Abstract
Background
Among Hispanic breast cancer (BC) survivors, we examined the long-term effects of a short-term culturally-based dietary intervention on increasing fruits/vegetables (F/V), decreasing fat and changing biomarkers associated with BC recurrence risk.
Methods
Spanish-speaking women (n=70) with a history of stage 0-III BC who completed treatment were randomized to ¡Cocinar Para Su Salud! (n=34), a culturally-based 9-session program (24 hours over 12 weeks, including nutrition education, cooking classes, and food-shopping field trips), or a control group (n=36, written dietary recommendations for BC survivors). Diet recalls, fasting blood, and anthropometric measures were collected at baseline, 6, and 12 months. We report changes between groups at 12 months in dietary intake and biomarkers using 2-sample Wilcoxon t-tests and Generalized Estimating Equation (GEE) models.
Results
At 12 months, the intervention group compared to the control group reported higher increases in mean daily F/V servings (total: +2.0 vs. −0.4; P=0.006), and non-significant decreases in percent calories from fat (−2.2% vs. −1.1%; P=0.69) and weight (−2.6 kg vs. −1.5 kg; P=0.56). Compared to controls, participants in the intervention group had higher increases in plasma lutein (+20.4% vs. −11.5%; P=0.002), and borderline significant increases in global DNA methylation (+0.8% vs. −0.5%; P=0.06).
Conclusions
The short-term ¡Cocinar Para Su Salud! program was effective at increasing long-term F/V intake in Hispanic BC survivors and changed biomarkers associated with BC recurrence risk.
Impact
It is possible for short-term behavioral interventions to have long-term effects on behaviors and biomarkers in minority cancer patient populations. Results can inform future study designs.
Keywords: dietary intervention, Hispanic, cancer survivors, breast cancer
INTRODUCTION
There are approximately 3.1 million breast cancer survivors in the U.S. today and over 230,000 women were newly diagnosed in 2015 (1). Scientific, clinical and patient advocacy communities continue to have considerable interest in understanding whether post-diagnosis lifestyle behaviors affect breast cancer outcomes (2–5). It has been hypothesized that a diet high in fruits and vegetables and low in energy dense foods, engagement in regular physical activity, and achieving and maintaining a healthy body weight will be associated with better breast cancer outcomes. The proposed mechanism is that these behaviors results in favorable inflammatory, hormonal, metabolic and DNA methylation changes that result in decreased tumor progression, decreased recurrence risk, and benefit other health outcomes (i.e., cardiovascular disease and diabetes). The American Cancer Society, the American Institute of Cancer Research and the American College of Sports Medicine have all issued guidelines in support of these behaviors largely based on observational data. Despite these guidelines, few breast cancer survivors meet the recommendations (6). In addition, few programs among cancer survivors have demonstrated health behavior changes and maintenance of these changes, while also showing measurable effects on biomarkers associated with cancer risk and recurrence (7, 8).
To date, the majority of the major behavioral intervention trials among breast cancer patient populations have predominantly included non-Hispanic white women (9–13). Hispanic breast cancer survivors are a growing population of cancer survivors with a clear health disparity. While Hispanic women have a lower incidence of breast cancer controlled to non-Hispanic whites, they are more likely to be diagnosed at later stages and are more likely to be diagnosed with larger and hormone receptor negative tumors, both of which are more difficult to treat (14). Currently available data conflict on whether Hispanic women have worse prognosis after controlling for these factors (15–18). The role of postdiagnosis lifestyle factors in this population has not been evaluated. Hispanics in the U.S. have higher rates of obesity and sedentary behavior, and are less likely to meet physical activity guidelines (19). In addition, Hispanic subgroups do not have uniform dietary patterns (20). For example, compared to non-Hispanic white populations, Mexican Americans have higher intake of fruits and vegetables, while Dominicans have much lower rates.
With the growing number and longevity of cancer survivors, there is a need for effective behavioral interventions that both address the risk of cancer recurrence and secondary cancers, as well as the risk of other chronic disease. Studies on maintaining behavioral change over time among non-cancer populations, mostly focused on weight loss, show that improvements are seldom maintained long term (21–24). Among the several diet and physical activity interventions conducted among cancer survivors (7), few resulted in maintenance of long-term behavioral change (25–28).
There are limited data on effective dietary interventions among minority cancer patient populations. To address this gap, ¡Cocinar Para Su Salud! (Cook For Your Health!) was designed as a randomized controlled trial to examine the effect of a 9-session, culturally-based dietary intervention on change in fruit/vegetable and fat intake among Hispanic breast cancer survivors. Primary objectives of the study were changes at 6 months in daily servings of fruits/vegetables intake, percent energy from fat, and anthropometric measures and have been previously reported (29). Here, we report data on secondary, long-term outcomes including change at 12 months in daily fruit/vegetable intake, percent energy from fat, anthropometric measures, plasma carotenoids, metabolic biomarkers, inflammatory biomarkers, and DNA methylation.
MATERIALS AND METHODS
Study Description
Details on the study design of ¡Cocinar Para Su Salud! (Cook For Your Health!) have been previously published (29, 30). Briefly, investigators from Columbia University partnered with the New York City-based nonprofit organization Cook For Your Life (31) to develop and test the effects of a culturally- and theory-based dietary intervention on achieving and maintaining dietary recommendations for cancer survivors among Hispanic breast cancer survivors. Nine classes (four nutrition roundtables, two food-shopping trips, and three cooking lessons) were conducted over 24 hours of class time over a 12 week period. The curriculum was tailored to Hispanic women by developing recipes based on traditional Latin American cuisine and incorporating the neighborhood food environment into behavioral recommendations regarding food shopping, cooking and eating out. All study staff were bilingual and study materials and assessments were in Spanish.
Study Participants
A description of study participants has been previously published (29). Briefly, between April 7, 2011 and March 30, 2012, 70 women were randomized into the intervention (n=34) and control (n=36) arms. Target participants were Spanish-speaking women with a history of stage 0-III breast cancer (≥3 months post-treatment including surgery, radiation or chemotherapy; current hormonal therapy allowed) and no evidence of metastatic disease. Additional eligibility criteria included: age ≥21 years; Hispanic descent and fluent in Spanish; no uncontrolled diabetes mellitus, defined as hemoglobin A1C >7%; no uncontrolled comorbidities (e.g., hypertension); currently a non-smoker (given the low likelihood of current smokers to engage in healthy lifestyle behaviors); average intake of <5 servings of fruits/vegetables per day as assessed by the Block Fruit/Vegetable/Fiber Screener (32); access to functional home or cell phone; and not currently active in a dietary change program. Women were screened and recruited from the Columbia University Medical Center (CUMC) Breast Oncology Clinic. A detailed screening interview was conducted to assess eligibility. The study was approved by institutional review boards of the participating institutions (ClinicalTrials.gov NCT01414062). All participants provided written informed consent.
Randomization and data collection
Once participants completed the screening questionnaire, eligible participants were contacted and scheduled for a baseline clinic visit two weeks before the dietary intervention program start date. Clinic visits took place at the Irving Center for Clinical and Translational Research at CUMC. Clinic visits included the following procedures: assessment of anthropometric measures (standing height, weight, waist and hip circumference); fasting blood collection for biomarker analysis; and a detailed questionnaire on health behaviors and psychosocial constructs. Baseline dietary intake was assessed using three 24-hour recall assessments (1 in-person and 2 telephone-based recalls) using the multiple pass approach (33) with the Nutrition Data System for Research (NDSR) developed by the University of Minnesota (one in-person during the baseline clinic visit, two by phone).
Upon completion of baseline data collection, participants were randomly assigned to the intervention group: the 9-session ¡Cocinar Para Su Salud! program, or a control group, which received standard of care written dietary recommendations for cancer survivors (29). Randomization used a permuted block design and stratified at enrollment based on menopausal status and current use of hormonal therapy. Classes were conducted in small groups of 4–12 participants.
Follow-up clinic visits were scheduled at 6 and 12 months after the initial clinic visit and included anthropometric measures, fasting blood draw, interviewer-administered questionnaires, and 24-hour dietary recalls (1 in-person and 2 telephone-based recalls).
Laboratory Methods
Serum carotenoids and retinol concentrations
Samples were analyzed in batches using HPLC methods that allow for the simultaneous determination of serum β-carotene, α-carotene, lycopene, β-cryptoxanthin, zeaxanthin, and all-trans retinol (34–36). The lower limits of detection for retinol, α-carotene, β-carotene, lycopene, β-cryptoxanthin and zeaxanthin are, respectively, 0.1, 10, 12, 8, 6, and 2 ng/ml for serum. The detection limits for these nutrients are very low and hence these compounds can be detected and quantified in extracts of human serum. The assay variability for assays performed on the same day is between 3–6%; and for assays performed on different days the variability is between 5–8% (37).
