Abstract
Compromised sleep and increased sympathetic nervous system (SNS) activity are implicated in the pathogenesis of, and disparities in, cardiovascular disease. Parasympathetic dominance during sleep may be important for cardiovascular health. Sleep and autonomic balance influence immune activity which impacts atherogenesis. We evaluated relationships between autonomic balance during sleep and morning levels of the immune activating cytokines, C-reactive protein (CRP) and Interleukin 6 (IL-6). Ninety four (59 female) young adult African Americans without medical conditions and substance use disorders spent two consecutive nights in a clinical research unit for sleep recordings and blood drawing on awakening. Cardiac tracings from the 2nd sleep recording were analyzed for heart rate variability (HRV). BMI was the only non-HRV measure correlated with cytokine levels. Indicators of SNS activity for the pre-sleep, and first non-REM and REM sleep periods were independently correlated with morning IL-6 levels. Altered autonomic balance during sleep may be a modifiable factor that influences immune activation.
Keywords: Autonomic nervous system, sympathetic nervous system, heart rate variability, sleep, proinflammatory cytokines
Compromised sleep (e.g. short sleep duration, insomnia, sleep apnea) is associated with adverse health consequences including obesity (Cappuccio et al., 2008), diabetes (Ayas, et al., 2003; Beihl et al., 2009), hypertension and cardiovascular disease (CVD) (Ayas, et al., 2003; Phillips, et al., 2007), and early mortality (Gallicchio et al., 2009; Kripke, et al., 2002). These conditions disproportionately affect African Americans (Center for Disease Control and Prevention, 2011). African Americans are more likely to report short sleep duration than whites, and this appears to be influenced by living in high density urban environments (Hale et al., 2007).
Autonomic balance is determined by the relative contributions of the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS). The contribution of the SNS increases during exercise and stress. Persistent increases in SNS activity is an important contributor to the negative effects of stress on cardiovascular health (Guzzetti et al., 2002; Kohara, et al., 1995; Phillips et al., 2000).
During sleep, particularly non-rapid-eye-movement (NREM) stages, there is an increase in PNS and decrease in SNS activity (Bonnet et al., 1997; Burgess et al., 2004; Trinder et al., 2001). Evidence of adverse consequences from shifts towards PNS dominance during sleep being compromised includes observations of increased nocturnal SNS activity with blood pressure non-dipping which is a well-established cardiovascular risk factor (Kohara et al., 1995), the association of sleep apnea with increased nocturnal SNS activity and hypertension (Narkiewicz et al., 1997), and observations of blunted nighttime reduction of SNS activity in individuals with histories of myocardial infarction (Guzzetti et al., 2002). These associations suggest that PNS dominance during sleep may be important toward maintaining cardiovascular health.
Heart rate variability (HRV) is a non-invasive method that analyzes patterns in time series of consecutive R-peaks in the cardiac cycle and is used to assess autonomic nervous system (ANS) activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Evidence that the shift during sleep toward PNS dominance can be compromised with insomnia and stress includes a study by Bonnet et al. (1998), in which the HRV index of parasympathetic tone (normalized high frequency, nHF) was reduced among participants with insomnia, who showed increased low frequency/high frequency (LF/HF) ratios, the standard index of SNS activity, compared with normal sleepers. Our group examined nocturnal ANS in PTSD and resilience (Kobayashi, et al., 2014) and found greater nHF power with resilience and a strong positive relationship between sleep duration and nHF and negative relationship to LF/HF in the resilient group but not in the PTSD group.
SNS influences on immune activity is now considered an important mechanism by which sustained SNS activity adversely affects cardiovascular health (Marvar, et al., 2011). Immune activation is often indexed by blood levels of pro-inflammatory cytokines including interleukin-6 (IL-6), interleukin-1, C-reactive protein (CRP), and tumor necrosis factor (TNF – α). Elevated levels of these cytokines have been associated with decreased HRV (Haarala et al., 2011), and increased risk of cardiovascular and metabolic disease (Cesari et al., 2003; Pai et al., 2004). In a recent comprehensive review and meta-analysis of investigations relating sleep duration, sleep disturbance, and experimental sleep deprivation with immune markers, Irwin et al. (2015) concluded that the evidence relates sleep disturbance and sleep duration to IL-6 and CRP levels (and not TNF – α).
