Summary
Approximately 100 trillion microorganisms exist in the oral cavity. For the commensal bacteria of the oral cavity, it is important to adapt to environmental stimuli, including human- or bacteria-derived antimicrobial agents. Recently, bacterial-specific signal transduction regulatory systems, called two-component systems (TCSs), which appear to be focused on sensing and adapting to the environment, were discovered. Streptococcus mutans is an oral commensal bacteria and is also known as a cariogenic bacteria. Although the virulence factors of S. mutans have been well demonstrated, the mechanism underlying the adaptation of the species to the oral cavity is poorly understood. S. mutans UA159 has 15 sets of TCSs. Among them, several have been demonstrated to be involved in acid tolerance, competence and biofilm formation. Recently, together with our findings, it was demonstrated that 5 TCSs were involved in resistance to antimicrobial agents. Furthermore, another TCS was associated with the production of bacteriocin. Six of 15 TCSs are associated with antimicrobial agents, implying that S. mutans can survive in the oral cavity by resisting various antimicrobial peptides.
In this review, we highlight the role of antimicrobial peptides in the oral cavity.
Keywords: Streptococcus mutans, Two-component system, Antimicrobial peptide, Bacteriocin, Defensin
1. Introduction
Approximately 100 trillion microorganisms exist in the human body; however, little information about the role and character of these commensal bacteria has been reported.
Recently, it was proposed that the human body establishes a symbiotic relationship that contributes to the maintenance of immune function by providing a niche for commensal bacteria called as microbiome [1], [2], [3]. Currently, the study of microbiomes in the human body is progressing via comprehensive gene analyses, using samples collected from saliva, skin, and feces [4], [5], [6]. Because the constitution patterns of the intestinal flora are of an infinite variety among humans, microbiomes may affect human physical and mental health because of their diversity [3], [7], [8]. It can be said that the study of microbiomes has changed the traditional concepts of microbial research.
In the process of indigenous flora formation, it is necessary to colonize the host by adapting to external stresses, including the immune system. Furthermore, bacteria must also compete with other commensal bacteria.
In the oral cavity, several hundreds of bacterial species exist, forming a complex bacterial floral community [9]. Oral flora, as well as intestinal flora, are also expected to exert various effects on the host. Although many studies of oral bacteria have focused on the pathogenesis of cariogenic bacteria and periodontal bacteria, future research on the association between oral flora and the host has attracted attention.
We first focused on Streptococcus mutans, a cariogenic bacteria, and sought to elucidate the colonization mechanism of this organism in the oral cavity. S. mutans plays a key role in the formation of dental plaque, as well as in tooth decay, via the production of acids [10], [11], [12]. Although the mechanism underlying caries development due to S. mutans is known, it is not clear why this bacterium is able to reside in the oral cavity. There are numerous antimicrobial agents in the oral cavity, and resistance to these antimicrobial agents is largely responsible for the colonization of S. mutans. To form the biofilm (dental plaque) physical barrier to resist the antimicrobial agents. Furthermore, bacteria including S. mutans possess two-component systems (TCSs) which sense the environmental stimuli including antimicrobial agents and regulate the expressions of several factors to be responsible for the adaptation to the stimuli.
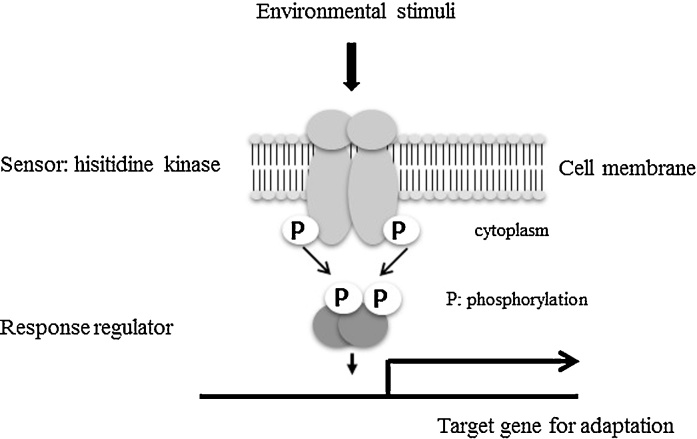
Two-component systems are prokaryote-specific signal transduction systems that comprise a sensory histidine kinase (HK) and a cognate response regulator (RR) [13]. The sensory HK undergoes autophosphorylation of a histidine residue in response to an environmental signal and relays the phosphate group to an aspartic acid residue on the cognate RR [14], [15]. The phosphorylated RR then binds to target DNA elements with strong affinity, activating or repressing transcription of target genes (Fig. 1). We focused on the relationship between TCSs and resistance to antimicrobial peptides. Through these results, we have gained new knowledge of the above phenomena.