Metabolic markers
Serum samples were analyzed in batches for metabolic tests including fasting insulin and fasting glucose. Serum insulin concentration was measured with the use of a Roche Diagnostics Elecsys 2010 automated analyzer and an Elecsys 1010/2010 insulin kit (no. 2017547). Serum glucose was measured on the COBAS INTEGRA 400 plus system (Roche Diagnostic, Montreal, Canada). Insulin resistance was calculated using the homeostasis model assessment (HOMA) index (38).
Markers of inflammation
Serum samples were batch analyzed for markers of inflammation. Interleukins (IL-1 α, IL-6, IL-8, IL-10), granulocyte macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α) were measured using a Luminex high sensitivity bead-based multiplex assay (EMD Millepore, Billerica, MA). High sensitivity C-reactive protein (CRPhg) was measured on the COBAS INTEGRA 400 plus system (Roche Diagnostic, Montreal, Canada).
Global DNA methylation
White blood cells (WBC) were batch analyzed at the completion of the study. Genomic DNA was extracted from the total WBC fraction by a standard salting out procedure. Following the manufacturer’s protocol, aliquots of DNA (500ng) were bisulfite-treated with the EZ DNA methylation kit (Zymo Research, Irvine, CA) and resuspended in 20 μL of distilled water and stored at −20°C until use. Pyrosequencing for Long Interspersed Nuclear Element 1 (LINE-1) methylation levels was performed using PCR and sequencing primers (39). Pyrosequencing was conducted using a PyroMark Q24 instrument (Qiagen, Hilden, Germany) with subsequent quantitation of methylation levels determined with PyroMark Q24 1.010 software. Three CpG sites were included in the analysis. Each set of amplifications included bisulfite-converted CpGenome universal methylated, unmethylated and non-template controls.
Statistical Analyses
Our a priori hypothesis to test was whether the ¡Cocinar Para Su Salud! program increased daily servings of fruits/vegetables and decreased fat as a percentage of daily calories for the intervention group compared to the control group at 12-months. Comparisons between the absolute and percent change in dietary, anthropometric, metabolic and inflammation outcomes from baseline to 12 months between groups were performed using 2-sample Wilcoxon signed-rank tests. Statistical tests used α=0.05 and 2-sided p-values. Differences in the changes in these outcomes over 12 months were estimated using Generalized Estimating Equation (GEE) models by fitting an interaction term between randomization arm and time, adjusting for menopausal status and use of hormonal therapy. In secondary analyses, we estimated percent changes in anthropometric measures, metabolic markers, inflammatory markers and global DNA methylation associated with every 10% increase in dietary factors using a GEE model, adjusted for the baseline value of the predictor of interest, randomization arm and stratification. All analyses were performed using R (40). The GEE models were fit using the R “gee” package (41).
RESULTS
Subject characteristics
A description of participant characteristics has been previously published (29). Briefly, at baseline, participants reported an intake of less than 4 servings of fruits/vegetables per day. No statistically significant differences between the intervention and control groups in demographic and clinical characteristics, including age, acculturation, education, income, use of government sponsored food programs, health literacy, stage of breast cancer diagnosis, time since diagnosis or body mass index (BMI). At baseline, participants were on average age 56.6 years (SD 9.7 years). All women self-identified as Hispanic and self-reported low levels of acculturation. Sixty percent of women reported a high school education or less, 40% reported working full-time or part-time, and 62.9% reported an annual household income of ≤$15,000. Approximately one quarter of participants had been diagnosed with ductal carcinoma in situ and one third had stage I tumors. Mean time since diagnosis was 3.4 years (range=0.3 to 15.6 years). Mean BMI of study participants was 30.9 (SD 6.0) (data not shown). At month 6, 61 women (87%) were retained (n=30, Intervention; n=31, Control), and at month 12, 58 women (83%) were retained (n=29, Intervention; n=29, Control). The main reasons for loss to follow-up included withdrawal from study, leaving the country, and family disapproval.
Change in dietary intake of fruits/vegetables
Change in fruit/vegetable intake at 3 and 6 months has been previously reported (29). At 12 months, participants who received the 9-session Cocinar Para Su Salud! intervention compared to participants in the control arm maintained significant increases from baseline in mean daily servings of total fruits/vegetables (+2 vs. −0.4; P=<0.01); total daily servings of vegetables (+1.6 vs. −0.2; P<0.01); targeted fruits/vegetables excluding juices, fried vegetables, potatoes and legumes (+2.3 vs. −0.1; P<0.01), and targeted vegetables (+1.6 vs. +0.1; P<0.01). Furthermore, at 12 months, participants in the intervention arm compared to the control arm maintained significantly higher intake from baseline of individual fruits/vegetables: citrus fruit (+0.1 vs. −0.2; P=0.01); dark-green vegetables (+0.5 vs. −0.1; P<0.01); and percent change in deep-yellow vegetables (+249.1% vs. −75.1%; P=0.02) (Table 1).
Table 1.
Change in dietary intake of fruits and vegetables at 12-months
| Baseline (BL)
|
12 months
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Δ from BL3
|
Percent Δ from BL3
|
||||||||||||||
| Group | N | Mean | SD | P values | N | Mean | SD | P values | Mean | SD | P values | Mean | SD | P values | |
|
|
|||||||||||||||
| Total fruit and vegetables1 | |||||||||||||||
| Daily fruits and vegetables servings | 0.12 | 0.10 | <0.01 | <0.01 | |||||||||||
| Intervention | 34 | 4.8 | 2.8 | 29 | 6.8 | 2.2 | 2.0 | 3.5 | 114.7 | 199.5 | |||||
| Control | 36 | 5.8 | 2.8 | 29 | 5.8 | 2.3 | −0.4 | 2.5 | 6.2 | 63.1 | |||||
| Fruits | 0.43 | 0.93 | 0.42 | 0.15 | |||||||||||
| Intervention | 34 | 2.2 | 2.2 | 29 | 2.7 | 1.5 | 0.4 | 2.7 | 218.1 | 689.2 | |||||
| Control | 36 | 2.6 | 2.0 | 29 | 2.8 | 1.9 | −0.2 | 2.1 | 17.2 | 83.0 | |||||
| Vegetables | 0.13 | 0.01 | <0.01 | 0.14 | |||||||||||
| Intervention | 34 | 2.5 | 1.8 | 29 | 4.1 | 1.4 | 1.6 | 2.2 | 496.2 | 1491.6 | |||||
| Control | 36 | 3.2 | 1.8 | 29 | 3.0 | 1.5 | −0.2 | 2.5 | 74.8 | 240.7 | |||||
| Restricted total2 | |||||||||||||||
| Daily fruits and vegetables servings | 0.37 | <0.01 | <0.01 | 0.01 | |||||||||||