Given the associations of disturbed or altered sleep, increased SNS activity, and elevated pro-inflammatory cytokine blood levels (all of which have been independently associated with cardiovascular disease risk), we hypothesized that ANS activity during sleep would contribute to sleep’s effect on immune activity. We evaluated this hypothesis by measuring CRP and IL-6 from morning blood samples and relating them to sleep measures, including HRV from the previous night, in young adult, urban residing African Americans, a population that is demographically at increased risk for future cardiovascular disease.
Method
Participants and Procedure
Participants of this study were the subsample of young adult African Americans who had completed a protocol consisting of two PSG recordings and blood drawing in the morning after the second PSG recording as a part of a larger study evaluating relationships of sleep, stress and nocturnal blood pressure. Participants were recruited from the Washington DC metropolitan region through flyers and referrals from previous participants. During initial phone and in-person evaluation, participants were screened for exclusionary criteria including: body mass index (BMI) ≥ 40, > 5 cups of coffee/day or equivalent levels with any other caffeinated beverages, > 20 cigarettes/day, > 14 alcoholic drinks/week for men, > 7 drinks/week for women, chronic medical conditions that can affect blood pressure or sleep and required continuous medication use, severe psychiatric illnesses (psychotic disorders, bipolar disorder, severe recurrent depression), and habitual bedtime and rise time after 2 AM and 10 AM, respectively, or habitual napping > 1 hour/day. Individuals who were eligible for the study were invited to the Clinical Research Unit at Howard University Hospital, and after participants were further informed about the study procedure, they signed informed consent documents approved by the Howard University Institutional Review Board.
A total of 543 participants completed a self-report survey packet including demographics and health questionnaires and a measure of posttraumatic stress disorder (PTSD) symptoms. One hundred eighty-five participants who completed self-report surveys were selected to participate in the laboratory phase. The selection was made to over-sample participants with probable PTSD and balance for gender and community representation (Mellman et al., 2015). All participants were assessed with the Clinician Administered PTSD Scale (CAPS). We defined subthreshold PTSD (DSM-IV) as meeting 2 of the 3 diagnostic symptom clusters. The laboratory phase included a diagnostic interview for psychiatric disorders, urine screening, height and weight measurement, and two consecutive overnight PSG recordings in the Clinical Research Unit. Additional exclusionary criteria including sleep breathing disorder, positive urine toxicology or observed intoxication were assessed during the laboratory phase. Of the 185 participants selected, 104 provided a blood sample. Participants were excluded due to positive urine toxicology (n = 2) and other protocol violations (n =2), and PSG data technical problems (n = 6); therefore, this analysis includes 94 participants (59 females; 35 males). Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics and sleep measures.
| n | Mean | SD | |
|---|---|---|---|
| Female | 59 (63%) | ||
|
| |||
| Male | 35 (37%) | ||
| Trauma exposure | 77 (82%) | ||
| Full PTSD | 11 (12%) | ||
| Subthreshold PTSD | 14 (15%) | ||
| PTSD Severity (CAPS)a | 19.30 | 19.99 | |
| Age | 22.57 | 4.50 | |
| Body mass index | 25.10 | 4.52 | |
| Total sleep time (min) | 386.10 | 74.40 | |
| WASO | 27.496 | 25.32 | |
| Sleep efficiency (%) | 86.03 | 10.08 | |
| N1 (%) | 2.43 | 1.68 | |
| N2 (%) | 52.24 | 7.89 | |
| N3 (%) | 24.18 | 9.15 | |
| REM sleep (%) | 21.12 | 5.90 | |
| REM latency (min) | 80.57 | 37.64 | |
| Pre-Sleep LF/HF | 1.69 | 1.92 | |
| First NREM LF/HF | .81 | .76 | |
| First REM LF/HF | 1.53 | 1.72 | |
| Last NREM LF/HF | .8649 | .8664 | |
| Last REM LF/HF | 1.44 | 2.08 | |
Note. N = 94. PTSD = posttraumatic stress disorder. REM = rapid-eye-movement. Subthreshold PTSD participants met diagnostic criteria for two of the three PTSD symptom clusters.
Among 77 with trauma exposure
Measures
Polysomnography (PSG)
Two consecutive overnight PSG recordings in the Clinical Research Unit were completed with the first night being for apnea screening and lab accommodation. Participants who had Apnea Hypopnia Index greater than ten were excluded from the study. Recordings were collected using an Embla (Denver, CO) titanium portable unit and included bilateral frontal, central, and occipital leads, 2 electrooculograms, and chin electromyograms. Independent scoring required 90% concordance of epoch scoring for at least 3 records. Scorers visually scored sleep records on a computer monitor and designated the sleep stage of each 30-second epoch applying the American Academy of Sleep Medicine scoring rules (American Academy of Sleep Medicine, 2007). The REM logic scoring system (Embla) calculated standard PSG measures.