Figure 1.
Scheme of the two-component system. The sensory histidine kinase undergoes autophosphorylation of a histidine residue in response to an environmental signal and relays the phosphate group to an aspartic acid residue on the cognate response regulator (RR). The phosphorylated RR then binds to target DNA elements with strong affinity, activating or repressing the transcription of target genes.
In this review, we present an overview of antimicrobial peptides and TCSs, including the results of our study regarding the acquisition of resistance mechanisms in S. mutans against human- and bacteria-derived antimicrobial peptides.
2. Human-derived antimicrobial peptides
In the oral cavity, there are many antimicrobial factors, such as antimicrobial peptides, lysozymes, hydrogen peroxide and lactoferrin [16], [17]. Among these antibacterial factors, antimicrobial peptides are believed to have bactericidal activity against various oral bacteria, including cariogenic and periodontopathogenic bacteria [17]. Human-derived antimicrobial peptides originate from various sources, such as the saliva, gingival epithelium, mucosa, neutrophils and gingival crevicular fluid [18], [19]. These peptides are considered the first defense against bacterial infections as a form of innate immunity. Fig. 2 shows the varieties of human-derived antimicrobial peptides.
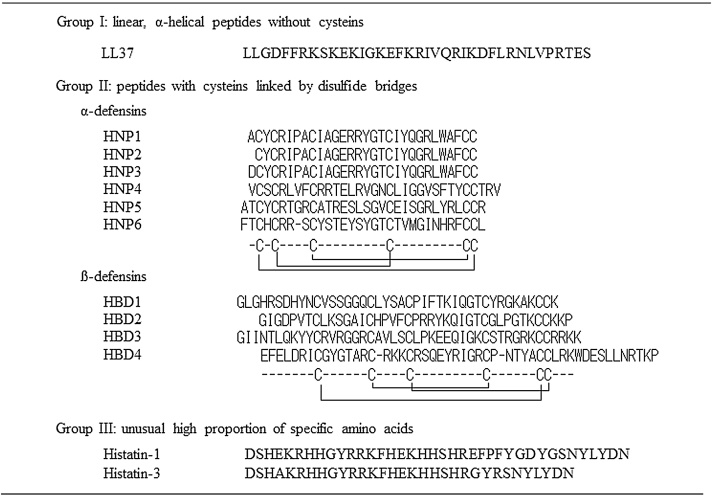
Figure 2.
Antimicrobial peptides in humans. LL37 has a linear form. Defensins produced by humans are classified into two types: alpha- and beta-defensins. Defensins have three disulfide bonds among 6 cysteines in peptides. Histatins are salivary antimicrobial peptides and are a family of histidine-rich cationic peptides produced by parotid and salivary duct cells. Histatins are known to have antifungal activity. Histatin 5, in particular, has strong activity against fungi, including Candida species.
2.1. Defensins
Defensins have three disulfide bonds among 6 cysteines in peptides, which distinguishes them from other antimicrobial peptides. Defensins are further classified into three types: alpha-, beta- and theta-defensins, although theta defensins are not expressed in humans [18], [20], [21]. Differences in producing cells and the arrangement of the 3 disulfide bonds distinguish alpha- and beta- defensins.
Several alpha- defensins (human neutrophil defensin: HNP) have been identified. HNP1-4 is mainly localized in the granules of neutrophils, whereas HNP5 and 6 are localized in the Paneth cells of the small intestine [22]. HNPs demonstrate broad antimicrobial activity against Gram-positive and -negative bacteria, fungi and viruses. The activity of these peptides is decreased by high NaCl concentrations. In addition to antimicrobial activity, several HNPs are reported to have chemotactic activity as cytokines.
To date, 4 beta-defensins (human neutrophil defensing 1–4: HBD1-4) have been reported. These peptides are expressed in various epithelial tissues, such as the skin, trachea, gingiva and saliva [22], [23], [24], [25]. In vitro experiments, HBD1 are constitutively expressed, while HBD2 and HBD3 are inducible [23], [24], [25], [26], [27], [28]. Additionally, HBD1 and HBD2 have been demonstrated to have strong activity against Gram-negative bacteria, but not Gram-positive bacteria, while HBD3 and HBD4 have strong activity against both types of bacteria [29], [30], [31], [32]. Like HNPs, HBDs also have chemotactic activity against human cells [33], [34].
