Abstract
Thirdhand smoke (THS) is the contamination that persists after secondhand tobacco smoke has been emitted into air. It refers to the tobacco-related gases and particles that become embedded in materials, such as the carpet, walls, furniture, blankets, and toys. THS is not strictly smoke, but chemicals that adhere to surfaces from which they can be released back into the air, undergo chemical transformations and/or accumulate. Currently, the hazards of THS are not as well documented as the hazards of secondhand smoke (SHS). In this Perspective, we describe the distribution and chemical changes that occur as SHS is transformed into THS, studies of environmental contamination by THS, human exposure studies, toxicology studies using animal models and in vitro systems, possible approaches for avoiding exposure, remediation of THS contamination, and priorities for further research.
Graphical abstract
1. Introduction
1.1. Definition
THS refers to tobacco residue and stale or aged secondhand smoke. THS is not strictly smoke but rather the residues left behind by smoking. It refers to the contamination of surfaces in contact with compounds emitted in SHS, the products generated by chemical transformations of these components, and the off-gassing of volatile components into the air.1,2 The phrase “the four Rs” provides a working definition of THS: tobacco chemicals (some toxic) that remain, react, re-emit, and/or are resuspended long after active smoking ends. THS constituents may remain adsorbed to surfaces and dust particles, often penetrating deep into materials such as wallboard or upholstery; as they persist they may react with atmospheric oxidants to yield potentially harmful byproducts. Both precursors and byproducts may be re-emitted back to the gas phase, and airborne particles that initially deposited onto indoor surfaces may be resuspended.
1.2. Differences between THS and SHS
THS is conceptually distinct from SHS, which is the aerosol (particle-bound and gas phase constituents) present while smoking is taking place. Nonsmokers' exposures to SHS are associated with freshly emitted smoke. Hence, the primary pathway is inhalation, and the time scales for exposure are relatively short (minutes to a few hours). By contrast, exposure pathways for THS include not only inhalation but also dermal uptake from contact with contaminated surfaces (potentially including the clothing of smokers) and ingestion of THS that is on the hands or perhaps food. For toddlers, mouthing of objects in their environment is another route of potential oral exposure to THS. The time scale for the presence of THS indoors will generally be much longer than that for SHS and could stretch to months.
1.3. Why Study THS?
Inhalation of tobacco smoke, both by those smoking actively and by nonsmokers involuntarily inhaling tobacco smoke, has been causally linked to a wide range of diseases and other adverse consequences.3 Interest in THS accelerated after the results of internal research at Phillip Morris in the 1980s were made public through litigation settlements.4 A researcher and coauthor of this paper (Schick) at the University of California San Francisco found records in Philip Morris papers showing that SHS can become more toxic as it ages. An analysis of unpublished results revealed that concentrations of carcinogenic tobacco-specific nitrosamines (TSNAs) increased over time in aging SHS. Soon after Schick's article appeared,4 a laboratory study5 showed that nicotine on surfaces can react with a common indoor pollutant to produce TSNAs under conditions that are commonly found in indoor environments. These high-impact discoveries directed attention to the concept that THS as a distinct entity poses health risks for children and adults. By 2011, both laboratory5,6 and field studies7,8 had produced sufficient evidence to warrant pursuing a programmatic research agenda to close gaps in our current understanding of the chemistry, exposure, toxicology, and health effects of THS, as well as its behavioral, economic, and sociocultural considerations and consequences.2 The California Consortium on Thirdhand Smoke was launched in 2011 and renewed in 2014 to carry out the research agenda.
1.4. Objectives for This Perspective
This Perspective describes progress made by the Consortium and other investigators during the past five years, updating the review published in 2011.2 This multidisciplinary Perspective covers THS chemistry, the occurrence of tobacco-derived substances in real world environments, including carcinogens, the toxicity of THS using in vitro and animal models, studies of human exposure using biomarkers, possible approaches to remediation of THS-contaminated environments, and how the results of research can influence public policy to reduce THS exposure. It is hoped that illuminating the toxic substance exposure potential of THS will encourage smoking cessation and tobacco control efforts.
1.5. Approach
The long-term goals of the California Consortium on Thirdhand Smoke are to identify the health effects of exposure to THS, develop environmental indicators and biomarkers of exposure to THS, and devise and disseminate evidence-based policies to prevent and remediate such exposures. The first three years (Phase I of the Consortium's collaborative multidisciplinary research) have led to sufficient understanding of exposure to and the mechanisms by which THS causes injury in order to lay the groundwork for more extensive investigation of its health effects and their policy implications. During the two years of Phase II, the Consortium has continued to use its highly successful collaborative structure to move the research toward addressing the question of how much harm THS causes to human health. The outcomes of the Consortium's research will be used to develop risk assessments as a basis for motivating and guiding policy development and implementation, particularly to those groups most likely to have the highest exposures.
2. Background
2.1. Early History
Harmful emissions from combustion of tobacco in cigarettes and other tobacco products have been studied for decades dating back to at least the mid 1930s when Roffo in Argentina identified benzo[a]pyrene in cigarette tar and showed that tar induced cancer in mice.9 Some of the earliest studies to link smoking and lung cancer appeared in 1939.10,11 The US Surgeon General Report of 196412 led to widespread recognition that smoking tobacco was harmful, and it described some of the mechanisms by which smoking causes disease. The Surgeon General Report of 198613 documented the health effects of secondhand smoke, focusing on inhalation by nonsmokers (passive smoking). The indoor pollution described in the first article to quantify nicotine in dust of smokers' homes14 would now be recognized as THS. By then studies of the indoor dynamic behavior of SHS had shown that many constituents of tobacco smoke sorb onto and desorb from indoor materials, based on their volatility and affinity for surfaces. Researchers had also found that rooms with THS-contaminated materials can exude nicotine and other compounds for long periods of time, long after smoking has ended.
2.2. Evidence of Human Exposure
The presence and amount of THS can be assessed through environmental sampling in air, in dust, and on surfaces. The study mentioned above14 found elevated levels of nicotine in dust collected in the homes of Danish smokers, and the researchers observed a strong positive association of nicotine with smoking level. They concluded that nonsmokers inhale nicotine and other tobacco smoke constituents from respirable dust, even if smoking does not occur while the nonsmokers are present. Matt et al.7 used a standardized dust sampling protocol in homes of smoking mothers of infants with and without indoor smoking bans.7 In addition to dust, they also observed elevated levels of nicotine on household surfaces (e.g., coffee table in living room, bedframe where the infant slept), and on the hands of the smoking mother. Compared to infants in homes where no smoking was allowed, concentrations of cotinine (a biomarker for exposure to nicotine) in the urine were much higher in infants whose parents smoked indoors. If the parents only smoked outdoors, the infants had lower cotinine levels, but still many times higher than infants of nonsmoking parents. By the time the California Consortium began functioning in 2011, Matt's group had also documented THS levels in nonsmokers' homes that had been recently occupied by smokers15 and in used cars.16,17 Since then, nicotine and other THS constituents have been found in virtually any indoor environment in which tobacco has been smoked regularly, as well as in nonsmoking indoor environments that are near areas frequented by smokers, which will be discussed in subsequent sections of this Perspective.
2.3. Dynamic Behavior of Tobacco Smoke Pollutants in Indoor Environments
Mechanical or natural ventilation is the main process by which harmful pollutant concentrations can be kept at acceptable levels.8 Typical ventilation (air exchange) rates in US residential and commercial buildings remove most airborne indoor pollutants over just a few hours by introducing cleaner outdoor air. However, ventilation alone cannot achieve acceptable indoor air quality if there is smoking.18 The residence time of many airborne SHS constituents in indoor air is usually short. By contrast, surface-bound THS constituents can remain in contact with indoor air for days, weeks, and months, thus providing ample time for chemical transformations to take place as THS on surfaces interact with reactive pollutants in indoor air. The reactive atmospheric species of outdoor origin that can drive these reactions are significantly depleted during the outdoor-to-indoor transit; e.g., indoor ozone levels are often 20–70% of the outdoor concentration measured simultaneously, and OH radicals can be reduced by more than an order of magnitude compared with outdoor air. However, these compounds are not completely removed from indoor air and often drive indoor chemistry.19–21 Indoor combustion sources, such as gas stoves, may generate other reactive species, including nitrous acid (HONO), hydrogen peroxide (H2O2), and free radicals. Even though direct sunlight is absent from many indoor settings, recent evidence indicates that the role of direct photolysis in the generation of indoor OH and NO3 radicals is more significant than originally thought.22,23 Thus, oxygen- and nitrogen-containing radicals, oxidants, and nitrosating species can be present at levels that can support reactions of THS compounds with indoor pollutants. With the long residence times observed for surface-bound THS constituents, there is potential for these constituents to be slowly transformed into various byproducts as they age.
Nicotine is one of the most prevalent constituents in tobacco smoke, and it is a critically important constituent in THS chemistry because of its high emission rate and its high concentrations and persistence on indoor surfaces.24,25 In contact with ozone, nicotine oxidizes, yielding numerous volatile and semivolatile species, as well as new ultrafine particles.26 Laboratory studies have revealed that several of the identified oxidation byproducts are multifunctional carbonyls, amides, N-oxides, and carboxylic acids that have an asthma hazard index higher than that of nicotine, indicating that oxidative aging may lead to more harmful residues in THS. In addition, reactive oxygen species were detected in secondary organic aerosol (SOA) formed by ozonation of nicotine.27,28
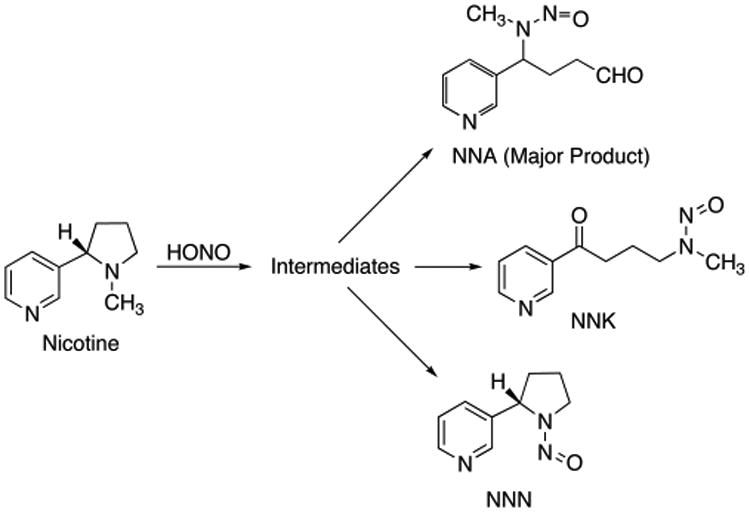
While ozone- and OH radical-driven oxidation is a major pathway for indoor chemistry, other reactions also lead to the formation of harmful byproducts. The nitrosation of nicotine by HONO emitted from combustion sources (including smoking) produced tobacco-specific nitrosamines (TSNAs) on indoor surfaces.5 These TSNAs included N′-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and 4-(methylnitrosamino)-4-(3-pyridyl)butanal (NNA), a TSNA that is specific to THS as it is not commonly found in fresh smoke (Scheme 1) and must be formed by reaction with HONO from the combustion sources. The mechanisms of nitrosamine formation are similar to those described for gas phase formation of volatile nitrosamines and formation of TSNAs in aqueous media.29,30 These studies replicated and extended unpublished research performed by Philip Morris in the 1980s, which revealed that TSNA concentrations increase over time and that secondhand smoke becomes more toxic as it ages.4 Interest in THS accelerated after these findings were uncovered because some TSNAs, in particular NNK and NNN, are highly carcinogenic.
Scheme 1. Formation of TSNAs from the Reaction of Nicotine and Nitrous Acid.
3. Progress and New Evidence
3.1. Chemistry of THS and Approaches to Exposure Assessment
3.1.1. THS Chemistry
Initial THS studies focused on oxidation and nitrosation processes that lead to the formation of semivolatile or nonvolatile byproducts that likely remain on indoor surfaces, as described above. More recently, the inhalable fraction of THS was also characterized in laboratory and field studies to provide more insights on inhalation exposure that remains highly relevant long after smoking has ended.31 More than 50 volatile organic compounds (VOCs) in THS were identified in a room-sized chamber operating at an air exchange rate of 0.14 h−1 over 18 h after smoking, including aliphatic and aromatic hydrocarbons, furans, carbonyls, terpenoids, and nitriles. However, amines, including nicotine, the tracer 3-ethenylpyridine (3EP), and several others, were quickly removed from air during the initial 2 h, probably through strong adsorption to surfaces. Three of the persistent VOCs exceeded levels considered harmful for 8-h exposures by the State of California during all or most of the 18-h period: acrolein, methacrolein, and acrylonitrile. Other compounds such as acetonitrile, 2-methylfuran, and 2,5-dimethylfuran are potentially useful candidates for THS tracers due to their persistence. Using a risk assessment approach, the concentration data were used to estimate the disability-adjusted life years (DALYs) lost by nonsmokers due to long-term exposure to THS, in order to assess the integrated health impacts. The assessment showed that particulate matter emitted during smoking and that stayed airborne over several hours (indexed by PM2.5) contributed the majority of the THS-associated disease burden, while acrolein, furan, acrylonitrile, and 1,3-butadiene were the most harmful VOCs among those for which epidemiological and/or toxicological data were available. It should be kept in mind that this approach carries a significant level of uncertainty, partly due to the fact that there are not enough data to inform the effects of hundreds of compounds present in THS. In addition, the disease burden of particulate matter is predicted from an integrated dose-response model that does not account for contributions of individual constituents. A time-resolved analysis for comparing SHS and THS contributions to DALYs was used to explore their relative impact. The analysis led to a finding that THS could be responsible for 5% to 60% of the predicted total disease burden, depending on where the arbitrary SHS/THS temporal transition is placed.31
3.1.2. Tracers for Tobacco Smoke-Derived Particulate Matter
Tobacco smoke is a complex mixture of particles and gases, which distribute themselves differently in indoor environments. The particles are small and distribute themselves widely as the air is mixed; more volatile and reactive gases are adsorbed onto surfaces. Numerous toxic substances are carried on the particulate matter (PM), but a tobacco-specific, environmentally stable tracer for tobacco smoke-derived particulate matter has been lacking. Since a model (discussed above) predicted that most of the toxicity of THS is due to fine particulate matter (PM2.5), a marker that could differentiate tobacco smoke-derived PM from PM derived from other sources, and could be used for source apportionment, would be valuable in THS studies.
Solanesol32 and scopoletin33,34 have been used as environmental tracers for tobacco smoke indoors (Chart 1), but both have nontobacco sources35 and stability issues. Solanesol is susceptible to oxidation by ozone or UV-induced decomposition.2,36,37 Scopoletin is a phenolic coumarin derivative that likewise would be expected to be susceptible to oxidation under environmental conditions, and it is present in numerous plant species. Long-chain hydrocarbons present in tobacco and its smoke, iso- and anteisoalkanes (C29–C34), have also been used as environmental tracers for tobacco smoke.38 These hydrocarbons have the advantage of stability but have nontobacco sources.39,40 Nicotine has been utilized as a tracer for the PM derived from tobacco smoke,33,34,41,42 but nicotine is volatile (calculated Log p = −1.52) and exists mainly in the gas phase of tobacco smoke collected indoors.41,43,44 If smoking at relatively constant levels has resulted in a steady state for its partitioning among the PM, the gas phase, and the surfaces to which it may be adsorbed, nicotine may perform well as a tracer for tobacco smoke-derived PM.33 This may not be the case in places where smoking is sporadic, as particles have different removal processes than gas phase nicotine. Ventilation, characteristics of the indoor space, such as composition furnishing and surfaces, could affect the partitioning of nicotine between the surfaces and the atmosphere.45,46
Chart 1. Tracers of Tobacco Smoke Particulate Matter.
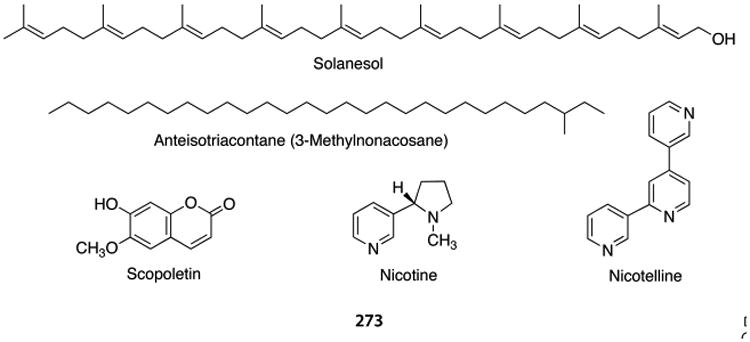
The tripyridine alkaloid nicotelline (Chart 1) has low volatility (calculated Log p = −6.05), is found almost entirely in the particulate matter (fp = 0.998), and its mass in aged cigarette smoke is highly correlated with the mass of PM (r2 = 0.95) (Figure 1). For these reasons, nicotelline has been proposed as a tracer for tobacco smoke-derived PM and should be applicable to the condensed phase in general (aerosols and stagnant surfaces).35 Nicotelline concentrations have been measured in a variety of materials, such as those being used in THS toxicity studies described elsewhere in this Perspective, in real-world samples, including house dust as described above, and in PM collected outdoors, as discussed below. Approaches for using nicotelline in PM source apportionment have been proposed.35
Figure 1.
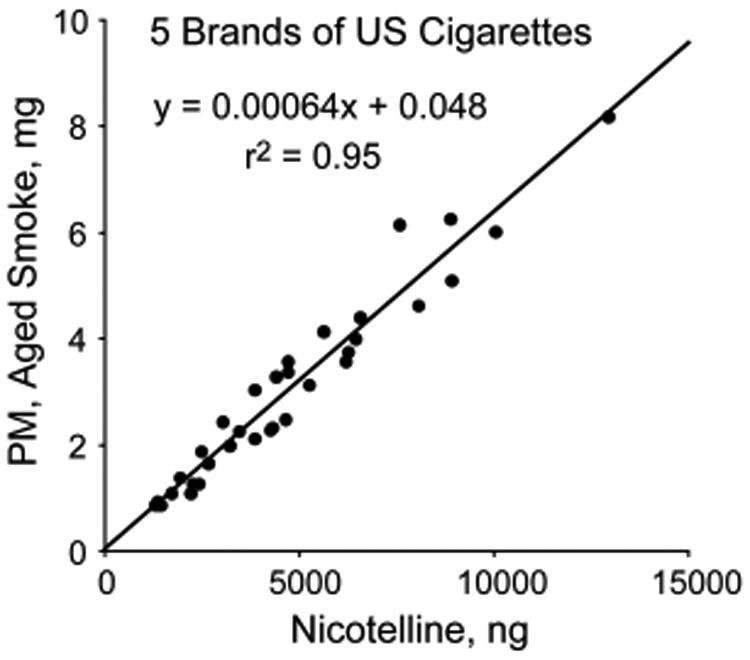
Correlation of nicotelline mass with PM mass in aged cigarette smoke from 5 US Brands. Smoke was generated from multiple cigarettes for each brand for times ranging from 2 to 6 h. Reproduced from ref 35. Copyright 2013 American Chemical Society.
