POA) shrink to one-eighth their volume and produce less GnRH mRNA and peptide, causing hypogonadism and loss of reproductive competence (~2 wk) (Davis and Fernald 1990; Francis et al. 1993). Similarly, androgen, estrogen, and GnRH receptor mRNA expression levels depend on social status (Au et al. 2006; Burmeister et al. 2007; Harbott et al. 2007), as do electrical properties of the GnRH neurons themselves (Greenwood and Fernald 2004).
ATTENTION HIERARCHIES IN MALE A. BURTONI
Animals in social groups monitor the behavior of conspecifics and use their observations to guide their ongoing behavior. For example, individuals may attend to male fighting, females choosing mates, or many other ongoing social interactions. In all social hierarchies, subordinate animals attend very closely to the behavior of dominant animals in what has been named an “attention hierarchy” (Chance and Larson 1976). Animals watch higher-ranking animals carefully presumably to calibrate their potential opportunities of social ascent. Attention hierarchies have been identified in humans, particularly in groups of children (Boulton and Smith 1990), where individuals modulate their behavior depending on their own status relative to that of others. Within a hierarchy, when a high-ranking individual threatens or attacks a lower-ranking individual, that individual often then subsequently attacks an individual of still lower rank (Vaughn and Waters 1981). In addition to humans, attention hierarchies have been described in baboons and mandrills (Emory 1976), as well as in reptiles (Summers et al. 2005).
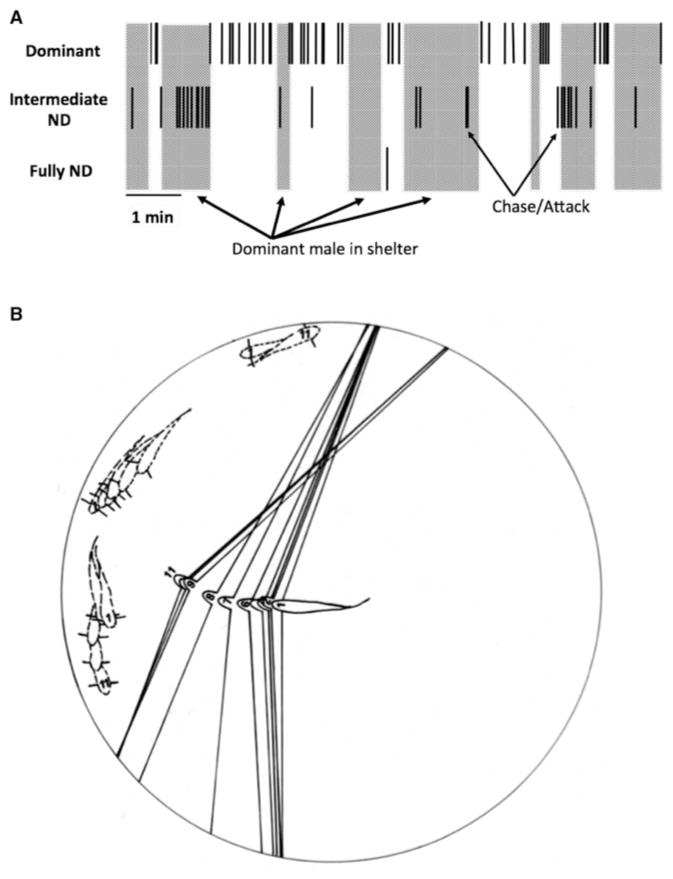
To understand attention hierarchies in A. burtoni, we videotaped groups of individually marked animals (n = 20/group; four replicates) and quantified interactions between dominant and nondominant males (Desjardins et al. 2012). We found that D and ND males never behaved aggressively at the same time. Even more interesting was that when the D male was out of view in a shelter, the ND males that were larger and attempting to ascend behaved aggressively and even courted females, behaviors that never occurred when the D male could see the ND male (Fig. 2).
Figure 2.
(A) Schematic illustration of typical dominant male behavior in the presence of an intermediate male attempting to ascend to dominance. Large rectangles represent the dominant male in his shelter, and small black bars show when an individual chases or attacks another fish. Note that intermediate males only attack other animals when they cannot be seen, because the dominant male is in his shelter (from data presented in Desjardins et al. 2012). (B) An example of eye movements during social interactions. Fish have been drawn from film images at 160-msec intervals with the first through 11th frames labeled. Relative eye locations of the dominant animal are shown by lines extending stalks attached to the eyes at an arbitrary angle behind the central axis of the eye. Note that the ND animals move out of the region being approached by the dominant male well before he arrives. (Redrawn from Fernald 1985.)