2.2. Cathelicidins
The cathelicidin family features characteristic conserved domains known as cathelins (cathepsin inhibitors) in their N-terminal and variable regions and C-termini with antimicrobial activity. In humans, only one cathelicidin, known as LL37, has been identified [19], [35]. LL37 is found in various cells, such as neutrophils, monocytes and various epithelial cells [35]. Unlike defensins, LL37 has no disulfide bonds and has a linear form. LL37 has strong antibacterial activity against Gram-negative and -positive bacteria [19], [36]. LL37 is also known as LPS neutralizing factor, which binds LPS and inhibits endotoxin activity [19], [37]. Moreover, LL37 has chemotactic activity against various cells, such as neutrophils, monocytes and mast cells [38].
2.3. Histatins
Histatins are salivary, cationic antimicrobial peptides and are a histidine-rich family produced by parotid and salivary duct cells [39]. Histatins are known to have antifungal activity. In particular, histatin 5 has strong activity against fungi, including Candida species [40].
3. Bacteria-derived antimicrobial peptides (bacteriocins)
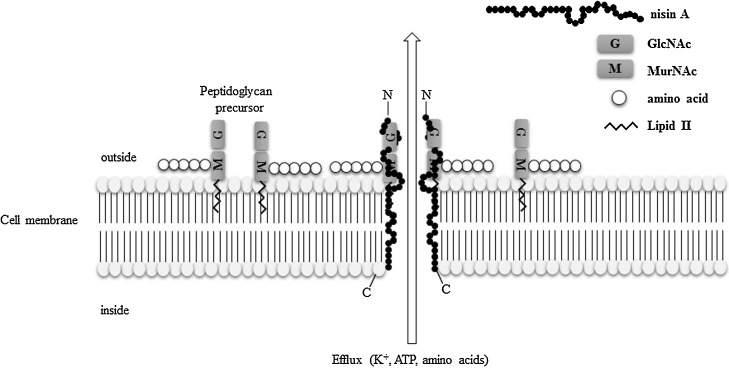
The antibacterial agents produced by bacteria are called bacteriocins. Bacteriocins are ribosomally synthesized peptides, or proteins that exhibit antibacterial activity, mostly against bacterial species that are closely related to the bacteriocin producer [41], [42]. Bacteriocins are mainly classified into classes I and II [43]. Class I bacteriocins (peptides <5 kDa) are called “lantibiotics” and contain a ring bridged by lanthionine and 3-methyllanthionine residues [44], whereas class II bacteriocins comprise unmodified amino acids [45]. Lantibiotics are further classified to Type A-I, Type A-II, and Type B by view of their similarities to peptides with established structures [43]. Table 1 shows the list of lantibiotics. Many bacteria produce bacteriocins to ensure their survival in this community [42], [46]. To persist in the oral cavity, bacteria must possess several factors to resist not only host immune factors but also bacteriocins from other microfloral species. The mode of action of bacteriocins is believed to involve pore formation in the cell membrane or inhibition of cell wall synthesis. Fig. 3 shows the antibacterial mechanism of nisin A, which is a typical bacteriocin produced by Lactococcus lactis. Nisin A can bind to the lipid II moiety, which is related to the biosynthesis of cell wall peptidoglycan [42], [43]. Therefore, the mode of action of nisin A is pore formation and inhibition of cell wall synthesis.
Table 1.
Classification of bacteriocins.
| Class | Characteristics | Representative bacteriocins |
|---|---|---|
| I | Lantibiotics, small (<5 kDa) heat-stable peptides containing unsaturated amino acids, lanthionine and 3-methyllanthionine | |
| AI | More elongated peptides than Type-AII | Nisin, mutacin I, II, III, 1140, streptin |
| AII | A linear N-terminus and a globular C-terminus | Lacticin 481, nukacin ISK-1, salivaricin, mutacin K8 |
| B | Globular peptide | Mersacidin, cinnamycin |
| others | Two peptide lantibiotics | Lacticin 3147, staphylococcin C55, Smb |
| II | Small (<10 kDa) heat-stable peptides formed by unmodified amino acids | |
| IIa | Anti-listerial peptides with a consensus sequence of YGNGVXC | Pediocin PA-1, Enterocin A |
| IIb | Two-peptide bactericins | Lactococcin G, Lactococcin Q, Enterocin 1071 |
| IIc | Other bacteriocins | Enterocin B, Lactococcin A |
| III | High-molecular-weight (>30kDa), heat-labile proteins | Helveticin J, enterolysin A |
| IV | Complex bacteriocins containing lipid or carbohydrate moieties | Leuconocin S, Lactocin 27 |
| V | Circular peptides | Enterocin AS-48, Lactocyclicin Q |
Figure 3.
