Abstract
Introduction
Low-income, low-literacy, limited English–proficient populations have low colorectal cancer (CRC) screening rates and experience poor patient–provider communication and decision-making processes around screening. The purpose of this study was to test the effect of a CRC screening decision aid on screening-related communication and decision making in primary care visits.
Study design
RCT with data collected from patients at baseline and immediately after the provider encounter.
Setting/participants
Patients aged 50–75 years, due for CRC screening, were recruited from two safety net clinics in North Carolina and New Mexico (data collection, January 2014–September 2015; analysis, 2015).
Intervention
Participants viewed a CRC screening decision aid or a food safety (control) video immediately before their provider encounter.
Main outcome measures
CRC screening–related knowledge, discussion, intent, test preferences, and test ordering.
Results
The study population (N=262) had a mean age of 58.3 years and was 66% female, 61% Latino, 17% non-Latino black, and 16% non-Latino white. Among Latino participants, 71% preferred Spanish. Compared with controls, intervention participants had greater screening-related knowledge (on average 4.6 vs 2.8 of six knowledge items correct, adjusted difference [AD]=1.8, 95% CI=1.5, 2.1) and were more likely to report screening discussion (71.0% vs 45.0%, AD=26.1%, 95% CI=14.3%, 38.0%) and high screening intent (93.1% vs 84.7%, AD=9.0%, 95% CI=2.0%, 16.0%). Intervention participants were more likely to indicate a specific screening test preference (93.1% vs 68.0%, AD=26.5%, 95% CI=17.2%, 35.8%) and to report having a test ordered (56.5% vs 32.1%, AD=25.8%, 95% CI=14.4%, 37.2%).
Conclusions
Viewing a CRC screening decision aid before a primary care encounter improves knowledge and shared decision making around screening in a racially, ethnically, and linguistically diverse safety net clinic population.
Trial registration
This study is registered at www.clinicaltrials.gov NCT02054598.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death in men and women in the U.S.1 CRC screening is effective at reducing CRC mortality. Expert groups, such as the U.S. Preventive Services Task Force, recommend a variety of tests for initial CRC screening, including fecal occult blood testing or fecal immunochemical testing (FOBT/FIT), with either guaiac-based or immunochemical tests, and endoscopic tests, typically with colonoscopy.2,3 Unfortunately, screening is underutilized, especially among vulnerable populations, including those with low income, low educational attainment, and limited English proficiency.4–10
Among the many barriers to screening in these populations are lack of patient awareness of screening options and not having a doctor recommend or discuss screening options during primary care visits.8,11–14 Studies also suggest that the way in which CRC screening is discussed and offered in clinical settings is important. When appropriately informed, primary care patients have distinct preferences for screening tests and are more likely to complete screening when their provider recommends a screening test that they prefer.15,16 However, studies also show that patients and physicians often have different screening test preferences, physicians are more likely than patients to prefer colonoscopy over stool-based tests, and physicians often misperceive or fail to acknowledge patients’ screening preferences, especially when they differ from their own.17–20 This suggests that improving informed decision making, through improved patient knowledge about CRC screening options, as well as shared decision making, through physician offering of a choice of tests and incorporation of patient test preference into the recommendation, may be effective at overcoming some barriers to screening.
Studies also suggest that improving screening-related communication by offering patients a choice of screening tests that includes FOBT/FIT may be especially important for vulnerable population subgroups such as Latinos and others served in safety net care settings. Hawley et al.17 showed that Latinos and those with lower educational attainment were more likely to prefer FOBT/FIT than non-Latino whites and those with more education. Inadomi and colleagues21 found that in a diverse, low-income population, participants for whom colonoscopy only was recommended were less likely to complete screening (38%) than participants receiving a recommendation for FOBT/FIT only (67%) or a choice between FOBT/FIT or colonoscopy (69%). They also found that Latino participants completed FOBT/FIT more often, whereas white participants completed colonoscopy more often. This demonstrated preference for FOBT/FIT among Latinos and those with lower educational attainment, juxtaposed with typical provider preference for colonoscopy, may contribute to lower screening completion rates. Based on these findings, experts in the field have emphasized the need to promote informed and shared decision making about CRC screening, which includes communication between patient and provider about screening and screening test options.22,23 However, this may be especially challenging in safety net care settings, where provider-level barriers, such as limited visit time and competing demands, are compounded by patient-level barriers that include language and literacy differences.24–29
Decision aids are useful in healthcare decisions where more than one reasonable option exists. They can improve the decision-making process and can lead to more informed, values-based choices.22 When delivered in a multimedia format before a primary care encounter, decision aids can mitigate literacy barriers and permit providers to use limited clinical time to clarify and act more specifically on informed patient preferences. CRC screening decision aids have been shown to increase screening knowledge, test ordering, intent to complete screening, discussion of screening, and (in some studies) test completion.30–33 However, no prior studies were found that have demonstrated that a CRC screening decision aid or educational video meaningfully increases discussion of more than one screening test option (a proxy for shared decision making).34 Further, although a few CRC decision aid studies have enrolled diverse, vulnerable patient populations,33,35 there is a need to identify screening interventions that are effective in Latino populations, who have substantially lower screening rates than the general U.S. population.36 However, no U.S. clinical trials of CRC screening decision aids conducted in Spanish-speaking populations were found.
