Abstract
Metastases remain the major cause of death from cancer. Recent molecular advances have highlighted the importance of metabolic alterations in cancer cells, including the Warburg effect that describes an increased glycolysis in cancer cells. However, how this altered metabolism contributes to tumour metastasis remains elusive. Here, we report that phosphorylation-induced activation of lactate dehydrogenase A (LDHA), an enzyme that catalyses the interconversion of pyruvate and lactate, promotes cancer cell invasion, anoikis resistance and tumour metastasis. We demonstrate that LDHA is phosphorylated at tyrosine 10 by upstream kinases, HER2 and Src. Targeting HER2 or Src attenuated LDH activity as well as invasive potential in head and neck cancer and breast cancer cells. Inhibition of LDH activity by small hairpin ribonucleic acid or expression of phospho-deficient LDHA Y10F sensitized the cancer cells to anoikis induction and resulted in attenuated cell invasion and elevated reactive oxygen species, whereas such phenotypes were reversed by its product lactate or antioxidant N-acetylcysteine, suggesting that Y10 phosphorylation-mediated LDHA activity promotes cancer cell invasion and anoikis resistance through redox homeostasis. In addition, LDHA knockdown or LDHA Y10F rescue expression in human cancer cells resulted in decreased tumour metastasis in xenograft mice. Furthermore, LDHA phosphorylation at Y10 positively correlated with progression of metastatic breast cancer in clinical patient tumour samples. Our findings demonstrate that LDHA phosphorylation and activation provide pro-invasive, anti-anoikis and pro-metastatic advantages to cancer cells, suggesting that Y10 phosphorylation of LDHA may represent a promising therapeutic target and a prognostic marker for metastatic human cancers.
INTRODUCTION
Metastasis is a multi-step cascading process that is tightly regulated by a group of cell signalling proteins and continues to cause more than 90% of human cancer deaths.1–7 Initiation of metastasis requires invasion, the process of cancer cells leaving the primary tumour and entering adjacent tissue. Anoikis describes the form of apoptotic cell death induced by detachment from the surrounding extracellular matrix.8 Since detachment changes occur during metastasis, metastatic tumour cells must be resistant to anoikis in order to disseminate. Breast cancer and head and neck cancer are common types of human cancers that frequently metastasize to distinct organs. Distant metastases to lung or bone usually represent incurable disease in breast cancer and squamous cell carcinoma of the head and neck (SCCHN). Therefore, new prognostic markers are needed to identify patients who are likely to develop metastases to improve prognosis and determine targets for therapy.9
Normal proliferating cells produce ATP through oxidative phosphorylation in the mitochondria. In contrast, cancer cells rely on aerobic glycolysis, in which cells take up and metabolize glucose more than normal tissue, but use less glucose for oxidative phosphorylation and favour glycolysis even in the presence of oxygen.10,11 However, the molecular mechanisms underlying this metabolic switch in cancer cells and how it contributes to tumour growth and tumour metastasis remain unknown. To explore how upregulated tyrosine kinase signalling regulates the Warburg effect in cancers, we performed a mass spectrometry-based proteomics study and identified a series of metabolic enzymes including lactate dehydrogenase A (LDHA), pyruvate kinase M2 isoform (PKM2), phosphoglycerate mutase 1 (PGAM1) and pyruvate dehydrogenase kinase (PDHK), which are tyrosine phosphorylated in cancer cells and provide metabolic advantages to tumour growth.12–15 Since it remains largely unclear whether tumour metabolism influences anoikis, invasion, and metastasis, we tested whether these metabolic enzymes are also important in tumour metastasis through a ‘mini-metabolomics’ study by investigating any positive correlation between their enzymatic activity and the invasive potential of cancer cells. We identified LDHA as a lead candidate, suggesting a potential functional role of LDHA in promoting the invasive and metastatic potential.
LDHA is an enzyme that catalyses conversion of pyruvate and NADH to lactate and NAD+ and plays a key role in regulating glycolysis.16 Cancer cells commonly have upregulated LDHA, which promotes a metabolic switch to aerobic glycolysis and generates lactate as a product. Several studies suggest the role of LDHA in tumour progression.12,17–22 We previously reported that tyrosine phosphorylation of LDHA promotes cancer cell metabolism and enhances growth of tumour by regulation of NADH/NAD+ redox homeostasis in leukemia cells and lung cancer cells harbouring dysregulated fibroblast growth factor receptor 1 (FGFR1).12 LDHA knockdown attenuates glycolysis and impacts mitochondrial physiology leading to severely decreased tumour growth in a breast cancer model.17 Downregulation of LDHA also suppresses tumour growth and metastasis of hepatocellular carcinoma cells and murine 4T1 breast tumour cells.23,24 Targeted downregulation of LDHA is known to induce reactive oxygen species (ROS) production and inhibit tumour progression, and these are partially reversed by the antioxidant N-acetylcysteine.25 Several LDHA inhibitors including gossypol and its derivative FX-11, galloflavin and N-hydroxyindole-based compounds are being tested for their anticancer activity.25–28 These findings provide evidence that LDHA plays a pivotal role in human cancers and therefore may be a promising target for the treatment of cancers.