3.1.3. Biomarkers to Distinguish THS from SHS Exposure: NNA Metabolites
A major challenge is developing a biomarker of exposure to distinguish THS exposure from SHS exposure. Ideally, for policy and mitigation purposes, this would be a substance unique to THS that is not present in significant amounts in SHS, one that is formed during the aging process as SHS is transformed into THS. In the pioneering studies by Sleiman et al., the major TSNA formed from the reaction of nicotine with nitrous acid, under conditions that modeled indoor environments, was 4-methylnitrosamino-4-(3-pyridyl)-butanal (NNA), as discussed above5 (Scheme 1). However, NNA is rarely detected in mainstream or sidestream tobacco smoke, probably because being an aldehyde, it is too reactive to survive during the combustion process. Therefore, NNA is a likely candidate for an environmental tracer and biomarker for THS that has reacted with HONO. To evaluate this possibility, an analytical method for the determination of NNA in THS samples produced in the laboratory and in environmental samples was developed, and NNA was administered to mice, and their urine was analyzed for metabolites that might serve as biomarkers.
Presumably due to its chemical reactivity, attempts to measure NNA directly in THS samples led to erratic results, and concentrations in aqueous solution declined over time during storage. In addition, chromatographic separation of NNA from its isomer NNK may be challenging, especially if using GC-based methods.47 Analytical methods for aldehydes often involve forming carbonyl adducts as derivatives to enhance stability. Conversion of NNA to its pentafluorophe-nylhydrazone derivative led to a satisfactory LC-MS/MS method that has been applied to THS extracts and to settled house dust48 (Scheme 2). Since mammalian metabolites of NNA had not been reported, NNA was administered to mice, and urine was collected to analyze for two likely metabolites resulting from the reduction or oxidation of the aldehyde moiety, 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanol (iso-NNAL) and the carboxylic acid 4-(methylnitrosamino)-4-(3-pyridyl)butyric acid (iso-NNAC), respectively49 (Scheme 3). The NNA was administered by application to the skin because another objective was to determine if transdermal absorption could occur since skin contact with THS-contaminated surfaces is a possible route of exposure to toxic substances present in THS. To minimize the possibility of exposure through the oral route, NNA was applied to shaved skin behind the head, and animals were housed individually to prevent cross-exposure among animals. The amount applied was 350 ng/cm2, resulting in a total dose of 1.4 μg per mouse.
Scheme 2. Conversion of NNA to Its Pentafluorophenylhydrazone Derivative.
Scheme 3. Metabolism of NNA to iso-NNAL and iso-NNAC.
LC-MS/MS methods for quantifying iso-NNAL and iso-NNAC in urine were developed. Determination of iso-NNAL was analogous to a previously reported method for determination of the isomeric compound NNAL, which is a metabolite/biomarker of the tobacco-specific lung carcinogen 4-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK).50 Determination of iso-NNAC was also performed by LC-MS/MS, following conversion to the pentafluorophenylhydrazide derivative (Scheme 4). Applying these methods to urine from 6 mice, both metabolites were detected and quantified in urine, demonstrating that dermal absorption of NNA had occurred and that the two predicted metabolites were indeed formed. iso-NNAC concentrations exceeded iso-NNAL concentrations by 2 to 3 orders of magnitude, which suggests that iso-NNAC would be the preferred biomarker of exposure49 (Table 1). The carcinogenic TSNA NNK was also administered to mice in similar fashion, and likewise, its metabolite NNAL was measured in urine, which demonstrated that both genotoxic TSNAs are absorbed through the skin.
Scheme 4. Derivatization of NNA Metabolites for LC-MS/MS Analysis.
Table 1. NNA Metabolites in Mouse Urine.
| unpublished data49 | ||
|---|---|---|
|
| ||
| sample | iso-NNAL (ng/mL) | iso-NNAC (ng/mL) |
| M-1 | 3.94 | 389 |
| M-2 | 4.16 | 496 |
| M-3 | 4.90 | 286 |
| M-4 | 6.05 | 367 |
| M-5 | 2.26 | 206 |
| M-6 | 4.00 | 150 |
However, developing NNA metabolites into biomarkers of exposure is a challenge. The dose administered to the mice was much higher than one would expect from human exposure in the real world. In settled house dust from smokers' homes analyzed for various tobacco alkaloids and TSNA, including NNA, measurable concentrations of NNA were found in house dust from 4 of the 6 homes sampled; the median NNA concentration was 0.46 ng/g.48 NNA was not detected in any dust samples from 20 nonsmokers' homes that were also analyzed for tobacco alkaloids and TSNA. This finding, along with the high recovery of the administered dose in mice suggests that iso-NNAC would be a useful biomarker of exposure in humans if an analytical method of sufficient sensitivity could be developed. However, even if a suitable method were developed, it would be necessary to demonstrate that SHS exposure does not result in excretion of iso-NNAC. In order to evaluate the possibility that iso-NNAC could be developed into a human biomarker, Consortium studies are in progress to (1) determine whether iso-NNAC is an in vitro human metabolite and (2) develop an analytical method with the anticipated sensitivity needed, low pg/mL or even subpg/mL sensitivity as was accomplished for NNAL.50
3.1.3.1. NNAL/Cotinine Ratio
As discussed above, a major driving force in generating interest in THS was the discovery that as SHS ages, TSNA concentrations increase4 and that this is likely due to the reaction of nicotine with ambient oxidant gases and nitrous acid.5 Furthermore, nicotine is considerably more volatile than the TSNAs. After smoking no longer takes place in an indoor environment, over time it would be expected that nicotine remaining on surfaces or incorporated into house dust would be removed by ventilation at a greater rate than the TSNAs. Therefore, as SHS ages to become THS, the ratio of TSNA/nicotine should increase, due to de novo formation of TSNA and faster removal of nicotine than the TSNAs. In people exposed to THS, it would be expected that the exposure to TSNAs relative to nicotine would be greater than that in people exposed to SHS. Well-validated biomarkers exist for both nicotine and the TSNA NNK, their metabolites cotinine51 and NNAL,52 respectively. In addition, highly sensitive methods for the determination of cotinine53,54 and NNAL50 in human urine are available. If indeed the ratio of NNK/nicotine in the environment increased over time, then the ratio of the biomarkers NNAL/cotinine might serve as a biomarker to assess the relative exposure to THS compared to SHS.
There are data consistent with this hypothesis. The NNAL/cotinine ratio in urine was significantly higher for passive smokers when compared with that for active smokers, which would be expected on the basis of increasing NNK/nicotine ratio as SHS ages.55 In a real-world environment, people exposed to SHS are generally exposed to THS as well. Young children, especially toddlers with parents who are smokers, would be expected to have relatively more THS exposure than adults living with smokers because they spend more time playing on the floor, may ingest house dust, tend to put objects in their mouths, and are likely to come in contact with parents' clothes. On this basis, it would be expected that the NNAL/cotinine ratio would be higher than that of adult nonsmokers exposed to SHS. Indeed, the NNAL/cotinine ratio of toddlers 6 months to 4 years in age was higher than that of adult nonsmokers exposed to SHS56,57 (Figure 2).
Figure 2.
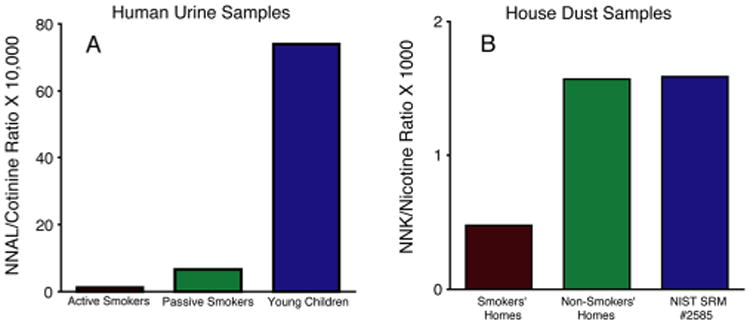
Possible approach to distinguishing THS exposure from SHS exposure: NNAL/cotinine ratio in urine. Panel A shows a higher ratio in smokers as compared to SHS-exposed nonsmokers, and a still higher ratio in infants and toddlers under the age of 2. Panel B shows a higher ratio of NNK/nicotine in nonsmokers' homes as compared to smokers' homes. These data are consistent with the loss of nicotine due to ventilation, and formation of NNK as smoke ages and SHS is transformed into THS. Smokers are exposed mainly to fresh smoke via inhalation. Nonsmokers are generally exposed to a relatively larger fraction of SHS/THS than are smokers, and toddlers are likely to be exposed to a relatively larger fraction of THS than adults due to hand to mouth behavior and contact with THS contaminated surfaces. Data are from Hovell et al.,56 Jacob et al.,57 and Whitehead et al.48
There are also real-world data suggesting that as tobacco smoke residues age, the NNK/nicotine ratio increases. Because of widespread contamination of the environment by tobacco smoke, tobacco alkaloids and TSNA can be detected in homes of nonsmokers and in the outdoor environment.48,58 In homes of nonsmokers, where nicotine and TSNAs come primarily from air and dust from outdoors, and from clothing of occupants exposed to smoke elsewhere, one would expect that the THS would have aged more than that in homes of smokers in which input of fresh smoke containing nicotine occurs on a regular basis. In a study in which nicotine and TSNAs were measured in homes of smokers and nonsmokers,48 the ratio of NNK/nicotine was higher in the homes of nonsmokers57 (Figure 2).
Consortium studies are in progress to further evaluate the NNAL/cotinine ratio as a biomarker in both field studies and in a laboratory study in which human subjects are being exposed to clothing impregnated with THS. Future studies will also explore the possibility that DNA adducts of NNA might serve as biomarkers, as proposed in the discussion on toxicology below.
3.1.4. Application of Conventional Tobacco Smoke Tracers and Biomarkers
In the absence of validated tracers and biomarkers that have specificity for THS, the current best approach in exposure assessment studies is to use conventional tracers, such as tobacco alkaloids and TSNAs, and biomarkers, such as nicotine, other tobacco alkaloids, and TSNA metabolites. Self-reports from study subjects might be used to provide an estimate their relative exposures to SHS and THS. Studies using conventional tracers and biomarkers are discussed in subsequent sections. An ongoing human laboratory study at the University of California, San Francisco will accomplish pure THS exposure, at least over a short time frame, by exposing the subjects to THS-contaminated clothing and measuring cotinine and NNAL excreted in urine.
3.2. Toxicology
3.2.1. Generation of THS Samples for Toxicology Studies
Generation and characterization of THS samples for toxicological studies has been a major endeavor of the California Consortium on Thirdhand Smoke. Different laboratories have successfully used a variety of methods for generating THS samples under controlled conditions.5,59,60 These test samples consist primarily of materials (fabrics or paper) that have been impregnated with cigarette smoke, either sidestream, or a mixture of sidestream and mainstream. Most methods employ flow cells, in which cigarette smoke flows through a chamber containing substrates. The important factors to consider when generating standardized THS samples include smoke concentration, flow rate, time, substrate, and storage conditions. Smoking machines generate either sidestream smoke or a mixture of sidestream and mainstream smoke, and they provide more consistent smoke than human smokers and can operate continuously. For exposure to materials, the machine-generated SS or SS+MS is diluted with particle free ambient air before exposure to materials. Flow rates can be set to match air exchange rates in homes and public buildings so that the air volume in the chamber turns over between 2 and 0.5 times per hour.61–64 The quantity of smoke can be tracked by simply counting cigarettes, or, more accurately, by measuring the change in concentration of aged SS for each episode of SS exposure, as measured by gravimetry. THS deposition on a sample of material will depend on the surface chemistry of the material and the total amount of THS PM that entered the exposure chamber in a long series of smoking episodes before the material was removed from the chamber. This method was used to derive the estimates of surface loading of THS (in μg·cm−2) that are included in the studies discussed in the genotoxicity and cytotoxicity sections of this Perspective.
Because chemical change is a defining characteristic of THS, time is a critical factor in generating THS samples. The duration of exposure and the elapsed time between the last smoke exposure and storage should be known. Experiments have shown that some of the toxins in THS on paper degrade when the samples are stored at room temperature.65 Some of the compounds in THS are volatile, labile, and/or sensitive to UV degradation, so samples should be protected from light and packed in containers impermeable to volatile organic compounds. Storage at −20 °C is recommended, but well-packaged specimens can be shipped at room temperature.
For in vitro studies with cell and tissue cultures, many investigators have extracted THS from paper or cloth samples by agitating the paper or cloth in cell culture media, squeezing out the liquid, and centrifuging to remove fibers. These aqueous extracts of THS contain only the subset of THS chemicals that are soluble in the media, which typically contain salts, nutrients, and proteins. For studies in animals, THS-exposed materials can be put into animal cages, or the entire cage and bedding can be exposed to cigarette smoke and then used to house the animals, as described below. Concentrations of selected tobacco smoke constituents, including nicotine, other tobacco alkaloids, and TSNAs are used as a measure of THS loading on fabrics or potency of extracts.
3.2.2. Genotoxicity
THS and its specific constituents, such as TSNAs, can cause significant molecular and cellular changes in vitro and in vivo at concentrations that are relevant to real world exposures. Sleiman et al.5 estimated the levels of TSNAs found on indoor surfaces: NNA, 2.2–3500 ng/m2, and NNK, 0.31–500 ng/m2, depending on the indoor matrix used for testing. Whereas in our studies using cell cultures the amounts of NNA added to the cells were at concentrations of 0.39–1.82 ng/mL, and for NNK 0.51–7.2 ng/mL, these values calculated on a per mL basis are comparable to the total amounts of NNA and NNK deposited per square meter of surface area and perhaps on the low end of the estimated range for indoor surface concentrations. Research on detection and identification of adducts and strand breaks in THS-treated DNA has revealed the genotoxic potential of THS exposure. Specific adduct(s) identified from the reactions with THS may prove useful as molecular biomarkers of exposure to THS.
3.2.2.1. THS-Induced Formation of DNA Strand Breaks
The genotoxic potential of THS and its known constituents was assessed in human cell lines using two in vitro assays.66 THS cellulose paper substrates were generated in both Lawrence Berkeley National Laboratory (LBNL) and the University of California, San Francisco (UCSF) using systems that simulated short (acute)- and long (chronic)-term exposures. The acute THS sample papers were exposed to cigarette smoke (SS + MS) for 1 day, followed by 32 h of aging in the smoking chamber. The estimated THS surface loading was <500 μg·cm−2. The chronic THS sample papers were exposed to cigarette smoke (SS + MS) for 258 h over 192 days, leading to an estimated THS loading of 3 μg·cm−2.60 The acute and chronic paper substrates were then extracted in cell culture medium. Twenty-four hour exposure of human HepG2 cells to samples either acutely exposed or chronically exposed to THS resulted in significant increases in DNA strand breaks in the alkaline Comet assay66 (Figure 3). Cell cultures exposed to NNA alone showed significantly higher levels of DNA strand breaks than controls in the same assay, similar to NNK in parallel experiments. Most recently, a phospho-H2AX (γ-H2AX) and p53BP1 colocalization approach was utilized to confirm the formation of double-strand breaks (DSBs) in cultured human BEAS-2B cells following exposure to THS exposure. Histone H2AX is specifically phosphorylated at the sites of DSBs, and γ-H2AX foci detection has been used as a very efficient method to demonstrate colocalization of other damage responsive proteins to DSBs, such as p53BP1. DSBs are the most harmful type of DNA damage, as both strands of the DNA duplex are compromised.
Figure 3.
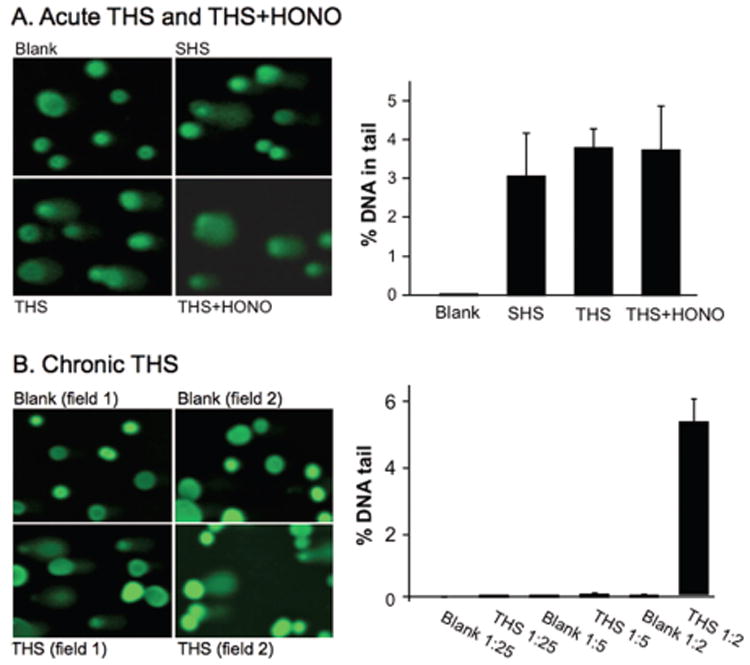
(A) Effect of acute THS and THS+HONO. HepG2 cells were exposed to samples at 37 °C for 24 h. The extent of DNA damage was analyzed by % DNA in the tail to total DNA from 90 cells. (B) HepG2 cells were exposed to chronic THS at varying dilutions under identical conditions as described above. HONO = nitrous acid. Reproduced with permission from ref 66. Copyright 2013 Bo Hang and Oxford University Press.
3.2.2.2. THS-Induced Oxidative DNA Damage
Using the long amplicon—qPCR (LA-qPCR) assay, aqueous extracts ofTHS on paper caused significantly higher levels of oxidative DNA damage in both HPRT and POLB genes in cultured human lung BEAS-2B cells than controls. LA-qPCR is highly sensitive to oxidative DNA damage when coupled with the repair enzyme formamidopyrimidine DNA glycosylase (Fpg) that is specific for incision of oxidized purine lesions. These results suggest that THS exposure may cause oxidative damage in DNA that could be an important contributing factor in THS-mediated cellular toxicity.66
To further confirm the effect of THS on the accumulation of oxidative DNA lesions, the oxidative stress-induced DNA damage in mouse skin wounds exposed to THS was measured using the LA-qPCR assay.68 THS exposure caused increased levels of oxidative DNA damage in mouse Polβ and β-Globin genes in the DNA samples from the THS-exposed skin wounds. This finding was in agreement with two other important observations in the same samples: (1) a significant increase in the levels of malondialdehyde (MDA), a marker for lipid peroxidation, and (2) high levels of 8-oxo-dG, a major oxidation product in DNA that causes G:C to TA transversion and is associated with many disease mechanisms (Figure 4). Overall, these findings suggest that THS exposure causes oxidative DNA damage in both in vitro and in vivo systems. Oxidative DNA damage can lead to mutations, which can in turn lead to cancer.
Figure 4.
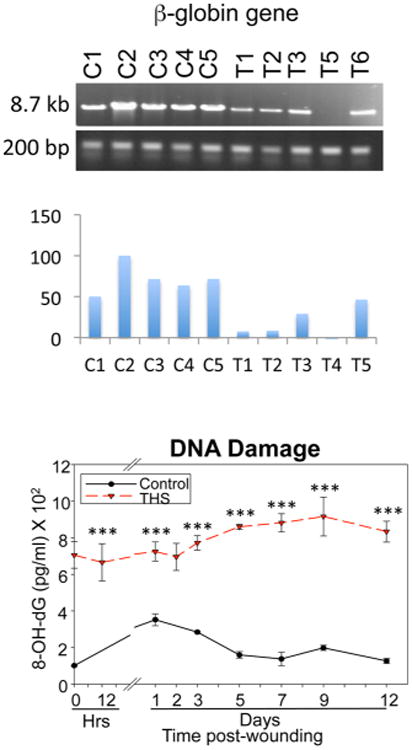
Oxidative DNA lesions induced by THS. Above: Formation of oxidative DNA damage in two mouse b-globin genes, as detected by LA-QPCR. Below: 8-oxo-dG detection using an 8-oxo-dG DNA damage assay. Adapted from ref 68. Copyright 2016 Dhall et al. Published by Portland Press Limited on behalf of the Biochemical Society.
