Abstract
The prevalence of HIV-Associated Neurocognitive Disorders (HAND) has not changed considerably in the last two decades. Potent antiretroviral therapy (ART) has shifted the severity of HAND to milder phenotypes, but excess morbidity and mortality continue to be associated with HAND. Changes in numerous markers of immune function, inflammation and cellular stress have been repeatedly associated with HAND but the underlying systems that drive these changes have not been identified. In this study we used systems informatics to interrogate the CSF proteomic content of longitudinal samples obtained from HIV-infected adults with stably unimpaired, stably impaired, worsening, or improving neurocognitive (NC) performance. The patterns of change in CSF protein content implicated the induction of acute phase and complement systems as important regulators of NC status. Worsening NC performance was preceded by induction of acute phase and complement systems, while improving NC performance was preceded by a downregulation of these systems.
Keywords: HIV, HAND, biomarker, CSF, proteomics, neurological impairment
INTRODUCTION
The widespread use of antiretroviral therapy (ART) in developed countries has modified HIV infection from a terminal illness to a chronic infection1. Considerable evidence suggests progressive damage occurs to multiple organ systems (including brain) despite viral suppression and immune recovery. Neurocognitive (NC) impairment in HIV-infected adults occurs as a spectrum of disorders that have been categorized as asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HIV-associated dementia (HAD)2. NC impairment remains a major cause of morbidity in the modern treatment era (prevalence between 15% and 55%) with the majority of NC impaired adults in the ANI and MND groups 3,4. Although ANI does not affect daily functioning, patients with ANI are two to six times more likely to progress to symptomatic MND or HAD 5.
HIV enters the central nervous system (CNS) soon after infection 6,7, and the brain is a site of chronic infection 8. ANI and MND are associated with immune 9–11 and cytokine activation 12,13, metabolic disturbances 14–18, accumulations of bioactive lipids such as ceramide, and sterol markers of cell stress 19,20; many of which worsen with age and duration of infection 11,18,19,21–34. Despite the considerable literature describing consequences of HIV infection in the CNS, few studies have determined the temporal relationship of inflammatory, stress and metabolic changes to NC performance. Here we used cerebrospinal fluid (CSF) to conduct an informatics analysis of the CSF protein content in groups of HIV+ adults selected to differ by longitudinal changes in NC performance, and found that upregulation of acute phase and complement systems preceded worsening cognitive function, while downregulation of acute phase and complement pathways preceded and was sustained during NC improvement.
MATERIALS AND METHODS
Subjects and Sample Collection
CSF samples were obtained from the Central Nervous System HIV Anti-Retroviral Therapy Effects Research (CHARTER) and the HIV Neurobehavioral Research Center (HNRC) studies. Subject selection was based on a case review of ~3,500 clinical visits from 430 study participants as previously described 35,36. Subjects had complete clinical and neuropsychological data available and were on stable ART for at least three months before the first visit and for the duration of the observation period (a single drug modification in ART was allowed if within the same drug class). Participants were demographically matched with the exception of group differences in current CD4 count, and CSF storage time (Table 1).
Table 1. Baseline Demographic and Clinical Characteristics.
ART=antiretroviral therapy, IQR=Interquartile range; Kruskal-Wallis test used for continuous variables and Fisher’s exact test used for categorical variables.
| Characteristic | Stably Unimpaired (N=25) | Worsening (N=22) | Stably Impaired (N=22) | Improving (N=20) | p-value |
|---|---|---|---|---|---|
| median (IQR)/N(%) | median (IQR)/N(%) | median (IQR)/N(%) | median (IQR)/N(%) | ||
| Age, years | 46.0 (38.0, 50.0) | 46.0 (40.0, 50.0) | 48.5 (41.0, 51.0) | 42.5 (37.5, 48.0) | 0.387 |
| Education, years | 13 (12, 15) | 12.5 (12, 14) | 13 (12, 16) | 12 (11, 16) | 0.875 |
| Sex (men) | 23 (92%) | 19 (86%) | 19 (86%) | 16 (80%) | 0.697 |
| Ethnicity (Caucasian) | 14 (56%) | 14 (64%) | 10 (45%) | 14(70%) | 0.613 |
| AIDS | 14 (56%) | 15 (68%) | 17(77%) | 17(85%) | 0.180 |
| HCV seropositive | 4 (16%) | 3 (14%) | 6 (27%) | 4 (20%) | 0.727 |
| Current CD4 count, cells/mm3 | 462 [312, 663] | 339 [189, 425] | 499.5 [370, 712] | 170.5 [101, 484] | 0.003 |
| Nadir CD4 count, cells/mm3 | 190 [102, 322] | 60 [18, 234] | 120 [41, 209] | 33 [7, 147] | 0.030 |
| ART Use | 19 (76%) | 14 (64%) | 19 (86%) | 15 (75%) | 0.231 |
| Among those taking ART | |||||
| Duration of current ART regimen, months | 9.2 [4.6, 31.2] | 9.6 (4.6, 24.6) | 10.9 (5.8, 37.9) | 11.8 (2.0, 29.5) | 0.876 |
| Plasma viral load ≤ 50 copies/mL | 10 (59%) | 8 (57%) | 12 (71%) | 3 (23%) | 0.072 |
| CSF viral load, ≤ 50 copies/mL | 14 (93%) | 11 (79%) | 15 (88%) | 11 (85%) | 0.754 |
Change in Neurocognitive Status
The neuropsychological testing battery was conducted by trained neuropsychometricians and consisted of tests covering seven cognitive domains including: executive function, learning and delayed recall, working memory, verbal fluency, speed of information processing, and motor skills 37 The best available normative standards were used to convert the scores to demographically-corrected standard scores (T-scores), which correct for effects of age, education, sex and ethnicity. The presence and severity of NCI was determined using the Global Deficit Score (GDS) approach, where a GDS≥0.5 was impaired38. All follow-up visits were corrected for practice effects.39. A change in status from the baseline visit to the second visit defined change in NC status and the third visit was used to confirm the trajectory of change as previously described 35. Based on changes in NC performance, participants were categorized as: Stably normal (no NC impairment at either visit, n=25), Improving (NC impairment at baseline that improved by visit 2, n=20), Worsening (normal cognitive status or NC impairment that then worsened over time, n=22) and Stably Impaired (NC impairment that was stable over time, n=22).
Standard Protocol Approvals, Registrations, and Patient Consents
The collection and use of human samples was approved by the Institutional Review Board at each study site. All participants provided informed consent for all study procedures, including future use of their stored specimens and data for research.
Sample Preparation and Mass Spectrometry
Immunodepletion of CSF was accomplished using the ProteoPrep® Immunoaffinity Albumin & IgG Depletion kit (Sigma PROTIA-1KT)40. Samples were methanol extracted, vortexed and stored in −80°C overnight to allow precipitation. Tubes were centrifuged for 5 minutes at 14,000 RPM (4°C), and the protein precipitate removed for digestion. Protein was trypsinized (Promega, Madison, WI) and purified using a 20μm filter (Pall Corporation, Ann Arbor, MI). Trypsinized peptides were purified using an Oasis mixed-mode weak cation exchange cartridge (Waters, Milford, MA). Samples were then dehydrated with a Savant ISS 110 SpeedVac Concentrator (Thermo Fisher, Rockford, IL), and resuspended in 7μl of 0.1% formic acid for LC-MS/MS analyses.