The primary objective of this study was to test the effect of a CRC screening decision aid, available in English31,37 and Spanish,38 and viewed before a primary care encounter, on patient-reported communication and decision-making outcomes in a racially and ethnically diverse safety net clinic population. To improve generalizability, study sites, described below, were selected in locations representative of new and established socio-historic immigration contexts.39,40 The hypothesis was that the decision aid would lead to improvements in outcomes relevant for informed and shared decision making, including screening-related knowledge, communication about CRC screening and test options, preference formation, and test ordering among the overall study population as well as the Latino subgroup.
Methods
Overview
Data were collected from January 2014 to September 2015, and analyzed in 2015, as part of the CHOICE/OPCIONES study. The study design is reported in detail elsewhere,41 but briefly, the trial was designed to test a two-part intervention including a CRC screening decision aid, delivered before the provider encounter, and patient navigation, delivered after the provider encounter. Outcomes reported here reflect the effect of the decision aid part of the intervention on communication and decision-making outcomes assessed via survey directly after the provider encounter (and prior to initiation of the patient navigation intervention). Screening completion outcomes (to be published separately) will be assessed by electronic health record review in 2016 and will reflect the additional effect of the second part of the intervention, delivered after the collection of the post-encounter survey measures reported here. This study was approved by the IRBs at the University of North Carolina at Chapel Hill, the University of New Mexico, and Carolinas HealthCare System.
Study Sites
Participants were recruited from two safety net clinic sites, one in Albuquerque, New Mexico, and one in Charlotte, North Carolina. The sites were selected because they serve diverse low-income communities that include substantial numbers of Latino patients. Additionally, the sites reflect two distinct immigration patterns within the U.S.: North Carolina is typical of U.S. regions where Latino immigration has been both new and rapid in recent decades, whereas New Mexico is representative of regions with established, multigenerational Latino populations.39,40 These sites were selected to improve generalizability of the findings across U.S. Latino populations.
Recruitment and Study Activities
Potentially eligible patients were identified by querying the practices’ appointment schedules. A research assistant reviewed electronic medical records for evidence of current CRC screening according to guidelines. The research assistant then attempted to contact potentially eligible patients before their upcoming visit or approached them on the day of the visit to invite them to participate. On the day of the physician visit, eligible and consented patients completed a baseline survey and were randomized to view the CRC screening decision aid or the control video before the physician encounter. After the provider encounter, participants completed a follow-up survey. All surveys were available in English and Spanish and were administered orally by a study team member; participants followed along and indicated their answer choice to the study team member (Appendix 1, available online).
Decision Aids
Development and prior testing of the Spanish (OPCIONES) and English (CHOICE) decision aids is described in detail in other publications.31,33,37,38,42 Both versions are approximately 14 minutes long and consist of three parts: (1) introduction and review of fecal testing (FOBT/FIT) and colonoscopy; (2) direct comparison of the features of the two testing options; and (3) screening readiness assessment, in which viewers are prompted to select one of three color-coded, printed brochures indicating their screening readiness.
Outcome Measures
Outcomes measured (Table 1) for this study included CRC screening–related knowledge, discussion, test preference, intent to be screened, and test ordering. Screening knowledge was assessed at baseline and post-encounter using six previously tested items.33,42 Screening discussion was measured using two items administered post-encounter follow-up survey. Intent to complete screening was assessed at baseline and post-encounter using a 5-point Likert-scale response that was dichotomized for analysis (bottom three versus top two categories). Screening test preference was assessed using one item on the post-encounter survey. Test ordering was assessed using three items on the post-encounter survey.
Table 1.