3.2.2.3. NNA-Induced Formation of DNA Adducts
It is well accepted that formation of DNA adducts, especially bulky adducts, plays a central role in smoking-induced mutagenesis and carcinogenesis. If DNA adducts are not repaired, they can cause miscoding during DNA replication, thus leading to mutations. 69–71 With the use of LC-ESI-MS/MS and 2D NMR, several adducts from the in vitro reaction of NNA with deoxyguanosine (dG) were identified (Figure 5).67 In additionto N1-, O6 -methyl-dG and 8-oxo-dG, two modifications that are novel in structure were identified. (1) 1,N2-NNA-dG: OnHPLC chromatography, this adduct was the major adduct (peak 34.264) (Figure 5). The UV spectrum showed λmax270 nm, 285 nm, and λmin 250 nm. ES-MS/MS revealed a product of m/z 455.17 for (M+1)+, which appears to result from the condensation of NNA and dG with the elimination of H2O and oxidative removal of hydrogen atoms occurring after addition of neutral C10H9N3 O to dG. The chemical structure of the adduct proposed in Figure 5 is based on ESI-MS/MS and 2D NMRexperiments.67 Given that NNA is highly selective for THS, this bulky covalent adduct would be a good candidate biomarker of THS exposure (see below). (2) 5′,3′-dimethyl-dG: NNA also causes novel sugar damage (Figure 5), which would lead to the breakage of the DNA backbone if this lesion were formed in THS-exposed cells. NNA reacts with deoxycytosine (dC) in vitro as well, forming several products on C18-HPLC (unpublished data).
Figure 5.
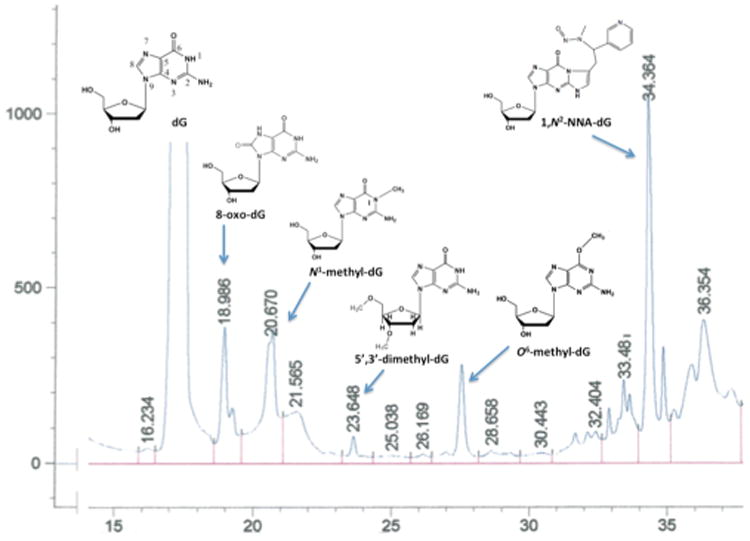
NNA reaction product with dG was separated by reverse phase (C18) chromatography and analyzed by UV spectroscopy, ESI-MS/MS and 2D-NMR. Data are from Hang et al.67
3.2.2.4. THS-Induced Metabolome Changes in Vitro
Exposure to THS extracts in two rodent male reproductive cell lines, GC-2 and TM-4, caused significant alterations in the metabolome.72 At low THS concentrations that yielded normal cell viability, cell cycle, apoptosis, and ROS production, glutathione metabolism in GC-2 cells and nucleic acid and ammonia metabolism in TM-4 cells were changed significantly. RT-PCR analyses of mRNAs for enzyme genes showed changes in the expression levels of genes that encode enzymes involved in glutathione, nucleic acid, and ammonia metabolism. A metabolomic approach could help identify biomarkers for exposure and risk assessment in THS-related research.
3.2.3. Studies in Cultured Cells: What Studies with Cultured Cells Can Tell Us about Human Health
Cells in culture can be used to study how environmental chemicals, such as THS, affect cellular processes, such as stress and survival, and can lead to a better understanding of how THS affects human health.73–75 Numerous assays can be done in vitro with different cell types, using different concentrations of THS, and different experimental conditions. Assays can be acute or chronic, end points can be single or multiplexed, and designs can be low- or high-throughput.76 Stem cells and differentiated cells from any organ can be compared, and THS can be studied independently from SHS. In vitro work can either establish a foundation upon which in vivo work with animals and humans can be based or it can provide a means to study results obtained in vivo in more depth.
There are relatively few in vitro studies on THS, in contrast to the vast literature dealing with mainstream (MS) and sidestream (SS) cigarette smoke.77 Most in vitro work has been done with extracts of THS from fabrics or other matrices exposed in controlled laboratory conditions or in some cases using samples that came from simulated field sites.66,78–81 It can be hypothesized that some of the effects that are well established for MS and SS smoke may also occur with exposure to THS.
3.2.3.1. Testing the Effect of THS on Cell Health Using Laboratory Controlled Conditions
Controlled laboratory experiments have been done to test the cytotoxicity of THS that was created and aged in a simulated car parked outdoors and in an exposure chamber that models an indoor space (such as an office) without windows.78 In the car experiment, both car seat cover fabric and floor carpeting were tested. Using live cell imaging, THS extracts inhibited cell proliferation. When mouse neural stem cells (mNSC) and human pulmonary fibroblasts (hPF) were tested in the Comet assay, which measures DNA strand breaks, both the seat cover and carpet extracts of THS increased the percentage of cells with DNA damage (Figure 6). Similar data have also been shown in HepG2 cells exposed to THS extracted from terry cloth in cell culture media.66
Figure 6.
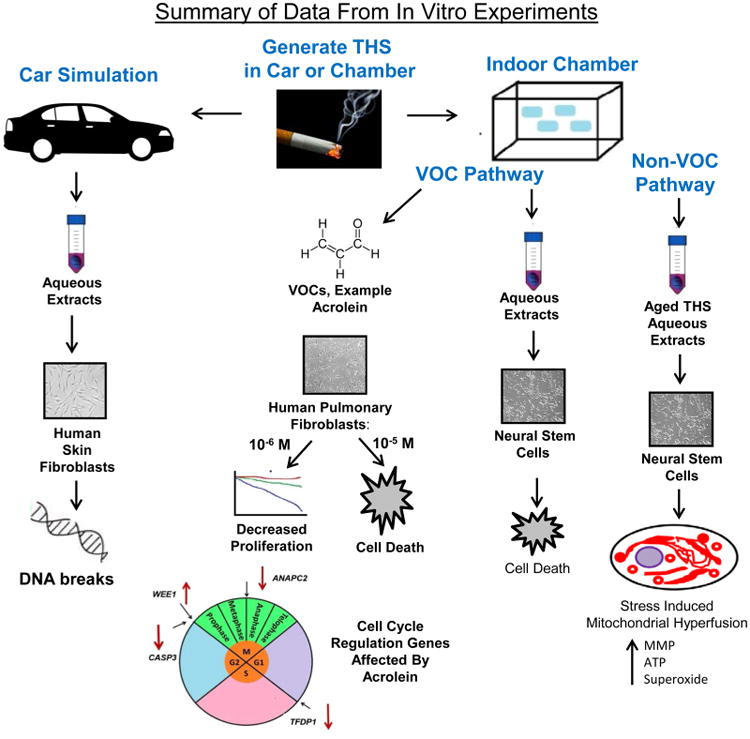
Summary of the effects of THS on cultured stem cells. In a simulated car experiment, THS extracts caused cell death and DNA strand breaks. In the VOC pathway, whole THS extracts and phenol, DMF, and acrolein killed various cell types at relatively high concentrations. Acrolein was the most potent of the three VOCs. Low concentrations of acrolein affected the expression of genes involved in the cell cycle in hPF resulting in decreased proliferation. In the non-VOC pathway, THS caused SIMH accompanied by downregulation of Fis1 and genes that decrease MMP. SIMH was accompanied by increase in MMP, ATP, and superoxide.
In an experimental chamber designed for exposures to cigarette smoke,60 terry cloth was exposed to cigarette smoke over a period of 16 months. The surface loading of THS on the terrycloth was 1.2 μg·cm–2. The effects of THS accumulation were assessed in the MTT assay, which measures mitochondrial reductase activity and can be used to assess cell survival and health (Figure 6).82 Toxicity was observed in extracts taken from terry cloth exposed to the equivalent of 54 cigarettes over an 8-month period. The surface loading of THS on the terry cloth was 0.7 μg·cm−2. This was considered a low dose of THS compared to the 4800 cigarettes that would be consumed by a pack-a-day smoker over an 8-month period. Toxicity returned to control values when smoking was stopped for several months, suggesting that the toxicity was due to volatile organic compounds (VOCs). However, when fabrics were extracted with culture medium containing serum protein, toxicity increased significantly, even in extracts that had been exposed to THS for only 4 months. Protein apparently removed a toxicant(s) that was not volatile. This idea was supported by the observation that extracts made with protein in the culture medium did not lose their toxicity when preincubated at 37 °C without cells for up to 72 h, which would be sufficient time for VOCs to escape. Fabrics that were aged for 2 months in the chamber without smoking had reduced cytotoxicity even when the extraction medium contained protein, suggesting that longer periods of aging do result in the loss of some of the protein extractable toxicants.
Similar results have been obtained using rat HepG2 cells exposed to THS extracts from laboratory experiments (1, 3, 5, 10, 15, or 20 cigarettes in a 27.6 L acrylic chamber) and samples collected from a smoker's home (60 cigarettes smoked over 3 days).81 Both cotton and paper samples were tested. The MTT assay, the neutral red uptake (NRU) assay, and trypan blue staining were used to assess cell viability when treated with aqueous THS extracts. Effects were observed in all assays indicating damage to the mitochondria (MTT), lysosomal compartment (NRU), and plasma membrane (trypan blue). Both laboratory-generated THS and samples collected from smokers' homes showed toxicity in these assays. Together, these results show that even low levels of THS contain volatile and semivolatile toxicants that build up on surfaces and that these toxicants can inhibit cell proliferation and impair cell survival in a dose dependent manner.
3.2.3.2. Identification of Volatile Organic Compounds in THS That Are Toxic to Cultured Cells
Cigarette smoke contains numerous volatile chemicals that cause harm to cells and interfere with cellular processes.83–86 Because VOCs desorb from sites of THS deposition28 and form from chemical reactions of THS on surfaces, they could induce harm in humans occupying indoor spaces where VOCs are being emitted. To test this possibility, pieces of terry cloth were exposed to THS from the equivalent of 133 cigarettes over an 11-month period (Figure 6).79 The estimated surface loading was 1.1 μg·cm−2. The fabric was aged 11 months in a sealed bag, then extracted and tested for cytoxicity using mouse neural stem cells (mNSC) in the MTT assay. Conditions were first optimized to extract THS-exposed cotton fabric (terry cloth). Extracts lost potency when the headspace of the extraction vessel was large or when extracts were allowed to age before testing, suggesting that VOCs were responsible for the observed cytotoxicity.
To understand how THS affects cells, live cell imaging experiments were done on cultured cells undergoing THS exposure. Analysis of time-lapse videos revealed that THS caused a concentration-dependent inhibition of cell growth, fragmentation of cells, vesiculation, and impaired motility. The effects on motility correlated with depolymerization of the actin filaments and microtubules by THS. This effect was lost when extracts of THS were aged before testing, again suggesting that VOCs were producing the effect. Cells were then screened for cytotoxicity using a library of 26 authentic standards of VOCs known to be present in cigarette smoke or THS (Figure 6).31,87 In the MTT assay, only three of the 26 chemicals in the screen (phenol, 2,5-dimethylfuran, and acrolein) showed significant cytotoxicity when tested with mNSC, human pulmonary fibroblasts (hPF), and human lung epithelial cells (A549). Toxicity was not increased when media containing test chemicals were replaced every 4 h for 24 h, suggesting that the toxic effects are exerted early in exposure and do not increase with addition of fresh test chemical. Since THS chemicals are normally presented to an exposed individual as a mixture, not as individual chemicals, the three toxic VOCs were tested in combination. The MTT dose–response curves were shifted to the left, indicating increased cytotoxicity when acrolein, phenol, and 2,5-dimethylfuran were tested together. These results further demonstrated the importance of testing mixtures of THS chemicals when evaluating cytotoxicity.
Acrolein, which was the most potent of the toxic VOCs, was further tested in a live cell imaging assay (Figure 6). Like THS, acrolein caused cell death at high concentrations (10−5 M) and inhibited proliferation at low concentrations (10−6 M). Taking into account the rapid removal of free acrolein by its binding to proteins in the culture medium, the effective concentration of acrolein in vitro would have been 10 to 100 lower than 10−6 M and would bracket the concentration of acrolein emitted from THS exposed materials.31 However, acrolein did not cause blebbing, fragmentation, vesiculation, or inhibition of motility, as was the case for THS, suggesting that there are additional, as yet unidentified, chemicals in THS that alter these processes.
Gene expression arrays were used to determine how acrolein slowed proliferation of cultured cells (Figure 6). Low concentrations of acrolein (10−6 M) inhibited expression of TFDP1, which functions in the transition from the G1 to S phase of mitosis. In addition, Casp3, which plays a role in transitioning of cells from G2 into the M phase of the cell cycle, was down regulated. Wee1 expression increased, which would inhibit transition from G2 into the M phase of the cycle, and AnaPC2 was down regulated, which would inhibit movement of chromosomes into anaphase during the M phase of division. Taken together, these data show that acrolein can target multiple steps in the cell cycle in a manner that would slow cell proliferation.
3.2.3.3. Stress-Induced Mitochondrial Hyperfusion
Mitochondria are vital organelles that perform numerous functions in cells. They have unique regenerative properties and can maintain their homeostasis by undergoing rounds of fission and fusion that enable unhealthy mitochondria to either be revived or targeted for mitophagy (destruction).88,89 Maintenance of a healthy pool of mitochondria is essential for cell health, and many diseases are due to mitochondrial malfunctioning.90 Recent work has shown that mNSC undergo a process called “stress-induced mitochondrial hyperfusion” (SIMH) in response to THS exposure.80 SIMH is characterized by the fusion of small round mitochondria into tubes, networks, and loops (Figure 6). The fused mitochondria differ from untreated controls in having an increased mitochondrial membrane potential (MMP), which can be visualized by labeling cells with Mitotracker Red (Figure 7). This effect is concentration dependent and can be observed when cells are treated with THS extracts from cloth that was exposed to as few as 11 cigarettes.80
Figure 7.
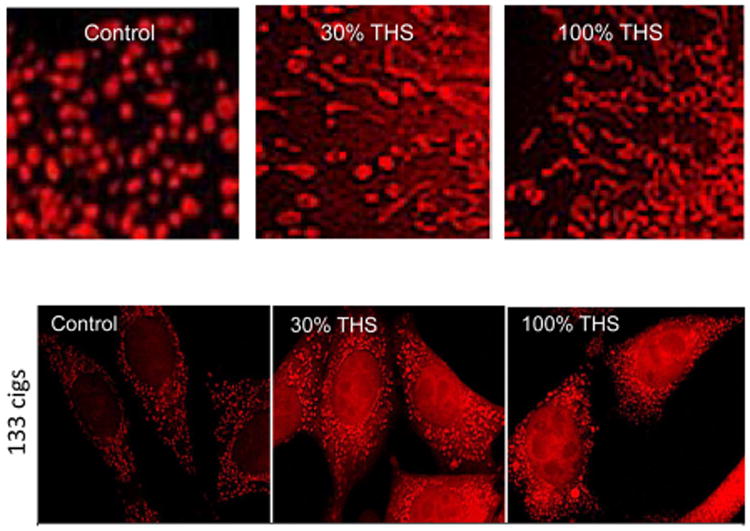
THS induces mitochondrial fusion. (Top panel) In controls, mNSC mitochondria are small and round in shape. In THS treatment groups (30 and 100%), mitochondria fuse together to form tubes, networks, and loops. THS induces an increase in mitochondrial membrane potential (MMP) (lower panel). mNSC treated with THS then incubated in Mitotracker Red show increased fluorescence relative to untreated controls, indicative of an increase in MMP. Reproduced with permission from ref 80. Copyright 2016 Oxford University Press.
The increase in MMP is accompanied by an increase in ATP production, the molecular fuel that provides energy for cellular processes (Figure 6).80 This increase in ATP may be necessary to cope with the stress induced by THS exposure. The increase in ATP was accompanied by an increase in reactive oxygen species (specifically superoxide), which are potentially dangerous and can damage cellular molecules. The Mitotimer protein, which localizes in mitochondria and fluoresces green in newly synthesized mitochondrial proteins but changes to red fluorescence as oxidation of protein increases was used to show that SIMH is accompanied by an increase in oxidation of mitochondrial proteins. While this does not immediately kill cells, it does signal that mitochondria are unhealthy and that they are slowly being damaged by THS exposure.
When cells were exposed to a relatively low dose of THS for 15 days, they maintained a strong MMP and increased their rate of cell proliferation.80 However, by 30 days of exposure, the same cells had lost their MMP, indicative of nonfunctional mitochondria, and cell proliferation had slowed. Gene expression data suggested that SIMH was brought about by the downregulation of (1) the Fis1 gene which is needed for mitochondrial fission; lack of sufficient Fis1 would favor mitochondrial fusion; (2) Ucp genes that function in reducing the MMP; down regulation of this group of genes would favor an increase in MMP; and (3) pro-apoptotic genes, such as Tspo and Bid, which would decrease the probably of apoptosis occurring. Suppression of these genes by THS treatment is consistent with the observed results of increased mitochondrial fusion, increased MMP, and decreased apoptosis during 15 days of treatment. All of these alterations in gene expression support the idea that SIMH is a pro-survival mechanism.
3.2.3.4. Relationship between in Vitro Studies and Human Health
In vitro studies on THS extracts have shown a dose– response relationship between exposure and response. The main responses observed to date at doses that do not kill cells have included the inhibition of proliferation, damage to DNA, alteration of the cytoskeleton and inhibition of motility, and induction of SIMH. Any of these responses could have effects on cell, organ, and human health. These effects may not be immediate, for example, SIMH may lead to cell death if THS stress is chronic; however, cells are not killed outright by SIMH, so effects on human health may take time to develop, as is also the case with conventional cigarette smoking. Any of these reported effects may contribute to morbidity which may worsen with longer exposures. Of particular concern would be changes to DNA, which if not properly repaired could eventually lead to cancer, one of the hallmarks of cigarette smoking.3 The accumulation of carcinogens, such as TSNAs, is also a concern and could promote unwanted changes in DNA. One study has shown that a toddler mouthing a small piece of cloth exposed to THS from about 133 cigarettes would receive a TSNA exposure about 16-fold higher than the inhalation exposure of a passive smoker.66 This model is based on the combined levels of NNK, NNN, and NNA, which may not be equally carcinogenic, which may change in concentration during aging, and which may be affected by ingestion as a mixture, rather than as isolated TSNAs.