Antibacterial mechanism of nisin A. Nisin A interferes with cell wall biosynthesis and forms complexes with lipid I and lipid II. Subsequently, nisin affects the cytoplasmic membrane of susceptible bacteria and is able to form short-lived pores in the cell membrane. This effect leads to an efflux of small molecules (potassium, ATP, and amino acids) and dissipation of the membrane potential, resulting in the arrest of all cellular biosynthesis.
4. Two-component systems
4.1. General characteristic of TCSs
Recently, TCSs were reported to be associated with resistance to antibacterial agents, such as human- or bacteria-derived antimicrobial peptides. bacteria are able to quickly adapt to the external environment by regulating gene expression. Generally, bacteria possess multiple TCSs. It is believed that TCSs are involved in the adaptation to external stimuli, such as osmotic pressure, pH and temperature and virulence factor expression.
4.2. S. mutans TCSs
S. mutans UA159 possesses 15 sets of TCSs (Table 2). Genome analysis of several S. mutans strains showed that more than 12 TCSs are conserved among S. mutans strains [47]. Each TCS is believed to have a role; however, the functions of some TCSs are unknown. ComDE is known as a quorum sensing system, which is a sensor of cell density in biofilm and is also related to competence [48], [49]. VicRK is also conserved among many bacterial species and is related to virulence [50], [51]. Due to the characteristics of S. mutans, the relationship between TCSs and biofilm formation and acid tolerance has been well-demonstrated [50], [52], [53], [54]. To date, several TCSs have been reported to be associated with acid tolerance and biofilm formation, although the precise mechanism underlying these linkages has not been elucidated. Regarding antimicrobial agents, it was reported that ComDE (SMU. 1916–17) is associated with production of the bacteriocin known as mutacin IV [48].
Table 2.
Two-component systems in S. mutans UA159.
| Gene ID | Gene name | Function |
|---|---|---|
| SMU_486-487 | liaRS | Envelope stress, acid torelance, biofilm formation |
| SMU_577-576 | unassigned | Unknown |
| SMU_660-659 | nsrRS | Nisin resistance |
| SMU_928-927 | unassigned | Low nutrition |
| SMU_1009-1008 | bceRS | Bacitracin resistance |
| SMU_1037-1038 | unassigned | Unknown |
| SMU_1128-1129 | ciaRH | Acid tolerance,competence, resistance against cationic agents |
| SMU_1145-1146 | lcrRS | Nukacin resistance |
| SMU_1516-1517 | vicRK | Biofilm, oxidative stress |
| SMU_1548-1547 | unassigned | Unknown |
| SMU_1814-1815 | unassigned | Oxidative stress |
| SMU_1916-1917 | comDE | MutacinIV production, competency |
| SMU_1965-1964 | unassigned | Unknown |
| SMU_1924 | gcrR | GbpC expression, biofilm formation |
| SMU_45-46 | unassigned | Oxidative Stress |
5. Association of TCS with antimicrobial susceptibility in S. mutans
5.1. Resistance to human antimicrobial agents
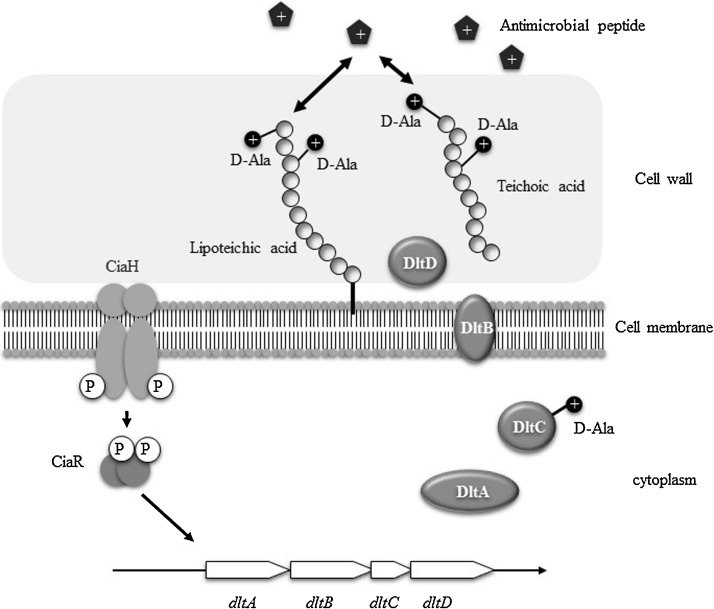