In this example, each time the dominant animal is out of view, the intermediate male attacks another ND male until the dominant male reappears. When the dominant male returns to the scene, he attacks within a few seconds but does not specifically target fish that have been aggressive to others in his absence (Desjardins et al. 2012).
Our data show that the ND males are attending to the D males and altering their behavior by acting aggressively, which is not possible when the D male is present. In addition, these males on occasion will approach and court females when the D male cannot see them, another behavior not possible when a D male is present. These behaviors reflect a sophisticated social calculus in which ND males are doing the most they can to increase their chances of becoming dominant. It is possible that they are also learning about being dominant through watching, a skill we have also reported elsewhere (Alcazar et al. 2014).
Based on the attention hierarchy described above, we reasoned that animals might alter their behavior when being observed by conspecifics, consistent with the notion that they could deceive other individuals that are watching them. To test this idea, we designed experiments in which two dominant male fish were in a tank, separated by a clear, watertight barrier, and both could be observed from a third compartment. When two size-matched dominant males are placed in these compartments, they will fight through the transparent barrier for the 20-min duration of the experiments while they are observed from a noninteracting audience. We asked how the behavior of the fighting animals would vary as a function of the composition of the audience and found that the animals fight harder when watched by a gravid female and less hard when watched by a larger male (Fig. 3; Desjardins et al. 2012).
Figure 3.
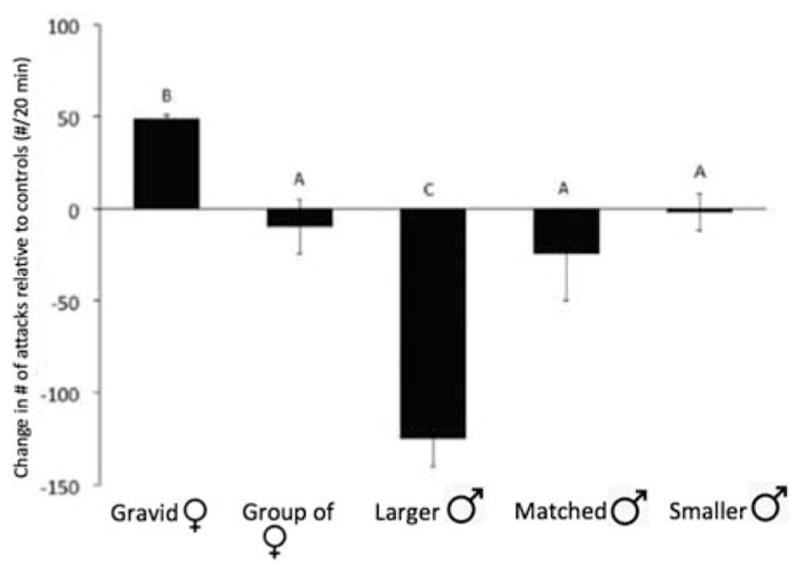
Aggressive acts (biting and ramming) displayed by a pair of males being watched by audiences composed of different kinds of conspecifics. Note that males were significantly (α = 0.05) more aggressive when being watched by a gravid female, and significantly less aggressive when being watched by a larger male (marked with B and C, respectively). In contrast, differences in aggression when being watched by a group of females, matched size male, or smaller males did not differ from control conditions (marked by A). (Redrawn from Desjardins et al. 2012.)
In an analysis of eye movements in A. burtoni, we observed that when a D male was in the presence of ND males and he oriented his eyes toward ND males, they would typically move out of his way (Fernald 1985). This anticipation of the actions of the D male contributes to the attention hierarchy of males within the social hierarchy and suggests that the ND males attend to specific attributes of the male behavior.
CAN MALES BE DECEPTIVE?