| Intervention | 34 | 3.4 | 2.2 | 29 | 5.8 | 1.9 | 2.3 | 2.9 | 184.4 | 312.8 | |||||
| Control | 36 | 3.9 | 2.2 | 29 | 3.9 | 1.8 | −0.1 | 1.9 | 20.1 | 77.0 | |||||
| Fruits | 0.31 | 0.22 | 0.08 | 0.18 | |||||||||||
| Intervention | 34 | 1.4 | 1.6 | 29 | 2.3 | 1.3 | 0.7 | 2.2 | 598.4 | 2123.0 | |||||
| Control | 36 | 1.8 | 1.4 | 29 | 1.8 | 1.6 | −0.2 | 1.6 | −9.0 | 59.0 | |||||
| Vegetables | 0.78 | <0.01 | <0.01 | 0.40 | |||||||||||
| Intervention | 34 | 2.0 | 1.5 | 29 | 3.5 | 1.4 | 1.6 | 2.3 | 543.7 | 1418.6 | |||||
| Control | 36 | 2.1 | 1.5 | 29 | 2.1 | 0.9 | 0.1 | 1.7 | 264.8 | 1026.8 | |||||
| Fruit sub-categories | |||||||||||||||
| Citrus juice | 0.54 | 0.39 | 0.40 | 0.35 | |||||||||||
| Intervention | 34 | 0.4 | 1.2 | 29 | 0.3 | 0.5 | −0.1 | 1.2 | 101.3 | 392.7 | |||||
| Control | 36 | 0.3 | 0.6 | 29 | 0.4 | 0.8 | 0.1 | 0.6 | −40.1 | 76.1 | |||||
| Fruit juice excluding citrus | 0.49 | 0.20 | 0.05 | 0.24 | |||||||||||
| Intervention | 34 | 0.2 | 0.4 | 29 | 0.1 | 0.4 | −0.1 | 0.6 | 8170.7 | 17768.8 | |||||
| Control | 36 | 0.1 | 0.4 | 29 | 0.3 | 0.7 | 0.3 | 0.7 | 77.8 | 307.9 | |||||
| Citrus fruit | 0.02 | 0.28 | 0.01 | 0.62 | |||||||||||
| Intervention | 34 | 0.0 | 0.1 | 29 | 0.1 | 0.3 | 0.1 | 0.3 | −75.6 | 42.3 | |||||
| Control | 36 | 0.2 | 0.5 | 29 | 0.1 | 0.2 | −0.2 | 0.6 | −46.1 | 134.9 | |||||
| Fruit, excluding citrus | 0.89 | 0.28 | 0.31 | 0.15 | |||||||||||
| Intervention | 34 | 1.4 | 1.5 | 29 | 2.1 | 1.3 | 0.6 | 2.2 | 163.9 | 467.9 | |||||
| Control | 36 | 1.4 | 1.3 | 29 | 1.7 | 1.6 | 0.1 | 1.6 | 11.9 | 84.2 | |||||
| Avocado and similar | 0.03 | 0.91 | 0.13 | 0.49 | |||||||||||
| Intervention | 34 | 0.0 | 0.2 | 29 | 0.1 | 0.2 | 0.0 | 0.2 | −23.8 | 68.9 | |||||
| Control | 36 | 0.2 | 0.3 | 29 | 0.1 | 0.1 | −0.1 | 0.3 | −72.3 | 50.3 | |||||
| Fried fruits | 0.26 | 0.13 | 0.58 | 0.22 | |||||||||||
| Intervention | 34 | 0.2 | 0.5 | 29 | 0.0 | 0.3 | −0.2 | 0.7 | −100.0 | 0.0 | |||||
| Control | 36 | 0.5 | 1.1 | 29 | 0.2 | 0.5 | −0.3 | 1.2 | −80.5 | 41.2 | |||||
| Vegetable sub-categories | |||||||||||||||
| Dark-green | 0.31 | <0.01 | <0.01 | 0.78 | |||||||||||
| Intervention | 34 | 0.1 | 0.2 | 29 | 0.6 | 0.6 | 0.5 | 0.6 | 313.9 | 360.5 | |||||
| Control | 36 | 0.2 | 0.3 | 29 | 0.1 | 0.2 | −0.1 | 0.4 | 407.3 | 1060.0 | |||||
| Deep-yellow | 0.37 | 0.56 | 0.96 | 0.02 | |||||||||||
| Intervention | 34 | 0.1 | 0.2 | 29 | 0.3 | 0.3 | 0.1 | 0.4 | 249.1 | 566.9 | |||||
| Control | 36 | 0.2 | 0.4 | 29 | 0.3 | 0.6 | 0.1 | 0.6 | −75.1 | 40.8 | |||||
| Tomato | 0.47 | 0.02 | 0.15 | 0.22 | |||||||||||
| Intervention | 34 | 0.3 | 0.4 | 29 | 0.5 | 0.4 | 0.1 | 0.5 | 641.0 | 2444.0 | |||||
| Control | 36 | 0.3 | 0.4 | 29 | 0.2 | 0.3 | 0.0 | 0.4 | 33.7 | 195.4 | |||||
| White potatoes | 0.05 | 0.16 | 0.12 | 0.74 | |||||||||||
| Intervention | 34 | 0.3 | 0.6 | 29 | 0.2 | 0.2 | −0.2 | 0.6 | −51.6 | 75.0 | |||||
| Control | 36 | 0.8 | 1.1 | 29 | 0.3 | 0.4 | −0.5 | 1.1 | −62.1 | 92.9 | |||||
| Other starchy vegetables | 0.27 | 0.01 | 0.40 | 0.33 | |||||||||||
| Intervention | 34 | 0.2 | 0.3 | 29 | 0.1 | 0.2 | 0.0 | 0.4 | 485.2 | 1445.7 | |||||
| Control | 36 | 0.3 | 0.4 | 29 | 0.4 | 0.5 | 0.1 | 0.7 | 1748.4 | 5788.3 | |||||
| Legumes (cooked dried beans) | 0.60 | 0.75 | 0.49 | 0.39 | |||||||||||
| Intervention | 34 | 0.5 | 0.6 | 29 | 0.9 | 0.8 | 0.4 | 0.9 | 17.0 | 142.8 | |||||
| Control | 36 | 0.6 | 0.8 | 29 | 0.9 | 0.6 | 0.2 | 0.9 | 69.4 | 204.9 | |||||
| Other vegetables | 0.74 | <0.01 | 0.06 | 0.35 | |||||||||||
| Intervention | 34 | 0.9 | 0.8 | 29 | 1.3 | 0.9 | 0.5 | 1.4 | 274.6 | 474.9 | |||||
| Control | 36 | 0.8 | 0.7 | 29 | 0.6 | 0.4 | −0.1 | 0.9 | 164.3 | 372.6 | |||||
Serving counts for both fruits and vegetables were compiled using the University of Minnesota Nutrition Data System for Research (NDSR) Nutrition Coordinating Center (NCC) food group serving count system.
Serving counts reported here exclude juices, potatoes, fried vegetables and legumes.
Mean absolute and percent changes are based on individual-level data.
Change in dietary intake of fat
Change in percent kcal from fat, saturated fat, monounsaturated fat, polyunsaturated fat and trans fats at 3 and 6 months has been previously reported (29). While participants in the intervention arm maintained a decrease in percent calories from fat and saturated fat from baseline to 12 months, the difference compared to the controls was no longer statistically significant (Table 2).
Table 2.
Changes in dietary fat intake at 12-months
| Baseline (BL)
|
12 months
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Δ from BL1
|
Percent Δ from BL1
|
||||||||||||||
| Group | N | Mean | SD | P values | N | Mean | SD | P values | Mean | SD | P values | Mean | SD | P values | |
|
|
|||||||||||||||
| Daily total caloric intake (kcal) | 0.76 | 0.07 | 0.35 | 0.28 | |||||||||||
| Intervention | 34 | 1577.6 | 403.4 | 29 | 1440.5 | 440.2 | −121.9 | 540.8 | −4.0 | 33.5 | |||||
| Control | 36 | 1608.1 | 423.6 | 29 | 1640.4 | 390.9 | 9.3 | 523.2 | 5.6 | 32.9 | |||||
| Fat consumption | |||||||||||||||
| Total fat, % of daily total calories | 0.43 | 0.89 | 0.69 | 0.29 | |||||||||||
| Intervention | 34 | 28.5 | 6.4 | 29 | 26.2 | 8.0 | −2.2 | 10.4 | −3.1 | 34.0 | |||||
| Control | 36 | 27.0 | 8.9 | 29 | 25.9 | 6.9 | −1.1 | 10.0 | 9.7 | 53.7 | |||||
| Saturated, % of daily total calories | 0.29 | 0.56 | 0.31 | 0.10 | |||||||||||
| Intervention | 34 | 9.9 | 3.2 | 29 | 8.8 | 3.4 | −0.9 | 4.2 | −1.6 | 44.1 | |||||
| Control | 36 | 8.9 | 4.0 | 29 | 9.3 | 3.0 | 0.2 | 4.0 | 27.9 | 83.8 | |||||
| Monounsaturated, % of daily total calories | 0.45 | 0.11 | 0.50 | 0.72 | |||||||||||
| Intervention | 34 | 10.9 | 3.4 | 29 | 11.4 | 4.1 | 0.6 | 5.1 | 14.2 | 49.6 | |||||
| Control | 36 | 10.2 | 4.2 | 29 | 9.9 | 3.1 | −0.3 | 5.0 | 20.0 | 69.2 | |||||
| Polyunsaturated, % of daily total calories | 0.68 | 0.89 | 0.93 | 0.87 | |||||||||||
| Intervention | 34 | 5.2 | 1.7 | 29 | 4.7 | 1.4 | −0.6 | 2.4 | 0.2 | 51.1 | |||||
| Control | 36 | 5.4 | 2.1 | 29 | 4.8 | 1.7 | −0.5 | 2.3 | −1.7 | 38.9 | |||||
| Trans fats, % of daily total calories | 0.53 | 0.67 | 0.72 | 0.33 | |||||||||||
| Intervention | 34 | 0.8 | 0.4 | 29 | 0.7 | 0.4 | 0.0 | 0.5 | 14.5 | 80.7 | |||||
| Control | 36 | 0.8 | 0.8 | 29 | 0.7 | 0.5 | −0.1 | 1.0 | 242.3 | 1226.6 | |||||
| Energy density (kcal/grams) | 0.65 | 0.04 | 0.29 | 0.24 | |||||||||||
| Intervention | 34 | 0.8 | 0.2 | 29 | 0.6 | 0.2 | −0.1 | 0.3 | −10.4 | 31.5 | |||||
| Control | 36 | 0.8 | 0.2 | 29 | 0.7 | 0.1 | 0.0 | 0.2 | −1.7 | 24.2 | |||||
Mean absolute and percent changes are based on individual-level data.