Mass spectrometry was conducted using a LTQ Velos system that included two alternating peptide traps and a PicoFrit C18 column emitter (New Objective, Woburn, MA). Samples were resuspended in water containing 0.1% formic acid and injected into the Velos with a linear gradient of acetonitrile from 0–100% over the course of 200 minutes. This was followed by two washes one at 0–40% ACN ramp up and the other linear gradient of ACN for 110 minutes 41–43.
Proteomic Analysis and Bioinformatics
Raw data was converted to mzXML format using MSConvert (ProteoWizard 3.0.6002), and proteomic data was searched with Mascot 2.4.1 44 using the SwissProt Human sequences from Uniprot (Version Year 2013). Results from the Mascot search engine were analyzed in Scaffold 4.0.5 (Proteome Software, Inc) using a subset search 45 with X!Tandem CYCLONE (2010.12.01.1) 46 and data filters of 5% peptide FDR (with 99% peptide confidence), 5% protein FDR (with 99% protein confidence) requiring a minimum of 2 peptides for protein identification. Protein identifications were further clustered to remove redundancy. Quantitative values were normalized by multiplying the spectral counts for each peptide by a ratio of the average for total spectra from all samples divided by the total spectra for each individual sample. Proteins were retained for further analyses if they were identified in 70% of the samples within each subject group.
Statistical Analyses
For descriptive analyses by group, Kruskall-Wallis tests were used for continuous variables and Fisher’s exact test for categorical variables. Paired two tailed t-tests were performed for calculating change CSF protein levels between baseline (visit 1) and follow up (visit 2). To account for multiple comparisons, we used the Benjamini-Hochberg correction method which resulted in p-values <= 0.0529 considered as statistical significant.
Predictive Modeling
Principal components analysis (PCA) was used to derive models that maximized the variance between CSF proteomes based on NC change group. PCA loadings were derived from spectral counts of proteins and missing values were set to 2 spectral counts (just below the lowest detectable limit). PCA was performed using R3.3.4 47.48,49. Heat maps and hierarchical cluster analysis were performed using the non-linear minimization package in R 50. GraphPad PRISM 6.07 and R-packages were used for statistical analysis and generation of figures. Ingenuity Pathway Analysis (IPA; Qiagen, USA) was performed using the relative fold change for each protein at baseline and follow-up based on quantitative data from proteomic mass spectrometry. The software was set to the following parameters: Reference set: Ingenuity Knowledge Base (Genes Only), Data Sources: All, Confidence: All, Species: All, Tissues & Cell Lines: All. Canonical pathways with few protein hits were excluded from further analysis. The ratio of the number of hits in a pathway to the total number of reference proteins in the IPA database, and p values were calculated by IPA.
RESULTS
Proteomic evidence for cellular stress, neural damage, and immune activation in HIV infected subjects with cognitive impairment
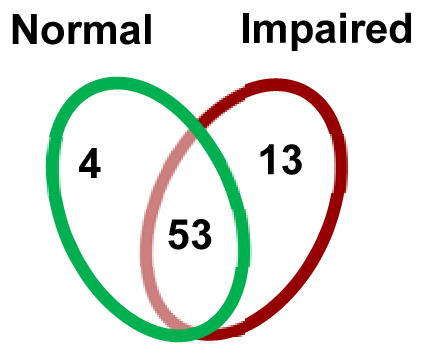
We first determined if there were cross sectional differences in CSF proteomic profiles of HIV-infected subjects classified as normal or impaired at the first visit. For these analyses, we only included proteins that were within detectable limits in a minimum of 70% of the patient samples within each group to ensure that the proteins identified as unique were indeed associated with the group, and not to a small number of individuals within a group. Using the specified criterion, 13 proteins were unique to the impaired group, 4 to the normal group, and 53 were common to both groups (Table 2). The 4 proteins found to be unique to the NC normal group were associated with immune function (Ig-γ-chain-2 C region), energy metabolism (Insulin like growth factor binding protein 6), and lipid metabolism (Ectonucleotide pyrophosphatase, Apolipoprotein A-II) (Table 2). The 13 proteins unique to the NC impaired group included markers of neuronal damage (Amyloid beta A4 precursor protein), immune regulation (Beta-2-microglobulin, and Ig-γ-chain-3C region, complement C4-B, and alpha-1-acidic glycoprotein), control of oxidative stress (Superoxide dismutase, Vitamin E binding protein), glycoproteins associated with cell adhesion (Vitronectin, Cadherin-13), structural integrity of muscle tissue (Dystroglycan), plasma and sterol transport (Afamin, Epididymal secretory protein E1, Haptoglobin-related protein), and RNA degradation (RNase I) (Table 2). Of the 53 proteins common between the normal and impaired cognition, 49 of these were decreased in the impaired group (Figure 1A). These data suggests that NC impairment is associated with alterations in the CSF proteome, characterized by induction of proteins associated with cellular stress, damage, and immune activation.
Table 2. CSF proteins unique to HIV-infected subjects with normal and impaired cognition.
Venn diagram shows the number of proteins unique to HIV-infected subjects with normal cognition (n=4), impaired cognition (n=13) and proteins common to both groups (n=53). Tables show protein and gene names for the specified groups of subjects.