First, we evaluated susceptibility to LL37 and β-defensins using 15 sets of TCS knockout mutants. As a result of this comprehensive analysis, one TCS (ciaRH) knockout mutant showed increased susceptibility to LL37 and beta-defensins (HBD1-3) in biofilm cells [55]. Further experiments showed that CiaRH regulates the dlt operon, which plays an important role in the acquisition of resistance to positively-charged antimicrobial agents, such as LL37 and β-defensins. In biofilm cells, dlt expression in a ciaRH knock-out mutant was decreased compared with the wild type. Normally, the bacterial cell surface is negatively charged, and positively charged antimicrobial peptides are attracted to the membrane. Dlt was reported to be a major contributor to the decreased negative charge of cell surfaces because the above factors are associated with the addition of alanine to teichoic acids [56], [57], [58]. Alanylation of teichoic acids in the cell wall conveys a positive charge to the bacterial cell surface, which results in a shift to a weak negative charge on the cell surface [57], [58]. Our results suggest that CiaRH is related to the expression of dlt in biofilm cells and contributes to resistance to positively-charged antibacterial agents by weakening the negative change of the cell surface (Fig. 4).
Figure 4.
Scheme of the resistance mechanism against antimicrobial peptides via the CiaRH-Dlt system in S. mutans. CiaRH is related to the expression of dlt in biofilm cells and contributes to resistance to positively-charged antibacterial agents by weakening thenegative charge of the cell surface.
5.2. Resistance to bacteria-derived antimicrobial agents (bacteriocins)
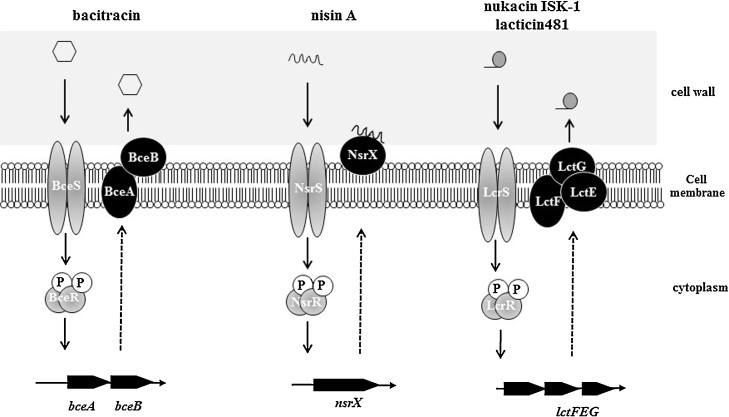
The susceptibility of the S. mutans wild type and its TCS knockout mutants was examined by direct methods using a variety of bacteriocin-producing bacteria. The susceptibility of three class I bacteriocins, including nisin A, nukacin ISK-1 and lacticin 481, and 3 class II bacteriocins, including types IIa-IIc, was evaluated. As a result, the knockouts of two unidentified TCSs (designated NsrRS and LcrRS) showed decreased susceptibility to bacteriocins, while the other mutants showed no change [59]. The knockout of the genes SMU.658–59, designated nsrRS, caused increased susceptibility to nisin A, a class I lantibiotic produced by L. lactis. In nsrRS mutants, the gene designated nsrX was not increased by the addition of nisin A, while nsrX expression was increased by nisin A in the wild type. Finally, we demonstrated that NsrX was expressed on the cell membrane and had binding affinity for nisin A, causing the trapping of nisin A to inhibit binding of nisin A to the target (Fig. 5).
Figure 5.
Proposed bacteriocin resistance mechanism mediated by TCSs in S. mutans. BceRS is related to bacitracin resistance by regulating BceAB ABC transporter. NsrRS is related to nisin A resistance by regulatiing NsrX which has ability to bind nisin A, suggesting trap nisin A. LcrRS is related to nukacin ISK-I and lacticin 481 resistance by regulating LctFEG ABC transporter.