Heart rate variability
Electrocardiogram signals collected during the PSG recordings were used to assess frequency-domain HRV parameters. The sampling rate for the ECG was 250Hz. Electrocardiogram data were imported to LabChart Pro (ADinstruments, Colorado Springs, CO) and visually inspected on a computer monitor to confirm or correct identification of R-peaks. Segments with ectopic beats or artifacts that did not allow for R-peak identification were excluded. The Lomb periodogram (Clifford et al., 2005; Lomb, 1976) was performed to compute frequency domain HRV parameters including low frequency power (LF 0.04 – 0.15 Hz), high frequency power (HF, 0.15 – 0.4 Hz), and LF/HF ratios for each non-overlapping 5-minute epoch. For the purpose of this study HRV parameters were computed for the 5-minute period prior to sleep onset and the first and last REM and NREM sleep periods that lasted ≥ 5 minutes.
IL-6 and CRP
Blood samples were obtained from participants by venipuncture within 15 minutes of awakening in the morning after the second night PSG recording. After clotting, blood samples were centrifuged and serum was collected and stored in a freezer at −70°C. IL-6 and CRP levels were detected and quantified using enzyme-linked immunosorbent assays (ELISA) (Quantikine HS ELISA Human IL-6 Immunoassay kit, R&D Systems, Minneapolis, MN and High sensitivity C-reactive protein ELISA kit, Calbiotech, Spring Valley, CA), and a microplate spectrophotometer.
Data Analyses
All data were analyzed using SPSS 22.0 (IBM), and alpha = .05 (two tailed) was applied for all analyses. Data were checked for normality. Log transformations were performed on IL-6 and CRP levels and pre-sleep LF/HF, first and last NREM LF/HF, and first and last REM LF/HF. Pearson correlations were computed between IL-6, CRP, HRV parameters, sleep measures, and participant characteristics. Hierarchical regression analyses were conducted to examine whether HRV parameters that were significantly correlated with IL-6 or CRP continued to predict IL-6 or CRP after controlling for BMI, which was significantly correlated with the cytokine levels. Separate regression analyses were performed for each predictor.
Results
Correlation coefficients between cytokines, HRV parameters, sleep measures and participant characteristics are presented in Table 2. BMI was the only clinical and non-HRV measure that was significantly correlated with IL-6 and CRP. In addition to the absence of a relationship to PTSD severity, t-tests did not reveal differences between IL-6 and CRP as a function of PTSD diagnosis (PTSD = 25, No PTSD = 69; t (1, 92) = 0.953; p = .343; t (1, 92) = 1.403, p = .164. Normalized HF was also not correlated with IL-6 or CRP. IL-6 was significantly positively correlated with LF/HF during the pre-sleep, first NREM, and first REM periods. CRP was significantly positively correlated with first REM LF/HF. LF/HF during the last NREM and REM sleep periods were not correlated with either IL-6 or CRP. The hierarchical regression analyses showed after controlling for BMI, pre-sleep, first NREM and REM LF/HF continued to be significantly associated with morning IL-6 levels (β = .252, p = .019, ΔR2 =.063; β = .260, p = .010, ΔR2 = .067; β = .204, p = .044, ΔR2 =.040; respectively) while LF/HF during the first REM period was no longer significantly associated with CRP (β = .166, p = .098, ΔR2 = .027). SDRR was only correlated with IL-6 during the pre-sleep period. When we added SDRR to the regression model, it did not significantly account for additional variance beyond BMI.
Table 2.
Pearson correlation coefficients between demographic, sleep measures, IL-6 and CRP, sleep HRV parameters.
| IL-6 | CRP | |
|---|---|---|
| Gender | −.184 | −.142 |
| Body mass index | .287** | .319** |
| Age | .192 | .122 |
| CAPS | −.017 | −.015 |
| TST | −.012 | −.034 |
| REM (%) | −.027 | −.098 |
| N3 (%) | −.012 | −.060 |
| Pre-sleep LF/HF | .376** | .154 |
| First NREM LF/HF | .347** | .085 |
| Last NREM LF/HF | .142 | .149 |
| First REM LF/HF | .349** | .237* |
| Last REM LF/HF | .026 | .137 |
| Pre-sleep SDRR | −.258** | −.152 |
| First NREM SDRR | −.124 | −.119 |
| First REM SDRR | −.032 | −.069 |
| Last NREM SDRR | −.070 | −.175 |
| Last REM SDRR | −.122 | −.145 |
Note.
p < .05,
p < .01,
< .001. n varied between 75 and 94 due to missing data. Gender code: 0 = women, 1= men IL-6 = interleukin-6. CRP = C-reactive protein. LF/HF = low frequency to high frequency ratio. SDRR = Standard deviation of R-R interval. TST = Total sleep time.