Change in anthropometric measures
Change in weight, BMI, waist circumference, hip circumference, and waist-to-hip ratio at 3 and 6 months has been previously reported (29). At 12 months, participants in the intervention arm maintained a numerical, but not statistically significant difference compared to the controls in percent weight change (−3.1% vs. −1.6%; P=0.50); BMI change (−2.8% vs. −1.6%; P=0.59); and waist circumference (+0.1% vs. +0.4%; P=0.89) (Table 3).
Table 3.
Changes in anthropometric measures at 12-months
| Baseline
|
12 months
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Δ from BL1
|
Percent Δ from BL1
|
||||||||||||||
| Group | N | Mean | SD | P values | N | Mean | SD | Mean | SD | P values | Mean | SD | P values | ||
|
|
|||||||||||||||
| Weight (kg) | 0.55 | 0.56 | 0.50 | ||||||||||||
| Intervention | 34 | 75.7 | 17.2 | 29 | 74.2 | 17.8 | −2.6 | 7.3 | −3.1 | 9.6 | |||||
| Control | 36 | 78.2 | 16.1 | 29 | 78.1 | 15.9 | −1.5 | 6.6 | −1.6 | 7.1 | |||||
| BMI (kg/m2) | 0.78 | 0.52 | 0.59 | ||||||||||||
| Intervention | 32 | 30.7 | 6.5 | 29 | 29.9 | 6.6 | −1.0 | 3.2 | −2.8 | 10.2 | |||||
| Control | 36 | 31.1 | 5.6 | 29 | 31.6 | 5.8 | −0.6 | 2.4 | −1.6 | 7.1 | |||||
| Waist circumference (cm) | 0.31 | 0.64 | 0.89 | ||||||||||||
| Intervention | 33 | 94.3 | 14.2 | 23 | 92.8 | 12.4 | −0.5 | 7.7 | 0.1 | 7.4 | |||||
| Control | 35 | 97.7 | 13.3 | 26 | 100.3 | 13.0 | 0.3 | 4.4 | 0.4 | 4.2 | |||||
| Hip circumference (cm) | 0.34 | 0.49 | 0.55 | ||||||||||||
| Intervention | 33 | 108.8 | 14.3 | 23 | 108.7 | 13.8 | 0.8 | 4.2 | 0.9 | 4.0 | |||||
| Control | 35 | 112.2 | 15.1 | 26 | 112.7 | 12.6 | −1.1 | 12.5 | −0.2 | 8.0 | |||||
| Waist-to-hip ratio | 0.27 | 0.41 | 0.38 | ||||||||||||
| Intervention | 26 | 0.9 | 0.1 | 33 | 0.9 | 0.1 | 0 | 0.1 | 0.6 | 9.6 | |||||
| Control | 25 | 0.9 | 0.1 | 35 | 0.9 | 0.1 | 0 | 0.1 | −1.7 | 9.6 | |||||
Mean absolute and percent changes are based on individual-level data.
Change in biomarkers: carotenoids and retinol, metabolic markers, markers of inflammation, DNA methylation
Carotenoids and retinol
At 6 months, participants in the intervention arm achieved significant percent change from baseline in plasma lutein (+13.8% vs. −9.7%; P<0.01); and α-carotene (+33.5% vs. +3.1%; P=0.04) compared to controls. At 12 months, participants in the intervention arm maintained a significant trend in differences compared to the controls in plasma lutein (+20.4% vs. −11.5%; P<0.01) (Table 4).
Table 4.
Changes in biomarkers at 12-months
| Baseline
|
12 months
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Δ from BL2
|
Percent Δ from BL2
|
||||||||||||||
| Group | N | Mean | SD | P values | N | Mean | SD | P values | Mean | SD | P values | Mean | SD | P values | |
|
|
|||||||||||||||
| Plasma Carotenoids | |||||||||||||||
| Total carotenoids (μg/L) | 0.48 | 0.96 | 0.76 | 0.24 | |||||||||||
| Intervention | 34 | 642.8 | 287.8 | 22 | 742.1 | 222.6 | 46.4 | 295.3 | 15.8 | 34.0 | |||||
| Control | 33 | 702.5 | 390.2 | 25 | 736.0 | 522.2 | 21.0 | 266.6 | 3.3 | 37.1 | |||||
| Lutein (μg/L) | 0.26 | 0.06 | <0.01 | <0.01 | |||||||||||
| Intervention | 34 | 109.5 | 51.0 | 22 | 132.3 | 48.9 | 15.2 | 41.6 | 20.4 | 37.8 | |||||
| Control | 33 | 125.5 | 64.0 | 25 | 105.3 | 47.2 | −21.3 | 45.0 | −11.5 | 24.2 | |||||
| Lycopene (μg/L) | 0.96 | 0.14 | 0.14 | 0.08 | |||||||||||
| Intervention | 34 | 226.6 | 98.2 | 22 | 253.6 | 138.2 | 17.8 | 103.2 | 16.2 | 42.1 | |||||
| Control | 33 | 225.3 | 107.6 | 25 | 198.3 | 107.3 | −20.1 | 60.9 | −5.6 | 39.1 | |||||
| α-carotene (μg/L) | 0.48 | 0.57 | 0.68 | 0.92 | |||||||||||
| Intervention | 34 | 79.8 | 52.8 | 22 | 102.4 | 86.9 | 15.4 | 62.6 | 23.7 | 44.8 | |||||
| Control | 33 | 95.7 | 117.5 | 25 | 125.6 | 180.6 | 24.9 | 88.8 | 21.4 | 104.3 | |||||
| β-carotene (μg/L) | 0.54 | 0.32 | 0.35 | 0.32 | |||||||||||
| Intervention | 34 | 117.3 | 181.3 | 22 | 132.8 | 92.2 | −6.6 | 179.1 | 1387.7 | 6234.1 | |||||
| Control | 33 | 147.5 | 215.3 | 25 | 200.4 | 318.3 | 40.6 | 153.6 | 39.7 | 144.6 | |||||
| β-cryptoxanthin (μg/L) | 0.87 | 0.06 | 0.19 | 0.17 | |||||||||||
| Intervention | 34 | 109.6 | 28.5 | 22 | 121.0 | 28.2 | 4.6 | 22.0 | 5.7 | 19.6 | |||||
| Control | 33 | 108.5 | 23.9 | 25 | 106.4 | 22.3 | −3.1 | 15.6 | −1.4 | 14.8 | |||||
| Retinol (μg/L) | 0.12 | 0.10 | 0.25 | 0.15 | |||||||||||
| Intervention | 34 | 589.9 | 128.2 | 22 | 575.3 | 124.5 | 18.5 | 97.9 | 4.7 | 17.2 | |||||
| Control | 33 | 644.8 | 153.2 | 25 | 650.0 | 174.3 | −14.0 | 89.1 | −2.1 | 13.4 | |||||
| Metabolic markers | |||||||||||||||
| Glucose (mg/dL) | 0.33 | 0.05 | 0.21 | 0.26 | |||||||||||
| Intervention | 34 | 102.8 | 23.9 | 23 | 99.1 | 16.8 | −1.0 | 12.3 | −0.1 | 10.0 | |||||
| Control | 33 | 108.6 | 24.9 | 25 | 117.0 | 39.9 | 6.2 | 25.2 | 4.9 | 19.1 | |||||
| Insulin (mIU/L) | 0.72 | 0.57 | 0.43 | 0.82 | |||||||||||
| Intervention | 32 | 17.0 | 13.2 | 22 | 15.1 | 7.9 | −1.5 | 9.2 | 10.0 | 50.1 | |||||
| Control | 33 | 18.4 | 18.5 | 24 | 16.5 | 9.5 | 0.5 | 6.8 | 6.8 | 40.3 | |||||
| HOMA-IR1 | 0.71 | 0.12 | 0.22 | 0.93 | |||||||||||
| Intervention | 32 | 4.8 | 5.1 | 22 | 3.7 | 2.0 | −0.6 | 3.1 | 12.6 | 53.7 | |||||
| Control | 33 | 5.3 | 6.9 | 24 | 5.0 | 3.7 | 0.5 | 2.6 | 14.0 | 50.7 | |||||
| Markers of inflammation | |||||||||||||||
| GM-CSF (pg/ml)1 | 0.38 | 0.37 | 0.41 | 0.59 | |||||||||||
| Intervention | 34 | 0.9 | 2.6 | 23 | 1.0 | 2.9 | 0.5 | 2.0 | 14.5 | 54.3 | |||||
| Control | 33 | 0.5 | 1.1 | 25 | 0.5 | 1.1 | 0.1 | 0.7 | 49.7 | 311.4 | |||||
| IL-1 α (pg/ml)1 | 0.34 | 0.28 | 0.56 | 0.67 | |||||||||||
| Intervention | 34 | 0.2 | 0.4 | 23 | 0.4 | 1.0 | 0.1 | 1.0 | 22.5 | 123.3 | |||||
| Control | 33 | 0.2 | 0.1 | 25 | 0.2 | 0.1 | 0.0 | 0.1 | 10.0 | 60.7 | |||||
| IL-6 (pg/ml) | 0.84 | 0.54 | 0.71 | 0.84 | |||||||||||
| Intervention | 34 | 10.3 | 30.1 | 23 | 8.3 | 18.9 | 2.1 | 10.1 | 3.8 | 55.5 | |||||
| Control | 33 | 12.0 | 39.1 | 25 | 16.6 | 64.3 | 3.8 | 20.3 | 7.4 | 63.8 | |||||
| IL-8 (pg/ml) | 0.74 | 0.84 | 0.66 | 0.45 | |||||||||||
| Intervention | 34 | 8.3 | 7.6 | 23 | 7.2 | 5.5 | 0.1 | 3.5 | 3.9 | 47.6 | |||||
| Control | 33 | 7.8 | 4.5 | 25 | 7.5 | 3.9 | 0.5 | 3.4 | 15.6 | 58.0 | |||||
| IL-10 (pg/ml) | 0.63 | 0.64 | 0.54 | 0.51 | |||||||||||