| |
|---|---|
| Cognitively Normal | Gene Name |
| Apolipoprotein A-II | APOA2 |
| Ectonucleotide pyrophosphatase | ENPP2 |
| Insulin-like growth factor-binding protein 7 | IGFBP7 |
| Ig lambda-2 chain C regions | IGLC2 |
| Cognitively Impaired | Gene Name |
|---|---|
| Alpha-1-acid glycoprotein 2 | ORM2 |
| Amyloid beta A4 protein | APP |
| Afamin | AFM |
| Beta-2-microglobulin | B2M |
| Cadherin-13 | CDH13 |
| Complement C4-B | C4B |
| Dystroglycan | DAG1 |
| Haptoglobin-related protein | HPR |
| Ig lambda-3 chain C regions | IGLC3 |
| Epididymal secretory protein E1 | NPC2 |
| Ribonuclease pancreatic | RNASE1 |
| Superoxide dismutase | SOD1 |
| Vitronectin | VTN |
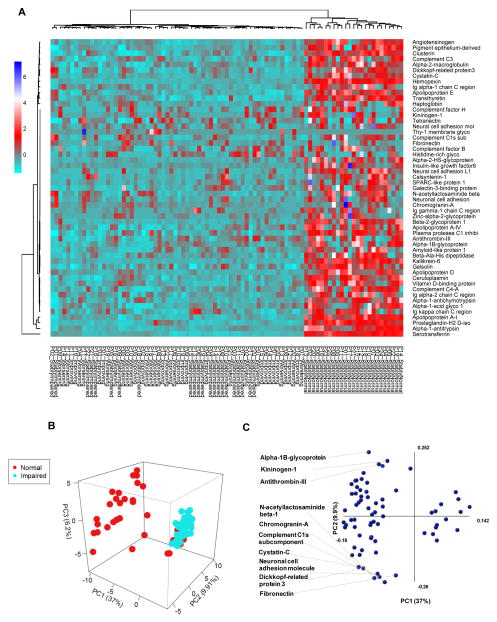
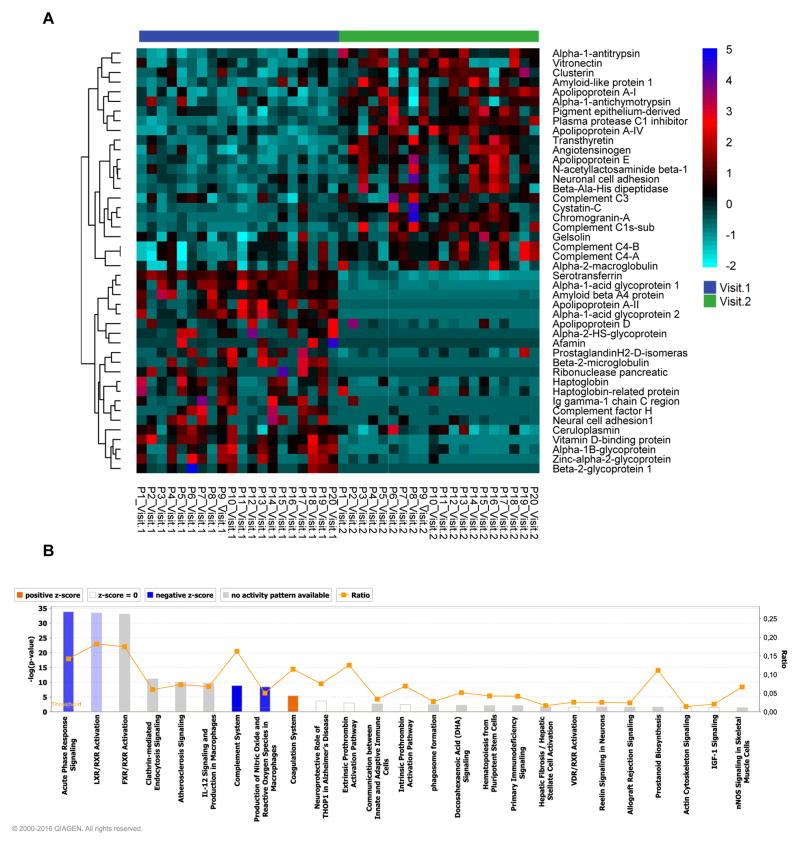
Figure 1. Relative baseline CSF protein expression in HIV-infected subjects with normal or impaired cognitive function.
Heat map and hierarchical clustering analysis showing relative baseline CSF proteomic profiles in subjects with normal cognition, stably impaired, worsening, or improving cognition. There is a general decrease in CSF proteins in subjects with impaired cognition compared to those with normal cognition. Protein identifications are on the right and patient group IDs are listed at the bottom of the figure. Heatmaps were generated using proteins common to subjects with normal and impaired cognition. Figure legend shows relationship of color to fold change in zeta score. B) Principal Components Analysis (PCA) of individual protein identifications showing separation between HIV-infected subjects with normal compared to impaired cognitive function. C) Protein components that contributed to PCA modeling are depicted in PCA1 and PCA2 axis. The top 10 proteins that contributed to the separation in PC1 are identified, and the axes show to what extent the proteins contribute to the first two principle components in the model.
To further explore the relationships between CSF proteomic profiles and NC status we used a principal components analysis (PCA) to determine if the entire CSF proteomic content could be used to separate groups based on NC status. A pronounced separation was present using a three-component PCA model (PC1=37.0%, PC2=9.9%, PC3=6.2%, PCA=53.1%) (Figure 1B). The primary contributors to PCA separation included proteins involved in wound healing and coagulation (Serpin C1, Fibronectin, and Kininogen-1), immune response (Complement component C1, Alpha-1B-glycoprotein), neuroendocrine function (Chromogranin A, and Cystatin 3), neuronal development (Dickkopf-3 and Neuronal Cell Adhesion Molecule), and glycosylation (B3GNT2) (Figure 1C). In this analysis, all impaired subjects were correctly classified, but 16 of 89 normal subjects were “incorrectly” clustered with the impaired group. When we stratified according to longitudinal change in cognitive status we found that all of the “incorrectly” classified normal subjects at baseline developed NC impairment within 6 months. This result suggests that baseline CSF protein profiles may be prognostic indicators for change in NC status.
Acute phase and complement cascades are implicated in changing cognitive status
We next stratified subjects as stably normal, worsening, improving or stably impaired based on change in cognitive function from the first to the second study visit (~6 months later). A third visit performed ~18 months following the baseline visit was used to validate the trajectory of change in cognitive status, as previously described 35,51. Using these stratifications we identified 2 proteins unique to the stably normal group, and 3 to the worsening group (Figure S1). The 2 proteins unique to the stably normal group were proteins involved in energy metabolism and signaling (Ectonucleotide pyrophosphatase, and Insulin-like growth factor-binding protein 7) (Figure S1). The 3 proteins unique to the worsening group were associated with cholesterol transport (Epididymal secretory protein E1), redox regulation (Superoxide dismutase), and cell adhesion (Thy-1 membrane glycoprotein) (Figure S1A,B). There were no proteins unique to the stably impaired or improving groups.
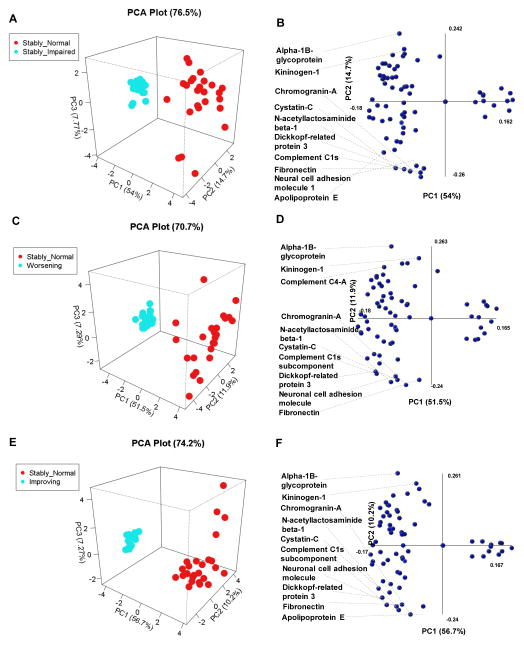
Using baseline CSF proteomics, PCA distinguished clear separations between the stably normal and the stably impaired (PC1=54.0%, PC2=14.7%, PC3=7.7%; PCA=76.4%) (Figure 2A), worsening (PC1=51.5%, PC2=11.9%, PC3=29.0; PCA=70.7%) (Figure 2C), and improving groups (PC1=56.7%, PC2=10.2%, PC3=7.2%; PCA=74.2%) (Figure 2E). The top 10 proteins that contributed to PCA separation between stably normal and stably impaired (Figure 2B) or stable normal and worsening (Figure 2C) were nearly identical and consisted of proteins involved in neurite development (NRCAM, DKK3), neuroendocrine signaling (Chromogranin-A and Cystatin-C), glycosylation (B3GNT2), immune response (Complement Component-1s, Alpha-1B-glycoprotein), coagulation and adhesion (Kininogen-1 and Fibronectin), lipid transport (Apolipoprotein-E). The single exception was a protein involved in immune response (Complement component C4-A) that replaced Apolipoprotein-E in the comparison to worsening. Interestingly five of these 10 proteins Alpha-1B-glycoprotein, B3GNT2, Chromagranin-A, Fibronectin and Kininogen-1) were found above to distinguish baseline NC function. Only a modest separation was possible between stably impaired and improved groups (Figure S2A); the major contributors to this separation shown in Figure S2B. Using PCA, we were not able to build a model that separated the improving and worsening groups using baseline CSF protein profiles (Figure S2C,D). This might be due to heterogeneity in the worsening group, which could be either normal or impaired at baseline. However, PCA was able to obtain good separation between the stably impaired and improving groups (Figure S2E,F), and the improving and worsening groups (Figure 2SG,H) using CSF proteomic profiles obtained from the follow-up visit. These data suggest that CSF protein profiles at baseline could distinguish subjects with impaired (or soon to be impaired) cognitive function from subjects with stably normal cognitive function, and the CSF protein profile at follow-up could further distinguish improving from declining cognitive function.