Discussion
The present study was conducted to examine relationships between nocturnal autonomic nervous system activity during sleep, evaluated by HRV indices, and morning levels of the pro-inflammatory biomarkers IL-6 and CRP. Findings of this study show that LF/HF ratios from pre-sleep, first NREM and first REM, but not from the last NREM or REM sleep periods were significant predictors of IL-6 after controlling for BMI. The nature of these relationships is consistent with the half-lives reported for the respective cytokines, 2–4 hours for IL-6 and 20 hours for CRP (Marino et al., 2008). Thus effects of autonomic activity on IL-6 secretion during the first half of the sleep period would likely be reflected by morning levels, whereas effects on longer enduring CRP would not necessarily be evident the next morning. Our findings are consistent with the emerging research linking immune activation to SNS arousal (Marvar et al., 2011). Given the absence of a relationship to standard PSG measures in our study, autonomic arousal may be a mechanism underlying the influence of compromised sleep on immune activation. The possible influence of nocturnal autonomic activity influencing immune activation is also supported by Irwin et al.’s finding a relationship between nocturnal circulating norepinephrine and compromised immune function with insomnia (Irwin, et al., 2003). These findings suggest the possibility that decreasing sympathetic nervous system activity prior to sleep, could reduce immune activation. It would be of interest to determine if recent popular approaches from complimentary medicine such as mindfulness, as well as increased daytime physical activity, favorably influence this mechanism.
Study limitations include utilizing only a single blood drawing and measurement of two cytokines, albeit the two most consistently linked to sleep (Irwin et al., 2015). Sleep was recorded and samples were obtained in a clinical laboratory setting in which sleep and related processes can vary from naturalistic settings. Further, interpretation of HRV parameters used in this study needs to be done with caution as it has been suggested that LF power is also contributed by parasympathetic nervous system (Reyes del Paso et al., 2013). In addition to this, menstrual cycle was not controlled for as this may have had an impact on autonomic nervous system balance. The present study focused on young adult African Americans which may limit generalizability to other populations, however, the study population is of high significance as the participants are demographically at elevated risk for adverse cardiovascular outcomes and interventions could have preventive benefits during early adulthood.
The possibility suggested by our preliminary findings, that autonomic activity during sleep influences cytokine activity, requires confirmation by more comprehensive evaluations, as there is still much variance that is not explained. Prospective studies are needed in order to evaluate nocturnal autonomic nervous system activity as a modifiable contributor to atherogenesis. The significance of further establishing such relationships is underscored by elevated autonomic arousal prior to sleep onset and during the early hours of sleep being a potentially modifiable contributor to processes that accelerate atherogenesis and related cardiovascular dysfunction in populations at risk.
Figure 1. Scatterplot for pre-sleep LF/HF and IL-6.
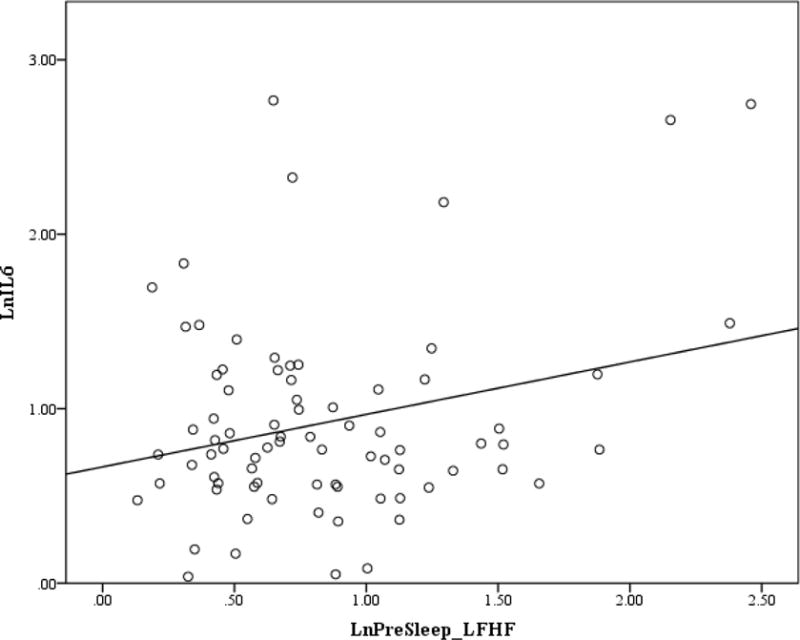
Note. Pre-sleep LF/HF and IL-6 were log transformed.