Although previous studies reported that targeting LDHA attenuates tumour growth and tumour metastasis, the molecular mechanisms by which LDHA is regulated to provide pro-invasive and pro-metastatic signals in human cancer remain largely unknown. Here we report that human epidermal growth factor receptor 2 (HER2) and avian sarcoma viral oncogene v-src homolog (Src) mediated tyrosine phosphorylation of LDHA at tyrosine 10, which is commonly upregulated in metastatic tumours compared to primary tumours, activates LDHA and provides an anti-anoikis, pro-invasive and pro-metastatic potential to cancer cells.
RESULTS
Targeted downregulation of LDHA inhibits cancer cell invasion and sensitizes cells to anoikis induction
To demonstrate the role of LDHA in pro-invasive actions, we first assessed the impact of targeting LDHA on the invasive capacity of cell lines derived from breast cancer and SCCHN, which are among the cancer types that most frequently metastasize to the distant organs. Lentiviral small hairpin ribonucleic acid (shRNA) clones of LDHA were used to stably knockdown LDHA in diverse invasive human cancer cell lines (Figure 1a). Cell lines from breast cancers included metastasized human breast adenocarcinoma MDA-MB231 derived from a breast cancer patient’s pleural effusion and SCP-28, a bone metastasized subline of MDA-MB231 established from in vivo selection of xenograft mice.29 SCCHN cell lines included 212LN, derived from a lymph node of an SCCHN patient, and UM-SCC1 from a human SCCHN primary tumour.30 Cells harbouring empty vector, scrambled shRNA and shRNA against green fluorescent protein (GFP), an engineered gene not present in the human genome, were used as negative controls. Knockdown of LDHA resulted in marked reduction of cell invasion (Figure 1b). To minimize the effects of proliferation and viability when assessing the invasive potential, the invasion assays were performed over 6–12 h. The effect of LDHA knockdown on cell invasion was independent of proliferation or viability since no significant difference was found between the groups with respect to proliferation and cell death within 6–12 h (Supplementary Figures S1a and b). Targeting LDHA on cancer cell invasion was further confirmed by matrigel invasion assay with cellular proliferation inhibited by mitomycin C, matrix metalloproteinase activity assay, scratch wound assay and tumour spheroid invasion assay (Supplementary Figures S1c–f). These data suggest that LDHA promotes cancer cell invasion.
Figure 1.

Knockdown of LDHA in metastatic cancer cells leads to significant reduction of cell invasion and sensitizes cells to anoikis induction. (a) Immunoblotting shows shRNA-mediated knockdown of endogenous LDHA in diverse cells. (b) LDHA stable knockdown attenuates cancer cell invasion. In vitro matrigel invasion assay was performed within 6 or 12 h. (c) Knockdown of LDHA sensitizes metastatic cancer cells to detachment-induced anoikis compared to control cells harbouring empty vector, scramble shRNA or GFP shRNA. Cells were cultured on 1% agar-treated dishes for 48 h and detachment-induced apoptotic cell death was assessed by FITC- or PE-annexin V staining. Data are means ± s.d. from three technical replicates of each sample. P values were determined using two-tailed Student’s t test (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001).
Anoikis describes the apoptotic cell death induced by detachment from the surrounding extracellular matrix. Since detachment changes occur during metastasis, it is necessary for metastatic tumour cells to overcome anoikis to disseminate. We also found that RNA interference-mediated downregulation of LDHA sensitizes breast cancer and SCCHN cells to anoikis induction, compared to cells not harbouring LDHA shRNA (Figure 1c and Supplementary Figure S2). These results together suggest that LDHA promotes cell invasion and provides anti-anoikis protection in cancer cells.
Y10 phosphorylation and LDHA enzyme activity correlate with cell invasive ability in diverse human cancer cell lines
To better characterize the role of LDHA in cancer cell invasion and tumour metastasis, we tested the expression and phosphorylation of LDHA in diverse human breast cancer and SCCHN cells with different invasive ability. We found that LDHA protein levels were not altered among diverse breast cancer and SCCHN cells. However, Y10 phosphorylation levels of LDHA were increased in invasive breast cancer cell lines MDA-MB231, SCP-28, SKBR3 and BT474 cells, as well as in invasive SCCHN 212LN and UM-SCC1 cells, compared to less invasive breast cancer MCF-7 cells, and SCCHN Tu686 and Tu212 cells, respectively (Figures 2a and b, Supplementary Figures S3a and b). We previously demonstrated that Y10 phosphorylation activates LDHA enzyme activity in vitro.12 In consonance with this observation, we found that Y10 phosphorylation levels of LDHA correlate with increased LDHA enzymatic activity in these cells (Figure 2c). The invasive breast cancer and SCCHN cells were also more resistant to detachment-induced anoikis than less invasive cells (Figure 2d and Supplementary Figure S3c). LDHA Y10 phosphorylation positively correlated with anoikis resistance or invasive ability in these cells, suggesting that Y10 phosphorylation-mediated LDHA activation may be related to anoikis protection and cell invasive potential in relevant cancer cells (Figures 2e and f).
Figure 2.