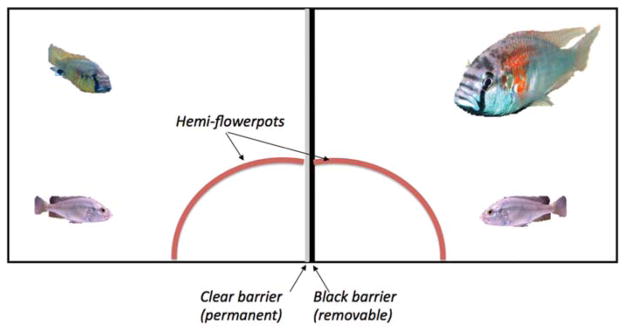
Can A. burtoni males be deceptive? This is an outstanding question in animal cognition because it is often taken as a hallmark of cognitive skills. To ask this question, we used a novel paradigm in which two differently sized males share a tank, divided in half by a clear, watertight divider and a black removable divider (Chen and Fernald 2011). The general idea is to see how a small D male responds to a larger D male when he can only see the larger fish. To do this, we placed a half terra-cotta pot, cut in half lengthwise, so that it allowed the males on each side to occupy a “shared” shelter, although with the black divider in place, neither animal knew the other animal was present (Fig. 4). One male was ~4 × larger than the other and each male had an appropriately sized female in their hemitank.
Figure 4.
Front view of the aquarium (45 L) divided in half with a watertight, clear divider (gray midline), and a removable opaque barrier (black midline). There is a small male fish (left compartment) and the large male fish (~4 × larger in the right compartment). The half terra cotta pot (red curved line) was cut so that both fish “shared” the same shelter, although they were not aware of each other’s presence. This “shared” shelter was hemisected by both center dividers. A layer of gravel covered the bottom of the tank.
Both the small and large fish were habituated to the tank with the opaque barrier in place for 2 d during which time each behaved like a normal dominant male in its territory: excavating gravel from the hemi-pot, courting the female in their half of the tank, and leading her back to the shelter and performing typical courtship and territorial male behaviors, all of which were quantified and exactly like normal D males. On the third day, the opaque barrier was lifted, and although there was no physical or chemical contact possible, the larger male made several “attacks” toward the small male, which quickly lost his coloration, including his eyebar. This is typical behavior for an animal losing his territorial status and was confirmed by quantifying their behavior. Indeed, the smaller males essentially abandoned their part of the shelter, digging a new pit remote from the site of the flowerpot shelter. Interestingly, this suppression of dominance, based entirely on visual signals, as reflected by the behavioral quantification (Chen and Fernald 2011), also reduced the expression of androgen hormones, but only for the first 3 d. Seven days after the black barrier was lifted, the smaller animals recovered their hormone expression levels and other brain markers of dominance while maintaining the coloration of an ND male. Moreover, they could be seen courting their females when out of view of the dominant male. So the effects of the visual suppression resulted in changes in the expression of aggressive, territorial behavioral responses by the smaller male but not in sustained physiological changes. This suggests that the smaller males uncoupled physiological changes in circulating hormones from their effects on outward appearance, seemingly presenting a false outward appearance not consonant with internal changes. This appears to be an example of deceptive behavior on the part of the male, allowing him to continue his courtship but not be influenced by the larger male. One can assume that the smaller animal had learned that the clear barrier prevented the larger male from actual physical attacks, and this recognition led the smaller animals to this novel strategy. The smaller ND male attended carefully to the D male when carrying out his behavior consistent with the attention hierarchy described above.
TRANSITIVE INFERENCE BY MALES
An ongoing goal of ND A. burtoni males is to ascend to dominance, triggering the physiological changes required to become reproductively competent. But how do ND males assess their chances to make this change? In their natural habitat, Lake Tanganyika, colonies of A. burtoni may range in size from a few dozen animals to more than 100, depending on the size of the feeding substrate (Fernald and Hirata 1977). ND males could, in principle, fight each D male in a colony to identify which might be likely targets for a takeover of their territories. However, fighting with tens of animals to find a territory holder weak enough to beat would obviously be prohibitive. As described above, the attention hierarchy evident during social encounters suggested A. burtoni might have observational skills that would allow them to predict the outcome of male–male encounters. That is, could males infer their chances of winning a fight simply from watching other animals fight? The logic of this process, known as transitive inference, is that if you know that A is taller than B and B is taller than C, you can infer that A is taller than C by constructing a virtual cognitive hierarchy without needing to see A, B, and C lined up for comparison. This ability was one of the developmental milestones first described by Piaget (1928) and since identified in humans older than 3, as well as in nonhuman primates (McGonigle and Chalmers 1977; Gillian 1981; Rapp et al. 1996), rats (Davis 1992; Roberts and Phelps 1994), and birds (von Fersen et al. 1991; Steirn et al. 1995; Bond et al. 2003; Weiss et al. 2010).