The knockout of the genes SMU.1146–47, designated lcrRS, caused increased susceptibility to nukacin ISK-1, which is a class I lantibiotic produced by Staphylococcus warneri. The expression of the ABC transporter lctFEG, which is located downstream of lcrRS, was inhibited by exposing nukacin ISK-1 in In lcrRS knockout mutants. This result suggested that the expression of LctFEG was induced by nukacin ISK-1 via LcrRS (Fig. 5). Therefore, the functions of NsrX and LctFEG are thought to be different: NsrX binds nisin A and captures nisin A to avoid attachment to the cell membrane; however, LctFEG may export nukacin ISK-1. In other reports, BraRS and LiaRS were demonstrated to be involved in bacitracin and vancomycin susceptibility, respectively [60], [61]. Also, ComDE was reported to be involved in Mutacin IV Production [48]. Therefore, totally 5 TCSs are associated with bacteriocin.
Deng et al. reported that VicRK, one of the TCSs in S. mutans, was associated with hydrogen peroxide (H2O2) stress [62]. Although this result was reconfirmed in our study, it is unclear which resistant factors are regulated by VicRK. It is known that Streptococcus sanguinis produces H2O2. This bacteria is one of the major commensal bacteria in the oral cavity. We expected that VicRK systems in S. mutans play an important role in resisting the H2O2 produced by S. sanguinis.
5.3. Bacteriocin affects the proportion of bacteria when two bacteria are co-cultured
To determine whether bacteriocins are important for persistent survival in complex bacterial communities, we established a co-culture assay to evaluate each bacterial proportion when two bacterial strains were mixed and cultured [59]. When wild type S. mutans was co-cultured with bacteriocin-producing or nonproducing strains, the proportion of S. mutans co-cultured with bacteriocin-producing strains was decreased compared to that co-cultured with bacteriocin-nonproducing strains. Additionally, when the respective TCS mutants were co-cultured with bacteriocin-producing strains, the proportion was significantly decreased compared to the WT. These results indicate that bacteriocin and TCS-mediated bacteriocin resistance are important for survival in complex bacterial communities.
6. Conclusions
In our study, 3 of the 15 TCSs identified in S. mutans were demonstrated to be associated with resistance to human- or bacteria-derived antimicrobial peptides [55], [59]. Furthermore, other researchers reported that 3 additional TCSs were associated with production of or resistance to antibacterial agents [48], [60], [61].
In particular, four TCSs have been identified as bacteriocin-related factors (Figure 4, Figure 5). These results highlight the role of bacteriocins in the interactions among different species of oral bacteria and the importance of TCSs in these interactions. In the oral cavity, TCSs play an important role in acquiring resistance to human- or bacteria-derived antimicrobial agents.
It is suggested that bacterial TCSs may be key factors in understanding the colonization mechanisms of commensal bacteria in humans. In recent years, it has been reported that intestinal bacterial microbiomes have a large impact on human health. In the process of microbiome formation, we considered that TCSs play an important role in adapting to the environmental stimuli that arise from interactions between bacteria and the human body. In the future, we expect to examine whether TCSs are involved in microbiome formation.
In addition, in this study, it was suggested that antimicrobial factors, including human- and bacteria-derived antimicrobial peptides, are strongly associated with commensal flora formation, including oral flora formation. Further examinations are needed to analyze microbiome formation.
Conflicts of interest
None.
Footnotes
Scientific field of dental sciences: oral bacteriology.
References
- 1.Lee Y.K., Mazmanian S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones M.L., Ganopolsky J.G., Martoni C.J., Labbé A., Prakash S. Emerging science of the human microbiome. Gut Microbes. 2014;5(4):446–457. doi: 10.4161/gmic.29810. [DOI] [PubMed] [Google Scholar]
- 3.Van Praet J.T., Donovan E., Vanassche I., Drennan M.B., Windels F., Dendooven A. Commensal microbiota influence systemic autoimmune responses. EMBO J. 2015;34(4):466–474. doi: 10.15252/embj.201489966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanahan F. The colonic microbiota in health and disease. Curr Opin Gastroenterol. 2013;29(1):49–54. doi: 10.1097/MOG.0b013e32835a3493. [DOI] [PubMed] [Google Scholar]