Figure 2. Principal components analyses of baseline CSF proteomic profiles for HIV-infected subjects grouped by temporal change in cognitive status.
A pairwise PCA was performed using all proteins detected in CSF of HIV-infected patients to compare the following groups: A–B) stably normal vs stably impaired cognition, C–D) stably normal vs worsening cognition, E–F) stably normal vs improving cognition. For each figure the left-hand panels show and the right panels show protein components that contributed to PCA modeling depicted in PCA1 and PCA2 axis. The top 10 proteins that contributed to the separation in PC1 are identified, and the axes show to what extent the proteins contribute to the first two principle components in the model.
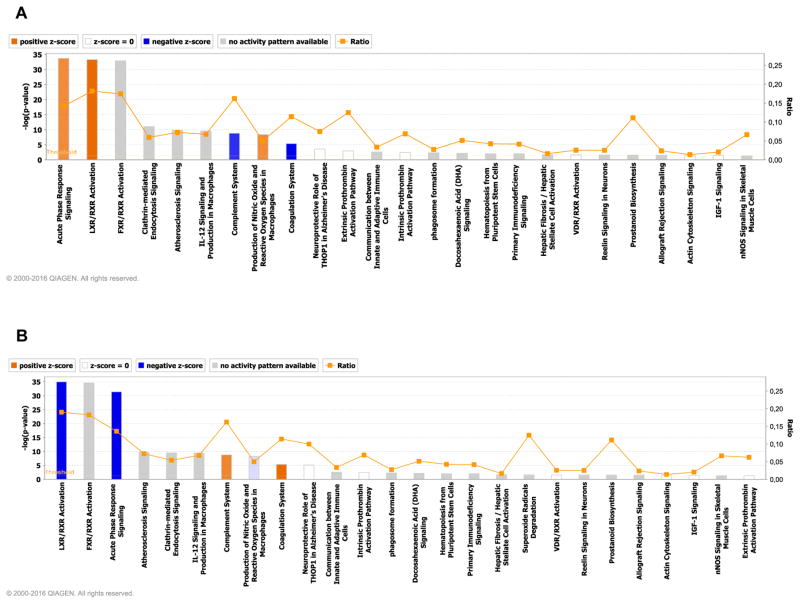
As complex modifications in the proteome are not easily interpreted by investigating the functions of individual proteins, we used IPA to interrogate the CSF proteomic content, and identified two integrated signaling pathways that were reciprocally regulated in the worsening and improving groups. In the worsening group, acute phase response and the complement pathway (Figure 3A; Figure S3,S4) were positively regulated while evidence of negative regulation of acute phase response and complement cascades was apparent in improving group (Figure 3B; Figure S5,S6). Induction of the acute phase response in the worsening group was suggested by the induction of proteins associated with the IL-6 response and the TNFα-associated acute phase response (Figure S3). Involvement of the alternate complement pathway was implicated by increases of serine proteases C3, and complement factor B (CBF) (Figure S5). Although C1 was also upregulated, the acute phase response protease inhibitor SERPING1 was also induced, and based on downstream protein expression, this appears to have successfully blocked induction of response elements downstream of C1 including C4 (Figure S5). In Improvers, acute phase and complement pathways were downregulated (Figure S4,S6). Although these analyses also implicated nuclear receptor signaling and macrophage reactive oxygen species pathways, the number of protein hits in these putative pathways were low, and largely overlapped with the acute phase or complement pathways (data not shown).
Figure 3.
CSF proteomics implicate acute phase and complement systems in changing cognitive status. for subjects with A) worsening, and B) improving cognitive status. The pathways are ranked according to their p value. Orange depicts pathways with increased activity, and blue pathways depict reduced pathway activity.
Longitudinal changes in CSF proteomic content
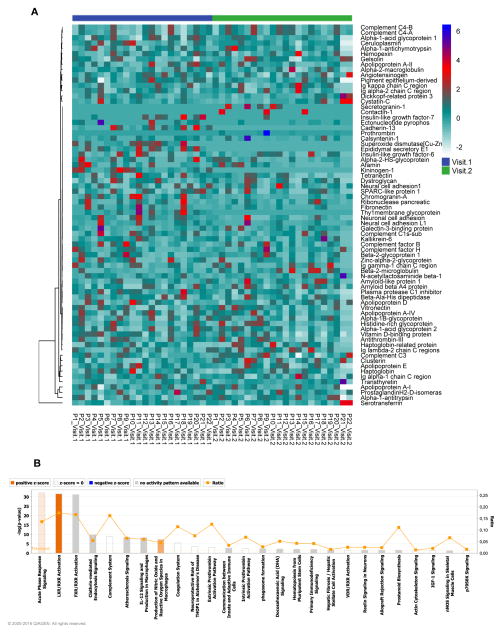
We used hierarchical clustering to arrange longitudinal changes in CSF protein content in the worsening group (Figure 4A). No groups of proteins that clustered from the first to the second visit were clearly evident, but seven proteins did significantly change between visits when comparing the worsening and stably normal groups. Proteins that increased in abundance were associated with immune function (β-2-microglobulin, Clusterin and Gelsolin), and proteins that decreased in abundance were associated with neuroendocrine function (Chromogranin-A), immune regulation (VitaminD-binding protein), complement pathway (Plasma protease-C1 inhibitor), and metabolism (insulin like growth factor binding protein-6) (Table 3;Figure S7). In addition to these proteins, a large number of proteins showed trends in the worsening group. Since each of these more subtle changes in protein expression can work together to modify cell signaling, we used IPA to interrogate the proteomic data obtained from analysis of CSF at the second visit. This analysis showed that induction of the of the acute phase and complement system that was apparent at the first visit (see Figure 3A), was largely downregulated at the second visit (Figs 4B). These results suggest that these pathways are transiently induced early in the progression of cognitive impairment.
Figure 4. Changes in CSF protein content during cognitive worsening.
A) Heat map and hierarchical clustering showing relative CSF protein expression in subjects with cognitive worsening. Each vertical lane shows relative amounts of the indicated protein for an individual subject. Subjects are identified at the bottom of the figure as P1–P22, and proteins are identified on the right. Blue line indicates the baseline visit (visit 1) and green indicates the follow-up visit (visit 2). Ingenuity Pathway analysis of the CSF protein content at the follow-up visit for subjects with worsening cognitive status. Combined bar charts (p value) and line graphs (ratio) show canonical pathways identified by IPA for subjects with A) worsening, and B) improving cognitive status. The pathways are ranked according to their p value. Orange depicts pathways with increased activity, and blue pathways depict reduced pathway activity.
