Abstract
Chemical and psychological stressors can exert long lasting changes in brain function and behavior. Changes in DNA methylation have been shown to be an important mechanism mediating long lasting changes in neural function and behavior, especially for anxiety-like or stress responses. Here we examined the effects of either a social or chemical stressor on DNA methyltransferase (DNMT) gene expression in the amygdala, an important brain region modulating stress responses and anxiety. In adult California mice (Peromyscus californicus) that were naïve to social defeat, females had higher levels of Dnmt1 expression in punch samples of the central amygdala (CeA) than males. In addition, social defeat stress reduced Dnmt1 and Dnmt3a expression in the CeA of females but not males. A second study using more anatomically specific punch samples replicated these effects for Dnmt1. Perinatal exposure, spanning from periconception through lactation, to bisphenol A or ethinyl estradiol (estrogens in birth control pills) also abolished sex differences in Dnmt1 expression in the CeA but not basolateral amygdala. These findings identify a robust sex difference in Dnmt1 expression in the CeA that is sensitive to both psychological and chemical stressors. Our results suggest that future studies should examine the impact of psychological and chemical stressors on DNA methylation in the CeA and that Dnmt1 may have an underappreciated role in plasticity in behavior.
Keywords: DNMT1, DNMT3A, estrogen receptor, California mouse, endocrine disruptors
Introduction
Neurobehavioral programming is the product of both intrinsic and extrinsic influences experienced across the lifespan. Experiences such as social interactions or dietary alterations can induce neuroadaptations that lead to long lasting changes in behavior. The methylation of CpG sites can be an important mechanism for mediating these experience-driven effects (1–3). This process can be controlled by DNA methyltransferases (DNMT) with Dnmt1 primarily targeting hemimethylated CpG and Dnmt3a which primarily targets unmethylated and hemimethylated CpG sites (4). The impact of DNMTs early in life is well documented (5–7), and there is growing evidence that DNMTs can also mediate the effects of experience in adults on brain function and behavior. For example, the infusion of a DNMT inhibitor in hippocampus blocked the formation of long-term fear memories (8). Social defeat stress induces anxiety-like and depression-like behaviors in male rodents, and infusions of a DNMT inhibitor into the nucleus accumbens (NAc) reduces the effects of defeat stress on behavior (9). This study also showed that defeat stress increased Dnmt3a expression, suggesting that experience can modulate DNMT activity, which in turn affects behavioral responses to stress (6, 7, 10). Exposure to endocrine disrupting chemicals (EDC) in the environment is another form of experience that can alter DNMT expression within the brain. Bisphenol A (BPA) is an EDC that binds weakly to estrogen receptors (ESR1 and 2) (11). In utero exposure to BPA increased expression of Dnmt3a as well as hypermethylation of Esr1 in the male mouse prefrontal cortex, while the same dose induced downregulation of Dnmt3a and hypomethylation of Esr1 in the female mouse prefrontal cortex. In the same study, Dnmt1 expression was decreased by BPA exposure in the female mouse hypothalamus. This suggests that BPA exposure disrupts sexually dimorphic methylation patterns (12). Prenatal BPA exposure also leads to increased expression of Dnmt1 in male mice hippocampus (13). There is growing evidence that exposure to EDCs such as BPA early in life can interact with stressors experienced later in life. This idea has been conceptualized as the “two hit” hypothesis, which poses that a combination of external hits can produce a phenotype that is greater than the sum of their individual effects (14, 15). As a first step towards testing this hypothesis, we examined the individual effects of developmental BPA exposure (or ethinyl estradiol: EE, positive estrogen control for BPA studies) or social defeat experienced at adulthood on DNMT gene expression.
We chose to study the California mouse (Peromyscus californicus), a monogamous, biparental rodent species in which both males and females are vulnerable to social defeat (10, 16). This approach allowed us to study the effect of defeat in both males and females, as sex differences in the effects of psychosocial stress on DNMT function have been observed (17). California mice are also sensitive to perinatal BPA exposure (18–21). We examined the effects of these stressors in the amygdala, which is sensitive to environmental stressors (10, 22, 23), and regulates behavioral responses to stress (24). In female rats perinatal BPA exposure reduced Dnmt1 expression in the basolateral amygdala (BLA) and increased anxiety-like behavior (25). Sex differences in Dnmt3a expression have been found in the developing amygdala (26), but to our knowledge adults have not been studied. Here, we examine Dnmt1 and Dnmt3a expression in the medial amygdala (MeA), central nucleus of the amygdala (CeA), and BLA. Two experiments were performed to examine the effects of social defeat stress on Dnmt expression in adult males and females, while a third experiment focused on developmental BPA exposure. Although specific subnuclei of the amygdala have not been examined, previous studies have generally found stronger effects of chemical (12) or psychosocial (17) stressors on Dnmt expression in females versus males. Based on these results we predicted that social defeat and BPA would have stronger effects on DNMT gene expression in females than males.
Methods
Animals
For experiments 1 and 2, California mice were obtained from a pathogen-free breeding colony at UC Davis. They were group housed (2–3 same-sex animals per cage) and maintained in a temperature-controlled room on a 16L-8D cycle with ad libitum water and food (Harlan Teklad 2016, Madison, WI). Cages were polycarbonate plastic with sanichip bedding, nestlets, and enviro-dri (27). All procedures were approved by the UC Davis Internal Animal Care and Use Committee (IACUC) and conformed to NIH guidelines.