Communication and Decision-Making Outcome Measures
| Survey Items | Response choices |
|---|---|
| Knowledge assessment itemsa | |
| There is only one way to get screened for colon cancer. | True, false,a don’t know |
| It is possible to do a colon cancer screening test at home. | True,a false, don’t know |
| A person can drive his or her car home immediately after a colonoscopy. | True, false,a don’t know |
| There is no risk during the colonoscopy procedure. | True, false,a don’t know |
| The (stool) fecal occult blood test, or FOBT, should be done every three years. | True, false,a don’t know |
| At what age is it recommended that a person start getting screened for colon cancer? | 35, 40, 45, 50,a 55 years |
| CRC screening communication assessment items | |
| Did you discuss colon cancer screening tests (such as colonoscopy or FOBT) with your doctor today? | Yes; no; don’t know |
| [If yes] Which colon cancer screening tests did you discuss with your doctor today? | FOBT only; colonoscopy only; FOBT and colonoscopy; don’t know |
| Intent to complete screening assessment item | |
| How much do you agree with the following statement? “I plan to be screened for colon cancer.” | Strongly disagree; disagree; neither agree nor disagree; agree; strongly agree |
| Test preference | |
| If you had to choose a colon cancer screening test, which test would you prefer? | FOBT; colonoscopy; haven’t decided which test; don’t have enough information to know |
| Test ordering | |
| Did any of the following happen TODAY with your medical team? Did you get an FOBT kit (home stool test for blood)? Did your doctor refer you for a colonoscopy? | Yes; no; don’t know |
Correct response for knowledge item.
CRC, colorectal cancer; FOBT, fecal occult blood testing.
Analytic Approach
Outcomes were compared using randomization-based nonparametric methods with a modified intention-to-treat approach that excluded all participants who provided no follow-up data. Although randomization was stratified by site, it was important to control for potential outcome variation across providers, who were nested within site. Therefore, analyses were conducted with stratification adjustment for providers, for which the data for lowest volume providers were combined in each site to ensure that each stratum included participants from each group (data were combined for three North Carolina providers, out of eight, who saw a total of 15 participants, and for six New Mexico providers, out of 27, who saw a total of eight participants). For screening discussion, test preference, and test ordering, which were only measured at follow-up, a stratified Mantel–Haenszel row mean score test was applied.43 For screening knowledge and intent, a stratified nonparametric ANCOVA, controlling for baseline value, was applied.43 Because these outcomes were pre-specified as secondary outcomes for this trial,41 each was tested using a two-sided 0.05 significance level, with no adjustments for multiple comparisons. Mantel–Haenszel weights were used to estimate adjusted differences along with 95% CIs. To explore the potential for heterogeneous effects across sites, generalized estimating equation methods were used to test site-by-group interactions at the 0.05 level; where interaction tests were significant, estimated effects within each site were reported. In a separate pre-planned analysis, the effect of the intervention among Latino participants only was tested. Analyses were conducted using SAS, version 9.4; nonparametric ANCOVA was conducted using the SAS macro NparCov3.44
Results
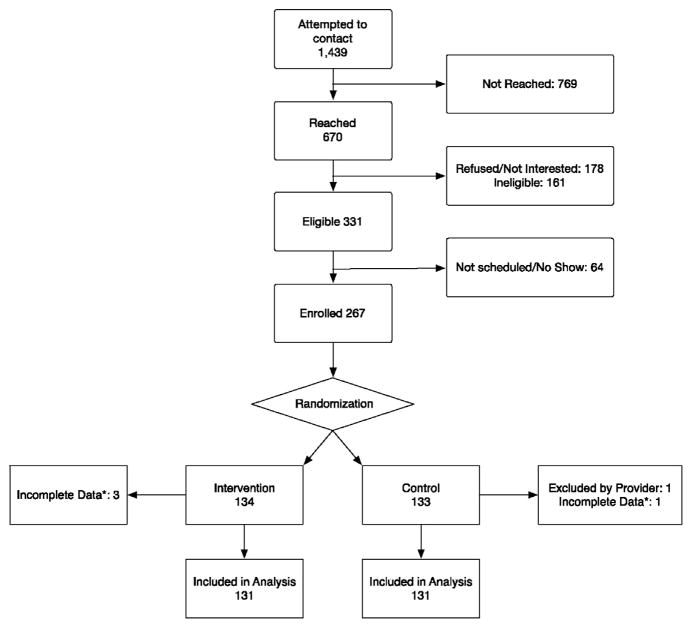
A total of 267 participants were enrolled and randomized (134 intervention, 133 control) between January 2014 and August 2015. Of these, 262 had complete data and were included in the analysis (Figure 1) (excluded were three intervention, two control). Participants had a mean age of 58.4 years, 65% were female, 61% were Latino, 17% non-Latino black, and 16% non-Latino white. Most (77%) had a household income <$20,000, 39% had a limited health literacy,45 and 34% were uninsured. Among Latino participants, 71% reported Spanish as the preferred language. There were no notable differences between groups at baseline (Table 2).