| Intervention | 34 | 16.5 | 19.2 | 23 | 25.0 | 61.2 | 6.9 | 42.4 | 30.9 | 140.3 | |||||
| Control | 33 | 19.2 | 27.0 | 25 | 18.2 | 30.1 | 1.3 | 8.9 | 9.7 | 64.2 | |||||
| TNF-α (pg/ml)1 | 0.92 | 0.92 | 0.60 | 0.90 | |||||||||||
| Intervention | 34 | 12.5 | 7.7 | 23 | 11.7 | 7.3 | −0.5 | 6.1 | 2.3 | 63.1 | |||||
| Control | 33 | 12.3 | 5.5 | 25 | 11.9 | 4.5 | 0.2 | 2.6 | 4.2 | 24.5 | |||||
| CRPhs (mg/L)1 | 0.67 | 0.78 | 0.75 | 0.62 | |||||||||||
| Intervention | 34 | 4.4 | 8.3 | 23 | 4.0 | 7.6 | 0.2 | 5.0 | 9.7 | 76.0 | |||||
| Control | 33 | 3.7 | 3.5 | 25 | 3.5 | 3.0 | −0.2 | 1.6 | 0.7 | 42.6 | |||||
| DNA Methylation | |||||||||||||||
| LINE-11 | 0.59 | 0.59 | 0.06 | 0.06 | |||||||||||
| Intervention | 32 | 69.5 | 2.6 | 23 | 69.9 | 2.2 | 0.8 | 2.3 | 1.2 | 3.5 | |||||
| Control | 32 | 69.9 | 3.2 | 24 | 69.5 | 2.7 | −0.5 | 2.5 | −0.7 | 3.8 | |||||
HOMA-IR, homeostasis model of assessment of insulin resistance; GM-CSF, granulated macrophage colony stimulating factor; IL, interleukin; TNF, tumor necrosis factor; CRPhs, high sensitivity C-reactive protein; LINE-1, long interspersed nucleotide element 1
Mean absolute and percent changes are based on individual-level data.
Metabolic markers
At 6 months, participants in the intervention had a non-significantly greater increase in fasting glucose, insulin, and HOMA-IR compared to controls. At 12 months, there was no significant difference in metabolic changes (Table 4).
Markers of inflammation
At 6 months, the intervention group had non-significant greater decreases in IL-1α, IL-6, IL-10, TNF-α, and CRPhs compared to the controls. At 12 months, the level of inflammatory markers increased in both groups, but the intervention group had non-significant lower increases in GM-CSF, IL-6, IL-8, and TNF-α compared to controls (Table 4).
DNA methylation
At 6 months, participants in the intervention arm achieved a non-significant increase from baseline in global DNA methylation (+0.9% vs. +0.5%; P=0.56); and maintained a borderline significant increase at 12 months (+0.8% vs. −0.5%; P=0.06) compared to the controls (Table 4).
GEE analyses of group differences in change from baseline to 6 and 12 months
At 6 months, compared to the controls, participants in the intervention arm achieved greater increases in daily servings of fruits/vegetables (+2.4; P<0.01); vegetables (+1.5; P<0.01); targeted fruits/vegetables (+2.3; P<0.01); targeted fruits (+1.1; P=0.01); and targeted vegetables (+1.2; P=0.01). Participants in the intervention arm had greater increases compared to the controls in intake of the following fruits/vegetables: avocado and similar (+0.1; P=0.04); dark-green vegetables (+0.6; P<0.01); deep-yellow vegetables (+0.3; P=0.01). In addition, participants in the intervention arm achieved a greater decrease in daily total caloric intake (−388.4 kcal; P<0.01) and a greater increase in plasma lutein (+33.9 μg/L; P<0.01) compared to the controls (Table 5).
Table 5.
GEE estimates of the difference in changes from baseline to 6 and 12 month between randomization groups.
| 6 months
|
12 months
|
|||
|---|---|---|---|---|
| β3 | P values4 | β3 | P values4 | |
|
|
||||
| Total fruit and vegetables1 | ||||
| Daily fruits and vegetables servings | 2.40 | <0.01 | 2.09 | 0.01 |
| Fruits | 0.82 | 0.12 | 0.28 | 0.66 |
| Vegetables | 1.54 | <0.01 | 1.74 | <0.01 |
| Restricted total2 | ||||
| Daily fruits and vegetables servings | 2.29 | <0.01 | 2.45 | <0.01 |
| Fruits | 1.12 | 0.01 | 0.69 | 0.17 |
| Vegetables | 1.16 | 0.01 | 1.74 | <0.01 |
| Fruit sub-categories | ||||
| Citrus juice | −0.27 | 0.24 | −0.19 | 0.42 |
| Fruit juice excluding citrus | −0.18 | 0.18 | −0.27 | 0.12 |
| Citrus fruit | 0.14 | 0.18 | 0.17 | 0.11 |
| Fruit, excluding citrus | 0.87 | 0.05 | 0.48 | 0.33 |
| Avocado and similar | 0.12 | 0.03 | 0.04 | 0.57 |
| Fried fruits | 0.17 | 0.46 | 0.08 | 0.73 |
| Vegetable sub-categories | ||||
| Dark-green | 0.63 | <0.01 | 0.59 | <0.01 |
| Deep-yellow | 0.34 | 0.01 | 0.02 | 0.89 |
| Tomato | 0.04 | 0.68 | 0.20 | 0.09 |
| White potatoes | 0.36 | 0.10 | 0.28 | 0.18 |
| Other starchy vegetables | 0.12 | 0.23 | −0.12 | 0.38 |
| Legumes (cooked dried beans) | −0.37 | 0.19 | 0.35 | 0.13 |
| Other vegetables | 0.52 | 0.06 | 0.58 | 0.05 |
| Daily total caloric intake (kcal) | −388.41 | <0.01 | −25.56 | 0.85 |
| Fat consumption | ||||
| Total fat, % of daily total calories | −3.79 | 0.10 | −2.99 | 0.23 |
| Saturated, % of daily total calories | −1.59 | 0.12 | −1.37 | 0.18 |
| Monounsaturated, % of daily total calories | −1.15 | 0.32 | −0.26 | 0.84 |
| Polyunsaturated, % of daily total calories | −1.10 | 0.09 | −0.22 | 0.71 |
| Trans fats, % of daily total calories | −0.14 | 0.44 | −0.04 | 0.82 |
| Energy density (kcal/grams) | −0.17 | <0.01 | −0.05 | 0.41 |
| Anthropometric measures | ||||
| Weight (kg) | −0.99 | 0.49 | −2.64 | 0.29 |
| BMI (kg/m2) | −0.82 | 0.26 | −1.08 | 0.48 |
| Waist circumference (cm) | −2.67 | 0.10 | −0.21 | 0.88 |
| Hip circumference (cm) | 0.86 | 0.71 | 1.31 | 0.40 |
| Waist-to-hip ratio | −0.02 | 0.30 | −0.02 | 0.28 |
| Plasma retinol and carotenoids | ||||
| Retinol (μg/L) | −4.33 | 0.82 | 8.56 | 0.76 |
| Lutein (μg/L) | 33.92 | <0.01 | 69.37 | <0.01 |
| Lycopene (μg/L) | 18.85 | 0.53 | 32.81 | 0.11 |
| α-carotene (μg/L) | 10.39 | 0.69 | 6.07 | 0.84 |
| β-carotene (μg/L) | −1.06 | 0.98 | −48.76 | 0.33 |
| β-Cryptoxathin (μg/L) | 4.97 | 0.34 | 6.82 | 0.19 |
| Total carotenoids (μg/L) | 69.50 | 0.30 | 19.11 | 0.80 |
| Metabolic markers | ||||
| Glucose (mg/dL) | −3.22 | 0.51 | −9.12 | 0.22 |
| Insulin (mIU/L) | 3.47 | 0.43 | 0.53 | 0.88 |
| HOMA-IR | 0.75 | 0.63 | −0.58 | 0.68 |
| Markers of inflammation | ||||
| GM-CSF (pg/ml) | −0.18 | 0.62 | 0.27 | 0.69 |
| IL-1 α (pg/ml) | −0.04 | 0.49 | 0.26 | 0.36 |
| IL-6 (pg/ml) | −1.69 | 0.76 | −12.53 | 0.38 |
| IL-8 (pg/ml) | 0.46 | 0.75 | 0.66 | 0.66 |
| IL-10 (pg/ml) | 5.87 | 0.42 | 32.82 | 0.32 |
| TNF-α (pg/ml) | −1.12 | 0.35 | −0.18 | 0.93 |
| CRPhs (mg/L) | −0.10 | 0.96 | −0.29 | 0.87 |
| DNA Methylation | ||||
| LINE-1 | 0.36 | 0.55 | 1.11 | 0.09 |
Serving counts for both fruits and vegetables were compiled using the University of Minnesota Nutrition Data System for Research (NDSR) Nutrition Coordinating Center (NCC) food group serving count system.