To discover if A. burtoni had the ability to infer fighting abilities from watching them fight, we asked whether bystander males could synthesize information from observed pairwise fights into an implied hierarchy of male fighting abilities. We did this by having bystander fish observe pairwise staged fights between five size-matched males (A–E) in which A>B, B>C, C>D, D>E with the implied hierarchy of A>B>C>D>E (Grosenick et al. 2007). Fights were staged by moving one rival into another rival’s territory, which resulted in the intruder animal losing. For fights among the control animals, there was no implied hierarchy (e.g., A = B = C = D = E). The bystander males were trained on pairwise fights, and we tested their preference between rivals they had never seen together before. That is, we tested whether the animal chose B or D as a winner. They consistently chose D as the weaker animal based on the prior data (Oliveira et al. 1998; Clement et al. 2005), showing that animals will indicate a choice by moving toward the rival they perceive to be weaker.
The fact that these fish can perform transitive inference in an important situation, choosing which male to attack, is consistent with the behavioral needs and ecological context in their natural habitat. In the temporary shore pools and estuaries of their native habitat, there is regular disruption of established territories by movement of hippopotamuses, wind, and predation (Fernald and Hirata 1977). So being able to judge their rivals based on featural representations, independent of context, would be invaluable for them to increase their chances of reproductive success. We have shown that social ascent upon gaining a territory is swift and activates many behavioral, physiological, and molecular processes, allowing the ascending male a chance at reproductive success (Burmeister et al. 2005; Maruska and Fernald 2014). It seems likely that transitive inference could be found in other colony-living animals that face similar constraints on reproduction. This would require designing experimental tests that exploit a natural context and behavioral elements related to behavioral acts in the animal’s natural life.
The experiments described above relied entirely on visual information being provided to the individual, although chemosensory information is also essential for many animal species including fish. We have also tested whether A. burtoni uses other sensory channels when communicating with conspecifics. Specifically, we injected dye into D males and tested whether these individuals used urine pulses as a part of their sensory signaling. We found that D males increased their urination along with territorial behaviors when they were visually exposed to another male (Fig. 5; Maruska and Fernald 2012). This study of contextual chemosensory urine signaling showed that males could distinguish female reproductive states using visual cues alone, suggesting that urine signals very likely play a complementary role to visual signaling.
Figure 5.

A urine pulse (arrow) released from a D male. A D male, injected with dye was exposed visually to another D male (after Maruska and Fernald 2012).
GENOMIC CONSEQUENCES OF FEMALE MATE CHOICE
For females of many species, information about potential mates changes their behavior dramatically, which makes sense because female mate choice is extremely important for the survival of their offspring. Given its importance, we wondered what cognitive activity might accompany behavioral and physiological changes in females in response to mate choice. Specifically, how does the female brain respond to social information about potential mates? This experimental question depends on deciding both how and where to look for a signal in the brain in response to female mate choice. We chose to measure brain sites that were marked by changes in gene expression using immediate-early genes (IEGs), egr-1 and c-fos, as a female chooses a mate. IEGs are inducible transcription factors that comprise a part of the first wave of gene expression induced in neurons being activated. Extensive experimental work has shown that a range of natural experiences including sensory stimuli can induce IEG expression and, consequently, it has been used in mammals and birds (e.g., Rusak et al. 1990; Mello et al. 1992) to identify social responses. More recently, we showed that egr-1 is highly conserved in A. burtoni and that functionally it responds robustly within 30 min of stimulation (Burmeister and Fernald 2005) and that c-fos is also a valuable genetic signal indicating brain responses in A. burtoni. As for the brain location, we hypothesized that the conserved vertebrate social behavior network (SBN) would be a logical place to look. The SBN was originally described by Newman (1999) as a collection of brain nuclei implicated in a variety of social behaviors including male mating behavior, female sexual behavior, parental behavior, and aggressive behavior. Anatomical homologs to this network have subsequently been identified in birds and fish (Goodson and Bass 2002; Goodson 2005) and are likely to exist in other vertebrates as well. It was unknown, however, whether they might also respond to social information as well as to behavioral actions. In previous experiments on female mate choice, we showed that reproductively ready (i.e., gravid) females prefer to associate with dominant, reproductively active males, whereas nongravid females prefer nondominant, nonreproductive males (Clement et al. 2005).