Figure 1.
CONSORT diagram.
*Missing one or more responses to outcome items
Table 2.
Participant Characteristics by Study Arm
| Factor | Intervention (n=131) | Control (n=131) | All (n=262) |
|---|---|---|---|
| Age (mean years) | 58.2 | 58.4 | 58.3 |
| Sex | |||
| Female | 67% | 64% | 66% |
| Race/ethnicity | |||
| Latino | 56% | 66% | 61% |
| Non-Latino black | 18% | 16% | 17% |
| Non-Latino white | 17% | 15% | 16% |
| Other | 9% | 3% | 6% |
| Language preference | |||
| Spanish | 40% | 47% | 44% |
| Education | |||
| Less than high school | 46% | 45% | 45% |
| High school or higher | 54% | 55% | 55% |
| Married | |||
| Married | 48% | 46% | 47% |
| Separated | 6% | 5% | 5% |
| Divorced | 12% | 22% | 17% |
| Widowed | 12% | 7% | 10% |
| Single/never | 22% | 20% | 21% |
| Insurance | |||
| None | 34% | 34% | 34% |
| Medicaid | 29% | 28% | 29% |
| Medicare | 23% | 27% | 25% |
| Private | 14% | 11% | 12% |
| Annual income | |||
| <$20,000 | 22% | 23% | 23% |
| ≥$20,000 | 78% | 77% | 78% |
| Employment | |||
| Not employed | 74% | 67% | 71% |
| Employed | 26% | 33% | 30% |
| Health literacya | |||
| Limited | 43% | 34% | 39% |
| Adequate | 57% | 66% | 62% |
From Chew et al. (2004).45
The participants enrolled in New Mexico (n=161) were predominantly Latino (75%) or non-Latino white (18%), whereas those recruited in North Carolina (n=101) were non-Latino black (42%), Latino (39%), or non-Latino white (12%). Additionally, participants in New Mexico (where Medicaid expansion was adopted) were more likely to have Medicaid than those in North Carolina (where Medicaid expansion was not adopted) (35% vs 18%, respectively). Otherwise, participants at the two sites were not notably different.
Intervention participants showed greater improvement in knowledge from baseline to follow-up than did control participants (Table 3). The mean adjusted difference (AD) in post-intervention knowledge was 1.8 knowledge items (95% CI=1.5, 2.1) correct on a 6-item scale. Improvement was observed for each of individual knowledge items as well (data not shown).
Table 3.
Patient-Reported Communication and Decision-Making Outcomes in Intervention Versus Control Participants
| Intervention (n=131) | Control (n=131) | Adjusted differencea | |
|---|---|---|---|
| Knowledgeb | |||
| Baseline | 2.6 | 2.5 | — |
| Follow-up | 4.6 | 2.8 | 1.8 (1.5, 2.1)*** |
| Screening discussion | |||
| Any discussion | 71.0% | 45.0% | 26.1% (14.3%, 38.0%)*** |
| FOBT only | 29.8% | 13.7% | |
| Colonoscopy only | 15.3% | 18.3% | |
| Both tests | 24.4% | 8.4% | |
| “Not sure which test” | 1.5% | 4.6% | |
| None | 29.0% | 55.0% | |
| Intent to be screenedc | |||
| Baseline | 70.2% | 71.8% | — |
| Follow-up | 93.1% | 84.7% | 9.0% (2.0%, 16.0%)* |
| Test preference | |||
| Any preference | 93.1% | 68.0% | 26.5% (17.2% 35.8%)*** |
| FOBT | 67.1% | 45.8% | |
| Colonoscopy | 26.0% | 22.2% | |
| “Haven’t decided which test” | 3.8% | 10.7% | |
| “Don’t have enough information to decide” | 3.1% | 21.4% | |
| Test ordering | |||
| Any test ordered | 56.5% | 32.1% | 25.8% (14.4%, 37.2%)*** |
| FOBT only | 35.9% | 18.3% | |
| Colonoscopy only | 17.6% | 13.0% | |
| Both tests | 3.0% | 0.8% | |
| None | 43.5% | 67.9% | |
All comparisons control for provider; comparisons for knowledge and intent also control for baseline. Boldface indicates statistical significance (*p<0.05; **p<0.001; ***p<0.001).