For experiment 3, outbred adult (60–90 days of age) founder California mouse females and males, free of common rodent pathogens, were purchased from the Peromyscus Genetic Stock Center (PGSC) at the University of South Carolina (Columbia, SC). They were placed in quarantine at the University of Missouri for a minimum of 8 weeks to ensure that they did not carry any transmittable and zoonotic diseases. From the time the animals had been captured between 1979 and 1987, P. californicus captive stocks have been bred by the PGSC to maintain their outbred status. Mice were housed in polypropylene cages (Allentown) with aspen shaving bedding (Bourn Feed, Columbia, MO) and nestlets. All experiments were approved by the University of Missouri Animal Care and Use Committee (ACUC) and confirmed to NIH guidelines.
Experiment 1: Effects of social defeat on gene expression in central and medial amygdala
Male and female California mice were randomly assigned to either social defeat stress or to remain naïve to defeat. Each mouse assigned to social defeat was placed in the cage of an aggressive same-sex mouse resident mouse (28). Each episode lasted 7 min or until the resident attacked the focal mouse 7 times (whichever occurred first), and this was repeated on 3 consecutive days with three different residents. Naïve mice were introduced into a clean cage for 7 min, again for 3 consecutive days. Immediately after defeat/control conditions mice were returned to their home cage. To focus on long term behavioral changes, behavior was assessed via a social interaction test two weeks after the last episode of defeat. Behavioral and neurobiological effects of defeat persist for at least 10 weeks (10, 29). The social interaction test consisted of 3 phases, 3 minutes each (30). In the open field phase (OF), animals were introduced into a large open field (89×63×60 cm). Durations within a center zone located 14cm from the sides were recorded using the Any-Maze video tracking system (Stoelting, Wood Dale, IL). During the acclimation phase a small wire cage was introduced against one side of the arena, the amount of time the mouse spent within 8 cm of the empty cage was recorded. During the social interaction phase an unfamiliar, same-sex virgin stimulus mouse was placed into the wire cage. We recorded the amount of time the focal mouse spent interacting with the wire cage and the duration spent in the two corners opposite the wire cage. Behavioral data for these mice have been published (Experiment 1, Greenberg et al. 2014). We did not include separate groups for different stages of the estrous cycle as previous studies observed that estrous cycle (10) and gonadectomy (28) had no effect on behavior after defeat.
Immediately after social interaction testing each mouse was lightly anesthetized with isoflurane and rapidly decapitated. Although it is possible that isoflurane exposure could affect gene expression, exposure was minimized (90 sec or less). Furthermore a recent study reported that 5 min of isoflurane exposure did not affect expression of 4 different genes in hippocampus (31). Although it is possible that the specific genes we quantified were affected by isoflurane, all experimental groups were euthanized in a consistent manner. Each brain was rapidly removed and placed in to a brain matrix chilled on ice to collect 2 mm slices (32). Punch samples of the MeA and CeA were collected with a 1 mm diameter punch tool (Fig. 1). The punches of the CeA may have contained a small amount of adjacent BLA. Samples were frozen on dry ice and stored at −40° C.
Figure 1.
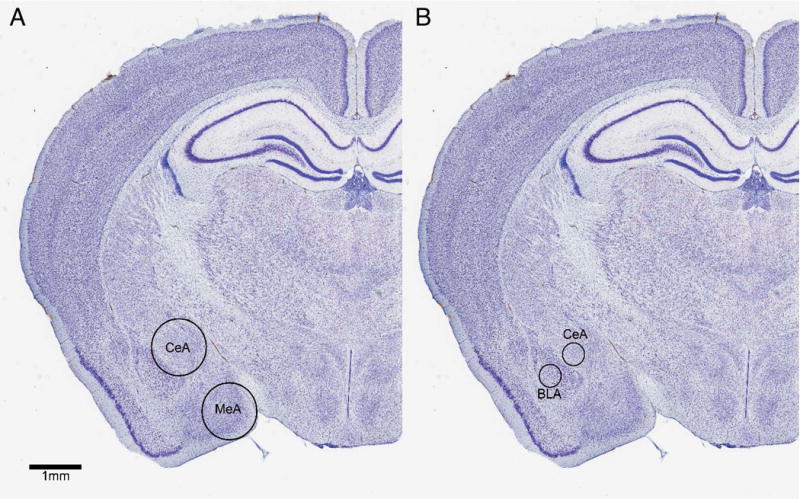
A) Diagram of 1mm punch sample taken from CeA and MeA in Experiment 1. B) Diagram of 0.36mm punch sample taken from CeA and BLA in Experiment 2. Nissl images from California mouse brain atlas at brainmaps.org. (CeA = central amygdala, MeA = medial amygdala, BLA = basolateral amygdala)
Experiment 2: Effects of social defeat on gene expression in central and basolateral amygdala
Male California mice were randomly assigned to defeat stress or control and then tested in the social interaction test as described in experiment 1. However, instead of euthanizing mice immediately after the social interaction test mice were euthanized by isoflurane anesthesia and decapitation 12–14 hours later. Brains were rapidly removed and a chilled brain matrix was used to collect 1 mm slices. Slices containing the amygdala were stored in RNAlater® (Ambion) overnight at 4° C and the next day bilateral punch samples were collected. Storing brain slices in this manner stabilizes RNA and results in a more rigid tissue that facilitates dissection. Here, we used a 0.36 mm diameter (20 gauge) punch tool to obtain non-overlapping samples of the CeA and BLA (Fig. 1). Samples were frozen on dry ice and stored at −40° C.