Using a similar paradigm, we placed females in a tank with one male at each end, behind a clear, watertight Plexiglas barrier so the female received only visual information from the males. We quantified the female’s preference based on her interactions and proximity to that male over a 20-min period. Following this, we staged a fight between the two males in one of the male compartments chosen at random. Subsequently, the chosen male either won or lost that fight. Our control condition was for the female to choose between two males and not see a subsequent fight. We hypothesized that different IEG expression patterns would be generated by the condition of females seeing their chosen male win or lose a fight. We then mapped brain gene expression patterns, comparing the mRNA levels of egr-1 and c-fos in six brain nuclei comprising the SBN (Desjardins et al. 2010).
Surprisingly, females seeing their selected male win or lose a fight produced dramatically different brain IEG expression patterns. Females who saw their preferred male win a fight activated brain nuclei associated with reproduction and reproductive behavior. Specifically the anterior hypothalamus, ventromedial hypothalamus, preoptic area, and the periaqueductal gray all had significantly more activation. In striking contrast, females who saw their preferred male lose a fight had a much higher expression of the IEGs that were in a part of the brain associated with anxiety, the lateral septum (Desjardins et al. 2010). A remarkable aspect of these results is that the females were responding to visual information alone, because they did not mate with the male, suggesting that the social information had caused changes in activation of key brain areas. It is important to remember that the IEG expression we measured is surely only a very small part of the total brain response and hence is just a glimpse of the genomic response to social information. However, the differential responses in specific brain areas show that females are activating their brains based on visual information and may use this to guide decisions about what to do.
One additional question is how this information might inform the female’s choice of a mate. In a separate experiment, we performed the same protocol, but after the female had chosen and seen the staged fight, she had to choose again. In this second choice, if she had seen her preferred male lose, she almost always switched her choice, and if he won, she rarely switched her choice (Fig. 6).
Figure 6.
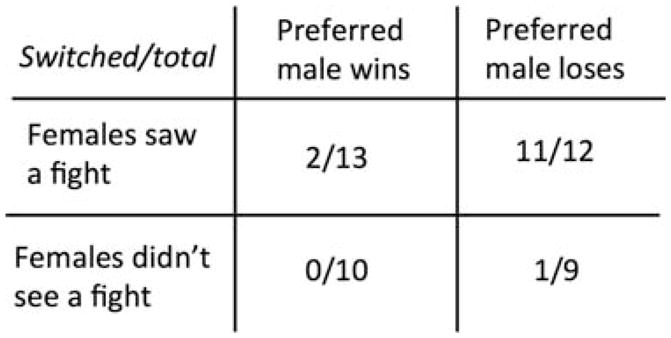
Tabulation of female A. burtoni choices after they saw their preferred male either win or lose a fight (top row) as compared with their choices after not seeing a fight (bottom row). After seeing her preferred male win a fight, the female rarely switches her choice (P = 0.0002; Fisher’s exact test), but after seeing her preferred male lose, she nearly always switches her choice (P = 0.0004; Fisher’s exact test) (Klausner et al. in prep.).
CONCLUSIONS
This review of research about the social behavior in A. burtoni has highlighted the important role of cognition in regulating the social life in this fish species. The A. burtoni social system is centered on a hierarchy of dominant males guarding a food resource, and we described some of the cognitive skills required for males and females to navigate this social landscape. Our observations in the field and in laboratory experiments have revealed that these animals collect information on conspecifics and use that information to decide what to do in particular circumstances. This is both surprising and yet, on reflection, obvious. Knowing what to do next in a social context is important. Both males and females use information gathered through observation to guide their behavior. We have followed this information into the brain to identify where it acts using immediate-early genes to mark active areas. As expected, males can change quickly what they do depending on what they perceive is happening in their surroundings, and these changes cause corresponding changes in the brain in specific cells, receptors, and circuits, thereby preparing the brain of the animal for a phase of life in a new status. How social information is transduced into cell and molecular changes in the brain, however, remains a mystery.
Acknowledgments
I am grateful for the many people in my laboratory over the years for their inspired contributions and hard work. This work was supported by NS 034950, NIMH 087930, and NSF IOS-0923588 to R.D.F.