Mean number of correct responses out of six items.
Higher intent to be screened (strongly agree/agree) versus lower (strongly disagree/disagree/neutral).
FOBT, fecal occult blood testing.
Intervention participants were more likely to report discussing any CRC screening test with their provider (AD=26.1%, 95% CI=14.3%, 38.0%). The increase was due principally to more discussion of FOBT/FIT and discussion of both tests (Table 3).
A substantial majority of participants expressed high screening intent at baseline (70.2% intervention vs 71.8% control), and the proportion expressing high intent increased in both groups at follow-up (93.1% intervention vs 84.7% control). The increase in the proportion with high intent to be screened was greater in the intervention group (AD=9.0%, 95% CI=2.0%, 16.0%).
Intervention participants were more likely to indicate a particular test preference than controls (AD=26.5%, 95% CI=17.2%, 35.8%). Preference for colonoscopy was similar across arms (26.0% intervention vs 22.2% control), whereas reported preference for FOBT/FIT was substantially greater among intervention patients (67.1% intervention vs 45.8% control).
Intervention participants were more likely to report that their provider ordered a CRC screening test (AD=25.8%, 95% CI=14.4%, 37.2%). Colonoscopy ordering was only modestly different across groups (17.6% intervention vs 13.0% control), whereas FOBT/FIT ordering was twice as high in the intervention arm (35.9% intervention vs 18.3% control). Similar effects on knowledge, discussions, intent, and test ordering were observed when the analysis was restricted to Latino participants only (Appendix 2, available online).
The intervention was effective at both sites. However, there was evidence of heterogeneity of effects across sites on knowledge (p<0.001; mean difference of 2.3 in North Carolina [95% CI=1.9, 2.7] and 1.5 in New Mexico [95% CI=1.2, 1.8]) and on screening discussion (p=0.015; mean difference of 41.6% in North Carolina [95% CI=29.1%, 54.1%] and 17.3% in New Mexico (95% CI=2.2%, 32.5%). There was no evidence of heterogeneity of effects across sites on intent to be screened (p=0.642); test preference (p=0.136); or test ordering (p=0.185).
Discussion
In this trial conducted in safety net clinics in the U.S., a CRC screening decision aid improved knowledge, communication, and decision-making outcomes. The finding that decision aid viewing increased knowledge about CRC screening is consistent with other U.S. decision aid studies in English-speaking populations30,35,37,46–48 and with the prior test of the Spanish language decision aid in a non-clinical setting.42 Although there is no consensus on what type of knowledge is necessary for “informed” decision making, the knowledge items assessed information that is decision relevant. For example, the items assess understanding about some of the key ways in which the two screening test options differ (e.g., testing frequency) and awareness that there is more than one test option available. Further, apart from higher knowledge scores, participants viewing the decision aid were more likely to be able to indicate a specific test preference and less likely to indicate that they “didn’t have enough information to decide.” Taken together, the findings suggest that the decision aid improved informed decision making about CRC screening.
Beyond showing that the decision aid leads to more-informed decision making, the findings also suggest that this intervention improves patient–provider communication about CRC screening. Specifically, intervention participants were more likely to report having a discussion about CRC with their providers, and were nearly three times as likely to report discussions about both fecal and endoscopic screening tests (a potential proxy for shared decision making). This suggests that using a decision aid in this context not only increases the frequency of screening-related communication, but also can improve the quality of that communication from a decision-making perspective. Although other CRC screening decision aid studies have assessed knowledge about CRC screening options and whether screening was discussed at all, this is the first study known to the authors to assess whether more than one screening option was discussed.