Serving counts reported here exclude juices, potatoes, fried vegetables and legumes.
β coefficients were group differences in the changes from baseline to 6 or 12-month, adjusted for stratification.
95% CIs and P values were calculated using the robust standard error from a GEE model.
Abbreviations: HOMA-IR, homeostasis model of assessment of insulin resistance; GM-CSF, granulated macrophage colony stimulating factor; IL, interleukin; TNF, tumor necrosis factor; CRPhs, high sensitive C-reactive protein; LINE-1, long interspersed nucleotide element 1
At 12 months, compared to the controls, participants in the intervention arm maintained greater increases in daily servings of fruits/vegetables (+2.1; P<0.01); vegetables (+1.7; P<0.01); targeted fruits/vegetables (+2.5; P<0.01); and targeted vegetables (+1.7; P<0.01), dark-green vegetables (+0.6; P<0.01), and lutein (+69.4 μg/L; P<0.01) (Table 5).
Association between changes in diet and changes in anthropometric, metabolic, inflammation and DNA methylation markers
We conducted hypothesis generating secondary analyses to understand how the changes in dietary components were associated with changes in cancer-related biomarkers. Figure 1 shows a heat map style figure indicating associations between dietary components and changes in anthropometric, metabolic, inflammatory and DNA methylation markers. We found that increases in fruit/vegetable intake was generally associated with beneficial changes in anthropometric, metabolic, inflammation and DNA methylation markers, while increases in fat intake were associated with worsening of these biomarkers. In addition, increases in both lutein and retinol were associated with beneficial changes in inflammatory markers. However, the increase in retinol was associated with unfavorable changes in some anthropometric outcomes (weight, BMI and waist circumference) (P<0.05, data not shown).
Figure 1. Heat map of associations between changes in dietary components and changes in anthropometric, metabolic, inflammatory and DNA methylation markers.
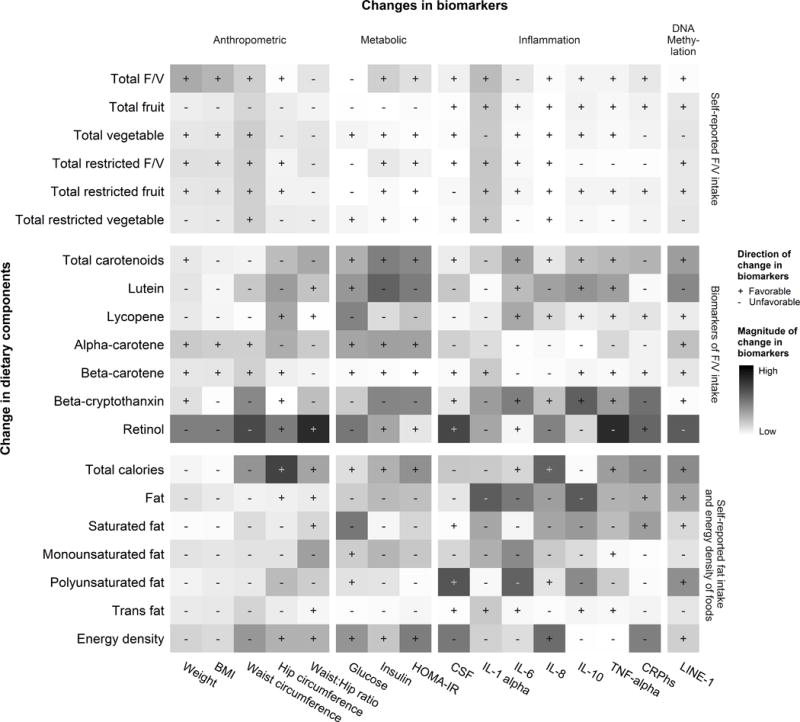
Each pixel represents the percent change in cancer-related biomarkers on the x-axis associated with every 10% change in dietary factors on the y-axis, adjusted for the baseline value of the dietary factor of interest, randomization arm and stratification. These estimated changes in cancer-related biomarkers were labelled as “+” if the change is in a favorable direction that reflects improvements in biomarkers, or “−” if otherwise. The darkness of color represents the magnitude of percent changes in cancer-related biomarkers. Our results showed that increases in fruit/vegetable intake were generally beneficial as measured by these cancer-related biomarkers, while increases in dietary fat generally led to unfavorable changes.
DISCUSSION
The 9-session (24 hours over 12 weeks) ¡Cocinar Para Su Salud! culturally-based dietary intervention successfully increased the combined intake of fruits/vegetables among urban Hispanic breast cancer survivors, the majority of whom were of low socioeconomic status, and the dietary changes persisted at 12 months. The intervention focused on teaching women how to achieve and maintain the dietary composition guidelines set forth by the AICR and ACS (3, 4). At 12 months, women in the intervention group consumed more daily servings of fruits/vegetables than women in the control group and, more importantly, they consumed more dark-green and deep-yellow vegetables. These self-reported results were confirmed by measured increases in plasma carotenoids, specifically lutein, a marker of green leafy vegetable intake. At 3 months, there was a decrease in the daily percent calories from fat in the intervention group compared with the control group, but this difference did not persist at 12 months, partially because the control group also changed their diet.
Among the several diet and physical activity interventions that have been conducted among cancer survivors, few resulted in maintenance of long-term behavioral change (25–28). Two large dietary interventions among breast cancer survivors were effective in long-term maintenance of dietary changes. Participants in the Women’s Intervention Nutrition Study (WINS) intervention were successful in maintaining significantly lower intake of dietary fat at 5 years and participants in the Women’s Healthy Eating and Living (WHEL) study reported significant increases in fruit and vegetable intake and decreases in dietary fat intake at 6 years (12, 42). However, both interventions were long, intensive and individualized, and included maintenance follow-up contacts throughout the duration of the studies, making it difficult to determine if observed dietary changes would be sustainable once behavior changes are no longer reinforced. In contrast, the ¡Cocinar Para Su Salud! short-term intervention was successful in promoting long-term increases in fruit and vegetable intake with minimal reinforcement.
The U.S. Hispanic population is heterogeneous and consists of both new immigrants and resident Hispanics. Previous findings have demonstrated that minorities are less likely to adhere to prevention programs or participate in clinical trials compared to non-Hispanic whites (43–46). Important barriers, including language, family support, and work constraints have been identified as potential barriers to adherence in minority populations (47–51). As such, there is a need for culturally-based dietary interventions and studies examining behavioral change specifically among minority breast cancer survivors, including Hispanics (52–54). Our study was unique in that it used a hands-on approach to address specific barriers related to Hispanics. All intervention procedures were conducted entirely in Spanish, minimizing potential barriers related to language. Additionally, lessons and other program procedures were conducted in small group settings, making it easier for women to be willing to participate and providing women with peer support.
Although our results showed that participants in the intervention arm had trends towards more favorable changes in biomarkers of interest compared to the control group, only the changes in lutein showed a statistically significant difference at 12 months. Obviously, in order for a behavioral intervention to affect cancer recurrence and survival outcomes, it is likely that long-term changes are needed in biomarkers along the carcinogenesis pathways. The fact that we did not observe long-term changes in many of the biomarkers could result from the intervention not being intensive enough. The lack of statistical significance may be also due to limited power to detect a difference, as sample size was determined based on dietary changes not change in biomarkers. Because most biomarkers have a smaller mean:standard deviation ratio compared to fruit/vegetable intake, a larger sample size would likely be needed to detect statistically significant differences in biomarkers.