In vitro studies have been done with various cell types, including stem cells, which were often more sensitive to THS exposure than differentiated cells. While very preliminary in nature, this observation should be pursued in the future as damage to stem cell populations may compromise health. Developing organisms are often more sensitive to environmental chemicals than adults, making studies on the prenatal and early postnatal periods of life particularly important.91 In considering how in vitro studies relate to human health, it is important to consider dosage, and much more evidence is needed on THS exposure in real-world settings. We do know that responses to THS in cell cultures are dose-dependent. In most in vitro work to date, concentrations of THS extracts have been quite low. For example, the aqueous extract of cloth exposed to the equivalent of 11 cigarettes produced cytotoxic effects even when diluted. The assays with acrolein produced effects at a 10−6 M concentration, and combining toxicants in THS increased the potency. Future in vitro studies will continue to establish a foundation upon which our understanding of THS can expand and will provide models for examining the molecular effects of THS in greater depth.
3.2.4. Animal Toxicity Studies
Separating THS exposure from SHS exposure is a challenge in both animal and human studies. An exposure system for mice that mimics exposure of humans to THS in homes of smokers has been developed (Figure 8). Using a smoking machine (Teague Enterprises, Inc., Woodland, CA92) designed for exposing rodents to cigarette smoke, common household fabrics are placed in empty mouse cages and subjected to SHS exposure.59 Cages contain materials commonly present in homes; 10 g of curtain material (cotton), 10 g of upholstery (cotton and fiber), and two 16 in2 pieces of carpet (fiber) to maintain equal exposure levels across experimental groups. Two packs of 3R4F research cigarettes were smoked each day, 5 days/week. All cigarettes were smoked and stored in accordance with the Federal Trade Commission (FTC) smoking regimen.93 Smoke was routed to a mixing compartment and distributed between two exposure chambers, each containing 4 cages with the materials. The materials were always exposed to the same level of SHS by adjusting the machine to deliver the same total particulate matter (TPM) to the chambers containing the cages with the materials. The levels of TPM were adjusted to fall within those found by the EPA in the homes of smokers (in the homes of smokers, 15–35 μg/m3; in our machine, 30± 5 μg/m3).
Figure 8.
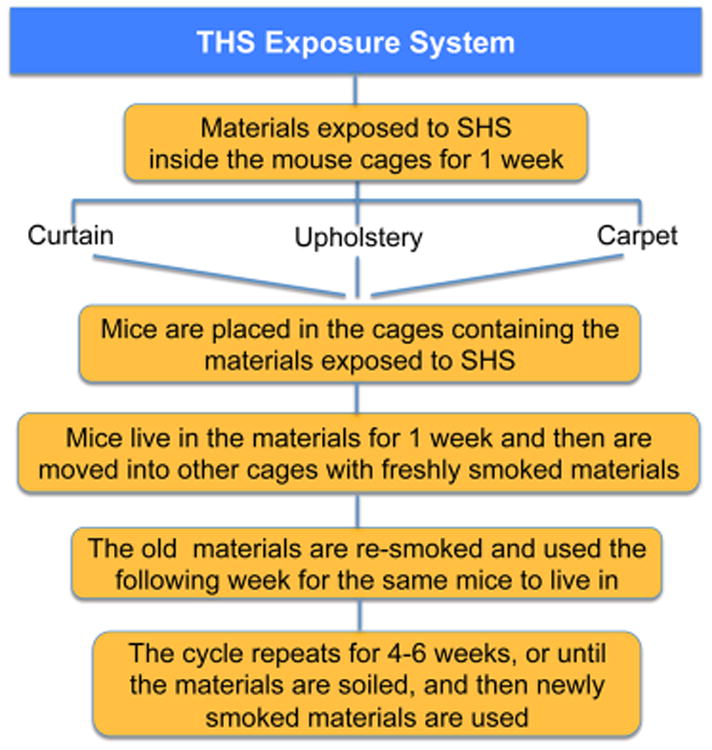
THS exposure system for studies in mice.
At the end of each week, cages were removed from the exposure chamber, bagged, and transported to the vivarium where mice were placed into the cages. For the next week, an identical set of cages and fabric was then prepared and exposed to smoke in the same way as that described above. Using two sets of cages and material, each of which was exposed on alternate weeks, ensured that mice always inhabited cages containing fabric that had been aged and with fresh THS at any given week (Figure 8). Throughout the exposure period, hair was removed weekly from the backs of the mice to mimic the bare skin of humans. This was done to mimic human skin that has very little hair. The back, rather than the belly was chosen because the belly is difficult to shave, and because mice burrow into the THS-exposed material, which is placed in the corner of the cage, there should be little effect on exposure. The experimental group was exposed to THS from right after weaning (3 weeks of age) to 24 weeks; the control group was never exposed to THS. The mice were fed a standard chow diet (percent calories: 58% carbohydrate, 28.5% protein, and 13.5% fat).
Using this system, the median NNAL level in the urine of THS-exposed mice was similar to that of a cohort of 50 infants/toddlers aged 0.5 to 4 years exposed to SHS, which suggests that the exposure system mimics exposure of children in the homes of smokers reasonably well.59 However, the possibility of differences in metabolism of NNK to NNAL in children as compared to adults is a factor that cannot be ruled out. When examining the effects of THS exposure on the mice under these conditions, several physiological functions were altered.59
3.2.4.1. THS Effects on Skin
It has long been known that smokers' wounds heal poorly.94 This is of particular concern for postsurgical wound healing. As a consequence, surgeons commonly recommend or require cessation of smoking for at least 4 weeks prior to surgery. The early effects of smoking that cause constriction of blood vessels are reversible in less than an hour after smoking, whereas the deficiencies in the inflammatory response do not return to normal until approximately 4 weeks after cessation,94 and it is not known how long it takes for the damage to cells to be reversed. The wounds of mice exposed to THS took longer to heal and showed characteristics that are conducive to reopening, such as heavy keratinization of the epithelium.59 The expression of numerous genes for keratins and keratin-associated proteins that are normally produced for hair and nails was increased. Also, the level of fibrillar collagen was greatly decreased in THS-exposed animals; the majority of collagen is not fibrillar and appears to be degraded, an observation consistent with gene array analysis showing a decrease in expression of tissue-inhibitor metalloproteinase 1 (TIMP1), an inhibitor of matrix metalloproteinases. The delay in wound closure is accompanied by decreased amounts of fibrillar collagen in the healing tissue and marked reduction of strength of wound tissue. This effect, in conjunction with the presence of keratins that convey rigidity to the epithelium and cells rich in contractile filaments, could cause or contribute to reopening of surgical wounds in smokers and, potentially, for those exposed to SHS and THS.
3.2.4.2. THS Effects on Lung
With THS exposure, the walls of the alveoli of the mice were disrupted and the alveoli contained secretions.59 Some areas of the respiratory bronchioles, the alveoli of the THS-exposed mice showed leukocyte infiltration, in particular macrophages, indicating inflammation. In the interstitial tissue, excessive disorganized collagen fibers suggesting fibrosis were observed.59 The elevated level of interstitial collagen, the thickened walls of some alveoli, the presence of macrophages in the walls of those alveoli and the increase in pro-inflammatory cytokines all suggest the possibility of increased risk for development of lung fibrosis in people who have been exposed to THS for prolonged periods of time. It is also possible that THS-exposed people have an increased susceptibility to toxicity from drugs that induce lung fibrosis.95–97
3.2.4.3. THS Effects on Liver
THS stimulates accumulation of fat in the hepatocytes (steatosis), giving the liver a pale red color compared to the deep red in normal liver, which was seen in 30% of the animals. The affected liver tissue contained large lipid droplets, whereas the droplets in control animals were very small. THS-exposed animals had greater amount of lipid with greater increase of triglycerides.59 Lipid elevation of more than 5% above normal fat indicates that steatosis has progressed to nonalcoholic fatty liver disease (NAFLD), a condition that, with prolonged exposure, can lead to fibrosis, cirrhosis, and cancer in humans. The blood of animals exposed to THS showed significantly elevated levels of triglycerides and low-density lipoprotein (LDL, bad cholesterol), whereas levels of high-density lipoprotein (HDL, good cholesterol) were significantly decreased.59 These changes in liver metabolism have potential implications for cardiovascular disease and stroke.98–103 It is also possible that THS-exposure may aggravate drug-induced damage (e.g., by acetaminophen) at doses that normally would not be damaging.
3.2.4.4. THS Effects on Metabolism
THS-exposed animals had elevated fasting glucose levels that would be classified as prediabetic, and they were significantly less efficient than control animals in using insulin to bring down blood glucose levels when an insulin tolerance test was performed.59,104 Similarly, glucose tolerance testing showed that THS-exposed mice handle the introduced glucose much less effectively than controls.59,104 The elevated triglycerides, increased LDL, decreased HDL and defects in insulin metabolism are elements of “metabolic syndrome,” a condition that predisposes humans to stroke, coronary artery disease and type 2 diabetes.99–103 These results are consistent with findings that show that tobacco smoke exposure and active smoking contribute to insulin resistance and could be associated with metabolic syndrome in US adolescent children.104
Subsequent studies were performed to examine the insulin signaling pathway that brings glucose into cells. Reduced levels of the insulin receptor, phosphoinositide 3-kinase (PI3K) and AKT (also known as protein kinase B), all important molecules in insulin signaling and glucose uptake by cells were observed.104 The inhibition of this signaling pathway results in the Glut4 (glucose) transporter remaining in the cytosol instead of being transported to the plasma membrane to allow the entrance of glucose into the cell. This will result in accumulation of glucose in the bloodstream (hyperglycemia). The effects on THS-induced insulin resistance were determined to be due to oxidative stress that causes damage to proteins, lipids, and DNA, key molecules in cellular function104 (Figure 9). To confirm that oxidative stress is important in the THS-induced insulin resistance, mice exposed to THS were treated with the antioxidants N-acetyl cysteine (NAC) and alphatocopherol (α-toc), which significantly reversed oxidative stress, molecular damage, and insulin resistance. Conversely, feeding the mice a western diet while exposing them to THS increased oxidative stress and aggravated hyperglycemia and hyperinsulinemia. These results indicate that THS exposure results in insulin resistance similar to nonobese type II diabetes (NODII) through oxidative stress.104
Figure 9.
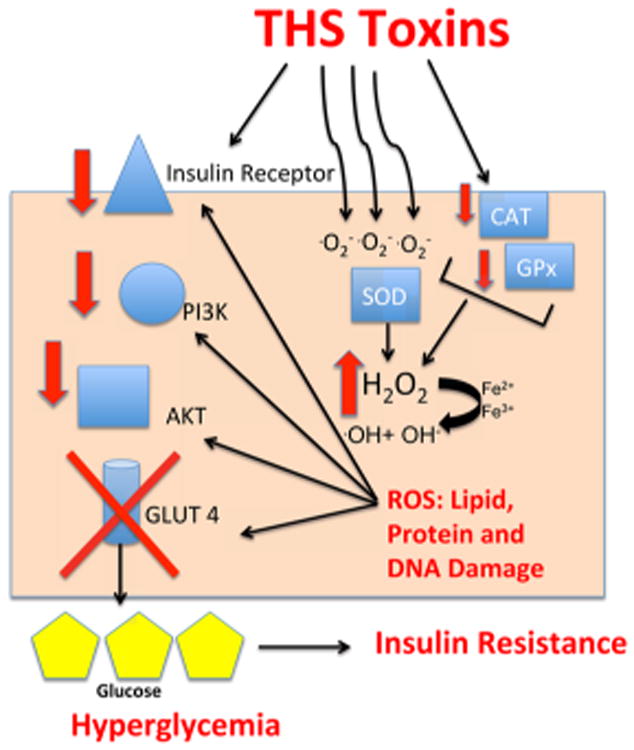
THS-induced insulin resistance.
3.2.4.5. THS Effects on Coagulation and Thrombosis
It is well-known that FHS and SHS increase the risk of coronary thrombosis.3 However, it is not known whether exposure to THS has similar effects. We found that mice exposed to THS as described above have enhanced platelet aggregation and secretion responses. Furthermore, THS increases the speed of coagulation, suggesting that this form of smoke increases the risk of thrombosis-related disease.105
3.2.4.6. THS Effects on Behavior
In initial studies, THS-exposed animals appeared hyperactive.59 To examine this in more detail, the mice were subjected to the Open Field test. Individual mice were placed in the Open Field, and walking, stationary, and rearing behaviors were assessed, as well as the frequency of transition from one of these behaviors to another. THS-exposed mice spent significantly more time walking, much less time standing still, and more time rearing than control mice. The frequency of transitions between these behaviors showed a similar pattern. In particular, THS-exposed mice were almost constantly in motion, whereas control mice were stationary for a considerable fraction of the time. Other exposed and nonexposed mice were studied in the Open Field test using Ethovision 7.1 video tracking software to track mice individually for an hour59 (Figure 10). Again, the THS-exposed mice covered longer distances at higher velocities and spent significantly more time in the periphery of the field. The difference in behavior between the two groups was particularly striking in the first 2 min during which the THS-exposed mice moved on average at high but decreasing velocity and the last 10 min of the hour in which the control mice showed on average little activity, whereas the THS-exposed mice remained very active. These studies indicate that THS-exposed mice are hyperactive.59
Figure 10.
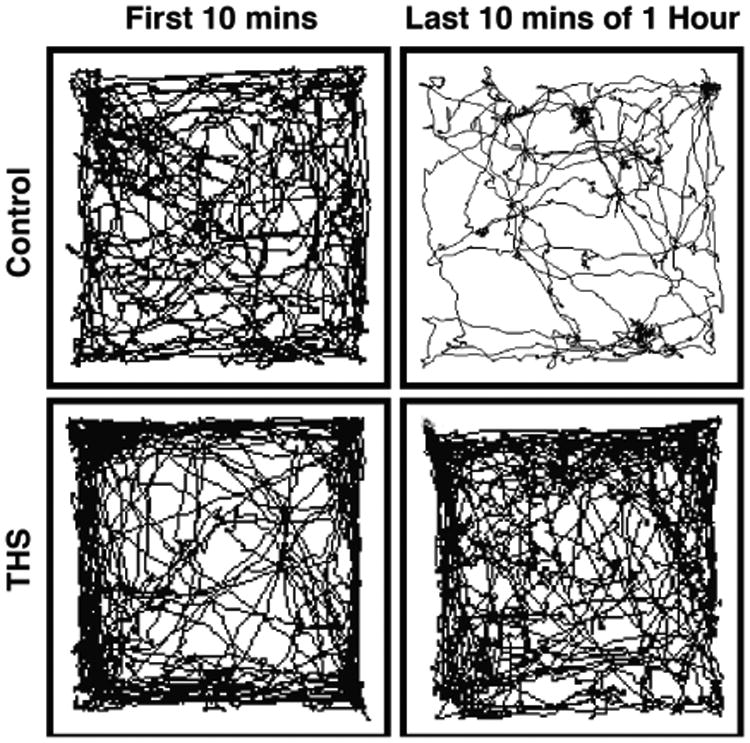
Effect of THS on mouse activity.
3.2.4.7. THS Effects on the Weight of Mice Exposed during Development and Weaning
The effects of THS exposure on the weight of mice exposed during pregnancy through weaning (postnatal day 21) and from birth until weaning were studied.106 In both cases, the THS-exposed mice weighed less than the nonexposed mice. This was seen in both males and females. When the mice were removed to a nonexposed setting and followed over time, the THS-exposed mice regained the weight to be similar to that of nonexposed mice. Furthermore, at 17 weeks of age, both males and females exposed to THS during the first 3 weeks of life had altered white blood counts.106 The eosinophil number was significantly higher in both genders, together with increased basophils in male mice and increased neutrophils in female mice. FACS analysis showed that early exposure to THS caused a significantly increased percentage of B-cells and T-suppressor cells, with decreased percentage of myeloid cells in adult mice. Equally remarkable is that exposure at the very young ages altered the white blood cell compartment leading to altered cell numbers in circulation. These results indicate that there is a window of susceptibility for some forms of cellular damage induced by THS-exposure. Damage that occurs during the very early stages of life can persist into adulthood. The results described in this section on the potential effects of THS exposure on human health are summarized in Figure 11.
Figure 11.
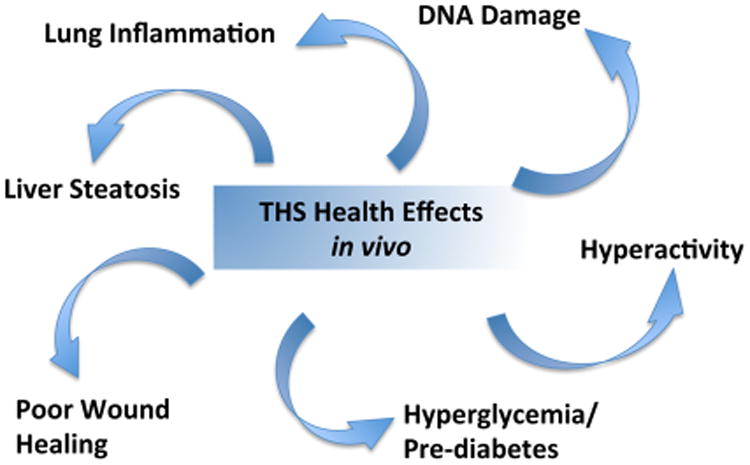
Potential health effects of THS exposure using a mouse model of exposure that mimics exposure of humans in their homes.
3.3. Human Exposure and Risk Assessment
3.3.1. Real-World Environmental Contamination
To evaluate the magnitude of the risks of THS to the general population, studies of the extent of environmental contamination by toxic substances present in THS are required, along with an understanding of the prevalence and magnitude of THS exposures to key populations. After the first reports of nicotine contamination in house dust by Hein et al.14 and of nicotine in house dust, surfaces, and on hands by Matt et al.,7 THS contamination has been measured in many public and private environments, private homes, and cars, hotels; casinos and rental cars; taxis, and even in neonatal intensive care units (NICUs).7,15,16,107–110 However, a systematic investigation of the percent of various environments such as homes and hospitality venues contaminated by THS has not been undertaken, as has been done for SHS. A striking feature of THS exposure is that exposure can be not only involuntary but also unknown, such as when an apartment has turnover of occupants and a new occupant moves into a THS contaminated space. Nicotine was found at significantly higher levels in dust and on surfaces from homes formerly occupied by smokers, even after being cleaned and occupied by nonsmokers a median of 62 days later.15 A study of THS contamination in homes of former smokers found that THS levels declined after smoking ceased but contamination was still higher than that in homes of nonsmokers after 6 months.111
3.3.1.1. THS in Air
Air measures of THS in real world environments to date have mainly quantified nicotine as a marker, though chamber studies have shown that other toxic volatile chemicals such as the irritating and toxic VOC acrolein are present during the aging of THS.31 Air samples collected in a large casino after a smoking ban showed measurable concentrations of nicotine months after the smoking stopped, demonstrating the magnitude of the reservoir of THS pollutants.112
3.3.1.2. THS on Surfaces
THS has also been measured on surfaces in homes, private and rental cars, hotels, and other public spaces (Figure 12).113 Nicotine and other semivolatile compounds from THS can rapidly sorb into and onto furnishings, walls, and other surfaces, which can then act as reservoirs, releasing the chemicals back into the environment over months and years.114 Nicotine levels can be as high on surfaces as in dust.15 Highly toxic and mutagenic TSNAs can also be found contaminating surfaces in homes of smokers.115An investigation of THS contamination of surfaces, in Nanjing, China, revealed widespread nicotine contamination in public places and in public transportation.116 Levels were very high compared to those of smoking rooms of hotels measured inCalifornia.117 Interestingly, levels in nonsmoking environments in Nanjing were also much higher than those in studies in California, where many of the first studies have been performed.113 Countries with high smoking rates probably have higher exposures and risks, and it is very important to perform field studies of THS in countries with high smoking rates.