The importance of promoting high-quality clinical communication about CRC screening is twofold. First, having a provider’s recommendation to complete a CRC screening test, that is, any screening communication, has repeatedly been shown to be an important factor in promoting screening completion.12,13,49 Second, accumulating evidence suggests that a discussion in which the provider incorporates a patient’s screening test preferences, rather than simply recommending screening, is also important in promoting screening completion.17,30,50
This study also enhances knowledge about the potential role of multimedia decision aids in addressing screening disparities for Latino populations. Although other U.S. CRC screening decision aid studies have enrolled minority patient populations,32,33,51 this is the first such trial known to the authors to enroll Spanish-speaking Latino patients. Improving screening in Latino populations is important because they are the largest and fastest-growing racial/ethnic minority group in the U.S.52 and have substantially lower screening rates than non-Latinos.36 This screening disparity is particularly striking for Spanish-speaking Latinos. In one national survey study, 33% of Latinos responding in Spanish reported having had a CRC screening test, compared with 51% of Latinos responding in English and 62% of non-Latinos.9 This study provides new evidence that a decision aid delivered in safety net care settings where racially, ethnically, and linguistically diverse patients are served can promote meaningful participation in informed and shared medical decision making about CRC screening.
Limitations
This study has limitations. First, research staff were not masked to the participants’ assigned study arm, which could lead to bias in measurement of study outcomes. Second, screening communication and test ordering outcomes were patient-reported and could be inaccurate. Third, randomization at the individual patient level within these clinics may have caused some providers to change their usual care with respect to CRC screening communication and decision making as a result of exposure to intervention patients. However, such contamination would bias results toward null effect.
This study also has several strengths. First, the RCT design increases the likelihood that these findings are internally valid. Second, the study included a racially, ethnically, and linguistically diverse sample of patients, who are understudied but reflective of populations cared for in safety net clinic settings. Third, the intervention was effective at both New Mexico and North Carolina sites. Thus, the potential generalizability of the findings to clinics serving Latino communities is enhanced by the fact that data were collected in states representing two U.S. regions that differ substantially with respect to Latino immigration history.39,40 Fourth, clinical communication and decision-making outcomes were assessed immediately after provider encounters, making the findings about provider communication more robust than population survey studies, which are more prone to recall bias. Finally, there was minimal loss to follow-up (2%), reducing potential for selection bias.
Conclusions
This study found that a CRC screening decision aid before a primary care visit led to improvements in decision-relevant knowledge, clinical communication, and decision-making processes in a racially, ethnically, and linguistically diverse patient population. Using a CRC screening decision aid in this care context can help vulnerable patient populations participate meaningfully in informed and shared decision making about screening. The decision aids used in this study can be accessed with permission of the authors. Future research should continue to explore methods for sustainable implementation of decision aids combined with patient navigation in safety net, primary care settings.
Supplementary Material
Acknowledgments
This study was funded by the American Cancer Society (Grant RSG-13-165-01–CPPB). Dr. Brenner was supported by the Association for Healthcare Research Quality’s National Research Service Award (Grant No. 5-T32HSHS000032). Dr. Weaver was also supported by the National Center for Advancing Translational Sciences, NIH (Grant No. 1UL1TR001111-01). Pilot work for this study was funded by University of New Mexico Clinical and Translational Science Center (Grant No. 8UL1TR000041) and the North Carolina Translational and Clinical Sciences Institute at the University of North Carolina (Grant No. 1UL1TR001111) and the University of North Carolina Lineberger Comprehensive Cancer Center. The authors thank the Colon Cancer Coalition for additional support. The authors also thank Anita Martinez, Diana Gutierrez, Miriam Espaillat, and Patricia Avraham for their work in the field.
Appendix Supplementary data
Supplementary data associated with this article can be found at http://dx.doi.org/10.1016/j.amepre.2016.03.025.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. http://dx.doi.org/10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.U. S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. http://dx.doi.org/10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. http://dx.doi.org/10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 4.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. http://dx.doi.org/10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph DA, King JB, Miller JW, Richardson LC CDC. Prevalence of colorectal cancer screening among adults— Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(suppl):51–56. [PubMed] [Google Scholar]
- 6.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. http://dx.doi.org/10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. http://dx.doi.org/10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46(3):228–236. doi: 10.1016/j.amepre.2013.10.023. http://dx.doi.org/10.1016/j.amepre.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Diaz JA, Roberts MB, Goldman RE, Weitzen S, Eaton CB. Effect of language on colorectal cancer screening among Latinos and non-Latinos. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2169–2173. doi: 10.1158/1055-9965.EPI-07-2692. http://dx.doi.org/10.1158/1055-9965.EPI-07-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Cancer Society. [Accessed June 27, 2014];Cancer prevention & early detection facts & figures. 2013 www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-037535.pdf.