We did observe a trend towards increased DNA methylation levels in the intervention but not the control arm. A growing body of literature suggests that high fruit/vegetable intake may be associated with high DNA methylation of LINE-1 (55–57). In addition, increased LINE-1 DNA methylation is associated with decreased genomic instability and less frequent nucleic acid sequence changes or chromosomal rearrangements (58, 59), which are important biological mechanisms underlying the cancer development and progression. Our findings support the hypothesis that higher fruit/vegetable intake may increase DNA methylation. However, the magnitude and duration of change in DNA methylation needed to affect cancer endpoints is unknown.
Unexpectedly, our hypothesis-generating analyses show that anthropometric measures were associated with circulating concentrations of retinol, indicating that as the subjects became leaner their circulating levels of retinol decreased. This is an intriguing observation because of the recent identification of retinol-binding protein (RBP4) as an adipokine, as well as RBP4’s association with breast cancer (60–62). RBP4 secreted from the liver is the sole transport protein for retinol in the blood, with circulating levels of retinol tightly correlating with circulating RBP4 levels (63, 64). Adipose tissue also synthesizes and secretes RBP4, with Yang et al. subsequently proposing that this RBP4 was an adipokine, providing a link between increased adiposity, insulin resistance and type 2 diabetes mellitus (60, 65). Although we did not measure RBP4 directly, we speculate that the association between decreasing levels of circulating retinol and anthropometric indicators of adiposity (i.e., body weight, BMI, and waist circumference) are reflective of decreased circulating RBP4 levels. As reviewed by Frey (66), some follow-up studies have confirmed the link between increased circulating levels of RBP4 and insulin resistance, while others have not seen a significant effect. The majority of these follow-up studies only reported circulating RBP4 levels and did not measure retinol, thus, our data is of particular interest because we show a correlation between circulating retinol levels and indicators of adiposity. Although we did not observe a significant association between retinol and the measured metabolic parameters (glucose, insulin, HOMA-IR), it is possible that the slight decrease in body weight and associated decrease in circulating retinol levels may be beneficial and reflect an improvement in insulin sensitivity. Further research with a focus on these parameters is required to definitively establish a link between markers of adiposity and insulin resistance, and circulating levels of RBP4 and retinol in this population. Our data also show that serum retinol levels are significantly associated with waist circumference, but not hip circumference. This observation is consistent with the fact that RBP4 is expressed at a higher level in visceral adipose tissue vs. subcutaneous adipose tissue, and is therefore a marker of intra-abdominal fat mass (67). Similarly, Lee et al. demonstrated that serum RBP4 levels are correlated with visceral adiposity but not subcutaneous fat area in women (68). Thus, the significant association we observed between retinol and waist circumference - but not hip circumference - is consistent with the literature regarding the known expression pattern of RBP4 in these different adipose tissue depots, and the link between visceral adiposity and circulating RBP4.
While this intervention did not actively examine dietary change in relation to cancer-related outcomes, our results are unique as there are scant data on behavioral change among Hispanic breast cancer survivors. Strengths of this study include its rigorous, randomized controlled design and the use of three, separate 24-hour dietary recalls. One limitation of this study is that the brief Block Fruit/Vegetable/Fiber questionnaire was used to assess fruit/vegetable intake prior to enrollment and may have resulted in an underrepresentation of intake at screening, as the baseline assessments using the 24-hour recalls reported higher intake. Additionally, because this study was conducted specifically among Hispanic cancer survivors in an urban environment it is likely that results are not generalizable to other populations of cancer survivors.
Using a theory-based, culturally-tailored curriculum design, the ¡Cocinar Para Su Salud! study was successful in improving fruit and vegetable intake, but not total fat intake, among a group of Hispanic breast cancer survivors and maintaining them at 12 months with minimal reinforcement. Such an intervention may be beneficial in other disease types where fruit/vegetable intake is important (i.e., colorectal cancer). However, the intervention had modest but provocative effects on biomarkers of interest. Dietary changes that address well-established risk factors for primary and secondary cancers, such as inadequate nutrition and obesity, are likely to help cancer survivors reduce their risk and improve their overall health. Our research can inform future community-based dietary interventions aiming to promote long-term behavioral change among Hispanics. Future studies can examine how best to promote and implement changes in other behaviors, including other dietary components, physical activity and weight management.
Acknowledgments
The authors would like to thank Maria Alvarez and Rossy Sandoval who assisted with study recruitment, and Monica Gonzalez, RDN, Lisa Zulig, MS, RDN, and health supportive chef Ela Guidon who assisted with developing and implementing the ¡Cocinar Para Su Salud! curriculum.
SUPPORT/FUNDING: Supported by the NCI/NIH R21CA152903 (H. Greenlee, A.O. Gaffney, A.C. Aycinena, P. Koch, I. Contento, J.M. Richardson, W.Y. Tsai, R.D. Clugston, D.L. Hershman), NIEHS/NIH P30 ES009089 (R. Santella), Herbert Irving Comprehensive Cancer Center Cancer Center Support Grant NCI/NIH 5P30 CA013696, and Columbia University’s Clinical and Translational Science Award grant UL1TR000040 from the National Center for Advancing Translational Sciences (W. Karmally, S. Cremers), National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: None
CLINICALTRIALS.GOV IDENTIFIER: NCT01414062
References
- 1.American Cancer Society. Breast Cancer Facts & Figures, 2015–2016. 2015 [cited 2015 21 Dec]; [Google Scholar]
- 2.Goodwin PJ, Ambrosone CB, CC H. Modifiable Lifestyle Factors and Breast Cancer Outcomes: Current Controversies and Research Recommendations. Adv Exp Med Biol. 2015:177–92. doi: 10.1007/978-3-319-16366-6_12. [DOI] [PubMed] [Google Scholar]
- 3.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 4.American Institute for Cancer Research. AICR’S Guidelines For Cancer Survivors: Recommendations to Reduce Your Cancer Risk. 2015 [cited 2015 21 Dec]; Available from: http://www.aicr.org/patients-survivors/aicrs-guidelines-for-cancer.html.
- 5.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard CM, Courneya KS, K S. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 7.Spark LC, Reeves MM, Fjeldsoe BS, Eakin EG. Physical activity and/or dietary interventions in breast cancer survivors: a systematic review of the maintenance of outcomes. Journal of cancer survivorship: research and practice. 2013;7:74–82. doi: 10.1007/s11764-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 8.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2014;15:749–68. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 9.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J Clin Oncol. 2015;33:3169–76. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32:2231–9. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 11.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. Journal of the National Cancer Institute. 2013;105:1821–32. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 12.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. Journal of the National Cancer Institute. 2006;98:1767–76. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2015–2017. 2015 [cited 2015 21 Dec]; Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046405.pdf.