Figure 12.
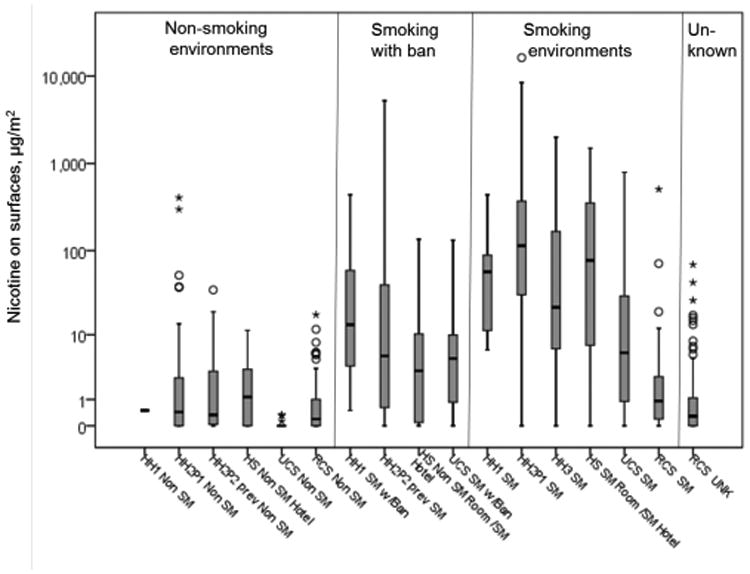
Surface nicotine measurements (μg nicotine/m2) from homes, cars and hotels, by type of environment. Abbreviations: SM, smoking environment; non-SM, nonsmoking environment; w/Ban, with a ban on smoking in the environment. Studies: HH1, Healthy Homes I, Matt et al., 2004. In homes of women with infants, HH1 examined smoking behavior of mother and effects of home smoking ban on protecting infant from exposure through tobacco smoke pollution on air dust and surfaces. HH2, Healthy Homes II, Matt et al.12 and Hoh et al.109 HH2 examined persistence of tobacco smoke contamination in homes of smokers who moved out and contamination and exposure in new occupants. HH2P1, Part 1 if HH2, before the occupants moved out. HH2P2, Part 2 of HH2, after cleaning and reoccupancy of same home.
3.3.1.3. THS in Indoor Dust
House dust from homes of smokers contains significantly higher levels of toxic contaminants including nicotine, PAHs, and TSNAs. The homes of smokers had higher nicotine concentrations per gram of dust and more dust loading (amount per surface area).15,111 This finding held, even in homes with young children where parents did not smoke in the presence of their children.7 Dust in cars in which smoking has taken place can also be highly contaminated with nicotine, indicating the presence of additional toxic THS compounds.16,17 PAHs form during combustion and some PAHs are known human carcinogens. Cigarette smoke contains PAHs, and THS can present an additional exposure risk. In homes of smokers, both the total PAH in dust and individual PAH loading were significantly greater than those in dust from nonsmokers' homes and were correlated with nicotine levels in the same sample, further implicating the role of smoking in elevating exposures to these ubiquitous and toxic pollutants.117
3.3.1.4. Effect of Smoking Bans on THS Contamination
Matt et al. examined private-party used cars for sales in San Diego, California.17 Cars offered for sale by smokers had significantly higher levels of nicotine (dust nicotine19.5 ug/g; surface nicotine 8.6 ug/m2) than cars for sale by nonsmokers (3.4 ug/g; 0.1 ug/m2), even when the smokers did not permit smoking in their cars (5.1 ug/g; 11.6 ug/m2). Thirdhand smoke is a particularly important problem in indoor settings that experience high occupancy turn over, like apartments, rental cars, and hotel rooms. Because smoking prevalence ranges from about 10–25% across the states, it is very probable that most indoor environments have been occupied by a smoker within the past few years. Matt et al. examined rental cars offered by national and local companies in San Diego, California.107 They found that regardless of their designation by rental companies as nonsmoking or smoking allowed, dust collected in the car cabins showed elevated levels of nicotine, with means ranging from 9.2 ug/g (designated nonsmoker cars rented from national companies) to 33.7ug/m2 (designated smoker cars rented from local companies). Matt et al. examined hotels with complete smoking bans and hotels that allowed smoking in some rooms.108 They found that compared with hotels with complete smoking bans, surface nicotine and air 3-ethenylpyr-idine were elevated in both nonsmoking and smoking rooms of the hotels that allowed smoking. Air nicotine levels in smoking rooms were significantly higher than those in nonsmoking rooms of hotels with and without complete smoking bans. Hallway surfaces outside of smoking rooms also showed higher levels of nicotine than those outside of nonsmoking rooms. Matt et al. examined homes of smokers after they moved out and nonsmokers moved in.15 They found that while dust surface and air nicotine levels decreased after change of occupancy, dust and surfaces continued to show higher levels compared to those of former nonsmokers homes. Most recently, Matt et al. measured nicotine and NNK in the homes of smokers who quit.111 The amount of nicotine on surfaces declined (baseline, 22.2 μg/m2; 1 week after cessation, 10.8 μg/m2), and the nicotine on fingers of nonsmoking residents declined (baseline, 29.1 ng/wipe; 1 week after cessation, 9.1 ng/wipe). However, there were no further decreases in the samples collected 1 and 3 months later. Concentrations of nicotine and NNK in dust did not change and remained near baseline levels after cessation (nicotine near 5 μg/g; NNK near 10 ng/g). Dust nicotine and NNK loadings, i.e., mass normalized to surface area, significantly increased immediately following cessation (nicotine baseline, 5.0 μg/m2; week 1 after cessation, 9.3 μg/m2; NNK baseline, 11.6 ng/m2; week 1 after cessation, 36.3 ng/m2) before returning to and remaining at near baseline levels.
3.3.1.5. THS Contamination of Smoke-Free Homes and Homes of Smokeless Tobacco Users
Carcinogenic tobacco-specific nitrosamines and various tobacco alkaloids have been found in dust from homes of nonsmokers as well as smokers, as discussed above.35,48,118 Results from 4 studies are listed in Table 2. Summary measures of TSNA concentrations varied considerably among the studies, likely due to the small number of observations and to differences in smoking practices between populations. For example, the relatively low TSNA concentrations in study 3 may be in part attributable to the fact that 5 of the 6 smokers' homes in study 3 had indoor smoking bans. The one home with indoor smoking had NNN and NNK concentrations of 8.43 ng/g and 19.4 ng/g, respectively. In addition, studies 1, 3, and 4 were conducted in California, whereas study 2 was conducted in Tarragona, in eastern Spain. In general, smoking prevalence is lower in California than in Spain, which results in a lower TSNA background. Other factors that may differ greatly between populations, such as home size and carpet coverage, might also affect the accumulation of dust/smoke or the dilution of TSNAs in the home. As expected, concentrations were higher in smokers' homes than in nonsmokers' homes. However, the fact that readily measurable concentrations of TSNAs were found in nonsmokers' homes indicates that tobacco smoke contamination is pervasive. A contributing factor to the presence of TSNA in homes of nonsmokers could be de novo formation from the reaction of HONO with nicotine and other tobacco alkaloids, as discussed above, in addition to transport into the homes from the outdoor environment. Tobacco alkaloids and TSNA have been measured in outdoor venues; their presence and implications will be discussed in a subsequent section.
Table 2. TSNAs in House Dust (ng/g).
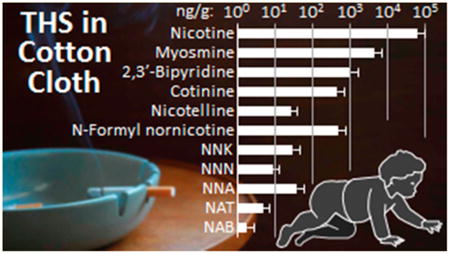
The study by Whitehead et al. found that house dust in homes of smokeless tobacco (oral snuff or chewing tobacco) users contained tobacco alkaloids and TSNAs (Chart 2), in concentrations statistically indistinguishable from homes of cigarette smokers and significantly higher than that in homes of nonsmokers (Table 3).48 These findings indicate that (1) living with smokeless tobacco users may result in exposure to carcinogenic tobacco-specific nitrosamines, as would living with smokers, and (2) high concentrations of tobacco alkaloids and TSNAs in homes indicate tobacco use in general, not just the use of combusted products. Whitehead et al. also found that the ratio of the tobacco alkaloids myosmine/nicotine can be used as an indicator of the source of tobacco contamination, distinguishing between the use of smokeless tobacco products and tobacco smoking (Figure 13). Concentrations of myosmine relative to nicotine were much higher in smokers' homes, presumably due to its formation from nicotine and nornicotine during combustion.
Chart 2. Tobacco Alkaloids and TSNAs Measured in House Dust.
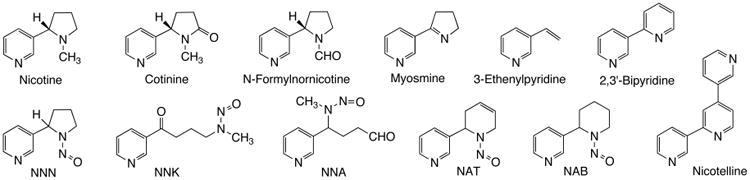
Table 3. Detection Frequencies and Median Concentrations (ng/g) of Tobacco Constituents in Vacuum Dust Collected from Homes Participating in the California Childhood Leukemia Study during the First and Second Sampling Rounds, by Tobacco Use at the Index Homea.
| tobacco constituent | first sampling round (2002–2007) | second sampling round (2010) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| smokeless tobacco users, N = 6 | active smokers, N =6 | tobacco-free homes, N = 20 | smokeless tobacco users, N = 5 | active smokers, N =6 | tobacco-free homes, N = 20 | |||||||
|
|
|
|
|
|
|
|||||||
| % det | median | % det | median | % det | median | % det | median | % det | median | % det | median | |
| nicotine | 100 | 14,000a | 100 | 7,000a | 90 | 520 | 100 | 15,000a | 100 | 7,800a | 95 | 510 |
| Tobacco-Specific Nitrosamines | ||||||||||||
| NNN | 67 | 5.7b | 67 | 1.6b | 5 | <1.4b | 80 | 4.3b | 83 | 2.9b | 10 | <1.4c |
| NNK | 100 | 6.3b | 100 | 3.7b | 40 | <0.45b | 100 | 3.4b | 100 | 5.8b | 65 | 0.51 |
| NNA | 50 | 1.2b | 67 | 0.46a | 0 | <0.45b | 40 | <0.45b,c | 50 | 0.60b | 5 | <0.45c |
| NAB | 50 | 0.28 | 33 | <0.15c | 20 | <0.15b | 60 | 0.29b | 50 | 0.18b | 10 | <0. Fifteenc |
| NAT | 50 | 3.6b | 0 | <4.2c | 0 | <4.2b | 40 | <4.2b | 0 | <4.2c | 5 | <4.2c |
| Minor Tobacco Alkaloids | ||||||||||||
| cotinine | 100 | 680b | 100 | 430b | 80 | 54 | 100 | 450b | 100 | 460b | 70 | 26 |
| myosmine | 100 | 310b | 100 | 440b | 85 | 54 | 100 | 140b | 100 | 700b | 80 | 45 |
| N-formylnornicotine | 100 | 370b | 100 | 480b | 90 | 43 | 100 | 200b | 100 | 660b | 75 | 30 |
| nicotelline | 100 | 5.5 | 100 | 8.0b | 95 | 1.0 | 100 | 2.9b | 100 | 7.1b | 100 | 0.62 |
| 2,3′-bipyridine | 83 | 76b | 100 | 74b | 60 | 6.2 | 100 | 52b | 100 | 72b | 75 | 5.9 |
Reproduced from ref 48. Copyright 2015 American Chemical Society. % Det, detection frequency; NNN, N′-nitrosonornicotine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNA, 4-(methylnitrosamino)-4-(3-pyridyl)butanal; NAB, N′-nitrosoanabasine; NAT, N′-nitro-soanatabine.
Significantly greater than concentrations of the tobacco constituents in dust samples collected during the same sampling round from tobacco-free homes, using the Wilcoxon two-sample Z-test, two-sided p < 0.05.
Lower limit of quantitation, determined as the lowest calibration standard for which back-calculated values were within ±20% of the expected concentration.
Figure 13.
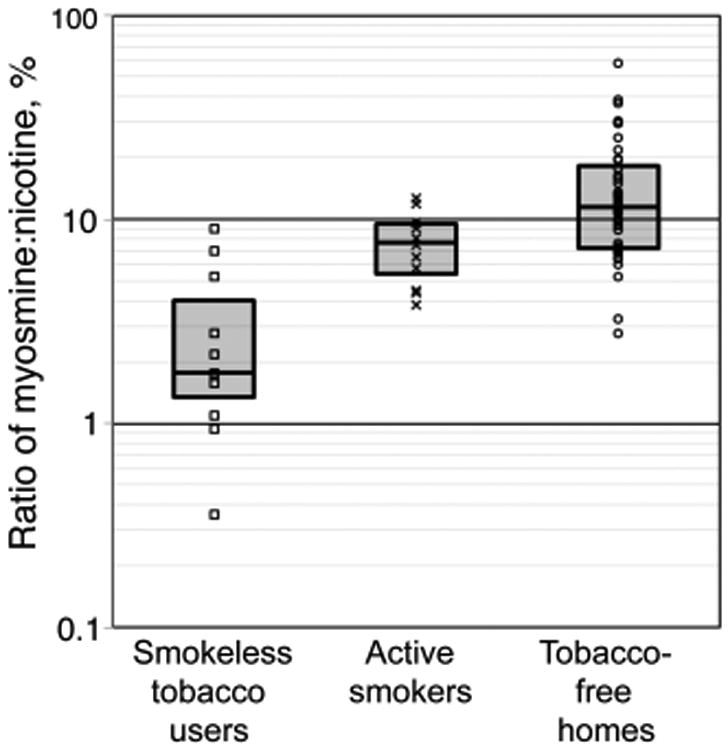
Myosmine-to-nicotine ratios (% nicotine) in vacuum dust samples collected from homes participating in the California Childhood Leukemia Study during the first (2002–2007) and second (2010) sampling rounds, by tobacco-use category. The vertical axis is shown on a logarithmic scale. Box plots represent the 25th, 50th, and 75th percentiles. Reproduced from ref 48. Copyright 2015 American Chemical Society.
3.3.2. THS Contamination Outdoors
About 6 trillion cigarettes are smoked worldwide each year.119 The sidestream smoke, i.e., the smoke from a smoldering cigarette not inhaled by the smoker, releases 10.5–34.4 mg of particulate matter (“tar”) and 1.9–5.3 mg of nicotine per cigarette into the environment.120 On the basis of the mean values of those ranges, cigarette smoking releases about 135 million kilograms of particulate matter (PM) and about 22 million kilograms of nicotine into the environment each year worldwide. Despite these rather large amounts, very little data have been published on concentrations of tobacco-derived substances in the outdoor environment. Nicotine concentrations have been measured in outdoor locations121,122 (Table 4), and increased levels of respirable fine particles (PM2.5) have been measured outdoors in the proximity of smokers.122,123 Using hydrocarbons present in cigarette smoke as tracers, it was reported that 1.0–1.3% of the fine particle mass concentration in the outdoor air in Los Angeles in 1982 was derived from cigarette smoke.38 In a recent study, tobacco-specific nitrosamines were detected in particulate matter collected outdoors in London (Table 4).58
Table 4. Tobacco Alkaloids, TSNAs, and Benzo[a]pyrene in Outdoor Air (pg/m3).
There are two main reasons for interest in outdoor contamination by tobacco smoke. One is to establish baseline levels of exposure. There is clear evidence that tobacco smoke-derived substances in the outdoor environment are transported indoors leading to readily detectible concentrations on surfaces and in house dust. Published studies have reported nicotine, other tobacco alkaloids, and TSNA in homes of nonsmokers, as discussed above. 35,48,113,118 As a nonsmoker whose home tested positive for nicotine and TSNAs said: “No one has ever smoked in my home. My neighbors don't smoke. Where is it coming from?” Outdoor air and dust is one source, as well as tobacco smoke-contaminated clothing and other articles brought into the home.
The second reason is to determine whether exposure outdoors poses a health risk. For people in the vicinity of smokers, it is reasonable to believe that exposure levels in air could be high enough to be unhealthful. St. Helen et al.125 reported exposure to secondhand smoke outside of a bar and a restaurant, measured by the biomarkers cotinine and NNAL, which were elevated in nonsmokers after outdoor exposure. It is not yet known whether exposure to THS-contaminated dust, air, or surfaces in outdoor microenvironments is a significant health risk. This will require further study.
Studies of atmospheric contamination by tobacco smoke constituents outdoors have been initiated at the University of California, San Francisco. The chemistry of outdoor THS would be expected to differ considerably from indoor THS due to more intense UV radiation, mixing with atmospheric constituents, and higher concentrations of reactive species outdoors such as ozone, other oxidants, and reactive nitrogen compounds. As a first step, airborne particulate matter (TPM) was collected on the roof of a building at a large urban hospital in San Francisco using a high-volume sampler, well away from active smokers. Airborne nicotine was also collected, using a sodium bisulfate treated filter, since nicotine is present largely in the gas phase.41,43,44 Filters were extracted and analyzed for nicotine, nicotelline, NNN, and NNK.48 Benzo[a]pyrene concentrations were also measured because it is a thoroughly studied, potent carcinogen that is ubiquitous in the environment and serves to put concentrations of the TSNA carcinogens, NNN and NNK, into perspective in terms of concentrations and possible health risk. Preliminary results are presented in Table 4. Outdoor nicotine concentrations were lower, by a factor of 5–10, than those reported in Rome. NNN and NNK concentrations were 3 orders of magnitude lower than those reported in London. These differences could be due to differences in smoking prevalence or to different atmospheric conditions (concentrations of reactive species, wind velocity, or humidity) that could affect decomposition and dispersion. Concentrations of nicotelline, a proposed tracer for tobacco smoke derived particulate matter,35 were about 7 pg/m3 and detectable in all samples analyzed. Benzo[α]pyrene concentrations averaged 23 pg/m3, which is lower than those generally found in large cities. Table 4 includes B[a]P data from Pallas, Finland,126 which like San Francisco has relatively low PAH levels in outdoor air.
Characterizing the extent of outdoor contamination has both public health and policy implications. Homes and motor vehicles owned by smokers sell for lower prices than those owned by nonsmokers, as discussed in section 3.4.1 below. If measuring tobacco-derived substances in homes and motor vehicles becomes an established method for assessing tobacco smoke contamination, cutpoints to distinguish contamination by smoking inside from contamination by outdoor sources will need to be established. Clearly, additional research on outdoor contamination by tobacco smoke is warranted.
3.3.3. Human Exposure Studies
The first study with the objective of measuring THS exposure in humans was reported by Matt et al. in 2004 who examined infants of smoking mothers with and without smoking bans in their homes.7 They compared cotinine levels in urine and nicotine and cotinine levels in the hair of infants. Average urinary cotinine levels for the infants were 15.5 ng/mL in those with smoking mothers in homes without smoking bans, 2.3 ng/mL in homes of smoking mothers with strict smoking bans, and 0.33 ng/mL in homes of nonsmoking mothers with strict smoking bans. For hair nicotine levels, the corresponding average nicotine concentrations were 5.9, 2.7, and 0.53 ng/g, respectively. Their findings showed that strict smoking bans and the absence of SHS exposure reduced but did not eliminate the exposure to tobacco smoke toxicants. The study also showed that cotinine levels in urine were associated with living room and bedroom nicotine levels in dust and on surfaces, as well as bedroom air nicotine levels. In their study of the persistence of THS in homes occupied by smokers, Matt et al.15 found that nonsmokers living in homes previously occupied by smokers showed higher levels of nicotine on their fingers than nonsmokers living in homes previously occupied by non-smokers (5.85 ng/wipe vs 0.75 ng/wipe). Nonsmokers living in homes previously occupied by smokers also showed significantly elevated levels of urine cotinine (0.61 vs 0.13 ng/mL). This was the case even though the homes had been cleaned before new people moved in and an average of 2 months had passed since the smokers had moved out.