- 11.Gupta S, Brenner AT, Ratanawongsa N, Inadomi JM. Patient trust in physician influences colorectal cancer screening in low-income patients. Am J Prev Med. 2014;47(4):417–423. doi: 10.1016/j.amepre.2014.04.020. http://dx.doi.org/10.1016/j.amepre.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shokar NK, Carlson CA, Weller SC. Factors associated with racial/ethnic differences in colorectal cancer screening. J Am Board Fam Med. 2008;21(5):414–426. doi: 10.3122/jabfm.2008.05.070266. http://dx.doi.org/10.3122/jabfm.2008.05.070266. [DOI] [PubMed] [Google Scholar]
- 13.Yepes-Rios M, Reimann JOF, Talavera AC, Ruiz de Esparza A, Talavera GA. Colorectal cancer screening among Mexican Americans at a community clinic. Am J Prev Med. 2006;30(3):204–210. doi: 10.1016/j.amepre.2005.11.002. http://dx.doi.org/10.1016/j.amepre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Holden D, Harris R, Porterfield DS, et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [Google Scholar]
- 15.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 suppl 1):S10–S16. doi: 10.1097/MLR.0b013e31817d932e. http://dx.doi.org/10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 16.Pignone MP, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14:432–437. doi: 10.1046/j.1525-1497.1999.00018.x. http://dx.doi.org/10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012;118(10):2726–2734. doi: 10.1002/cncr.26551. http://dx.doi.org/10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. http://dx.doi.org/10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafata JE, Divine G, Moon C, Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med. 2006;31(3):202–209. doi: 10.1016/j.amepre.2006.04.010. http://dx.doi.org/10.1016/j.amepre.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQueen A, Bartholomew LK, Greisinger AJ, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med. 2009;24(11):1228–1235. doi: 10.1007/s11606-009-1108-4. http://dx.doi.org/10.1007/s11606-009-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. http://dx.doi.org/10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. http://dx.doi.org/10.1002/14651858.cd001431.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. doi: 10.1056/NEJMp1109283. http://dx.doi.org/10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 24.Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Med Care. 2008;46(7):738–745. doi: 10.1097/MLR.0b013e318178935a. http://dx.doi.org/10.1097/MLR.0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- 25.Holden D, Jonas DE, Porterfield DSDS, Reuland DS, Harris R. Systematic review: enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. http://dx.doi.org/10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 26.Napoles AM, Santoyo-Olsson J, Stewart AL, et al. Physician counseling on colorectal cancer screening and receipt of screening among Latino patients. J Gen Intern Med. 2015;30(4):483–489. doi: 10.1007/s11606-014-3126-0. http://dx.doi.org/10.1007/s11606-014-3126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson-Kozlow M, Roussos S, Rovniak L, Hovell M. Colorectal cancer test use among Californians of Mexican origin: influence of language barriers. Ethn Dis. 2009;19(3):315–322. [PMC free article] [PubMed] [Google Scholar]
- 28.Jerant AF, Arellanes RE, Franks P. Factors associated with Hispanic/non-Hispanic white colorectal cancer screening disparities. J Gen Intern Med. 2008;23(8):1241–1245. doi: 10.1007/s11606-008-0666-1. http://dx.doi.org/10.1007/s11606-008-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afable-Munsuz A, Liang SY, Ponce NA, Walsh JME. Acculturation and colorectal cancer screening among older Latino adults: differential associations by national origin. J Gen Intern Med. 2009;24(8):963–970. doi: 10.1007/s11606-009-1022-9. http://dx.doi.org/10.1007/s11606-009-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroy PC, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31(1):93–107. doi: 10.1177/0272989X10369007. http://dx.doi.org/10.1177/0272989X10369007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pignone MP, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. Ann Intern Med. 2000;133(10):761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. http://dx.doi.org/10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CL, Brenner AT, Griffith JM, Pignone MP. The uptake and effect of a mailed multi-modal colon cancer screening intervention: a pilot controlled trial. Implement Sci. 2008;3:32. doi: 10.1186/1748-5908-3-32. http://dx.doi.org/10.1186/1748-5908-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller DP, Spangler JG, Case LD, Goff DC, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40(6):608–615. doi: 10.1016/j.amepre.2011.02.019. http://dx.doi.org/10.1016/j.amepre.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolan NC, Ramirez-Zohfeld V, Rademaker AW, et al. The effectiveness of a physician-only and physician-patient intervention on colorectal cancer screening discussions between providers and African American and Latino patients. J Gen Intern Med. 2015;30(12):1780–1787. doi: 10.1007/s11606-015-3381-8. http://dx.doi.org/10.1007/s11606-015-3381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroy PC, Emmons KM, Peters E, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43(6):573–583. doi: 10.1016/j.amepre.2012.08.018. http://dx.doi.org/10.1016/j.amepre.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Cancer Society. Cancer facts and figures for Hispanics/Latinos: 2012–2014. 2012. [Google Scholar]