Table 3. CSF proteins showing increases (left), and decreases (right) in abundance during cognitive improvement (right).
Benjamini-Hochberg corrected p-value from paired two tailed t-test.
| Protein | p-value | Up | Protein | p-value | Down |
|---|---|---|---|---|---|
| Regulation of Protein Folding and Proteolysis | Lipolysis | ||||
| Clusterin | 0.037885 | ↑ | Zinc-alpha-2-glycoprotein | 0.00088 | ↓ |
| Vitronectin | 0.003026 | ↑ | Plasma Transport | ||
| Alpha-1-antitrypsin | 0.000185 | ↑ | Vitamin D-binding protein | 0.000001 | ↓ |
| Plasma protease C1 inhibitor | 0.000001 | ↑ | Beta-2-glycoprotein 1 | 0.006946 | ↓ |
| Alpha-1-antichymotrypsin | 0.015667 | ↑ | Alpha-1B-glycoprotein | 0.000035 | ↓ |
| Alpha-2-microglobulin | 0.041462 | ↑ | Ceruloplasmin | 0.051851 | ↓ |
| Beta-Ala-His didpeptidase | 0.000896 | ↑ | Afamin | 0.048041 | ↓ |
| Cystatin C | 0.003981 | ↑ | Apolipoprotein D | 0.052998 | ↓ |
| Gelsolin | 0.0203 | ↑ | Apolipoprotein A-II | 0.000035 | ↓ |
| Plasma Transport | Serotransferrin | 0.000000000068 | ↓ | ||
| Transthyretin | 0.000017 | ↑ | Alpha-1-acid glycoprotein 2 | 0.000035 | ↓ |
| Lipid Metabolism and Transport | Alpha-2-HS-glycoprotein | 0.003551 | ↓ | ||
| Apolipoprotein E | 0.000243 | ↑ | Haptoglobin | 0.000744 | ↓ |
| Apolipoprotein A-I | 0.000248 | ↑ | Haptoglobin-related protein | 0.020599 | ↓ |
| Apolipoprotein A-IV | 0.000035 | ↑ | Neurite Outgrowth | ||
| Neuronal Development and Neurotrophic Functions | Neural cell adhesion molecule 1 | 0.00882 | ↓ | ||
| Neuronal cell adhesion molecule | 0.00882 | ↑ | Prostaglandin-H2 D-isomerase | 0.020458 | ↓ |
| Pigment epithelium-derived factor | 0.000896 | ↑ | Amyloid beta A4 protein | 0.003981 | ↓ |
| Amyloid-like protein 1 | 0.003981 | ↑ | Immune and Acute Phase Response | ||
| Neuroendocrine Signaling | |||||
| Angiotensinogen | 0.000185 | ↑ | Beta-2-microglobulin | 0.001393 | ↓ |
| Chromogranin-A | 0.003375 | ↑ | Ig gamma-1 chain C region | 0.017997 | ↓ |
| Immune Regulation | Complement factor H | 0.003981 | ↓ | ||
| Complement C4-A | 0.001569 | ↑ | Alpha-1-acid glycoprotein 1 | 0.000001 | ↓ |
| Complement C4-B | 0.001615 | ↑ | RNA Degradation | ||
| Complement C3 | 0.036589 | ↑ | Ribonuclease pancreatic | 0.009935 | ↓ |
| Complement C1s subcomponent | 0.000195 | ↑ | |||
| Glycosylation | |||||
| N-acetyllactosaminide beta-1,3-N-acetylglucosaminyltransferase | 0.00128 | ↑ | |||
A robust change in CSF protein content was apparent in the improving group (Figure 5A) with 23 proteins increased, and 21 decreased at the second visit (Table 3; Figure S8, S9). Proteins increased in abundance were associated with the regulation of protein folding and proteolysis, plasma transport, lipid metabolism and transport, neuronal development and neurotrophic functions, neuroendocrine signaling and immune regulation (Figure S8A–W). The 21 proteins decreased in abundance at the second visit in the improving group included proteins involved in lipolysis, plasma transport, neurite outgrowth, immune and acute phase response, and RNA degradation (Figure S9A–U). IPA of the CSF proteomic content at the second for subjects with improving cognitive function showed a negative regulation of acute phase and complement systems (Figure 5B), suggesting that an active inhibition of these systems begins before measurable improvements in cognitive status, and continues after cognitive status has improved.
Figure 5. Changes in CSF protein content during cognitive improvement.
A) Heat map and hierarchical clustering showing CSF proteomes from subjects with improving cognitive status. Vertical lanes show the relative amounts of the indicated protein for individual subjects. Subjects are identified as P1–P20, and proteins are identified on the left. Blue labels indicate the baseline visit (visit 1) and green indicates the follow-up visit (visit 2). B) Ingenuity Pathway analysis of the CSF protein content at the follow-up visit for subjects with improving cognitive status. Combined bar charts (p value) and line graphs (ratio) show canonical pathways identified by IPA for subjects with A) worsening, and B) improving cognitive status. The pathways are ranked according to their p value. Orange depicts pathways with increased activity, and blue pathways depict reduced pathway activity.
DISCUSSION
An informatics analysis of the baseline CSF protein content stratified by longitudinal change in NC performance identified modifications in acute phase and complement pathways as early events that may regulate NC changes in HIV+ adults. The complement system plays key roles in regulating the host-defense to pathogens. Depending on the involvement of particular molecular activators, the complement system is induced through three distinct pathways known as the classical, alternative and mannose-binding lectin pathways 52,53. All pathways converge on the activation of C3, a central component that is critical for the formation of the terminal membrane attack complex (MAC). The MAC forms a lytic pore in the lipid bilayer of membranes that allows free passage of solutes and water to lyse foreign pathogens and cells. Almost all components of the complement system are produced locally in the brain, but expression in the CNS is tightly controlled to avoid inflammation-associated tissue damage 54,55. Complement C1q, C3 and C4 have previously been detected in the CSF of HIV infected patients 56–59, and gene expression studies using postmortem human brain tissues have suggested that complement components are strongly upregulated in the neocortex of patients with cognitive impairment and HIV encephalitis, but not in cognitively impaired patients without encephalitis 60. Our data suggest that complement activation in the CNS of HIV infected adults may be an early event that promotes neuronal dysfunction. While determining the precise underlying cause for the induction of the complement system is not possible, intact HIV virions, Nef and gp41 (but not Tat, gp120 and gp160) induce complement expression in astrocytes, and promote export of C3 61. In the SIV model of HIV infection, high levels of C1q and C3 were measured in brain of infected non-human primates, and both were found deposited on the membrane of neurons, a prerequisite for formation of the MAC. These data support a model in which blips of HIV replication in the CNS may be responsible for induction of the complement system with subsequent neural damage, and worsening cognitive function.