In their study of hotels,108 Matt et al. found that nonsmokers who stayed one night in hotels without complete smoking bans showed higher levels of finger nicotine (nonsmoker room, 11.94 ng/wipe; in smoker room, 60.3 ng/wipe) and urine cotinine (smoker room, 0.63 ng/mL) than those staying in hotels with complete smoking bans (2.5 ng/wipe nicotine; 0.14 ng/mL urine cotinine). The nonsmokers who stayed in the 10 most polluted rooms identified in the study also showed significant increases in urinary NNAL (0.86 vs 1.25 ng NNAL/mg creatinine).
The long-term exposure risks to THS were further demonstrated in a study of smokers after successful smoking cessation.111 Nonsmoking cohabitants continued to show elevated levels of cotinine and NNAL up to six months after the smoker had quit and no additional tobacco had been smoked in the home. Compared to baseline levels before the smokers quit (4.6 ng/mL cotinine; 10.0 pg/mL NNAL), cotinine and NNAL in urine had significant initial declines (1 week and 1 month after cessation: 1.3 and 1.6 ng/mL cotinine; 4.2 and 6.0 pg/mL NNAL) without further significant changes until 6 months after cessation.
Even in places where smoking bans are strictly enforced, such as neontatal intensive care units in hospitals, THS can be found. Northrup et al. collected data on THS levels and infant exposure in a neonatal ICU (NICU), using surface nicotine samples from the fingers of smoking mothers, nicotine on the surfaces of infants' crib/incubator and hospital-provided furniture, and by measuring cotinine and NNAL concentrations in infants' urine. Incubators/cribs and other furniture had detectable surface nicotine, and both cotinine and NNAL were detected in the infants' urine. The authors concluded that THS appears to be ubiquitous, even in highly controlled, smoke-free settings.110
3.3.4. Biomarkers of Harm
To study THS exposure and carry out a targeted risk assessment, it is necessary to develop specific biomarkers that can be linked to risk. This is currently a very active research area. As discussed above, Consortium studies are in progress on the identification and development of such biomarkers in mammalian systems, based on specific DNA adducts and metabolites derived from NNA, a unique compound in THS. The detection of a specific NNA-DNA adduct in human tissue or peripheral blood cells could be the basis for a definitive biomarker of THS exposure, as well as harm (genotoxicity), which could be utilized in human studies.
3.3.5. THS and Human Disease
Although numerous adverse health effects of active smoking and SHS are well documented, the dangers of THS are poorly under-stood.77,127–129 As discussed above, many of the constituents of THS have the potential to have serious adverse health effects, but it is not clear if concentrations in indoor environments are sufficient to result in such effects. Epidemiological studies have yet to be conducted on the relationship between THS and health. Such studies will be challenging because most people who are exposed to THS are also exposed to SHS. The diseases most likely to be caused by THS are those that are already known to be caused by SHS exposure, particularly respiratory tract infections and asthma. Animal toxicology studies reviewed elsewhere in this Perspective suggest that THS exposure may also contribute to cancer, impaired wound healing, lung fibrosis, hepatic steatosis, insulin resistance and diabetes, lipid abnormalities, metabolic syndrome, behavior hyperactivity, and impaired immune responses. Dose–response modeling studies indicate a possible association between nitrosamines in THS and cancer.118
3.3.6. Vulnerable Populations
Children, especially infants and very young children, are likely to be among the most vulnerable populations in regard to both exposure to and effects of THS.1,2,5,7,17,26,130 Nearly 88 million US nonsmokers ≥3 years-old living in homes of smokers have ≥0.05 ng/mL serum cotinine (a metabolite of nicotine) and TSNAs in urine.1Young children may be highly exposed to THS in house dust and surfaces through the following routes: orally, dermally, and through inhalation.131 Oral exposure is enhanced in children by frequent hand to mouth behaviors.132 Dermal exposure is enhanced by crawling and touching behaviors. Children also have thinner skin than adults. Inhalation exposures are enhanced though their short stature and active play near the floor, where they can resuspend fine house dust which can then be inhaled or settle on skin. Kilogram for kilogram, children breathe more air than adults, so that children have greater exposures even in the same environment than do adults. Children also have a larger surface area to body weight ratio than do adults.133 Children may be more vulnerable to the toxic effects of THS due to the fact that their organ systems are rapidly developing, and children can differ from adults in their ability to detoxify pollutants.131 For example, newborn children have little ability to detoxify organophosphate pesticides ascompared to adults.134 Younger children also stay close to their parents and caregivers, which means that they cannot avoid SHS and THS if the caregivers smoke. Take-home or para-occupational exposures have been shown to be a significant source of environmental exposures for children of workers exposed to other pollutants such as lead, which can enter the home on the clothes of parents and significantly expose theirchildren.135 It is likely that the children of nonsmokers who are exposed to SHS at work (such as a casino card dealer) are exposed to THS this way.77 The importance of house dust as an exposure route has been well established for other environmental pollutants, such as lead and flame retardants.136,137 Dermal exposure has also been shown as an important route of exposure to pesticides for young children.138,139 Therefore, it is likely that para-occupational exposures and dust are significant sources of THS exposure.
3.4. THS Toxicity and Policy Implications
3.4.1. Policy
Policies have evolved over more than three decades to limit SHS exposure, which has declined greatly in the United States and some other countries.3 Banning smoking indoors eliminates SHS and potentially THS as well. However, evidence reviewed here shows that THS can persist in a space after smoking is no longer taking place. We lack evidence, however, on how long THS persists in indoor environments, and whether it ever clears from fabrics and construction materials in buildings where smoking took place for long periods of time, e.g., decades. On the basis of knowledge of the components of THS and their toxicity and on the emerging in vivo and in vitro evidence reported in this Perspective, THS can be assumed to pose a hazard to human health. However, we lack a population-based body of data on the concentrations of THS in various environments and the range of exposures received by the population and particularly by susceptible infants and young children who may experience the highest exposures. We also lack epidemiological evidence on the health risks posed by THS, disentangled from the risks of SHS exposure.
Nonetheless, data collected previously by the Consortium's investigators indicate that THS is considered a problem in some sectors: real estate and motor vehicle sales and the hotel/motel business3,140 Nonsmokers do not want to purchase homes where smokers have lived, particularly if they have children. In a publication on strategies for SHS control, Samet and colleagues140 collected information from a convenience sample of individuals working in these sectors, confirming that THS was problematic, although not recognized specifically by the designation of THS.45 The odor of tobacco smoke, signaling the presence of prior smoking, was problematic for the real estate and used car sales sectors.
Approaches to managing several indoor pollutants were considered as policy models: lead, asbestos, and radon. Each has known hazard, and for each, the presence of the contaminant can be documented by a measurement made according to a standard and accepted protocol. The control strategies are mandated at the federal level for asbestos and lead, while the Environmental Protection Agency, which lacks regulatory jurisdiction for indoor environments, has published a guideline value for indoor radon concentration. Extended to THS, a parallel approach would require a marker for its presence and a risk-based target concentration for its mitigation. The work of the Consortium is developing the foundation for elaborating such an approach.
3.4.2. Avoiding Exposure and Remediation
In the US, diverse companies perform restoration services for home and commercial buildings affected by tobacco smoke, mold, fire, and flood damage. In general, treatments combine removing severely affected materials (such as carpets) or using liquid cleaners. Ammonia-based cleaners are recommended to remove tobacco odors. Restoration companies also use ozone generators to remove intense tobacco odors. The ozone concentrations generated are several orders of magnitude higher than typical levels found in urban atmospheres. There has been very little scientific research to date on how well these treatments work or on the toxic reaction products that may be created. Evidence from ozonation of nicotine suggests that several asthmagens can be found among the byproducts.26 More research is needed in order to better assess the potential risks associated with the use of high levels of ozone for THS remediation.
4. Conclusions and Priorities for Further Research
There is much that remains to be learned about THS, particularly related to the magnitude of the health risk that THS poses to humans. However, based on research carried out over the past few years, by this consortium and others, some conclusions can be made: (1) Tobacco smoke contamination in the form of THS is pervasive. Tobacco-derived substances, including alkaloids and their pyrolysis products, and tobacco specific nitrosamine (TSNA) carcinogens can be measured in homes, other indoor venues such as casinos, and in particulate matter collected outdoors. (2) Laboratory studies have demonstrated that the chemical reactions that take place during the aging of tobacco smoke residues produce secondary organic pollutants, including de novo formation of TSNAs and release of VOCs. Using concentration data and standard methods for the estimation of disability-adjusted life years (DALYs) lost by nonsmokers due to long-term exposure to THS, particulate matter (indexed by PM2.5) was estimated to contribute to a majority of the THS-associated disease burden, and acrolein, furan, acrylonitrile, and 1,3-butadiene were the most harmful VOCs among those for which epidemiological and/or toxicological data were available. (3) In studies using in vitro systems, THS extracts inhibited cell proliferation, caused a concentration dependent inhibition of cell growth, fragmentation of cells, vesiculation, impaired motility, and caused damage to DNA and mitochondria. In vitro evidence for the genotoxicity of THS includes the formation of DNA strand breaks, oxidative damage to DNA, and characterization of NNA adducts with DNA. (4) Toxicology studies using mouse models demonstrated numerous deleterious effects of THS on organ and cellular systems, including delay in wound healing, lung and liver damage, metabolic effects, including elevated triglycerides, increased LDL, decreased HDL defects in insulin metabolism, and permanent changes in peripheral blood immune cell composition. THS-exposed animals showed behavior that was indicative of hyperactivity. Transdermal absorption of the TSNAs NNA and NNK in mice showed the likelihood of this route occurring in humans, a special concern for children who are more likely than adults to be in contact with contaminated surfaces, such as parents' clothes and skin. (5) Biomarkers measured in nonsmokers have documented THS exposure in nonsmokers living in homes of former smokers, nonsmokers staying in hotel rooms with no smoking bans, and subjects visiting casinos after smoking was banned, and infants in a neonatal ICU are exposed to THS toxins. (6) THS is considered a problem in some sectors of the general public, in real estate and motor vehicle sales, and the hotel and motel business.
An overarching goal is to provide a reasonable assessment of the health risks caused by THS exposure. More extensive field studies of THS exposure are needed that incorporate specific markers. A major challenge is to distinguish THS exposure from SHS exposure. For most people exposed to THS, especially adults, it would be hard to rule out SHS exposure outside of their THS-contaminated homes where smoking has ceased to occur, unless a THS-specific biomarker could be found. For infants and very young children, accurate reports from parents might largely rule out SHS exposure. Another approach is laboratory studies in which human subjects are exposed to THS-contaminated articles, such as clothing, with biomarker measurements to assess exposure. A Consortium study is underway in which subjects are being exposed to THS-contaminated clothes, with urinary cotinine and NNAL being used to assess exposure. Although progress has been made, further studies of the chemistry of THS and the extent of environmental contamination are needed to determine which substances are likely to be a health risk. Animal and in vitro toxicology studies, especially those with next generation risk assessment approaches, coupled with more extensive human exposure data might be used to develop more extensive models for estimating human health risk.
Acknowledgments
Many colleagues, too numerous to list here, made important contributions to the research described in this Perspective. Their contributions are documented in cited publications. We thank Dr. Anwer Mujeeb of the State of California Tobacco-Related Disease Research Program, Program Officer for the California Consortium on Thirdhand Smoke, who has offered invaluable guidance and support to the Consortium over many years, and Tyson Douglass for editorial assistance.
Funding
We thank the California Tobacco-Related Disease Research Program (TRDRP) for support of most of the studies described in this Perspective: TRDRP Grant 20PT-0184 - Thirdhand Tobacco Smoke Exposure and Health Risk Assessment (Phase-1); TRDRP Grant 23PT-0013 - Impacts of Thirdhand Smoke on Public Health (Phase-2). Support from the National Institute on Drug Abuse, P30 DA012393, and from the National Center for Research Resources, S10 RR026437, for laboratory resources at the University of California, San Francisco; support from the U.S. Department of Energy, DE-AC02-05CH11231, and from TRDRP Grants 19CA-0164, 24RT-0038, 20KT-0051, 19XT-0070, 18FT-0105 for research at Lawrence Berkeley National Laboratory; from TRDRP Grants 25IP-0023, 22RT-0139, 19CA-0164, 17RT-0162H and from the U.S. Department of Housing and Urban Development, CAHHU0028-15, for research at San Diego State University; support from TRDRP Grants 24RT-0037H, 23DT-0103, 22DT-0002, 22RT-0141H, 19XT-0151H, 19XT-0166 for research at University of California Riverside; support from TRDRP Grants 25ST-0038, 24RT-0039, and from the Flight Attendant Medical Institute (UCSF Bland Lane Center of Excellence on Secondhand Smoke) for research at University of California San Francisco is gratefully acknowledged.
Abbreviations
- B[a]P
benzo[a]pyrene
- DALYs
disability-adjusted life years
- HDL
high-density lipoprotein
- HONO
nitrous acid
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- iso-NNAC
4-(methylnitrosamino)-4-(3-pyridyl)butyric acid
- iso-NNAL
4-(methylnitrosamino)-4-(3-pyridyl)-1-butanol
- LDL
low-density lipoprotein
- NNA
4-(methylnitrosamino)-4-(3-pyridyl)butanal
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N′-nitrosonornicotine
- NRU
neutral red uptake
- PAHs
polycyclic aromatic hydrocarbons
- PM
particulate matter
- PM2.5
fine particulate matter
- ROS
reactive oxygen species
- SHS
secondhand smoke
- SIMH
stress-induced mitochondrial hyperfusion
- THS
thirdhand smoke
- TSNA
tobacco-specific nitrosamine
- VOCs
volatile organic compounds
Biographies
Peyton Jacob, III is a Research Chemist in the Division of Clinical Pharmacology at the University of California, San Francisco. His expertise is in the areas of organic and analytical chemistry, in particular chromatography—mass spectrometry methods. He has carried out research on the pharmacology and toxicology of tobacco for over 30 years, including the development of environmental tracers and biomarkers of exposure to carcinogens and other toxic substances, the development of methods for quantitative analysis organic small molecules, and synthesis of stable isotope-labeled tobacco alkaloids used for metabolic studies in humans. His laboratory provides a resource to numerous investigators throughout the US and abroad, for tobacco biomarker analyses in support of collaborative projects.
Neal Benowitz, M.D. is Professor of Medicine and Bioengineering & Therapeutic Sciences and Chief of the Division of Clinical Pharmacology at the University of California San Francisco. He serves as the PI of the California Consortium on Thirdhand Smoke. Dr. Benowitz's research includes studies of biomarkers of exposure and health effects of tobacco products and secondhand smoke.
Hugo Destaillats, Ph.D., is a Staff Scientist and Deputy Leader of the Indoor Environment Group at the Lawrence Berkeley National Laboratory. He holds a Ph.D. in Chemistry from the University of Buenos Aires, Argentina. His research focus is environmental chemistry of the built environment. In particular, Dr. Destaillats has investigated the sources, fate, and transport of indoor pollutants, including those associated with secondhand and thirdhand smoke and electronic cigarettes. He has also developed and characterized novel air cleaning and remediation technologies.
Lara A. Gundel, Ph.D., is a Staff Scientist in the Indoor Environment Group at Lawrence Berkeley National Laboratory. She also serves as the Co–PI and Science Coordinator for the California Consortium on Thirdhand Smoke. Her research focuses on heterogeneous physical chemistry that affects the dynamic behavior of toxicants indoors, particularly secondhand smoke and thirdhand smoke. She spearheaded the groundbreaking measurements of surface-bound nicotine that laid the foundation for LBNL researchers to quantify and model the sorption and desorption behavior of labile organic compounds in SHS as it ages to THS. With Dr. Destaillats she led the pioneering study that showed that adsorbed nicotine reacts with nitrous acid, a common combustion byproduct indoors, to form carcinogenic tobacco specific nitrosamines. Her publications are approximately evenly distributed among the fields of atmospheric science, complex mixtures of toxicants in indoor and outdoor settings, and new instrumentation.
Bo Hang, Ph.D., is a Staff Scientist at the Lawrence Berkeley National Laboratory. He has been working in the field of DNA damage and repair and their roles in environmental mutagenesis and carcinogenesis for more than 25 years. His recent interest is in understanding the biological effects of exposure to thirdhand smoke (THS) and related mechanisms using NexGen risk assessment approaches, including delineating THS genotoxic effects using in vitro assays, identifying novel biomarkers of THS exposure such as DNA adducts, and investigating THS impact on health in mouse models and human cohort studies. Dr. Hang is a member of the CA Consortium on Thirdhand Smoke Exposure and Health Effects.
Manuela Martins-Green, Ph.D., is Professor of Cell Biology in the Department of Cell Biology and Neuroscience at the University of California, Riverside. Professor Martins-Green came to the US from Portugal on a Fulbright Fellowship and received a Ph.D. in Zoology with emphasis in Developmental Biology from the University of California, Davis in December 1987. She held a postdoctoral fellowship at the Lawrence Berkeley National Laboratory, a National Research Service Award from NIH, and was Adjunct Assistant Professor at Rockefeller University before joining the UC Riverside faculty in 1993. She is an internationally recognized researcher in the field of response to injury and wound healing. In the past decade, she has been working on understanding how the body responds to injury caused by toxins from tobacco. Recently, her research has concentrated on the effects of toxins from thirdhand smoke (THS) and has shown that mice exposed to THS under conditions that mimic exposure of humans have numerous health problems.
Georg E. Matt, Ph.D., is Professor and Chair of the Department of Psychology, College of Sciences at San Diego State University. His research concerns identifying loopholes in the protection of nonsmokers from exposure to tobacco smoke toxicants. This includes investigating thirdhand smoke pollution and exposure in actual field settings, including, homes, cars, hospitality industry, multiunit housing, and pediatric care. A goal is to develop and evaluate specific measures and general policies to protect nonsmokers from secondhand and thirdhand smoke.
Penelope, J.E. Quintana, Ph.D., received her Ph.D. in Environmental Health Sciences from the University of California, Berkeley and her M.P.H. in Occupational and Environmental Health from San Diego State University (SDSU). She is currently a Professor in the division of Environmental Health at the Graduate School of Public Health at SDSU. She has a research focus on exposures to children and vulnerable populations at the US-Mexico border. She has assessed children's exposure to toxicants in house dust and on surfaces, especially residual tobacco toxicants remaining after smoking has taken place, known as third-hand smoke. She has studied exposure to traffic pollutants as an environmental justice issue at the US–Mexico border, including exposures to pedestrians waiting in lines to cross the US– Mexico border. She is a Scientific Guidance Panel member for the California Environmental Contaminant Biomonitoring Program.
Jonathan M. Samet, M.D., M.S., is Distinguished Professor and Flora L. Thornton Chair of the Department of Preventive Medicine of the University of Southern California. His research has addressed the health risks of active and passive smoking, air pollution, and ionizing radiation. He has also been engaged in activities related to risk assessment and policy.