- 37.Pignone M, Winquist A, Schild LA, et al. Effectiveness of a patient and practice-level colorectal cancer screening intervention in health plan members: the CHOICE trial. Cancer. 2011;117(15):3352–3362. doi: 10.1002/cncr.25924. http://dx.doi.org/10.1002/cncr.25924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko L, Reuland D, Jolles M, Clay R, Pignone M. Cultural and linguistic adaptation of a multimedia colorectal cancer screening decision aid for Spanish speaking Latinos. J Health Commun. 2014;19(2):192–209. doi: 10.1080/10810730.2013.811325. http://dx.doi.org/10.1080/10810730.2013.811325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perreira KM. Mexican families in North Carolina: the socio-historical contexts of exit and settlement. Southeast Geogr. 2011;51(2):260–286. http://dx.doi.org/10.1353/sgo.2011.0014. [Google Scholar]
- 40.Crowley M, Lichter DT. Social disorganization in new Latino destinations. Rural Sociol. 2009;74(4):573–604. http://dx.doi.org/10.1526/003601109789864026. [Google Scholar]
- 41.Brenner AT, Getrich CM, Pignone M, et al. Comparing the effect of a decision aid plus patient navigation with usual care on colorectal cancer screening completion in vulnerable populations: study protocol for a randomized controlled trial. Trials. 2014;15(1):275. doi: 10.1186/1745-6215-15-275. http://dx.doi.org/10.1186/1745-6215-15-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuland DS, Ko LK, Fernandez A, Braswell LC, Pignone MP. Testing a Spanish-language colorectal cancer screening decision aid in Latinos with limited English proficiency: results from a pre-post trial and four month follow-up survey. BMC Med Inform Decis Mak. 2012;12:53. doi: 10.1186/1472-6947-12-53. http://dx.doi.org/10.1186/1472-6947-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaVange LM, Durham TA, Koch GG. Randomization-based nonparametric methods for the analysis of multicentre trials. Stat Methods Med Res. 2005;14(3):281–301. doi: 10.1191/0962280205sm397oa. http://dx.doi.org/10.1191/0962280205sm397oa. [DOI] [PubMed] [Google Scholar]
- 44.Zink RC, Koch GG. NParCov3: A SAS/IML macro for nonparametric randomization-based analysis of covariance. J Stat Softw. 2012;50(3) http://dx.doi.org/10.18637/jss.v050.i03. [Google Scholar]
- 45.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 46.Jerant AF, Kravitz RL, Rooney M, Amerson S, Kreuter MW, Franks P. Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices. Patient Educ Couns. 2007;66(1):67–74. doi: 10.1016/j.pec.2006.10.009. http://dx.doi.org/10.1016/j.pec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Frosch DL, Legare F, Mangione CM. Using decision aids in community-based primary care: a theory-driven evaluation with ethnically diverse patients. Patient Educ Couns. 2008;73(3):490–496. doi: 10.1016/j.pec.2008.07.040. http://dx.doi.org/10.1016/j.pec.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffith JM, Lewis CL, Brenner AT, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis Mak. 2008;8:4–4. doi: 10.1186/1472-6947-8-4. http://dx.doi.org/10.1186/1472-6947-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cronan TA, Devoscomby L, Villalta I, Gallagher R. Ethnic differences in colorectal cancer screening. J Psychosoc Oncol. 2008;26(2):63–86. doi: 10.1300/j077v26n02_05. http://dx.doi.org/10.1300/J077v26n02_05. [DOI] [PubMed] [Google Scholar]
- 50.Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests. J Gen Intern Med. 2001;16(12):822–830. doi: 10.1111/j.1525-1497.2001.10337.x. http://dx.doi.org/10.1046/j.1525-1497.2001.10337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruffin MTt, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45(4):267–273. doi: 10.1016/j.ypmed.2007.07.003. http://dx.doi.org/10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Brown A. U.S. Hispanic and Asian populations growing, but for different reasons. [Accessed September 17, 2015];Facttank. www.pewresearch.org/fact-tank/2014/06/26/u-s-hispanic-and-asian-populations-growing-but-for-different-reasons/. Published June 26, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.