Experiment 3: Effects of BPA exposure on gene expression in central and basolateral amygdala
Two weeks prior to breeding, virgin females (8–12 weeks of age) were randomly assigned to receive one of three diets: 1) a low phytoestrogen AIN 93G diet supplemented with 7% by wt corn oil to minimize potential phytoestrogenic contamination that would otherwise be present with inclusion of soybean oil in the diet, 2) a diet supplemented with 50 mg BPA/kg feed weight, which we have documented to lead to internal serum concentrations close to those measured in pregnant women unknowingly exposed to this chemical (33), 3) a control diet supplemented with 0.1 ppb feed weight served as an estrogen positive control, as requested by the FDA for any BPA study that may be considered to guide policy decisions. Females were maintained on these diets throughout gestation and lactation, as described previously (21, 33, 34). Diets were assigned at the time males were paired with females and maintained until the offspring were weaned. Thus, this exposure regimen to BPA or EE is unlikely to affect the caudal epididymal spermatozoa, which developed and matured prior to being placed on the diets. However, it is possible that direct exposure to EDCs might have affected paternal care, as discussed below. At weaning (30 days of age), F1 male and female offspring were placed on the AIN control diet, and they were maintained on this diet throughout the remainder of the study. At adulthood, which ranged from 60–194 days of age, mice were euthanized by cervical dislocation. Each brain was rapidly removed and then frozen on dry ice and stored −80°C.
Frozen brains were shipped to UC Davis where they were sliced on a cryostat at a thickness of 500 μm at −10°C, and immediately immersed into RNAlater® for approximately 24 hours at 4°C (35). A 0.36 mm diameter punch tool was used to collect bilateral samples of BLA and CeA. Samples were then kept frozen at −80°C until RNA extraction.
RNA extraction and real-time PCR
RNA was extracted from punch samples by using RNAqueous®-Micro Total RNA Isolation Kit (Ambion™ AM1931), and underwent a DNase I treatment before reverse transcription. For each sample the RNA concentration and quality was assessed using a Nanodrop spectrophotometer. Samples were reverse transcribed by using the iScript™ cDNA Synthesis Kit (Bio-Rad #1708891).
For real-time polymerase chain reaction (PCR) analysis, SYBR green chemistry was used to detect genes of interest. For experiment 1, specific primers for Dnmt1a and Dnmt3a were used for both CeA and MeA samples. In addition, Bdnf was also quantified for CeA. We also examined Esr1 (estrogen receptor 1) and Esr2 (estrogen receptor 2) in MeA due to the high abundance of these transcripts in MeA. In experiments 2 and 3, Dnmt1and Bdnf were quantified for both BLA and CeA samples. Due to technical difficulties, Dnmt3a was not examined in samples for Experiment 2. The small amount of RNA recovered from the smaller punch size prevented us from re-running this analysis. All gene expression measurements were normalized to B2m1 expression and normalized to control males as described previously (29). Primer sequences can be referenced in Table 1.
Table 1.
Primer sequences from all qPCR ran in Experiments 1–3.
| Genbank Accession | Forward Primer | Reverse Primer | |
|---|---|---|---|
| Bdnf | JX977026 | CCA TAA GGA CGC GGA CTT GTA T | GCA GAG GAG GCT CCA AAG G |
| Dnmt1 | XM_006987030.2 | AGC CGG AGA GCA GAA ATG GC | ACT GTC CGA CTT GCT TCT CC |
| Dnmt3a | XM_006981412.2 | TCT TGA GTC CAA CCC CGT GAT G | CCT CAC TTT GCT GAA CTT GGC T |
| Esr1 | XM_015996803.1 | TGC ACC AGA TCC AAG GGA AC | TCG GGG TAG TTG AAC ACA GC |
| Esr2 | XM_006973152.2 | CAC GCT TCG AGG GTA CAA GT | AGG CAG CCA TAA GAT GAC GC |
| B2m | XM_3006995122.1 | TCT AGT GGG AGG TCC TGT GG | TGC GTT AGA CCA GCA GAA GG |
Data analysis
Gene expression data were analyzed with two-way ANOVA for experiments 1 and 2 followed by planned comparisons testing (36) for effects of sex or stress using SPSS. Planned comparisons were limited to comparing control males and females (baseline sex difference) and the effects of stress within each sex (3 comparisons total per variable). In experiment 3, we tested for effects of diet on gene expression data (within sex) using Kruskal-Wallis nonparametric analyses due to heterogeneous variability between experimental groups (as indicated by Levene’s test). Mann-Whitney tests were used for pair-wise comparisons in all three experiments. For experiment 1 we also used Spearman nonparametric correlations to correlate gene expression data with previously published behavior data. Behavioral variables considered included total distance in an open field test, time spent in the center of the open field, time spent within 8 cm of an empty cage, and time spent within 8 cm of a cage containing an unfamiliar same-sex mouse.