References
- Alcazar RM, Hilliard AT, Becker L, Bernaba M, Fernald RD. Brains over brawn: Experience overcomes a size disadvantage in fish social hierarchies. J Exp Biol. 2014;217:1462–1468. doi: 10.1242/jeb.097527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo JJ, McKaye KR, van den Berghe EP. Parental defense of young by the convict cichlid, Archocentrus nigrofasciatus, in Lake Xiloá, Nicaragua. J Aquaric Aquat Sci. 2001;9:208–228. [Google Scholar]
- Au TM, Greenwood AK, Fernald RD. Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behav Brain Res. 2006;170:342–346. doi: 10.1016/j.bbr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Balshine-Earn S, Neat FC, Reid H, Taborsky M. Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav Ecol. 1998;9:432–438. [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Anim Behav. 2003;65:479–487. [Google Scholar]
- Boulton MJ, Smith PK. Affective bias in children’s perceptions of dominance relationships. Child Dev. 1990;61:221–229. doi: 10.1111/j.1467-8624.1990.tb02774.x. [DOI] [PubMed] [Google Scholar]
- Brännäs E, Eriksson T. Floating, switching, or nonswitching as different behaviours when Arctic charr (Salvelinus alpinus) are visiting two feeding tanks. Can J Fish Aquat Sci. 1999;56:1068–1077. [Google Scholar]
- Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, Simakov O, Ng AY, Lim ZW, Bezault E, et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 2014;513:375–381. doi: 10.1038/nature13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Fernald RD. Evolutionary conservation of the egr-1 immediate-early gene response in a teleost. J Comp Neurol. 2005;481:220–232. doi: 10.1002/cne.20380. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Horm Behav. 2007;51:164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance MRA, Larsen RR, editors. The social structure of attention. Wiley; New York: 1976. [Google Scholar]
- Chen CC, Fernald RD. Visual information alone changes behavior and physiology during social interactions in a cichlid fish (Astatotilapia burtoni) PLoS ONE. 2011;6:e20313. doi: 10.1371/journal.pone.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement TS, Parikh V, Schrumpf M, Fernald RD. Behavioral coping strategies in a cichlid fish: The role of social status and acute stress response in direct and displaced aggression. Horm Behav. 2005;47:336–342. doi: 10.1016/j.yhbeh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Davis H. Transitive inference in rats (Rattus norvegicus) J Comp Psychol. 1992;106:342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- Davis MR, Fernald RD. Social control of neuronal soma size. J Neurobiol. 1990;21:1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- Desjardins JK, Klausner JQ, Fernald RD. Female genomic response to mate information. Proc Natl Acad Sci. 2010;107:21176–21180. doi: 10.1073/pnas.1010442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Hofmann HA, Fernald RD. Social context influences aggressive and courtship behavior in a cichlid fish. PLoS ONE. 2012;7:e32781. doi: 10.1371/journal.pone.0032781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant A, Shpigel M. Interspecific feeding associations of groupers (Teleostei: Serranidae) with octopuses and moray eels in the Gulf of Eilat (Aqaba) Environ Biol Fishes. 1985;13:153–159. [Google Scholar]
- Emory GR. Aspects of attention, orientation, and status hierarchy in mandrills (Mandrillus sphinx) and Gelada baboons (Theropithecus gelada) Behaviour. 1976;59:70–87. [Google Scholar]
- Fernald RD. Eye movements in the African cichlid fish, Haplochromis burtoni. J Comp Physiol A. 1985;156:199–208. doi: 10.1007/BF01350031. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Social control of the brain. Annu Rev Neurosci. 2012;35:133–151. doi: 10.1146/annurev-neuro-062111-150520. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: Habitats and co-habitants. Environ Biol Fishes. 1977;2:299–308. [Google Scholar]
- Francis RC, Soma K, Fernald RD. Social regulation of the brain-pituitary-gonadal axis. Proc Natl Acad Sci. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillian DJ. Reasoning in the chimpanzee: II. Transitive inference. J Exp Psychol Anim Behav Process. 1981;7:87–108. [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Fernald RD. Social regulation of the electrical properties of gonadotropin-releasing hormone neurons in a cichlid fish (Astatotilapia burtoni) Biol Reprod. 2004;71:909–918. doi: 10.1095/biolreprod.104.030072. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Gross MR, Sargent RC. The evolution of male and female parental care in fishes. Am Zool. 1985;25:807–822. [Google Scholar]