- 15.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127:729–38. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. Am J Epidemiol. 2002;155:534–45. doi: 10.1093/aje/155.6.534. [DOI] [PubMed] [Google Scholar]
- 17.Boone SD, Baumgartner KB, Joste NE, Pinkston CM, Yang D, Baumgartner RN. The joint contribution of tumor phenotype and education to breast cancer survival disparity between Hispanic and non-Hispanic white women. Cancer Causes Control. 2014;25:273–82. doi: 10.1007/s10552-013-0329-3. [DOI] [PubMed] [Google Scholar]
- 18.Warner ET, Tamimi RM, Hughes ME. Mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33:2254–61. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. NCHS Health E-Stat. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 20.Siega-Riz AM, Sotres-Alvarez D, Ayala GX, Ginsberg M, Himes JH, Liu K, et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99:1487–98. doi: 10.3945/ajcn.113.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumanyika SK, Van Horn L, Bowen D, Perri MG, Rolls BJ, Czajkowski SM, et al. Maintenance of dietary behavior change. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2000;19:42–56. doi: 10.1037/0278-6133.19.suppl1.42. [DOI] [PubMed] [Google Scholar]
- 22.Wing RR, Papandonatos G, Fava JL, Gorin AA, Phelan S, McCaffery J, et al. Maintaining large weight losses: the role of behavioral and psychological factors. Journal of consulting and clinical psychology. 2008;76:1015–21. doi: 10.1037/a0014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. The New England journal of medicine. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 24.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL, Machan J. STOP regain: are there negative effects of daily weighing? Journal of consulting and clinical psychology. 2007;75:652–6. doi: 10.1037/0022-006X.75.4.652. [DOI] [PubMed] [Google Scholar]
- 25.Cantarero-Villanueva I, Fernandez-Lao C, Del Moral-Avila R, Fernandez-de-Las-Penas C, Feriche-Fernandez-Castanys MB, Arroyo-Morales M. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evidence-based complementary and alternative medicine: eCAM. 2012;2012:620619. doi: 10.1155/2012/620619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–18. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 27.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. The journal of supportive oncology. 2009;7:158–67. [PMC free article] [PubMed] [Google Scholar]
- 28.Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ (Clinical research ed) 2007;334:517. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenlee H, Gaffney AO, Aycinena AC, Koch P, Contento I, Karmally W, et al. ¡Cocinar Para Su Salud!: Randomized Controlled Trial of a Culturally Based Dietary Intervention among Hispanic Breast Cancer Survivors. Journal of the Academy of Nutrition and Dietetics. 2015;115:709–23 e3. doi: 10.1016/j.jand.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aycinena AC, Jennings KA, Gaffney AO, Koch P, Contento I, Gonzalez M, et al. ¡Cocinar Para Su Salud!: Development of a culturally-based nutrition education curriculum for Hispanic breast cancer survivors using a theory-driven procedural model. Health Educ Behav. 2016 doi: 10.1177/1090198116642236. In press. [DOI] [PubMed] [Google Scholar]
- 31.Cook For Your Life. 2016 [cited 2016 May 13]; Available from: http://cookforyourlife.org/
- 32.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. American journal of preventive medicine. 2000;18:284–8. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 33.Dwyer J, Picciano MF, Raiten DJ. Collection of food and dietary supplement intake data: What we eat in America-NHANES. J Nutr. 2003;133:590S–600S. doi: 10.1093/jn/133.2.590S. [DOI] [PubMed] [Google Scholar]
- 34.Mills JL, Tuomilehto J, Yu F, Colman N, Blaner WS, Koskela P, et al. Maternal vitamin levels during pregnancies producing infants with neural tube defects. J Pediatr. 1992;120:863–71. doi: 10.1016/s0022-3476(05)81951-1. [DOI] [PubMed] [Google Scholar]
- 35.Redlich CA, Grauer JN, VanBennekum AM, Clever SL, Ponn RB, Blaner WS. Characterization of carotenoid, vitamin A, and alpha-tocopherol levels in human lung tissue and pulmonary macrophages. Am J Respir Crit Care Med. 1996;154:1436–43. doi: 10.1164/ajrccm.154.5.8912761. [DOI] [PubMed] [Google Scholar]
- 36.Kim YK, Quadro L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol Biol. 2010;652:263–75. doi: 10.1007/978-1-60327-325-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redlich CA, Blaner WS, VanBennekum AM, Chung JS, Clever SL, Holm CT, et al. Effect of supplementation with beta-carotene and vitamin A on lung nutrient levels. Cancer Epidemiol Biomarkers Prev. 1998;7:211–4. [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B-cell function from fasting glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R-Core-Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 41.Carey VJ, Lumley T, Ripley B. gee: Generalized Estimation Equation solver. 2012 R package version 4.13-18ed. [Google Scholar]
- 42.Blackburn GL, Wang KA. Dietary fat reduction and breast cancer outcome: results from the Women’s Intervention Nutrition Study (WINS) Am J Clin Nutr. 2007;86:s878–81. doi: 10.1093/ajcn/86.3.878S. [DOI] [PubMed] [Google Scholar]
- 43.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 44.Grann VR, Jacobson JS, Troxel AB, Hershman D, Karp J, Myers C, et al. Barriers to minority participation in breast carcinoma prevention trials. Cancer. 2005;104:372–9. doi: 10.1002/cncr.21164. [DOI] [PubMed] [Google Scholar]
- 45.Mandelblatt J, Kaufman E, Sheppard VB, Pomeroy J, Kavanaugh J, Canar J, et al. Breast cancer prevention in community clinics: will low-income Latina patients participate in clinical trials? Prev Med. 2005;40:611–8. doi: 10.1016/j.ypmed.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore) 2008;87:1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- 47.American Institute for Cancer Research. Recommendations for Cancer Prevention. 2014 [cited 2015 October 30]; Available from: http://www.aicr.org/reduce-your-cancer-risk/recommendations-for-cancer-prevention/
- 48.Wallington SF, Dash C, Sheppard VB, Goode TD, Oppong BA, Dodson EE, et al. Enrolling Minority and Underserved Populations in Cancer Clinical Research. American journal of preventive medicine. 2015;50:111–7. doi: 10.1016/j.amepre.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.London L, Hurtado-de-Mendoza A, Song M, Nagirimadugu A, Luta G, Sheppard VB. Motivators and barriers to Latinas’ participation in clinical trials: the role of contextual factors. Contemp Clin Trials. 2015;40:74–80. doi: 10.1016/j.cct.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao J, Tan T, Li S, Meghani SH, Glanz K, Bruner D. Attitudes and barriers towards participation in an acupuncture trial among breast cancer patients: a survey study. BMC Complement Altern Med. 2014;14:7. doi: 10.1186/1472-6882-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernard-Davila B, Aycinena AC, Richardson J, Gaffney AO, Koch P, Contento I, et al. Barriers and facilitators to recruitment to a culturally-based dietary intervention among urban Hispanic breast cancer survivors. J Racial Ethn Health Disparities. 2015;2:244–55. doi: 10.1007/s40615-014-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berry LL, Mirabito AM. Partnering for Prevention With Workplace Health Promotion Programs. Mayo Clinic proceedings Mayo Clinic. 2011;86:335–7. doi: 10.4065/mcp.2010.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mama SK, Song J, Ortiz A, Tirado-Gomez M, Palacios C, Hughes DC, et al. Longitudinal social cognitive influences on physical activity and sedentary time in Hispanic breast cancer survivors. Psychooncology. 2015 doi: 10.1002/pon.4026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meneses K, Gisiger-Camata S, Schoenberger YM, Weech-Maldonado R, McNees P. Adapting an evidence-based survivorship intervention for Latina breast cancer survivors. Womens Health (Lond Engl) 2015;11:109–19. doi: 10.2217/whe.14.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang FF, Morabia A, Carroll J, Gonzalez K, Fulda K, Kaur M, et al. Dietary patterns are associated with levels of global genomic DNA methylation in a cancer-free population. J Nutr. 2011;141:1165–71. doi: 10.3945/jn.110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scoccianti C, Ricceri F, Ferrari P, Cuenin C, Sacerdote C, Polidoro S, et al. Methylation patterns in sentinel genes in peripheral blood cells of heavy smokers: Influence of cruciferous vegetables in an intervention study. Epigenetics. 2011;6:1114–9. doi: 10.4161/epi.6.9.16515. [DOI] [PubMed] [Google Scholar]
- 57.Delgado-Cruzata L, Zhang W, McDonald JA, Tsai WY, Valdovinos C, Falci L, et al. Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J Nutr. 2015;145:783–90. doi: 10.3945/jn.114.202853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulis M, Esteller M. DNA methylation and cancer. Advances in genetics. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 59.Toyota M, Suzuki H. Epigenetic drivers of genetic alterations. Advances in genetics. 2010;70:309–23. doi: 10.1016/B978-0-12-380866-0.60011-3. [DOI] [PubMed] [Google Scholar]
- 60.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 61.Noy N, Li L, Abola MV, Berger NA. Is retinol binding protein 4 a link between adiposity and cancer? Hormone molecular biology and clinical investigation. 2015;23:39–46. doi: 10.1515/hmbci-2015-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fedders R, Muenzner M, Schupp M. Retinol binding protein 4 and its membrane receptors: a metabolic perspective. Hormone molecular biology and clinical investigation. 2015;22:27–37. doi: 10.1515/hmbci-2015-0013. [DOI] [PubMed] [Google Scholar]
- 63.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, et al. Retinoids and retinoid-binding protein expression in rat adipocytes. The Journal of biological chemistry. 1992;267:1805–10. [PubMed] [Google Scholar]
- 64.Gamble MV, Ramakrishnan R, Palafox NA, Briand K, Berglund L, Blaner WS. Retinol binding protein as a surrogate measure for serum retinol: studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am J Clin Nutr. 2001;73:594–601. doi: 10.1093/ajcn/73.3.594. [DOI] [PubMed] [Google Scholar]
- 65.Wolf G. Serum retinol-binding protein: a link between obesity, insulin resistance, and type 2 diabetes. Nutrition reviews. 2007;65:251–6. doi: 10.1111/j.1753-4887.2007.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 66.Frey SK, Vogel S. Vitamin A metabolism and adipose tissue biology. Nutrients. 2011;3:27–39. doi: 10.3390/nu3010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell metabolism. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Lee JW, Im JA, Lee HR, Shim JY, Youn BS, Lee DC. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity. 2007;15:2225–32. doi: 10.1038/oby.2007.264. [DOI] [PubMed] [Google Scholar]