- 5.Wade W.G. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.E., Tsao H. The skin microbiome: current perspectives and future challenges. J Am Acad Dermatol. 2013;69(1):143–155. doi: 10.1016/j.jaad.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridaura V., Belkaid Y. Gut microbiota: the link to your second brain. Cell. 2015;161(2):193–194. doi: 10.1016/j.cell.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Grice E.A., Segre J.A. The human microbiome: our second genome. Annu Rev Genom Hum Genet. 2012;13:151–170. doi: 10.1146/annurev-genom-090711-163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada S., Slade H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi N., Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 12.Hamada S., Slade H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitrophanov A.Y., Groisman E.A. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22:2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampersaud A., Harlocker S.L., Inouye M. The OmpR protein of Escherichia coli binds to sites in the ompF promoter region in a hierarchical manner determined by its degree of phosphorylation. J Biol Chem. 1994;269:12559–12566. [PubMed] [Google Scholar]
- 15.Hoch J.A. Two-component and phosphorelay signal transduction. Curr Opin Microbiol. 2000;3:165–170. doi: 10.1016/s1369-5274(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 16.Tenovu J., Lumikari M., Soukka T. Salivary lysozyme, lactoferrin and peroxidases: antibacterial effects on cariogenic bacteria and clinical applications in preventive dentistry. Proc Finn Dent Soc. 1991;87:197–208. [PubMed] [Google Scholar]
- 17.Dale B.A., Fredericks L.P. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2005;7(2):119–133. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz T., Lehrer R.I. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 19.Larrick J.W., Hirata M., Balint R.F., Lee J., Zhong J., Wright S.C. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganz T., Selsted M.E., Szklarek D., Harwig S.S., Daher K., Bainton D.F. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunliffe R.N. Alpha-defensins in the gastrointestinal tract. Mol Immunol. 2003;40:463–467. doi: 10.1016/s0161-5890(03)00157-3. [DOI] [PubMed] [Google Scholar]
- 22.Mathews M., Jia H.P., Guthmiller J.M., Losh G., Graham S., Johnson G.K. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67(6):2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W.L., Bevins C.L. Tracheal antimicrobial peptide, a cysteine rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolzenberg E.D., Anderson G.M., Ackermann M.R., Whitlock R.H., Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci U S A. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krisanaprakornkit S., Weinberg A., Perez C.N., Dale B.A. Expression of the peptide antibiotic human beta defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valore E.V., Park C.H., Quayle A.J., Wiles K.R., McCray P.B., Jr., Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krisanaprakornkit K., Kimball J.R., Weinberg A., Darveau R.P., Bainbridge B.W., Dale B.A. Inducible expression of human β-defensin-2 (hBD-2) by Fusobacterium nucleatumin oral epithelial cells: multiple signaling pathways and the role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harder J., Bartels J., Christophers E., Schroder J.M. Isolation and characterization of human betadefensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 29.Harder J., Bartels J., Christophers E., Schröder J.M. A peptide antibiotic from human skin. Nature. 1997;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 30.Hoover D.M., Rajashankar K.R., Blumenthal R., Puri A., Oppenheim J.J., Chertov O. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275(42):32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 31.Hoover D.M., Chertov O., Lubkowski J. The structure of human beta-defensin-1: new insights into structural properties of beta-defensins. J Biol Chem. 2001;276(42):39021–39026. doi: 10.1074/jbc.M103830200. [DOI] [PubMed] [Google Scholar]
- 32.Komatsuzawa H., Ouhara K., Kawai T., Yamada S., Fujiwara T., Shiba H. Susceptibility of periodontopathogenic and cariogenic bacteria to defensins and potential therapeutic use of defensins in oral diseases. Curr Pharm Des. 2007;13(30):3084–3095. doi: 10.2174/138161207782110426. [DOI] [PubMed] [Google Scholar]
- 33.Niyonsaba F., Someya A., Hirata M., Ogawa H., Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31(4):1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Yang D., Biragyn A., Kwak L.W., Oppenheim J.J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23(6):291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 35.Frohm Nilsson M., Sandstedt B., Sorensen O., Weber G., Borregaard N., Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaiou M., Gallo R.L. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med (Berl) 2002;80(9):549–561. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 37.Nagaoka I., Hirota S., Niyonsaba F., Hirata M., Adachi Y., Tamura H. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol. 2002;9(5):972–982. doi: 10.1128/CDLI.9.5.972-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokumaru S., Sayama K., Shirakata Y., Komatsuzawa H., Ouhara K., Hanakawa Y. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175(7):4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 39.Troxler R.F., Offner G.D., Xu T., Vanderspek J.C., Oppenheim F.G. Structural relationship between human salivary histatins. J Dent Res. 1990;69(1):2–6. doi: 10.1177/00220345900690010101. [DOI] [PubMed] [Google Scholar]