Figure 2. Scatterplot for first Non-REM LF/HF and IL-6.
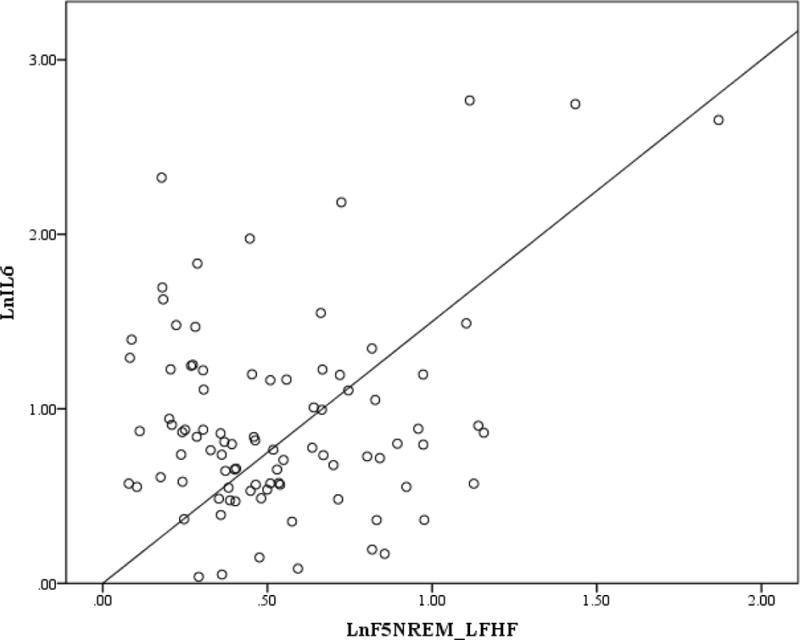
Note. First non-REM LF/HF and IL-6 were log transformed.
Figure 3. Scatterplot for First REM LF/HF and IL-6.
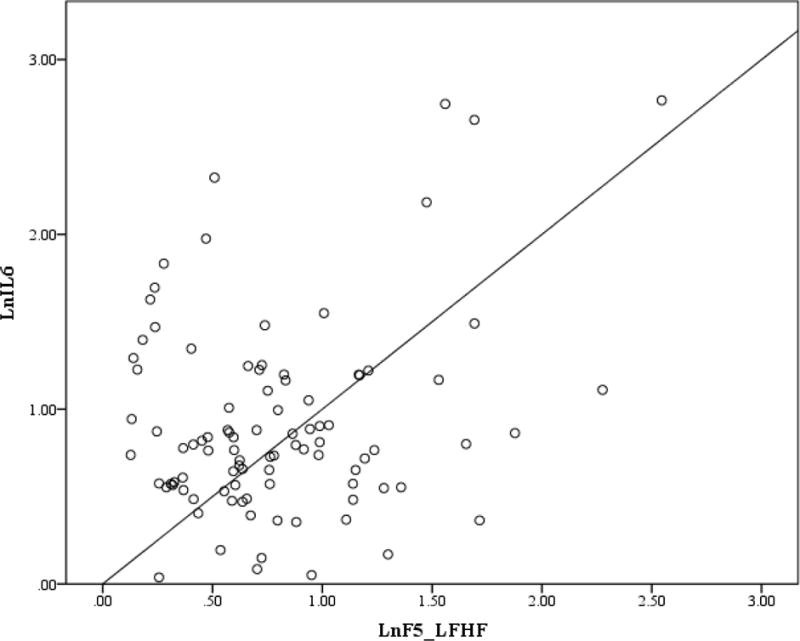
Note. First REM LF/HF and IL-6 were log transformed.