Results
Experiment 1
When 1 mm punches were used to collect CeA samples, main effects of sex were detected for Dnmt1 (Fig. 2A, F1,25=4.8, p < 0.05) and Dnmt3a (Fig. 2B, F1,25=6.5, p < 0.05). Although there was no significant sex by stress interaction for either transcript, planned comparisons revealed that control females had higher Dnmt1a (p<0.05) and Dnmt3a (p<0.05) expression than control males whereas these sex differences were not significant in stressed mice. There were no differences in Bdnf expression. In MeA samples, there were no differences in Dnmt1, Dnmt3a, or Esr1 expression (Fig. 3, all p’s > 0.2). However, there was a significant main effect of sex for Esr2 in the MeA (Fig. 3D, F1,25=11.5, p <0.01). Again, while the interaction between sex and stress was not significant, planned comparisons showed that control males had higher Esr2 expression than control females (p < 0.001) whereas this difference was not significant in stressed mice. In correlational analyses, Esr2 in the MeA was negatively correlated with total distance in the open field for females (ρ = −0.61, p = 0.01) but not males (ρ = −0.17, p = 0.58). Gene expression data for Dnmt1, Dnmt3a, or Bdnf were not significantly correlated with behavior in the social interaction test.
Figure 2.
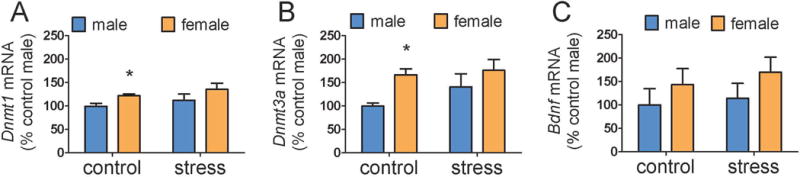
Effects of sex and social defeat on gene expression in 1mm diameter punch samples of CeA. Control females (n=7) had higher Dnmt1 and Dnmt3a expression than control males (n=7). No sex differences were observed between stressed males (n=7) and stressed females (n=8). There were no differences in Bdnf expression. * p < 0.05 vs. control male. (CeA = central amygdala, Dnmt1 = DNA methyltransferase 1, Dnmt3a = DNA methyltransferase 3a, Bdnf = brain-derived neurotrophic growth factor)
Figure 3.
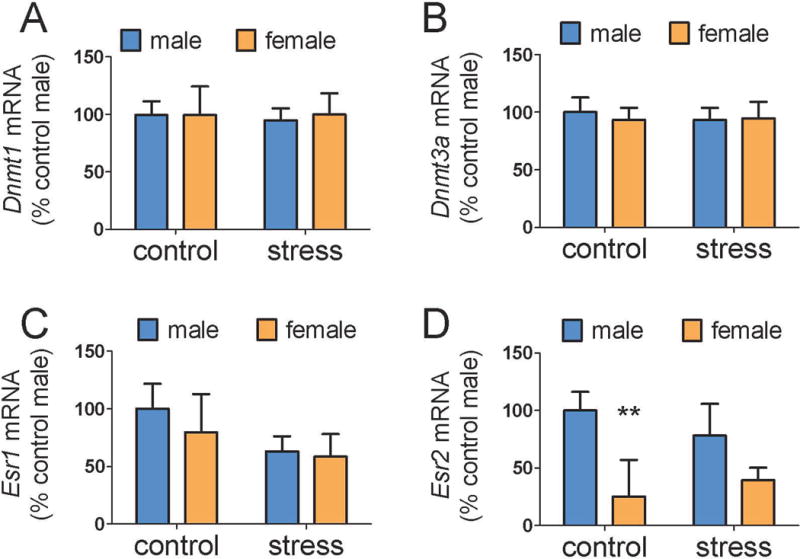
Effects of sex and social defeat on gene expression in the MeA. There were no differences in Dnmt1 (A), Dnmt3a (B), or Esr1 (C) expression. Control males (n=8) had significantly more Esr2 expression (D) than control females (n=8) but this difference was not detected between stressed males (n=7) and stressed females (n=8). ** p < 0.01 vs. control male. (MeA = medial amygdala, Dnmt1 = DNA methyltransferase 1, Dnmt3a = DNA methyltransferase 3a, Esr1 = estrogen receptor 1, Esr2= estrogen receptor 2)
Experiment 2
When a smaller punch was used to collect the CeA, a main effect of sex on Dnmt1 was detected in the CeA region (Fig. 4A, F1,31=4.21, p < 0.05). As in experiment 1, although sex by treatment interaction was not significant, planned comparisons detected increased Dnmt1 expression in control females compared to control males whereas no sex difference was detected in stressed mice. In addition, stress reduced Dnmt1 expression in females (Fig. 4A, p<0.05) but not males. There were no differences in Bdnf expression in the CeA (Fig. 4B, all p’s > 0.07). Analyses of punch samples from the BLA showed no differences in Dnmt1a or Bdnf (all p’s > 0.14).
Figure 4.