- 40.Oppenheim F.G., Xu T., McMillian F.M., Levitz S.M., Diamond R.D., Offner G.D. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263(16):7472–7477. [PubMed] [Google Scholar]
- 41.Jack R.W., Tagg J.R., Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nissen-Meyer J., Nes I.F. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167:67–77. [PubMed] [Google Scholar]
- 43.Bierbaum G., Sahl H.G. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr Pharm Biotechnol. 2009;10:2–18. doi: 10.2174/138920109787048616. [DOI] [PubMed] [Google Scholar]
- 44.Nagao J., Asaduzzaman S.M., Aso Y., Okuda K., Nakayama J., Sonomoto K. Lantibiotics: insight and foresight for new paradigm. J Biosci Bioeng. 2006;102:139–149. doi: 10.1263/jbb.102.139. [DOI] [PubMed] [Google Scholar]
- 45.Nes I.F., Holo H. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers. 2000;55:50–61. doi: 10.1002/1097-0282(2000)55:1<50::AID-BIP50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Cotter P.D., Hill C., Ross R.P. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 47.Song L., Sudhakar P., Wang W., Conrads G., Brock A., Sun J. A genome-wide study of two-component signal transduction systems in eight newly sequenced mutans streptococci strains. BMC Genom. 2012;4(13):128. doi: 10.1186/1471-2164-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Ploeg J.R. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J Bacteriol. 2005;187(12):3980–3989. doi: 10.1128/JB.187.12.3980-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemme A., Gröbe L., Reck M., Tomasch J., Wagner-Döbler I. Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J Bacteriol. 2011;193(8):1863–1877. doi: 10.1128/JB.01363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senadheera M.D., Guggenheim B., Spatafora G.A., Huang Y.C., Choi J., Hung D.C. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J Bacteriol. 2005;187(12):4064–4076. doi: 10.1128/JB.187.12.4064-4076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senadheera D.B., Cordova M., Ayala E.A., Chávez de Paz L.E., Singh K., Downey J.S. Regulation of bacteriocin production and cell death by the VicRK signaling system in Streptococcus mutans. J Bacteriol. 2012;194(6):1307–1316. doi: 10.1128/JB.06071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn S.J., Burne R.A. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J Bacteriol. 2007;189(17):6293–6302. doi: 10.1128/JB.00546-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lévesque C.M., Mair R.W., Perry J.A., Lau P.C., Li Y.H., Cvitkovitch D.G. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett Appl Microbiol. 2007;45(4):398–404. doi: 10.1111/j.1472-765X.2007.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Burne R.A. Multiple two-component systems modulate alkali generation in Streptococcus gordonii in response to environmental stresses. J Bacteriol. 2009;191(23):7353–7362. doi: 10.1128/JB.01053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazda Y., Kawada-Matsuo M., Kanbara K., Oogai Y., Shibata Y., Yamashita Y. Association of CiaRH with resistance of Streptococcus mutans to antimicrobial peptides in biofilms. Mol Oral Microbiol. 2012;27(2):124–135. doi: 10.1111/j.2041-1014.2012.00637.x. [DOI] [PubMed] [Google Scholar]
- 56.Peschel A., Otto M., Jack R.W., Kalbacher H., Jung G., Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 57.Peschel A., Jack R.W., Otto M., Collins L.V., Staubitz P., Nicholson G. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins L.V., Kristian S.A., Weidenmaier C., Faigle M., Van Kessel K.P., Van Strijp J.A. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis. 2002;186:214–219. doi: 10.1086/341454. [DOI] [PubMed] [Google Scholar]
- 59.Kawada-Matsuo M., Oogai Y., Zendo T., Nagao J., Shibata Y., Yamashita Y. Involvement of the novel two-component NsrRS and LcrRS systems in distinct resistance pathways against nisin A and nukacin ISK-1 in Streptococcus mutans. Appl Environ Microbiol. 2013;79(15):4751–4755. doi: 10.1128/AEM.00780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suntharalingam P., Senadheera M.D., Mair R.W., Lévesque C.M., Cvitkovitch D.G. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J Bacteriol. 2009;191(9):2973–2984. doi: 10.1128/JB.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouyang J., Tian X.L., Versey J., Wishart A., Li Y.H. The BceABRS four-component system regulates the bacitracin-induced cell envelope stress response in Streptococcus mutans. Antimicrob Agents Chemother. 2010;54(9):3895–3906. doi: 10.1128/AAC.01802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng D.M., Liu M.J., ten Cate J.M., Crielaard W. The VicRK system of Streptococcus mutans responds to oxidative stress. J Dent Res. 2007;86(7):606–610. doi: 10.1177/154405910708600705. [DOI] [PubMed] [Google Scholar]