Acknowledgments
This research was supported by National Heart, Lung, and Blood Institute grant R01HL087995 to Thomas A. Mellman and National Center for Advancing Translational Sciences grant UL1RR031975 for the Georgetown-Howard Universities Center for Clinical and Translational Science. Kimberly A. Bell and Yuanxiu Chen do not have any conflicts of interest to disclose. Ihori Kobayashi and Thomas A. Mellman receive research funding from Merck & Co. Thomas A. Mellman also served as a consultant and a speaker for Merck & Co.
Contributor Information
Kimberly A. Bell, Department of Psychology, Howard University
Ihori Kobayashi, Department of Psychiatry and Behavioral Sciences, Howard University.
Yuanxiu Chen, Department of Community Health and Family Medicine, Howard University.
Thomas A. Mellman, Department of Psychiatry and Behavioral Sciences, Howard University
References
- American Academy of Sleep Medicine. AASM manual for the scoring of sleep and associated events. American Academy of Sleep Medicine; 2007. [Google Scholar]
- Ayas NT, White DP, Al-Delaimy, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–84. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arc Int Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann of Epidemiol. 2009;19:351–57. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Heart rate variability: Sleep stage, time of night, and arousal influences. Electroencephalogro Clin Neurophysiol. 1997;102:390–96. doi: 10.1016/S0921-884X(96)96070-1. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–15. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Penev PD, Schneider R, Van Cauter E. Estimating cardiac autonomic activity during sleep: Impedance cardiography, spectral analysis, and poincare plots. Clin Neurophys. 2004;115:19–28. doi: 10.1016/S1388-2457(03)00312-2. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. CDC health disparities and inequalities report — United States. 2011;2011(60) [Google Scholar]
- Cesari M, Penninx BWJH, Newman AB, et al. Inflammatory markers and cardiovascular disease (the health, aging and body composition [health ABC] study) Am J Cardiol. 2003;92:522–28. doi: 10.1016/s0002-9149(03)00718-5. doi: http://dx.doi.org/10.1016/S0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Clifford GD, Tarassenko L. Quantifying errors in spectral estimates of HRV due to beat replacement and resampling. IEEE Trans Biomed Eng. 2005;52:630–638. doi: 10.1109/TBME.2005.844028. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Kalesan B. Sleep duration and mortality: A systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Spyrou N, Rosen SD, et al. Low frequency spectral component of heart rate variability and myocardial beta-adrenoceptor density after acute myocardial infarction. Basic Res in Cardiol. 2002;97:97–104. doi: 10.1007/s395-002-8392-8. [DOI] [PubMed] [Google Scholar]
- Haarala A, Kähönen M, Eklund C, et al. Heart rate variability is independently associated with C-reactive protein but not with serum amyloid A. the cardiovascular risk in young Finns study. Eur J Clin Invest. 2011;41:951–57. doi: 10.1111/j.1365-2362.2011.02485.x. [DOI] [PubMed] [Google Scholar]
- Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–03. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.014. doi: http://dx.doi.org/10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed]
- Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav, and Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. doi: http://dx.doi.org/10.1016/S0889-1591(03)00031-X. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Lavela J, Mellman TA. Nocturnal autonomic balance and sleep in PTSD and resilience. J Trauma Stress. 2014;27:712–16. doi: 10.1002/jts.21973. [DOI] [PubMed] [Google Scholar]
- Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic nervous function in non-dipper essential hypertensive subjects: Evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26:808–14. doi: 10.1161/01.HYP.26.5.808. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arc Gen Psych. 2002;59:131–36. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Lomb NR. Least-squares frequency analysis of unequally spaced data. Astrophys Space Sci. 1976;39:447–62. [Google Scholar]
- Marino A, Giotta N. Cinacalcet, fetuin-A and interleukin-6. Nephrol Dial Transplant. 2008;23:1460–61. doi: 10.1093/ndt/gfm856. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin in Pharmacol. 2011;11:156–61. doi: 10.1016/j.coph.2010.12.001. doi: http://dx.doi.org/10.1016/j.coph.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Brown TSH, Kobayashi I, et al. Blood pressure dipping and urban stressors in young adult african americans. Ann of Behav Med. 2015;49:622–27. doi: 10.1007/s12160-014-9684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: Implications for hypertension. J Hypertens. 1997;15:1613–19. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Eng J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- Phillips RA, Sheinart KF, Godbold JH, Mahboob R, Tuhrim S. The association of blunted nocturnal blood pressure dip and stroke in a multiethnic population. Am J Hypertens. 2000;13:1250–55. doi: 10.1016/S0895-7061(00)01217-6. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–87. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]