Suzaynn Schick, Ph.D. is an environmental scientist who studies the health effects of air pollutants. She received her Ph.D. in Biomedical Sciences from the University of California, San Francisco in 2001. She published some of the first data showing that the respiratory toxicity of secondhand smoke is greater than that of the smoke that smokers inhale and that the chemical compounds in secondhand smoke can react to create new, potentially more carcinogenic compounds. She has shown that the majority of the particulate material, nicotine, tobacco-specific nitrosamines, and polycyclic aromatic hydrocarbons in secondhand smoke deposit on indoor surfaces before they can be removed by ventilation. Her lab is a Core for the California Thirdhand Smoke Consortium and produces standardized thirdhand smoke samples for research in laboratories around the world. She studies the cardiovascular and respiratory effects of exposure to secondhand cigarette smoke, thirdhand cigarette smoke, and wood smoke in human subjects. Her clinical research has shown that very short exposures to secondhand smoke cause vascular dysfunction and nasal congestion.
Prue Talbot, Ph.D., is a Professor of Cell Biology and the Director of the UCR Stem Cell Center and Core and the Inland Empire Stem Cell Consortium. Her current position is in the Department of Cell Biology & Neuroscience at the University of California, Riverside, CA. Her laboratory studies the effect of tobacco products/residues, including thirdhand smoke and electronic cigarettes, on human health. Dr. Talbot uses human embryonic stem cells to determine how in utero exposure to nicotine and other chemicals in tobacco products/residues alters prenatal development. Her laboratory also uses a variety of in vitro assays to screen chemicals in tobacco products/residues for cytotoxicity and genotoxicity. Her overall goal is to contribute to a better understanding of how thirdhand smoke and electronic cigarettes affect adult and neonatal health.
Noel J. Aquilina, Ph.D., holds a Senior Lecturer position within the Department of Geosciences at the University of Malta, Malta. His Ph.D. at the University of Birmingham, UK focused on personal exposure and microenvironment measurements of VOC and PAH and the development of personal exposure models of the same air toxics. His research interests include improving sampling protocols of airborne tobacco-related carcinogens and contributing to the understanding of their atmospheric behavior in indoor and outdoor microenvironments.
Melbourne Hovell, Ph.D., MPH is Professor in the Graduate School of Public Health, San Diego State University and Director of the Center for Behavioral Epidemiology and Community Health. He has completed 100s of studies and reports, including the first Second Hand Smoke exposure trial demonstrating that children with asthma could be protected from their parents' smoke. This study launched a program of second hand smoke studies and resulted in the identification of unusual patterns of child cotinine assays. This led to the discovery of Thirdhand Smoke with leadership from Georg Matt, Neal Benowitz, and Peyton Jacob. His current research is focused on protecting children from both second- and thirdhand smoke.
Jian-Hua Mao, Ph.D. is a Geneticist Career Staff Scientist in Lawrence Berkeley National Laboratory. He received his Ph.D. at Department of Radiation Oncology, University of Glasgow, UK, and completed his postdoctoral training at Department of Medical Oncology, the University of Glasgow. Jian-Hua Mao is known for innovative studies on identifying the combinations of genes and their functional polymorphisms that affect the susceptibility to tumor development after environmental exposure, discovering genetic alterations in tumors using recently developed high throughput technologies, such as CGH microarray, SNP microarray, gene expression microarray, and next generation sequencing, and studying the functional and mechanistic role of new discovered genes in tumor development using genetic engineering mice. Dr. Mao has authored more than 120 peer-reviewed publications.
Todd Whitehead, Ph.D., holds an Assistant Researcher position in the School of Public Health at the University of California, Berkeley. His Ph.D. at UC Berkeley focused on assessing children's exposure to indoor contaminants, including constituents of thirdhand smoke. His research interests involve improving exposure science to identify causes of childhood cancer and reducing its incidence.
Footnotes
Notes: The authors declare the following competing financial interest(s): Dr Benowitz had served as a paid expert witness in litigation against tobacco companies
References
- 1.Winickoff JP, Friebely J, Tanski SE, Sherrod C, Matt GE, Hovell MF, McMillen RC. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics. 2009;123:e74–9. doi: 10.1542/peds.2008-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matt GE, Quintana PJ, Destaillats H, Gundel LA, Sleiman M, Singer BC, Jacob P, Benowitz N, Winickoff JP, Rehan V, Talbot P, Schick S, Samet J, Wang Y, Hang B, Martins-Green M, Pankow JF, Hovell MF. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119:1218–26. doi: 10.1289/ehp.1103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Office of the Surgeon General; Washington, DC: 2014. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/ [Google Scholar]
- 4.Schick SF, Glantz S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in side-stream cigarette smoke increase after release into indoor air: results from unpublished tobacco industry research. Cancer Epidemiol, Biomarkers Prev. 2007;16:1547–53. doi: 10.1158/1055-9965.EPI-07-0210. [DOI] [PubMed] [Google Scholar]
- 5.Sleiman M, Gundel LA, Pankow JF, Jacob P, 3rd, Singer BC, Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci U S A. 2010;107:6576–81. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrick LM, Svidovsky A, Dubowski Y. Thirdhand Smoke: Heterogeneous Oxidation of Nicotine and Secondary Aerosol Formation in the Indoor Environment. Environ Sci Technol. 2011;45:328–333. doi: 10.1021/es102060v. [DOI] [PubMed] [Google Scholar]
- 7.Matt GE, Quintana PJE, Hovell MF, Bernert JT, Song S, Novianti N, Juarez T, Floro J, Gehrman C, Garcia M, Larson S. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tobacco Control. 2004;13:29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundell J, Levin H, Nazaroff WW, Cain WS, Fisk WJ, Grimsrud DT, Gyntelberg F, Li Y, Persily AK, Pickering AC, Samet JM, Spengler JD, Taylor ST, Weschler CJ. Ventilation rates and health: multidisciplinary review of the scientific literature. Indoor Air. 2011;21:191–204. doi: 10.1111/j.1600-0668.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 9.Roffo AH. Krebserzeugendes Benzpyren, gewonnen aus Tabakteer. J Cancer Res Clin Oncol. 1939;49:588–597. [Google Scholar]
- 10.Ochsner A, DeBakey M. Primary Pulmonary Malignancy: Treatment by Total Pneumonectomy: Analysis of 79 Collected Cases and Presentation of 7 Personal Cases. Ochsner J. 1999;1:109–125. [PMC free article] [PubMed] [Google Scholar]
- 11.Muller FH. Tabakmissbrauch und Lungencarcinom. J Cancer Res Clin Oncol. 1940;49:57–85. [Google Scholar]
- 12.US-DHHS. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Office of the Surgeon General; Washington, DC: 1964. [Google Scholar]
- 13.US-DHHS. The Health Consequences of Involuntary Smoking: A Report to the Surgeon General, United States Public Health Service. Office of the Surgeon General; Washington, DC: 1986. [Google Scholar]
- 14.Hein HO, Suadicani P, Skov P, Gyntelberg F. Indoor dust exposure: an unnoticed aspect of involuntary smoking. Arch Environ Health. 1991;46:98–101. doi: 10.1080/00039896.1991.9937435. [DOI] [PubMed] [Google Scholar]
- 15.Matt GE, Quintana PJ, Zakarian JM, Fortmann AL, Chatfield DA, Hoh E, Uribe AM, Hovell MF. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control. 2011;20:e1. doi: 10.1136/tc.2010.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortmann AL, Romero RA, Sklar M, Pham V, Zakarian J, Quintana PJ, Chatfield D, Matt GE. Residual tobacco smoke in used cars: futile efforts and persistent pollutants. Nicotine Tob Res. 2010;12:1029–36. doi: 10.1093/ntr/ntq144. [DOI] [PubMed] [Google Scholar]
- 17.Matt GE, Quintana PJ, Hovell MF, Chatfield D, Ma DS, Romero R, Uribe A. Residual tobacco smoke pollution in used cars for sale: air, dust, and surfaces. Nicotine Tob Res. 2008;10:1467–75. doi: 10.1080/14622200802279898. [DOI] [PubMed] [Google Scholar]
- 18.ASHRAE. Position Document on Environmental Tobacco Smoke. American Society of Heating, Refrigerating and Air-Conditioning Engineers; Atlanta, GA: 2013. [Google Scholar]
- 19.Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10:269–88. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- 20.Weschler CJ. Ozone's impact on public health: contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ Health Perspect. 2006;114:1489–96. doi: 10.1289/ehp.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weschler CJ, Shields HC. Measurements of the Hydroxyl Radical in a Manipulated but Realistic Indoor Environment. Environ Sci Technol. 1997;31:3719–3722. [Google Scholar]
- 22.Gómez Alvarez E, Amedro D, Afif C, Gligorovski S, Schoemaecker C, Fittschen C, Doussin JF, Wortham H. Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid. Proc Natl Acad Sci U S A. 2013;110:13294–13299. doi: 10.1073/pnas.1308310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez Alvarez E, Sörgel M, Gligorovski S, Bassil S, Bartolomei V, Coulomb B, Zetzsch C, Wortham H. Light-induced nitrous acid (HONO) production from NO2 heterogeneous reactions on household chemicals. Atmos Environ. 2014;95:391–399. [Google Scholar]
- 24.Singer BC, Hodgson AT, Nazaroff WW. Gasphase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ. 2003;37(39–40):5551–5561. [Google Scholar]
- 25.Singer BC, Revzan KL, Hotchi T, Hodgson AT, Brown NJ. Sorption of organic gases in a furnished room. Atmos Environ. 2004;38:2483–2494. [Google Scholar]
- 26.Sleiman M, Destaillats H, Smith JD, Liu CL, Ahmed M, Wilson KR, Gundel LA. Secondary organic aerosol formation from ozone-initiated reactions with nicotine and secondhand tobacco smoke. Atmos Environ. 2010;44:4191–4198. [Google Scholar]
- 27.Sleiman M, Destaillats H, Gundel LA. Solid-phase supported profluorescent nitroxide probe for the determination of aerosol-borne reactive oxygen species. Talanta. 2013;116:1033–9. doi: 10.1016/j.talanta.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Destaillats H, Singer BC, Lee SK, Gundel LA. Effect of Ozone on Nicotine Desorption from Model Surfaces: Evidence for Heterogeneous Chemistry. Environ Sci Technol. 2006;40:1799–1805. doi: 10.1021/es050914r. [DOI] [PubMed] [Google Scholar]
- 29.Hecht SS, Chen CHB, Ornaf RM, Jacobs E, Adams JD, Hoffmann D. Chemical studies on tobacco smoke. 52. Reaction of nicotine and sodium nitrite: formation of nitrosamines and fragmentation of the pyrrolidine ring. J Org Chem. 1978;43:72–76. doi: 10.1021/jo00395a017. [DOI] [PubMed] [Google Scholar]
- 30.Pitts JN, Grosjean D, Van Cauwenberghe K, Schmid JP, Fitz DR. Photooxidation of aliphatic amines under simulated atmospheric conditions: formation of nitrosamines, nitr-amines, amides, and photochemical oxidant. Environ Sci Technol. 1978;12:946–953. [Google Scholar]
- 31.Sleiman M, Logue JM, Luo W, Pankow JF, Gundel LA, Destaillats H. Inhalable constituents of thirdhand tobacco smoke: chemical characterization and health impact considerations. Environ Sci Technol. 2014;48:13093–101. doi: 10.1021/es5036333. [DOI] [PubMed] [Google Scholar]
- 32.Tang H, Richards G, Benner CL, Tuominen JP, Lee ML, Lewis EA, Hansen LD, Eatough DJ. Solanesol: a tracer for environmental tobacco smoke particles. Environ Sci Technol. 1990;24:848–852. [Google Scholar]
- 33.Daisey JM. Tracers for assessing exposure to environmental tobacco smoke: what are they tracing? Environ Health Perspect. 1999;107(Suppl 2):319–27. doi: 10.1289/ehp.99107s2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douce DS, Clench MR, Frost B. Variations in the estimation of the contribution of environmental tobacco smoke (ETS) to respirable indoor air particulates obtained by the use of different analytical methods. J Environ Monit. 2001;3:295–301. doi: 10.1039/b008212k. [DOI] [PubMed] [Google Scholar]
- 35.Jacob P, 3r, Goniewicz ML, Havel CM, Schick SF, Benowitz NL. Nicotelline: a proposed biomarker and environmental tracer for particulate matter derived from tobacco smoke. Chem Res Toxicol. 2013;26:1615–31. doi: 10.1021/tx400094y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogden MW, Richardson JD. Effect of lighting and storage conditions on the stability of ultraviolet particulate matter, flourescent particulate matter, and solanesol. Tob Sci. 1998;42:10–15. [Google Scholar]
- 37.Tucker SP, Pretty JR. Identification of oxidation products of solanesol produced during air sampling for tobacco smoke by electrospray mass spectrometry and HPLC. Analyst. 2005;130:1414–1424. doi: 10.1039/b505328e. [DOI] [PubMed] [Google Scholar]
- 38.Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of Fine Organic Aerosol. 6. Cigaret Smoke in the Urban Atmosphere. Environ Sci Technol. 1994;28:1375–1388. doi: 10.1021/es00056a030. [DOI] [PubMed] [Google Scholar]
- 39.Radulovic NS, Blagojevic PD. Volatile secondary metabolites of Micromeria dalmatica Benth. (Lamiaceae): biosynthetical and chemotaxonomical aspects. Chem Biodiversity. 2012;9:1303–19. doi: 10.1002/cbdv.201100429. [DOI] [PubMed] [Google Scholar]
- 40.Audino M, Grice K, Alexander R, Boreham CJ, Kagi RI. Unusual distribution of monomethylalkanes in Botryococcus braunii–rich samples: origin and significance. Geochim Cosmochim Acta. 2001;65:1995–2006. [Google Scholar]
- 41.Hammond SK, Leaderer BP, Roche AC, Schenker M. Characterization of Contaminant Emissions from Indoor Sources Collection and analysis of Nicotine as a marker for environmental tobacco smoke. Atmos Environ. 1987;21:457–462. [Google Scholar]
- 42.Repace J, Al-Delaimy WK, Bernert JT. Correlating atmospheric and biological markers in studies of secondhand tobacco smoke exposure and dose in children and adults. J Occup Environ Med. 2006;48:181–94. doi: 10.1097/01.jom.0000184883.72902.d4. [DOI] [PubMed] [Google Scholar]
- 43.Pankow JF, Mader BT, Isabelle LM, Luo W, Pavlick A, Liang C. Conversion of Nicotine in Tobacco Smoke to Its Volatile and Available Free-Base Form through the Action of Gaseous Ammonia. Environ Sci Technol. 1997;31:2428–2433. [Google Scholar]
- 44.Loefroth G. Phase Distribution of Nicotine in Real Environments As Determined by Two Sampling Methods. Environ Sci Technol. 1995;29:975–978. doi: 10.1021/es00004a017. [DOI] [PubMed] [Google Scholar]
- 45.Apelberg BJ, Hepp LM, Avila-Tang E, Gundel L, Hammond SK, Hovell MF, Hyland A, Klepeis NE, Madsen CC, Navas-Acien A, Repace J, Samet JM, Breysse PN. Environmental monitoring of secondhand smoke exposure. Tob Control. 2013;22:147–55. doi: 10.1136/tobaccocontrol-2011-050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Loy MD, Riley WJ, Daisey JM, Nazaroff WW. Dynamic Behavior of Semivolatile Organic Compounds in Indoor Air. 2. Nicotine and Phenanthrene with Carpet and Wallboard. Environ Sci Technol. 2001;35:560–567. doi: 10.1021/es001372a. [DOI] [PubMed] [Google Scholar]
- 47.Sleiman M, Maddalena RL, Gundel LA, Destaillats H. Rapid and sensitive gas chromatography ion-trap mass spectrometry method for the determination of tobacco-specific N-nitrosamines in secondhand smoke. J Chromatography A. 2009;1216:7899–7905. doi: 10.1016/j.chroma.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead TP, Havel C, Metayer C, Benowitz NL, Jacob P., 3rd Tobacco alkaloids and tobacco-specific nitrosamines in dust from homes of smokeless tobacco users, active smokers, and nontobacco users. Chem Res Toxicol. 2015;28:1007–14. doi: 10.1021/acs.chemrestox.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacob P, III, Havel C, Yu L, Adhami N, Flores C, Martins-Green M, Benowitz N. Determination of Two Metabolites of the Tobacco-Specific Nitrosamine NNA (4-(methylnitrosamino)-4-(3-pyridyl)butanal) in Mouse Urine. Application to a Dermal Absorption Study in Mice. Presented at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco (SRNT; February 5–8; Seattle WA, USA. Society For Research On Nicotine and Tobacco, Madison, WI, USA. 2014. [Google Scholar]
- 50.Jacob P, 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80:8115–21. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 52.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 53.Bernert JT, Jacob P, 3rd, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, Feyerabend C, Aldous KM, Sharifi M, Kellogg MD, Langman LJ. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009;11:1458–66. doi: 10.1093/ntr/ntp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B: Anal Technol Biomed Life Sci. 2011;879:267–76. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benowitz N, Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P., 3rd Urine cotinine underestimates exposure to the tobacco-derived lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in passive compared with active smokers. Cancer Epidemiol, Biomarkers Prev. 2010;19:2795–800. doi: 10.1158/1055-9965.EPI-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hovell MF, Zakarian JM, Matt GE, Liles S, Jones JA, Hofstetter CR, Larson SN, Benowitz NL. Counseling to reduce children's secondhand smoke exposure and help parents quit smoking: A controlled trial. Nicotine Tob Res. 2009;11:1383–94. doi: 10.1093/ntr/ntp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob P, III, Havel C, Yu L, Bahl V, Talbot P, Schick S, Hovell M, Whitehead T, Benowitz N. In Assessing Exposure to Thirdhand Smoke (THS) in Humans: The Need for a Comprehensive Approach. Presented at “Tobacco Control, Research and Education: Joining Forces to Address New Challenges”California Tobacco-Related Disease Research Program; October 27–29; Sacramento CA, USA, 2015. Tobacco-Related Disease Research Program (TRDRP), University of California, Office of the President, Oakland, CA. 2015. [Google Scholar]
- 58.Farren NJ, Ramirez N, Lee JD, Finessi E, Lewis AC, Hamilton JF. Estimated Exposure Risks from Carcinogenic Nitrosamines in Urban Airborne Particulate Matter. Environ Sci Technol. 2015;49:9648–56. doi: 10.1021/acs.est.5b01620. [DOI] [PubMed] [Google Scholar]