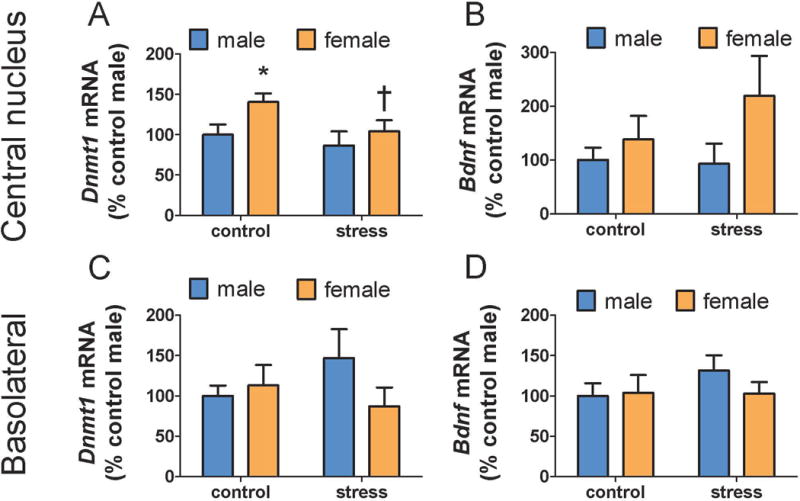
Effects of stress and social defeat in 0.36 mm diameter punch samples of CeA and BLA. Control females (n=9) had significantly more Dnmt1 expression in the CeA compared to control males (n=12, A). Dnmt1 expression was reduced in stressed females (n=7) compared to control females. There was no difference in Dnmt1 expression between stressed males (n=7) and control males. No differences in Dnmt1 were observed in BLA (C). No differences in Bdnf were observed in CeA (B) or BLA (D). * p < 0.05 vs. control male. † p < 0.05 vs. control female. (CeA = central amygdala, BLA = basolateral amygdala, Dnmt1 = DNA methyltransferase 1, Bdnf = brain-derived neurotrophic growth factor)
Experiment 3
Developmental exposure to one of the two EDCs led to sex and anatomically specific effects on gene expression. First, Kruskal-Wallis analyses showed significant effects of diet on Dnmt1a expression in the CeA in males (Fig. 5A, Kruskal-Wallis, H=6.1, p < 0.05) but not females (Fig. 5A, Kruskal-Wallis, H=1.1, p = 0.56). Pairwise comparisons showed that males raised by EE exposed dams had significantly higher Dnmt1 expression than males derived from dams on the control diet (p<0.05). No effect of diet was observed in Dnmt3a or Bdnf expression in the CeA (Fig 5, all p’s > 0.23). The BLA Kruskal-Wallis analyses showed significant effects of treatment on Bdnf expression in males (Fig. 5F, Kruskal-Wallis, H=6.1, p < 0.05) but not females (Fig. 5A, Kruskal-Wallis, H=6.1, p < 0.05). Pairwise comparisons showed that males raised by EE exposed dams had significantly lower Bdnf expression than males raised by dams on the control diet (p<0.05).
Figure 5.
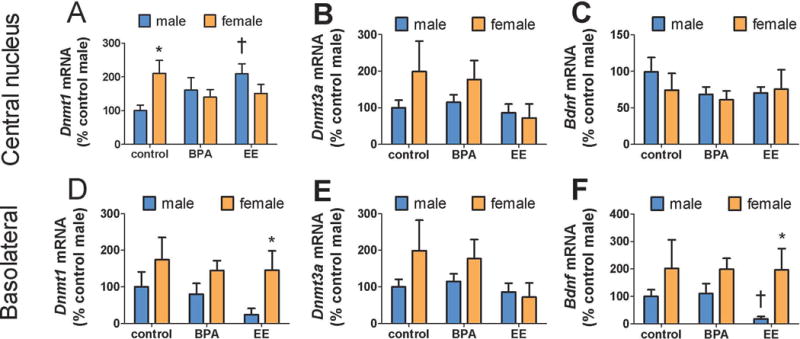
Effects of sex, BPA, and EE on gene expression in the CeA and BLA. Control females (n=11) had higher Dnmt1 expression in CeA than control males (n=11, A), but this differences was absent in BPA (females, n = 10, males, n = 9) and EE treated mice. EE diet increased Dnmt1 expression in males. In the BLA, females treated with EE (n = 9) had higher Dnmt1 expression than EE treated males (n= 7, D). No differences in Dnmt3a were observed in CeA (B) or BLA (E). The EE diet reduced male Bdnf expression in the BLA (F) but not CeA (C). * p < 0.05 effect of sex within diet, † p < 0.05 effect of diet within sex. (BPA = bisphenol A, EE = ethinyl estradiol, CeA = central amygdala, BLA = basolateral amygdala, Dnmt1 = DNA methyltransferase 1, Bdnf = brain-derived neurotrophic growth factor)
For mice raised by dams on the control diet, females had significantly higher Dnmt1 expression than males in the CeA (Fig. 5A, Mann-Whitney, U=80, p<0.05) but not BLA (Fig. 5D). In mice raised by dams on the EE diet, females had higher Dnmt1 (Fig. 5D, Mann-Whitney U=39, p<0.01) and Bdnf (Fig. 5F, Mann-Whitney, U=37, p<0.05) expression in the BLA. For these same mice, no sex differences were observed in the CeA (all p’s>0.1). Intriguingly, no sex differences were observed in either the CeA or BLA for mice raised by dams on the BPA diet. Thus, developmental exposure to BPA reduced the sex differences otherwise observed in the CeA region for Dnmt1.