The complement response to viral infection is qualitatively different from the acute phase response elicited by bacterial infections. HIV infection is accompanied by a higher concentration and faster synthesis of positive acute phase proteins, but not by lower concentrations and slower synthesis of negative acute phase proteins 62. For example, the positive acute phase proteins haptoglobin and haptoglobin-hemoglobin scavenger receptor CD163 levels in HIV-infected patients are positively correlated with viral load 63, and with the progression of HIV-infection 64. Likewise, ceruloplasmin is increased in HIV infected patients 65, in association with cognitive impairment 66, and with decreased oxidative damage in untreated HIV-positive subjects 67. This peculiar acute phase response to viral infection that lacks induction of “negative” acute phase proteins to limit the inflammatory response may contribute to the low level chronic inflammation frequently observed in brain and CSF of HIV-infected patients, even when viral replication is suppressed by ART 68,69.
Inflammatory cascades are known to cross-talk with cellular stress responses, and with the unfolded protein response to create an integrated stress response that can inhibit protein translation, cellular homeostasis, and cellular response to stress 70–72. In the current study we found a decreased CSF protein content in HIV-infected subjects, and recent postmortem studies of brain tissues from HIV-infected patients with cognitive impairment found elevated levels of ER stress proteins BiP, and ATF6 73,74. However, other studies have shown that CSF pleocytosis, and/or increased CSF protein content is apparent in advanced AIDS 75, in patients with acute CSF viral escape 76, or with the onset of neurological symptoms 77. In cases of advanced AIDS, or when evidence for CNS viral replication is apparent, this increased CSF protein content likely reflects damage to the blood brain barrier, and increased intrathecal albumin and/or IgG 78. In the current study we depleted albumin and IgG before proteomic analysis to facilitate the detection of less abundant proteins. Thus, the lower amounts of many CSF proteins in the cognitively impaired individuals in our study most likely reflects lower brain protein production that could be the result of an integrated CNS stress response.
These data were obtained from a relatively small group of subjects selected from ~3000 case reviews to be clinically and demographically matched. While this approach provides a considerable advantage to identify biomarkers that specifically relate to changes in the cognitive status of HIV infected adults, these findings need to be replicated in a larger cohort to determine if variations in demographic and clinical factors modulate the CSF protein biomarkers used to identify acute phase and complement systems.
Our informatics analysis of longitudinal changes CSF protein content suggests that changes in markers of immune function, cell stress, and inflammation in HIV-infected patients with HAND may reflect the induction of acute phase and complement systems. The induction of these systems is associated with declines in cognitive function, while the downregulation of acute phase and complement systems was associated with improvements in cognitive function, suggesting that modulation of these pathways may be beneficial in HIV-infected patients with HAND.
Supplementary Material
Figure S1. Venn diagram of baseline total protein identifications grouped by change in cognitive status. A) Numbers of unique and overlapping protein identifications for the indicated subject groups. B) Protein identifications that are unique to stably normaland worsening groups.
Figure S2. Principal components analyses of baseline CSF proteomic profiles for HIV-infected subjects grouped by temporal change in cognitive status. A pairwise PCA was performed using all proteins detected in CSF of HIV-infected patients to compare the following groups: A–B) stably impaired vs improving cognition at baseline, C–D) worsening vs improving cognition at baseline, E–F) stably impaired vs improving cognition at the follow up visit. G–H) Improving vs. worsening at the follow up visit. For each figure the left-hand panels show PCA loadings and the right panels show protein components that contributed to PCA modeling depicted in PCA1 and PCA2 axis. The top 10 proteins that contributed to each model are indicated, and the axes show how well the proteins correlated with each other.
Figure S3. Worsening neurocognitive performance is associated with induction of the acute phase response at baseline. IPA canonical pathway for the acute phase response shows proteins detected in CSF associated with the acute phase response that are increased (red) or decreased (green) in CSF of patients with worsening neurocognitive status.
Figure S4. Improving neurocognitive performance is associated with a decrease in acute phase response proteins at baseline. IPA canonical pathway for the acute phase response showing baseline CSF proteins decreased (green), and increased (red) in subjects with improving neurocognitive status.
Figure S5. Worsening cognitive performance is associated with an induction of the alternative compliment pathway at baseline. IPA canonical pathway for the acute phase response showing baseline CSF proteins decreased (green), and increased (red) in subjects with worsening neurocognitive status.
Figure S6. Improving cognitive performance is associated with suppression of activity in both the classical and alternative compliment pathways at baseline. IPA canonical pathway for the acute phase response showing baseline CSF proteins decreased (green), and increased (red) in subjects with improving neurocognitive status.
Figure S7. Changes in CSF protein abundance for HIV-infected subjects with worsening cognitive function. Pairwise analysis of CSF protein levels at baseline (visit 1) and follow up (visit 2) for proteins with A–C) significant (p<0.05 or better) increases from baseline to follow-up visit and D–F), significant (p<0.05 or better) decreases from the baseline to follow-up visit. Protein identifications and exact p values are shown in insets. All p values were adjusted using a Benjamini-Hochberg correction (p <= 0.0487827).
Figure S8. CSF proteins showing increased abundance during cognitive improvement. A–W) Paired two-tailed and non-parametric analysis of CSF protein levels at baseline (visit 1) and follow up (visit 2) for proteins exhibiting significant (p<0.05 or better) increase from the baseline to follow-up visit. Protein identifications and exact p values are shown in insets. All p values were adjusted using a Benjamini-Hochberg correction (p <= 0.041461)
Figure S9. CSF proteins showing decreased abundance during cognitive improvement. A–W) Pairwise analysis of CSF protein levels at baseline (visit 1) and follow up (visit 2) for proteins exhibiting significant (p<0.05 or better) decrease from the baseline to follow-up visit. Protein identifications and exact p values are shown in insets. All p values were adjusted using a Benjamini-Hochberg correction (p <= 0.041461)
Acknowledgments
The authors acknowledge the stellar efforts of faculty and staff associated with each study site and the efforts of the research subjects who generously provided their time and samples. This work was supported by the National Institutes of Health awards AA0017408, MH077542, MH075673, AG034849, MH103985, (NJH and JCM), MH071150 (NS), P30 MH62512 (RH), N01 MH22005 and HHSN271201000036C (CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) Study (IG), and a joint biomarker supplement MH110582 (NJH, HF, RH).