- 59.Martins-Green M, Adhami N, Frankos M, Valdez M, Goodwin B, Lyubovitsky J, Dhall S, Garcia M, Egiebor I, Martinez B, Green HW, Havel C, Yu L, Liles S, Matt G, Destaillats H, Sleiman M, Gundel LA, Benowitz N, Jacob P, 3rd, Hovell M, Winickoff JP, Curras-Collazo M. Cigarette smoke toxins deposited on surfaces: implications for human health. PLoS One. 2014;9:e86391. doi: 10.1371/journal.pone.0086391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schick SF, Farraro KF, Perrino C, Sleiman M, van de Vossenberg G, Trinh MP, Hammond SK, Jenkins BM, Balmes J. Thirdhand cigarette smoke in an experimental chamber: evidence of surface deposition of nicotine, nitrosamines and polycyclic aromatic hydrocarbons and de novo formation of NNK. Tob Control. 2014;23:152–9. doi: 10.1136/tobaccocontrol-2012-050915. [DOI] [PubMed] [Google Scholar]
- 61.Murray DM, Burmaster DE. Residential air exchange rates in the United States: Empirical and estimated parametric distributions by season and climatic region. Risk Anal. 1995;15:459–465. [Google Scholar]
- 62.Pandian MD, Ott WR, Behar JV. Residential air exchange rates for use in indoor air and exposure modeling studies. J Expo Anal Environ Epidemiol. 1993;3:407–416. [PubMed] [Google Scholar]
- 63.Yamamoto N, Shendell DG, Winer AM, Zhang J. Residential air exchange rates in three major US metropolitan areas: results from the Relationship Among Indoor, Outdoor, and Personal Air Study 1999–2001. Indoor Air. 2010;20:85–90. doi: 10.1111/j.1600-0668.2009.00622.x. [DOI] [PubMed] [Google Scholar]
- 64.Pandian MD, Ott WR, Behar JV. Residential air exchange rates for use in indoor air and exposure modeling studies. J Expo Anal Environ Epidemiol. 1993;3:407–16. [PubMed] [Google Scholar]
- 65.Bahl V, Jacob P, 3rd, Havel C, Schick SF, Talbot P. Thirdhand cigarette smoke: factors affecting exposure and remediation. PLoS One. 2014;9:e108258. doi: 10.1371/journal.pone.0108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hang B, Sarker AH, Havel C, Saha S, Hazra TK, Schick S, Jacob P, 3rd, Rehan VK, Chenna A, Sharan D, Sleiman M, Destaillats H, Gundel LA. Thirdhand smoke causes DNA damage in human cells. Mutagenesis. 2013;28:381–91. doi: 10.1093/mutage/get013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hang B, Lavarone A, Havel C, Jacob P, III, Villalta P, Matter B, Sharan D, Hang M, Sleiman M, Destaillats H, Gundel LA, Chenna A. 247th National Meeting of the American Chemical Society (ACS) with Press Release, Dallas, 2014. American Chemical Society; Washington, DC: 2014. NNA, a Thirdhand Smoke Constituent, Induces DNA Damage in Vitro and in Human Cells. [Google Scholar]
- 68.Dhall S, Alamat R, Castro A, Sarker AH, Mao JH, Chan A, Hang B, Martins-Green M. Tobacco toxins deposited on surfaces (third hand smoke) impair wound healing. Clin Sci. 2016;130:1269–84. doi: 10.1042/CS20160236. [DOI] [PubMed] [Google Scholar]
- 69.Hang B. Formation and Repair of Tobacco Carcinogen-Derived Bulky DNA Adducts. J Nucleic Acids. 2010;2010:1–29. doi: 10.4061/2010/709521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–71. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu B, Chen M, Yao M, Ji X, Mao Z, Tang W, Qiao S, Schick SF, Mao JH, Hang B, Xia Y. Metabolomics reveals metabolic changes in male reproductive cells exposed to thirdhand smoke. Sci Rep. 2015;5:15512. doi: 10.1038/srep15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talbot P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res, Part C. 2008;84:61–72. doi: 10.1002/bdrc.20120. [DOI] [PubMed] [Google Scholar]
- 74.Talbot P, Lin S. The effect of cigarette smoke on fertilization and pre-implantation development: assessment using animal models, clinical data, and stem cells. Biol Res. 2011;44:189–94. [PubMed] [Google Scholar]
- 75.Talbot P, Lin S. Mouse and human embryonic stem cells: can they improve human health by preventing disease? Curr Top Med Chem. 2011;11:1638–52. doi: 10.2174/156802611796117621. [DOI] [PubMed] [Google Scholar]
- 76.Zahedi A, On V, Lin SC, Bays BC, Omaiye E, Bhanu B, Talbot P. Evaluating Cell Processes, Quality, and Biomarkers in Pluripotent Stem Cells Using Video Bioinformatics. PLoS One. 2016;11:e0148642. doi: 10.1371/journal.pone.0148642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.US-DHHS. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Center for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2006. [PubMed] [Google Scholar]
- 78.Bahl V, Shim HJ, Jacob P, 3rd, Dias K, Schick SF, Talbot P. Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol In Vitro. 2016;32:220–231. doi: 10.1016/j.tiv.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bahl V, Weng NJ, Schick SF, Sleiman M, Whitehead J, Ibarra A, Talbot P. Cytotoxicity of Thirdhand Smoke and Identification of Acrolein as a Volatile Thirdhand Smoke Chemical That Inhibits Cell Proliferation. Toxicol Sci. 2016;150:234–46. doi: 10.1093/toxsci/kfv327. [DOI] [PubMed] [Google Scholar]
- 80.Bahl V, Johnson K, Phandthong R, Zahedi A, Schick SF, Talbot P. Thirdhand Cigarette Smoke Causes Stress-Induced Mitochondrial Hyperfusion and Alters the Transcriptional Profile of Stem Cells. Toxicol Sci. 2016;153:55–69. doi: 10.1093/toxsci/kfw102. [DOI] [PubMed] [Google Scholar]
- 81.Figueiro LR, Dantas DC, Linden R, Ziulkoski AL. Thirdhand tobacco smoke: procedures to evaluate cytotoxicity in cell cultures. Toxicol Mech Methods. 2016;26:355–61. doi: 10.1080/15376516.2016.1188190. [DOI] [PubMed] [Google Scholar]
- 82.Mossman BT. In vitro approaches for determining mechanisms of toxicity and carcinogenicity by asbestos in the gastrointestinal and respiratory tracts. Environ Health Perspect. 1983;53:155–61. doi: 10.1289/ehp.8353155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riveles K, Iv M, Arey J, Talbot P. Pyridines in cigarette smoke inhibit hamster oviductal functioning in picomolar doses. Reprod Toxicol. 2003;17:191–202. doi: 10.1016/s0890-6238(02)00150-8. [DOI] [PubMed] [Google Scholar]
- 84.Riveles K, Roza R, Arey J, Talbot P. Pyrazine derivatives in cigarette smoke inhibit hamster oviductal functioning. Reprod Biol Endocrinol. 2004;2:23. doi: 10.1186/1477-7827-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riveles K, Roza R, Talbot P. Phenols, quinolines, indoles, benzene, and 2-cyclopenten-1-ones are oviductal toxicants in cigarette smoke. Toxicol Sci. 2005;86:141–51. doi: 10.1093/toxsci/kfi112. [DOI] [PubMed] [Google Scholar]
- 86.Yu R, Wu M, Lin S, Talbot P. Cigarette Smoke Toxicants Alter Growth and Survival of Cultured Mammalian Cells. Toxicol Sci. 2006;93:82–95. doi: 10.1093/toxsci/kfl047. [DOI] [PubMed] [Google Scholar]
- 87.Ueta I, Saito Y, Teraoka K, Miura T, Jinno K. Determination of volatile organic compounds for a systematic evaluation of third-hand smoking. Anal Sci. 2010;26(5):569–74. doi: 10.2116/analsci.26.569. [DOI] [PubMed] [Google Scholar]
- 88.van der Bliek AM. Fussy mitochondria fuse in response to stress. EMBO J. 2009;28:1533–4. doi: 10.1038/emboj.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for MTDNA stability in skeletal muscle and tolerance of MTDNA mutations. Cell. 2010;141:280–9. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–116. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grandjean P, Bellinger D, Bergman A, Cordier S, Davey-Smith G, Eskenazi B, Gee D, Gray K, Hanson M, van den Hazel P, Heindel JJ, Heinzow B, Hertz-Picciotto I, Hu H, Huang TT, Jensen TK, Landrigan PJ, McMillen IC, Murata K, Ritz B, Schoeters G, Skakkebaek NE, Skerfving S, Weihe P. The faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2008;102:73–5. doi: 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 92.Teague-Enterprises Inhalation exposure apparatus. available at: http://www.teague-ent.com/equipment/smoking-machines http://www.teague-ent.com/equipment/smoking-machines (Sep 1, 2016)
- 93.NIH The FTC Cigarette Test Method for Determining Tar, Nicotine, and Carbon Monoxide Yields of U.S. Cigarettes. http://cancercontrol.cancer.gov/brp/TCRB/monographs/7/
- 94.Sorensen L, Gottrup F. Smoking and postsurgical wound healing – an update on mechanism and biological factors. Adv Wound Care. 2009;1 [Google Scholar]
- 95.Cutroneo KR, White SL, Phan SH, Ehrlich HP. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211:585–9. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 96.Azambuja E, Fleck JF, Batista RG, Menna Barreto SS. Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther. 2005;18:363–6. doi: 10.1016/j.pupt.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Kupiainen H, Kinnula VL, Lindqvist A, Postma DS, Boezen HM, Laitinen T, Kilpelainen M. Successful Smoking Cessation in COPD: Association with Comorbidities and Mortality. Pulm Med. 2012;2012:725024. doi: 10.1155/2012/725024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan H, Shyy JYJ, Martins-Green M. Secondhand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. 2009;51:535–47. doi: 10.1016/j.jhep.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007;115:990–5. doi: 10.1161/CIRCULATIONAHA.106.648469. [DOI] [PubMed] [Google Scholar]
- 100.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 101.Raupach T, Schafer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J. 2006;27:386–92. doi: 10.1093/eurheartj/ehi601. [DOI] [PubMed] [Google Scholar]
- 102.Anthony HM. Reactive changes in the blood of smokers and the development of arterial diseases and COPD, a review: evidence of associations between changes and subsequent disease with implications for the evaluation of harmful effects of cigarettes, and for susceptibility to the chronic effects of inhaled pollutants. Rev Environ Health. 1989;8:25–86. [PubMed] [Google Scholar]
- 103.Kisfali P, Polgar N, Safrany E, Sumegi K, Melegh BI, Bene J, Weber A, Hetyesy K, Melegh B. Triglyceride level affecting shared susceptibility genes in metabolic syndrome and coronary artery disease. Curr Med Chem. 2010;17(30):3533–41. doi: 10.2174/092986710792927822. [DOI] [PubMed] [Google Scholar]
- 104.Adhami N, Starck SR, Flores C, Martins Green M. A health threat to bystanders living in the homes of smokers: how smoke toxins deposited on surfaces can cause insulin resistance. PLoS One. 2016;11:e0149510. doi: 10.1371/journal.pone.0149510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karim ZA, Alshbool FZ, Vemana HP, Adhami N, Dhall S, Espinosa EV, Martins Green M, Khasawneh FT. Third-hand smoke: impact on hemostasis and thrombogenesis. J Cardiovasc Pharmacol. 2015;66:177–182. doi: 10.1097/FJC.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 106.Hang B, et al. Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci Rep. doi: 10.1038/srep41915. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matt GE, Fortmann AL, Quintana PJ, Zakarian JM, Romero RA, Chatfield DA, Hoh E, Hovell MF. Towards smoke-free rental cars: an evaluation of voluntary smoking restrictions in California. Tob Control. 2013;22:201–7. doi: 10.1136/tobaccocontrol-2011-050231. [DOI] [PubMed] [Google Scholar]
- 108.Matt GE, Quintana PJ, Fortmann AL, Zakarian JM, Galaviz VE, Chatfield DA, Hoh E, Hovell MF, Winston C. Thirdhand smoke and exposure in California hotels: nonsmoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control. 2014;23:264–72. doi: 10.1136/tobaccocontrol-2012-050824. [DOI] [PubMed] [Google Scholar]
- 109.Kassem NO, Daffa RM, Liles S, Jackson SR, Kassem NO, Younis MA, Mehta S, Chen M, Jacob P, 3rd, Carmella SG, Chatfield DA, Benowitz NL, Matt GE, Hecht SS, Hovell MF. Children's exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tob Res. 2014;16:961–75. doi: 10.1093/ntr/ntu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Northrup TF, Khan AM, Jacob P, Benowitz NL, Hoh E, Hovell MF, Matt GE, Stotts AL. Thirdhand smoke contamination in hospital settings: assessing exposure risk for vulnerable paediatric patients. Tobacco Control. 2015 doi: 10.1136/tobaccocontrol-2015-052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matt GE, Quintana P, Zakarian J, Hoh E, Hovell MF, Mahabee-Gittens M, Chatfield D. When Smokers Quit: Exposure to Nicotine and Carcinogen Persists from Thirdhand Smoke Population. Tobacco Control. 2016 doi: 10.1136/tobaccocontrol-2016-053119. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quintana PJE, Matt GE, Hoh E, Zakarian J, Destaillats H, Dhaliwal N, Chowdhury Z, Watanabe K, Sleiman M, Gundel LA, Russell ML, Theweny T, Arceo J, Muniz M, Flores V, Klepeis N. Thirdhand Tobacco Smoke Pollutants in a Casino Measured before and after Smoke-Free Status. International Society of Exposure Science (ISES) Conference; Henderson, NV. October, 2015; Herndon, VA, USA: International Society of Exposure Science ISES Secretariat; 2015. http://www.ises2015.org/ [Google Scholar]
- 113.Quintana PJ, Matt GE, Chatfield D, Zakarian JM, Fortmann AL, Hoh E. Wipe sampling for nicotine as a marker of thirdhand tobacco smoke contamination on surfaces in homes, cars, and hotels. Nicotine Tob Res. 2013;15:1555–63. doi: 10.1093/ntr/ntt014. [DOI] [PubMed] [Google Scholar]
- 114.Van Loy MD, Nazaroff WW, Daisey JM. Nicotine as a Marker for Environmental Tobacco Smoke: Implications of Sorption on Indoor Surface Materials. J Air Waste Manage Assoc. 1998;48:959–968. doi: 10.1080/10473289.1998.10463742. [DOI] [PubMed] [Google Scholar]
- 115.Thomas JL, Hecht SS, Luo X, Ming X, Ahluwalia JS, Carmella SG. Thirdhand Tobacco Smoke: A Tobacco-Specific Lung Carcinogen on Surfaces in Smokers' Homes. Nicotine Tob Res. 2014;16:26–32. doi: 10.1093/ntr/ntt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang S, Qiao S, Chen M, Xia Y, Hang B, Cheng S. An investigation of thirdhand smoke pollution in 3 types of places of Nanjing, 2014. Zhonghua Yu Fang Yi Xue Za Zhi (Chinese Journal of Preventive Medicine) 2015;49:31–5. [PubMed] [Google Scholar]
- 117.Hoh E, Hunt RN, Quintana PJ, Zakarian JM, Chatfield DA, Wittry BC, Rodriguez E, Matt GE. Environmental tobacco smoke as a source of polycyclic aromatic hydrocarbons in settled household dust. Environ Sci Technol. 2012;46:4174–83. doi: 10.1021/es300267g. [DOI] [PubMed] [Google Scholar]
- 118.Ramirez N, Ozel MZ, Lewis AC, Marce RM, Borrull F, Hamilton JF. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ Int. 2014;71:139–47. doi: 10.1016/j.envint.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 119.The Tobacco Atlas; 2015. World-Lung-Foundation, Cigarette Consumption. available at http://www.tobaccoatlas.org/topic/cigarette-use-globally/ [Google Scholar]
- 120.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1200. available at http://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf. [PMC free article] [PubMed] [Google Scholar]
- 121.Cecinato A, Balducci C, Romagnoli P, Perilli M. Airborne psychotropic substances in eight Italian big cities: burdens and behaviours. Environ Pollut. 2012;171:140–7. doi: 10.1016/j.envpol.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 122.Lopez MJ, Fernandez E, Gorini G, Moshammer H, Polanska K, Clancy L, Dautzenberg B, Delrieu A, Invernizzi G, Munoz G, Precioso J, Ruprecht A, Stansty P, Hanke W, Nebot M. Exposure to secondhand smoke in terraces and other outdoor areas of hospitality venues in eight European countries. PLoS One. 2012;7:e42130. doi: 10.1371/journal.pone.0042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sureda X, Martinez-Sanchez JM, Lopez MJ, Fu M, Aguero F, Salto E, Nebot M, Fernandez E. Secondhand smoke levels in public building main entrances: outdoor and indoor PM2.5 assessment. Tobacco Control. 2012;21:543–8. doi: 10.1136/tobaccocontrol-2011-050040. [DOI] [PubMed] [Google Scholar]
- 124.Hood NE, Ferketich AK, Klein EG, Pirie P, Wewers ME. Associations between self-reported in-home smoking behaviours and surface nicotine concentrations in multiunit subsidised housing. Tobacco Control. 2014;23:27–32. doi: 10.1136/tobaccocontrol-2012-050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.St Helen G, Bernert JT, Hall DB, Sosnoff CS, Xia Y, Balmes JR, Vena JE, Wang JS, Holland NT, Naeher LP. Exposure to secondhand smoke outside of a bar and a restaurant and tobacco exposure biomarkers in nonsmokers. Environ Health Perspect. 2012;120:1010–6. doi: 10.1289/ehp.1104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, andguidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–88. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, Peto R, Zatonski W, Hsia J, Morton J, Palipudi KM, Asma S. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 128.US-FDA Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. available at http://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm297786.htm (April 2012)
- 129.Asotra K. Presented at the International Symposium on Cancer Chemoprevention and Translational Research, School of Life Sciences. Jawaharlal Nehru University; New Delhi, India: 2009. Thirdhand Smoke Exposure: An Unappreciated Public Health Hazard. [Google Scholar]
- 130.Drehmer JE, Ossip DJ, Rigotti NA, Nabi-Burza E, Woo H, Wasserman RC, Chang Y, Winickoff JP. Pediatrician interventions and thirdhand smoke beliefs of parents. Am J Prev Med. 2012;43:533–6. doi: 10.1016/j.amepre.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.WHO Children's Health and the Environment. available at http://www.who.int/ceh/capacity/Children_are_not_little_adults.pdf (July 2008)
- 132.US-EPA. Child-Specific Exposure Factors Handbook (Final Report) EPA/600/R-06/096F, U.S. Environmental Protection Agency; Washington, DC: 2008. [Google Scholar]
- 133.WHO Summary of Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals. 2011 available at http://www.who.int/ceh/publications/health_risks_exposure_chemicals/en/
- 134.Huen K, Bradman A, Harley K, Yousefi P, Boyd Barr D, Eskenazi B, Holland N. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environ Res. 2012;117:8–16. doi: 10.1016/j.envres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.CDC. Take-home lead exposure among children with relatives employed at a battery recycling facility - Puerto Rico, 2011. Centers for Disease Control and Prevention. MMWR. 2012;61(47):967–70. [PubMed] [Google Scholar]
- 136.Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39:5121–30. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- 137.Lanphear BP, Matte TD, Rogers J, Clickner RP, Dietz B, Bornschein RL, Succop P, Mahaffey KR, Dixon S, Galke W, Rabinowitz M, Farfel M, Rohde C, Schwartz J, Ashley P, Jacobs DE. The contribution of lead-contaminated house dust and residential soil to children's blood lead levels. A pooled analysis of 12 epidemiologic studies. Environ Res. 1998;79:51–68. doi: 10.1006/enrs.1998.3859. [DOI] [PubMed] [Google Scholar]
- 138.Quandt SA, Arcury TA, Rao P, Snively BM, Camann DE, Doran AM, Yau AY, Hoppin JA, Jackson DS. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ Health Perspect. 2004;112:382–7. doi: 10.1289/ehp.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Exposure Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- 140.Samet JM, Chanson D, Wipfli H. The Challenges of Limiting Exposure to THS in Vulnerable Populations. Curr Environ Health Rep. 2015;2:215–25. doi: 10.1007/s40572-015-0060-1. [DOI] [PubMed] [Google Scholar]