- Harbott LK, Burmeister SS, White RB, Vagell M, Fernald RD. Androgen receptors in a cichlid fish, Astatotilapia burtoni: Structure, localization, and expression levels. J Comp Neurol. 2007;504:57–73. doi: 10.1002/cne.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourigan TF. Environmental determinants of butterflyfish social systems. Environ Biol Fishes. 1989;25:61–78. [Google Scholar]
- Hughes RN, Blight CM. Algorithmic behaviour and spatial memory are used by two intertidal fish species to solve the radial maze. Anim Behav. 1999;58:601–613. doi: 10.1006/anbe.1999.1193. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Contextual chemosensory signaling in an African cichlid fish. J Exp Biol. 2012;215:68–74. doi: 10.1242/jeb.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Social regulation of gene expression in the African cichlid fish Astatotilapia burtoni. In: Canli T, editor. The Oxford handbook of molecular psychology. Oxford University Press; Oxford: 2014. pp. 1–22. [Google Scholar]
- McGonigle BO, Chalmers M. Are monkeys logical? Nature. 1977;267:694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Mars RB, Neubert FX, O’Reilly JX, Andersson JL, Mitchell AS, Bell AH, Miller KL, Rushworth MF. A neural circuit covarying with social hierarchy in macaques. PLoS Biol. 2014;12:e1001940. doi: 10.1371/journal.pbio.1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, MPK, Burford FRL, Custódio MR, Latruffe C. Functions of mudballing behaviour in the European fiddler crab Uca tangeri. Anim Behav. 1998;55:1299–1309. doi: 10.1006/anbe.1997.0685. [DOI] [PubMed] [Google Scholar]
- Paśko L. Tool-like behavior in the sixbar wrasse, Thalassoma hardwicke (Bennett, 1830) Zoo Biol. 2010;29:767–773. doi: 10.1002/zoo.20307. [DOI] [PubMed] [Google Scholar]
- Piaget J. Judgment and reasoning in the child. Kegan, Paul, Trench & Trubner; London: 1928. [Google Scholar]
- Pitcher TJ, Green DA, Magurran AE. Dicing with death: Predator inspection behavior in minnow Phoxinus phoxinus shoals. J Fish Biol. 1986;28:439–448. [Google Scholar]
- Rapp PR, Kansky MT, Eichenbaum H. Learning and memory for hierarchical relationships in the monkey: Effects of aging. Behav Neurosci. 1996;110:887–897. doi: 10.1037//0735-7044.110.5.887. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Phelps MT. Transitive inference in rats—A test of the spatial coding hypothesis. Psychol Sci. 1994;5:368–374. [Google Scholar]
- Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Steirn JN, Weaver JE, Zentall TR. Transitive inference in pigeons: Simplified procedures and a test of value transfer theory. Anim Learn Behav. 1995;23:76–82. [Google Scholar]
- Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Overli O, Höglund E, Ronan PJ, Summers TR, Renner KJ, et al. Dynamics and mechanics of social rank reversal. J Comp Phys A Neuroethol Sens Neural Behav Physiol. 2005;191:241–252. doi: 10.1007/s00359-004-0554-z. [DOI] [PubMed] [Google Scholar]
- Timms AM, Keenleyside MH. The reproductive behavior of Aequidens paraguayensis (Pisces, Cichlidae) Z Tierpsychol. 1975;39:8–23. doi: 10.1111/j.1439-0310.1975.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Vail AL, Manica A, Bshary R. Referential gestures in fish collaborative hunting. Nat Commun. 2013;4:1765. doi: 10.1038/ncomms2781. [DOI] [PubMed] [Google Scholar]
- Van den Berghe EP, McKaye KR. Reproductive success of maternal and biparental care in a Nicaraguan cichlid fish, Parachromis dovii. J Aquaricult Aquat Sci. 2001;9:49–65. [Google Scholar]
- Vaughn BE, Waters E. Attention structure, sociometric status and dominance: Interrelations, behavioral correlates and relationships to social competence. Dev Psychol. 1981;17:275–288. [Google Scholar]
- von Fersen L, Wynne CDL, Delius JD, Staddon JER. Transitive inference formation in pigeons. J Exp Psychol Anim Behav Process. 1991;17:334–341. [Google Scholar]
- Weiss BM, Kehmeier S, Schloegl C. Transitive inference in free-living greylag geese, Anser anser. Anim Behav. 2010;79:1277–1283. [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J Exp Biol. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: Neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]