References
- 1.Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nature reviews Neurology. 2016;12(5):309. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. 01.WNL.0000287431.88658.8b [pii] [published Online First: 2007/10/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–52. doi: 10.1212/wnl.43.11.2245. [published Online First: 1993/11/01] [DOI] [PubMed] [Google Scholar]
- 4.Heaton R, Franklin DO, Woods S, et al. HIV-associated neurocognitive disorders (HAND) persist in the era of potent antiretroviral therapy: The CHARTER Study. Neurology. 2010 doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant I, Franklin DR, Jr, Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–62. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. The Journal of infectious diseases. 2012;206(2):275–82. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kore I, Ananworanich J, Valcour V, et al. Neuropsychological Impairment in Acute HIV and the Effect of Immediate Antiretroviral Therapy. Journal of acquired immune deficiency syndromes. 2015;70(4):393–9. doi: 10.1097/QAI.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fois AF, Brew BJ. The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Current HIV/AIDS reports. 2015;12(2):299–303. doi: 10.1007/s11904-015-0257-9. [DOI] [PubMed] [Google Scholar]
- 9.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. Journal of acquired immune deficiency syndromes (1999) 2011;57(5):371. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdo TH, Weiffenbach A, Woods SP, et al. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS (London, England) 2013;27(9) doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes (1999) 2012;60(3):234. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacktor N, Miyahara S, Evans S, et al. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. J Neurovirol. 2014;20(6):620–26. doi: 10.1007/s13365-014-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan L, Qiao L, Wei F, et al. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol. 2013;19(2):144–49. doi: 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed MA, Barker PB, Skolasky RL, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magnetic Resonance Imaging. 2010;28(9):1251–57. doi: 10.1016/j.mri.2010.06.007. doi: http://dx.doi.org/10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towgood KJ, Pitkanen M, Kulasegaram R, et al. Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Human brain mapping. 2013;34(10):2484–93. doi: 10.1002/hbm.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55(2):257–67. doi: 10.1002/ana.10828. [published Online First: 2004/02/03] [DOI] [PubMed] [Google Scholar]
- 17.Dickens AM, Anthony DC, Deutsch R, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29(5):559–69. doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassol E, Misra V, Dutta A, et al. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28(11):1579–91. doi: 10.1097/QAD.0000000000000303. [published Online First: 2014/04/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandaru VVR, Mielke MM, Sacktor N, et al. A lipid storage–like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81(17):1492–99. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandaru VV, McArthur JC, Sacktor N, et al. Associative and predictive biomarkers of dementia in HIV-1–infected patients. Neurology. 2007;68(18):1481–87. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst T, Jiang CS, Nakama H, et al. Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV-associated neurocognitive disorder. Journal of Magnetic Resonance Imaging. 2010;32(5):1045–53. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gongvatana A, Harezlak J, Buchthal S, et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. 2013;19(3):209–18. doi: 10.1007/s13365-013-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L, Lee P, Yiannoutsos C, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23(4):1336–47. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Mielke MM, Bandaru VV, McArthur JC, et al. Disturbance in cerebral spinal fluid sphingolipid content is associated with memory impairment in subjects infected with the human immunodeficiency virus. J Neurovirol. 2010;16(6):445–56. doi: 10.3109/13550284.2010.525599. [published Online First: 2010/11/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh J, Sinclair E, Peterson J, et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. Journal of neuroinflammation. 2014;11(1):199. doi: 10.1186/s12974-014-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuire JL, Gill AJ, Douglas SD, et al. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol. 2015:1–10. doi: 10.1007/s13365-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassol E, Misra V, Holman A, et al. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC infectious diseases. 2013;13:203. doi: 10.1186/1471-2334-13-203. [published Online First: 2013/05/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Archibald S, McCutchan J, Sanders C, et al. Brain morphometric correlates of metabolic variables in HIV: the CHARTER study. J Neurovirol. 2014;20(6):603–11. doi: 10.1007/s13365-014-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seider TR, Gongvatana A, Woods AJ, et al. Age exacerbates HIV-associated white matter abnormalities. Journal of neurovirology. 2015 doi: 10.1007/s13365-015-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, Jiang C, Cunningham E, et al. Effects of APOE epsilon4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014;82(24):2213–22. doi: 10.1212/WNL.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canizares S, Cherner M, Ellis RJ. HIV and aging: effects on the central nervous system. Seminars in neurology. 2014;34(1):27–34. doi: 10.1055/s-0034-1372340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfefferbaum A, Rogosa DA, Rosenbloom MJ, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiology of aging. 2014;35(7):1755–68. doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas JB, Brier MR, Snyder AZ, et al. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–93. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012;18(4):291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcotte TD, Deutsch R, Michael BD, et al. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(5):1123–35. doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickens AM, Anthony DC, Deutsch R, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. Aids. 2015 doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. 75/23/2087 [pii] [published Online First: 2010/12/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of clinical and experimental neuropsychology. 2004;26(3):307–19. doi: 10.1080/13803390490510031. [published Online First: 2004/10/30] [DOI] [PubMed] [Google Scholar]
- 39.Cysique LA, Franklin D, Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. Journal of clinical and experimental neuropsychology. 2011;33(5):505–22. doi: 10.1080/13803395.2010.535504. 934517506 [pii] [published Online First: 2011/03/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendyala G, Fox HS. Proteomic and metabolomic strategies to investigate HIV-associated neurocognitive disorders. Genome medicine. 2010;2(3):22. doi: 10.1186/gm143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisniewski JR, Zougman A, Nagaraj N, et al. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6(5):359–62. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 42.Stauch KL, Purnell PR, Fox HS. Quantitative proteomics of synaptic and nonsynaptic mitochondria: insights for synaptic mitochondrial vulnerability. J Proteome Res. 2014;13(5):2620–36. doi: 10.1021/pr500295n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stauch KL, Purnell PR, Fox HS. Aging synaptic mitochondria exhibit dynamic proteomic changes while maintaining bioenergetic function. Aging-Us. 2014;6(4):320–34. doi: 10.18632/aging.100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perkins DN, Pappin DJC, Creasy DM, et al. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20(18):3551–67. doi: 10.1002/(Sici)1522-2683(19991201)20:18<3551::Aid-Elps3551>3.0.Co;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Tharakan R, Edwards N, Graham DRM. Data maximization by multipass analysis of protein mass spectra. Proteomics. 2010;10(6):1160–71. doi: 10.1002/pmic.200900433. [DOI] [PubMed] [Google Scholar]
- 46.Fenyo D, Beavis RC. A method for assessing the statistical significance of mass spectrometry-based protein identifications using general scoring schemes. Anal Chem. 2003;75(4):768–74. doi: 10.1021/ac0258709. [DOI] [PubMed] [Google Scholar]
- 47.Team RC. R: A Language and Environment for Statistical Computing. 2016. [Google Scholar]
- 48.Troyanskaya O, Cantor M, Sherlock G, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17(6):520–5. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 49.Aittokallio T. Dealing with missing values in large-scale studies: microarray data imputation and beyond. Brief Bioinform. 2010;11(2):253–64. doi: 10.1093/bib/bbp059. [DOI] [PubMed] [Google Scholar]