Discussion
Across the three experiments, we observed that Dnmt1 expression in the CeA was higher in adult females compared to adult males. Furthermore, this sex difference was reduced in mice assigned to social defeat stress after sexual maturity or BPA exposure during development. This difference was anatomically specific, as no baseline sex differences or changes in Dnmt1 expression were observed in MeA or the adjacent BLA. The sub-regions of the amygdala have different anatomical connections and cell types (37), which may contribute to the anatomically specific patterns in Dnmt expression. This may explain why a previous study, which examined samples of the entire amygdala, observed no sex difference in Dnmt1 expression (26). An intriguing finding of this study was that differences in Dnmt1 expression were long lasting, detected weeks after the termination of BPA exposure or social defeat.
A consistent finding was that females had higher Dnmt1 expression in the CeA than males. This sex difference in Dnmt1 expression was observed in California mice raised in colonies at different universities, with varying early life experiences, and contrasting experimental procedures. This is despite the inevitable differences in husbandry practices that are present between different animal facilities. This difference in expression is a robust sex difference and does appear not dependent on factors such as husbandry (39). If these differences in mRNA expression influence DNMT activity, this would suggest that there could be important sex differences in DNA methylation patterns in the CeA. This in turn could affect the transcription of other genes. Social defeat reduced Dnmt1 expression in females, yet we found no significant correlations between Dnmt1 expression and behavior in the social interaction test. One possible explanation for the lack of correlations is that DNMT1 may act immediately after episodes of defeat to facilitate long term changes in behavior. If this hypothesis is correct, then DNMT inhibitors administered at the time of defeat would have stronger effects on behavior than inhibitors administered after defeat. This would follow the same pattern as DNMT inhibition in the hippocampus, where the behavioral effects of fear conditioning are blocked when a DNMT inhibitor (targeting both DNMT1 and DNMT3a) is given immediately after a conditioning episode, but not when administered six hours later (8).
Traditionally Dnmt1 has been considered to play a more defining role during the early developmental stages, due to its role in maintaining DNA methylation patterns (40). However, Dnmt1 has important functions in the adult brain. When a conditional knockout mouse was used to inactivate Dnmt1 in the forebrain (including amygdala) 2–3 weeks after birth, synaptic plasticity in the hippocampus and spatial memory were impaired (41). Male conditional Dnmt1 knockout mice also had reduced anxiety-like behavior in the open field and increased social interaction behavior (38). The loss of Dnmt1 may have broad effects on transcription that could contribute to increases in anxiety-like behavior. Our results suggest Dnmt1 inhibition may have even more robust effects in females. Further study is needed to determine the impact of decreases in DNMT1 activity in the adult brain on transcription. It is likely that effects of DNMT1 on methylation and transcription will be dependent on social/environmental stress exposure.
BPA exposure elicited the same changes to Dnmt1 expression that social defeat did. Developmental exposure to BPA or EE reduced sex differences in Dnmt1 expression in the CeA. Developmental exposure to BPA also reduced sex differences in behavior by decreasing exploratory behavior in females and reducing territorial marking behavior in males (21). These results support the hypothesis the BPA exposure reduces sexually dimorphic characteristics. Effects of developmental EE exposures were stronger than BPA, driven primarily by increased Dnmt1 expression in males exposed to EE. It is unclear how EE can have such sex-dependent effects on Dnmt1 expression in the CeA, a region of the amygdala with few nuclear estrogen receptors (42, 43). One possibility is that EE acts in other nuclei with inputs into the CeA. For example, the bed nucleus of the stria terminalis (BNST) has abundant estrogen receptor α and estrogen receptor β expression in California mice (44) and sends dense projections to the CeA (45).
When interpreting the effects of developmental exposure to BPA or EE in the Experiment 3, it is important to consider the potential impact these diets may have had on parental behavior. Although developmental exposure to BPA and EE can affect parental behavior of adult male and female California mice (19), it remains to be determined whether adult exposure to these diets induces similar disruptions. Cross-fostering approaches could be useful in teasing apart the direct effects of developmental BPA/EE exposure on gene expression versus indirect effects of BPA/EE on parental care. For example, one study showed that mice exposed to BPA during gestation and reared by a foster dam demonstrated increases in anxiety-like behavior compared to controls (53). Yet even cross-fostered offspring might also bear a permanent stamp due to prenatal exposure that may be sensed by the foster parents. This could in turn affect parental investment. Thus, there is currently no ideal way to dissect the neurobehavioral effects attributed to direct exposure to EDCs versus those potentially arising from altered parental care.
Data from the other amygdala subregions differed from findings in the CeA. In the MeA, we observed that males had higher expression of Esr2 than females, and that this difference was weakened by defeat stress. Currently, it is unclear whether sex differences in Esr2 in the MeA are evolutionarily conserved. One study in rats reported increased Esr2 mRNA expression in males versus females in the MeA (46) but this difference was not replicated (47). Quantitative analysis of estrogen receptor β (ERβ) protein has been hampered by cross-reactivity of ERβ antibodies in mice (48). The development of Esr2 reporter mice is a creative solution to this problem, but no sex differences in ERβ-enhanced green fluorescent protein were observed in the MeA between PND 0 and 21 (49). Work with Syrian hamsters shows strong evidence that Esr1 acting in the MeA has important effects on social behaviors (50), but almost nothing is known about the role of Esr2 in the MeA. In the CeA and BLA, we did not observe differences in Bdnf expression. Previous work in Syrian hamsters showed that two hours after experiencing social defeat Bdnf expression in BLA was increased (51). In experiment 2, brains were collected two weeks after defeat, suggesting that elevated Bdnf transcription after defeat may be transitory. Another possibility is that BDNF protein levels could be elevated in the absence of Bdnf mRNA (30, 52).