- 50.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11:367. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickens AM, Anthony DC, Deutsch R, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. Aids. 2015;29(5):559–69. doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spector SA, Singh KK, Gupta S, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. Aids. 2010;24(10):1471–9. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwok JY, Vaida F, Augst RM, et al. Mannose binding lectin mediated complement pathway in multiple sclerosis. Journal of neuroimmunology. 2011;239(1–2):98–100. doi: 10.1016/j.jneuroim.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speth C, Dierich MP, Gasque P. Neuroinvasion by pathogens: a key role of the complement system. Mol Immunol. 2002;38(9):669–79. doi: 10.1016/s0161-5890(01)00104-3. [DOI] [PubMed] [Google Scholar]
- 55.Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol Immunol. 2005;42(2):213–28. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Reboul J, Schuller E, Pialoux G, et al. Immunoglobulins and complement components in 37 patients infected by HIV-1 virus: comparison of general (systemic) and intrathecal immunity. Journal of the neurological sciences. 1989;89(2–3):243–52. doi: 10.1016/0022-510x(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 57.Jongen PJ, Doesburg WH, Ibrahim-Stappers JL, et al. Cerebrospinal fluid C3 and C4 indexes in immunological disorders of the central nervous system. Acta neurologica Scandinavica. 2000;101(2):116–21. doi: 10.1034/j.1600-0404.2000.101002116.x. [DOI] [PubMed] [Google Scholar]
- 58.Rozek W, Ricardo-Dukelow M, Holloway S, et al. Cerebrospinal fluid proteomic profiling of HIV-1-infected patients with cognitive impairment. Journal of proteome research. 2007;6(11):4189–99. doi: 10.1021/pr070220c. [DOI] [PubMed] [Google Scholar]
- 59.McGuire JL, Gill AJ, Douglas SD, et al. The complement system, neuronal injury, and cognitive function in horizontally-acquired HIV-infected youth. Journal of neurovirology. 2016;22(6):823–30. doi: 10.1007/s13365-016-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelman BB, Chen T, Lisinicchia JG, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS one. 2012;7(9):e46178. doi: 10.1371/journal.pone.0046178. PONE-D-11-14842 [pii] [published Online First: 2012/10/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Speth C, Schabetsberger T, Mohsenipour I, et al. Mechanism of human immunodeficiency virus-induced complement expression in astrocytes and neurons. Journal of virology. 2002;76(7):3179–88. doi: 10.1128/JVI.76.7.3179-3188.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jahoor F, Jackson A, Gazzard B, et al. Erythrocyte glutathione deficiency in symptom-free HIV infection is associated with decreased synthesis rate. Am J Physiol. 1999;276(1 Pt 1):E205–11. doi: 10.1152/ajpendo.1999.276.1.E205. [DOI] [PubMed] [Google Scholar]
- 63.Spitsin S, Stevens KE, Douglas SD. Expression of substance P, neurokinin-1 receptor and immune markers in the brains of individuals with HIV-associated neuropathology. Journal of the neurological sciences. 2013;334(1–2):18–23. doi: 10.1016/j.jns.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Treitinger A, Spada C, da Silva LM, et al. Lipid and acute-phase protein alterations in HIV-1 infected patients in the early stages of infection: correlation with CD4+ lymphocytes. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases. 2001;5(4):192–9. doi: 10.1590/s1413-86702001000400005. [DOI] [PubMed] [Google Scholar]
- 65.McLemore JL, Beeley P, Thorton K, et al. Rapid automated determination of lipid hydroperoxide concentrations and total antioxidant status of serum samples from patients infected with HIV: elevated lipid hydroperoxide concentrations and depleted total antioxidant capacity of serum samples. American journal of clinical pathology. 1998;109(3):268–73. doi: 10.1093/ajcp/109.3.268. [DOI] [PubMed] [Google Scholar]
- 66.Rozek W, Horning J, Anderson J, et al. Sera proteomic biomarker profiling in HIV-1 infected subjects with cognitive impairment. Proteomics Clinical applications. 2008;2(10–11):1498–507. doi: 10.1002/prca.200780114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephenson J. Heart risk rises with long-term HIV drugs. Jama. 2005;293(17):2081. doi: 10.1001/jama.293.17.2081. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. Am J Pathol. 2010;176(2):893–902. doi: 10.2353/ajpath.2010.090390. [published Online First: 2009/12/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. The Journal of infectious diseases. 2013;207(11):1703–12. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrington DA, Gregerson DS. Immunoproteasomes: structure, function, and antigen presentation. Prog Mol Biol Transl Sci. 2012;109:75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 73.Lindl KA, Akay C, Wang Y, et al. Expression of the endoplasmic reticulum stress response marker, BiP, in the central nervous system of HIV-positive individuals. Neuropathology and applied neurobiology. 2007;33(6):658–69. doi: 10.1111/j.1365-2990.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 74.Akay C, Lindl KA, Shyam N, et al. Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex. Neuropathology and applied neurobiology. 2012;38(2):175–200. doi: 10.1111/j.1365-2990.2011.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elovaara I, Iivanainen M, Valle SL, et al. CSF protein and cellular profiles in various stages of HIV infection related to neurological manifestations. Journal of the neurological sciences. 1987;78(3):331–42. doi: 10.1016/0022-510x(87)90046-3. [DOI] [PubMed] [Google Scholar]
- 76.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. Aids. 2012;26(14):1765–74. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(5):773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 78.Calcagno A, Alberione MC, Romito A, et al. Prevalence and predictors of blood-brain barrier damage in the HAART era. Journal of neurovirology. 2014;20(5):521–5. doi: 10.1007/s13365-014-0266-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Venn diagram of baseline total protein identifications grouped by change in cognitive status. A) Numbers of unique and overlapping protein identifications for the indicated subject groups. B) Protein identifications that are unique to stably normaland worsening groups.
Figure S2. Principal components analyses of baseline CSF proteomic profiles for HIV-infected subjects grouped by temporal change in cognitive status. A pairwise PCA was performed using all proteins detected in CSF of HIV-infected patients to compare the following groups: A–B) stably impaired vs improving cognition at baseline, C–D) worsening vs improving cognition at baseline, E–F) stably impaired vs improving cognition at the follow up visit. G–H) Improving vs. worsening at the follow up visit. For each figure the left-hand panels show PCA loadings and the right panels show protein components that contributed to PCA modeling depicted in PCA1 and PCA2 axis. The top 10 proteins that contributed to each model are indicated, and the axes show how well the proteins correlated with each other.
Figure S3. Worsening neurocognitive performance is associated with induction of the acute phase response at baseline. IPA canonical pathway for the acute phase response shows proteins detected in CSF associated with the acute phase response that are increased (red) or decreased (green) in CSF of patients with worsening neurocognitive status.
Figure S4. Improving neurocognitive performance is associated with a decrease in acute phase response proteins at baseline. IPA canonical pathway for the acute phase response showing baseline CSF proteins decreased (green), and increased (red) in subjects with improving neurocognitive status.
Figure S5. Worsening cognitive performance is associated with an induction of the alternative compliment pathway at baseline. IPA canonical pathway for the acute phase response showing baseline CSF proteins decreased (green), and increased (red) in subjects with worsening neurocognitive status.
Figure S6. Improving cognitive performance is associated with suppression of activity in both the classical and alternative compliment pathways at baseline. IPA canonical pathway for the acute phase response showing baseline CSF proteins decreased (green), and increased (red) in subjects with improving neurocognitive status.
Figure S7. Changes in CSF protein abundance for HIV-infected subjects with worsening cognitive function. Pairwise analysis of CSF protein levels at baseline (visit 1) and follow up (visit 2) for proteins with A–C) significant (p<0.05 or better) increases from baseline to follow-up visit and D–F), significant (p<0.05 or better) decreases from the baseline to follow-up visit. Protein identifications and exact p values are shown in insets. All p values were adjusted using a Benjamini-Hochberg correction (p <= 0.0487827).
Figure S8. CSF proteins showing increased abundance during cognitive improvement. A–W) Paired two-tailed and non-parametric analysis of CSF protein levels at baseline (visit 1) and follow up (visit 2) for proteins exhibiting significant (p<0.05 or better) increase from the baseline to follow-up visit. Protein identifications and exact p values are shown in insets. All p values were adjusted using a Benjamini-Hochberg correction (p <= 0.041461)
Figure S9. CSF proteins showing decreased abundance during cognitive improvement. A–W) Pairwise analysis of CSF protein levels at baseline (visit 1) and follow up (visit 2) for proteins exhibiting significant (p<0.05 or better) decrease from the baseline to follow-up visit. Protein identifications and exact p values are shown in insets. All p values were adjusted using a Benjamini-Hochberg correction (p <= 0.041461)