Conclusions
A consistent finding across all three experiments is that females had higher expression of Dnmt1 in the CeA than males. This difference was robust across different experiments and procedures. Although DNA methylation is considered an important mechanism for inducing behavioral and neurobiological plasticity, it is usually assumed that in the adult brain Dnmt3a plays a larger role in this process than Dnmt1. Our results suggest that further study of Dnmt1 function in the amygdala of adults may be warranted. Interestingly, both BPA and defeat stress weakened sex differences in Dnmt1 expression in the CeA, which supports the idea that in some respects BPA acts as a chemical stressor. Even though these stressors occurred during different developmental time points, they both manifested with a similar molecular change in adults. Currently, it is unclear whether developmental BPA exposure would alter the impact of social defeat on brain function and behavior. An environmental stressor experienced during development might render an individual to being more susceptible to a second extrinsic stressor experienced later in life, as predicted by the two-hit hypothesis (15, 54–56). Our results indicate that future research investigating epigenetic changes in the amygdala following stress will need to consider the possibility that sex differences in DNMT expression may lead to sex-specific transcriptional responses to stress.
Acknowledgments
The authors thank Angela B. Javurek for technical assistance. This work is supported by NIH R01MH103322 to BCT and 5R21ES023150 to CSR.
References
- 1.Van den Veyver IB. Genetic effects of methylation diets. Annual review of nutrition. 2002;22:255–82. doi: 10.1146/annurev.nutr.22.010402.102932. [DOI] [PubMed] [Google Scholar]
- 2.Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA Methylation Regulates Cocaine-Induced Behavioral Sensitization in Mice. Neuropsychopharmacology. 2010;35(12):2450–61. doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain, Behavior, and Immunity. 2011;25(7):1299–304. doi: 10.1016/j.bbi.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20(24):3139–55. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 5.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological psychiatry. 2009;65(9):760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11b-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PloS One. 2012;7:e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–94. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller CA, Sweatt JD. Covalent Modification of DNA Regulates Memory Formation. Neuron. 2007;53(6):857–69. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 9.LaPlant Q, Vialou V, Covington HE, Dumitriu D, Feng J, Warren B, Maze I, Dietz DM, Watts EL, Iñiguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolaños CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature neuroscience. 2010;13(9):1137–43. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex Differences in Social Interaction Behavior Following Social Defeat Stress in the Monogamous California Mouse (<italic>Peromyscus californicus</italic>) PLoS ONE. 2011;6(2):e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocrine Reviews. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proceedings of the National Academy of Sciences. 2013;110(24):9956–61. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences. 2015;112(22):6807–13. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews D, Gore AC. Life Imprints: Living in a Contaminated World. Environmental Health Perspectives. 2011;119(9):1208–10. doi: 10.1289/ehp.1103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenfeld CS, Trainor BC. Environmental Health Factors and Sexually Dimorphic Differences in Behavioral Disruptions. Current Environmental Health Reports. 2014;1(4):287–301. doi: 10.1007/s40572-014-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman MQ, Trainor BC. Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Semin Cell Dev Biol. 2017;61:92–8. doi: 10.1016/j.semcdb.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Ménard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. The Journal of Neuroscience. 2015;35(50):16362–76. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bomert KH, Givan SA, Rosenfeld CS. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7:471–85. doi: 10.1080/19490976.2016.1234657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson SA, Javurek AB, Painter MS, Peritore MP, Ellersieck MR, Roberts RM, Rosenfeld CS. Disruption of Parenting Behaviors in California Mice, a Monogamous Rodent Species, by Endocrine Disrupting Chemicals. PLoS ONE. 2015;10(6):e0126284. doi: 10.1371/journal.pone.0126284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SA, Painter MS, Javurek AB, Ellersieck MR, Wiedmeyer CE, Thyfault JP, Rosenfeld CS. Sex-dependent effects of developmental exposure to bisphenol A and ethinyl estradiol on metabolic parameters and voluntary physical activity. Journal of Developmental Origins of Health and Disease. 2015;6(06):539–52. doi: 10.1017/S2040174415001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS. Effects of Developmental Bisphenol A Exposure on Reproductive-Related Behaviors in California Mice (<italic>Peromyscus californicus</italic>): A Monogamous Animal Model. PLoS ONE. 2013;8(2):e55698. doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou R, Bai Y, Yang R, Zhu Y, Chi X, Li L, Chen L, Sokabe M, Chen L. Abnormal synaptic plasticity in basolateral amygdala may account for hyperactivity and attention-deficit in male rat exposed perinatally to low-dose bisphenol-A. Neuropharmacology. 2011;60(5):789–98. doi: 10.1016/j.neuropharm.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Yu CJ, Fang QQ, Tai FD. Pubertal BPA exposure changes central ERα levels in female mice. Environmental Toxicology and Pharmacology. 2015;40(2):606–14. doi: 10.1016/j.etap.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26(3):321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhou R, Chen F, Chang F, Bai Y, Chen L. Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. Journal of Psychiatric Research. 2013;47(10):1535–44. doi: 10.1016/j.jpsychires.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Kolodkin MH, Auger AP. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. Journal of neuroendocrinology. 2011;23(7):577–83. doi: 10.1111/j.1365-2826.2011.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, Trainor BC. Anxiolytic effects of vasopressin V1a receptor in the medioventral bed nucleus of the stria terminalis: sex specific effects in social and nonsocial contexts. Neuropharmacology. 2016;110:59–68. doi: 10.1016/j.neuropharm.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Hormones and behavior. 2013;63(3):543–50. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EL, Walch K, Bales KL, Trainor BC. Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry. 2016;80:406–14. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Frontiers in behavioral neuroscience. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekhbat M, Merrill L, Kelly SD, Lee VK, Neigh GN. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: Implications for tissue collection methods. Behavioural Brain Research. 2016;305:122–5. doi: 10.1016/j.bbr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78(1):36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Jr, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(28):11715–20. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jasarevic E, Williams SA, Vandas GM, Ellersieck MR, Liao C, Kannan K, Roberts RM, Geary DC, Rosenfeld CS. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Hormones and Behavior. 2013;63:180–9. doi: 10.1016/j.yhbeh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perea-Rodriguez JP, Takahashi EY, Amador TM, Hao R, Saltzman W, Trainor BC. Effects of reproductive status on central expression of progesterone, oxytocin, and vasopressin V1a receptors in male California mice (Peromyscus californicus) 27:2015245–52. doi: 10.1111/jne.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letner MBT. Experimental design and analysis. Valley, Blacksburg, VA: Valley Book Company; 1993. [Google Scholar]
- 37.Swanson LW, Petrovich GD. What is the amygdala? Trends in Neurosciences. 1998;21(8):323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 38.Morris MJ, Na ES, Autry AE, Monteggia LM. Impact of DNMT1 and DNMT3a forebrain knockout on depressive- and anxiety like behavior in mice. Neurobiology of Learning and Memory. 2016;135:139–45. doi: 10.1016/j.nlm.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villalon Landeros R, Yoo HJ, Morisseau C, Fu S, Hammock BD, Trainor BC. Corncob bedding reverses the effects of estrogens on aggressive behavior and reduces estrogen receptor alpha expression in the brain. Endocrinology. 2012;153:949–53. doi: 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Current Opinion in Neurobiology. 2009;19(2):207–12. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng J, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–30. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shughrue P, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. The Journal of comparative neurology. 2001;436(1):64–81. [PubMed] [Google Scholar]
- 43.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Roher SP, Schaefer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 44.Trainor BC, Finy MS, Nelson RJ. Paternal aggression in a biparental mouse: parallels with maternal aggression. Horm Behav. 2008;53:200–7. doi: 10.1016/j.yhbeh.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. Journal of Comparative Neurology. 2001;436(4):430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 46.Cao J, Patisaul HB. Sex Specific Expression of Estrogen Receptors α and β and Kiss1 in the Postnatal Rat Amygdala. The Journal of comparative neurology. 2013;521(2):465–78. doi: 10.1002/cne.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao J, Joyner L, Mickens JA, Leyrer SM, Patisaul HB. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction (Cambridge, England) 2014;147(4):537–54. doi: 10.1530/REP-13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. Journal of neuroscience methods. 2010;188(2):226–34. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor beta expression in the mouse forebrain: age and sex differences. The Journal of comparative neurology. 2014;522(2):358–71. doi: 10.1002/cne.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: Rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Taylor SL, Stanek LM, Ressler KJ, Huhman KL. Differential BDNF expression in limbic brain regions following social defeat or territorial aggression. Behavioral neuroscience. 2011;125(6):911–20. doi: 10.1037/a0026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tropea D, Capsoni S, Tongiorgi E, Giannotta S, Cattaneo A, Domenici L. Mismatch between BDNF mRNA and protein expression in the developing visual cortex: the role of visual experience. The European journal of neuroscience. 2001;13(4):709–21. doi: 10.1046/j.0953-816x.2000.01436.x. [DOI] [PubMed] [Google Scholar]
- 53.Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58(5):754–61. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lesse A, Rether K, Groger N, Braun K, Bock J. Chronic Postnatal Stress Induces Depressive-like Behavior in Male Mice and Programs second-Hit Stress-Induced Gene Expression Patterns of OxtR and AvpR1a in Adulthood. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-0043-8. [DOI] [PubMed] [Google Scholar]
- 55.Groger N, Bock J, Goehler D, Blume N, Lisson N, Poeggel G, Braun K. Stress in utero alters neonatal stress-induced regulation of the synaptic plasticity proteins Arc and Egr1 in a sex-specific manner. Brain structure & function. 2016;221(1):679–85. doi: 10.1007/s00429-014-0889-3. [DOI] [PubMed] [Google Scholar]
- 56.Novak G, Fan T, O’Dowd BF, George SR. Postnatal maternal deprivation and pubertal stress have additive effects on dopamine D2 receptor and CaMKII beta expression in the striatum. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2013;31(3):189–95. doi: 10.1016/j.ijdevneu.2013.01.001. [DOI] [PubMed] [Google Scholar]