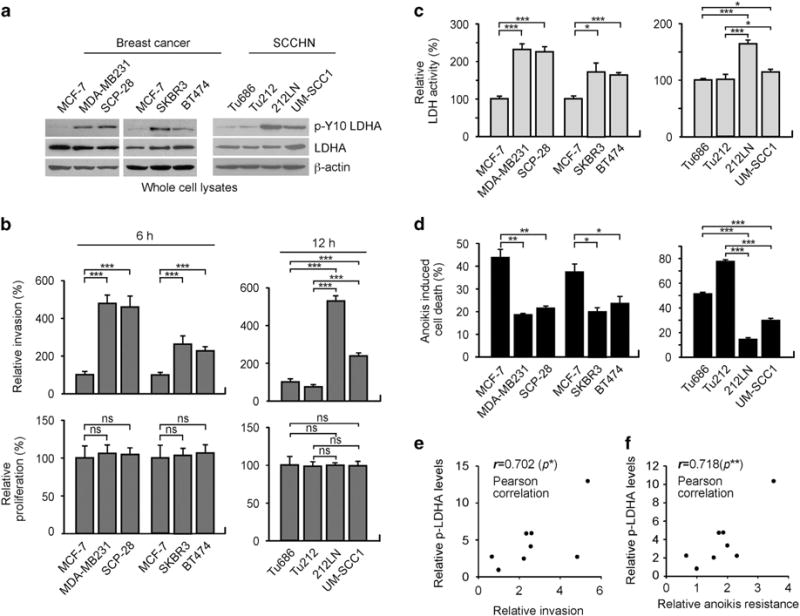
LDHA is phosphorylated at Y10 and activated in a group of highly invasive and anoikis resistant human cancer cell lines. (a) Expression and Y10 phosphorylation levels of LDHA in breast cancer cells (MCF-7, MDA-MB231, SCP-28, SKBR3, BT474) and SCCHN cells (Tu686, Tu212, 212LN, UM-SCC1) shown by western blot analyses. (b) In vitro matrigel invasion assay demonstrates different invasive ability at 6 or 12 h. Proliferation rates at the corresponding time points are shown at the bottom. (c) LDH activity was determined by measuring the oxidation of NADH. (d) Anoikis assay was performed by detecting detachment-induced apoptotic cells using annexin V staining. Cells were cultured on 1% agar-treated dishes for 48 h and detachment-induced cell death was determined by FITC- or PE-annexin V staining. Data are mean ± s.d. from three technical replicates of each sample. P values were determined using two-tailed Student’s t-test for (b–d). (e–f) Pearson correlation between LDHA Y10 phosphorylation and invasive potential (e) or anoikis resistance (f) in the breast cancer and SCCHN cell lines (ns: not significant, *0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001).
LDHA is phosphorylated and activated by HER2 and Src in breast cancer and SCCHN cells
To elucidate the molecular mechanism by which LDHA is phosphorylated and activated to confer pro-metastatic potential in cancer cells, we first examined the expression and activation levels of FGFR1 in cell lines of diverse cancer types including breast cancer, SCCHN and lung cancer, based on our previous finding that FGFR1 phosphorylates and activates LDHA. FGFR1 expression was not detectable in the cell lines tested except SCCHN 212LN and control lung cancer cell H1299 (Supplementary Figure S4a). Although inhibition of FGFR1 using TKI258 or FGFR1 shRNA attenuated LDHA phosphorylation and LDH activity in H1299 cells, invasive potential was not altered by FGFR1 inhibition suggesting that the FGFR1-LDHA axis is not a crucial factor that controls cell invasion in lung cancer H1299 cells (Supplementary Figures S4b and c). Moreover, inhibition of FGFR1 did not alter Y10 LDHA phosphorylation and activation levels or invasive potential in 212LN cells (Supplementary Figures S4d and e). This suggests that although FGFR1 is present in 212LN cells, it is not a predominant kinase that contributes to phosphorylation and activation of LDHA and consequently cancer cell invasion.
To explore upstream kinases that contribute to LDHA phosphorylation/activation in breast cancer and SCCHN cells, we performed in vitro kinase assays using tyrosine kinases, which are often dysregulated in breast cancer and SCCHN including HER2, epidermal growth factor receptor (EGFR) and their downstream effector Src. Purified recombinant proteins, LDHA wild type (WT) and phospho-deficient Y10F mutant were incubated with recombinant active HER2, Src or EGFR. We found HER2 and Src, but not EGFR, are upstream kinases that phosphorylate LDHA at Y10 (Figure 3a). We previously reported that phosphorylation of LDHA at Y10 enhances its activity by tetramer formation and at Y82 by enhancing cofactor NADH binding.12 Therefore, we tested whether phosphorylation of LDHA by HER2 or Src at Y10 enhances LDH activity by enhancing tetramer formation. Indeed, recombinant LDHA WT but not Y10F formed tetramer when phosphorylation was induced by recombinant active HER2 or Src (Figure 3b). We next assessed the effect of targeting HER or Src on Y10 LDHA phosphorylation, LDH activity, and cell invasion in breast cancer and SCCHN cells. Two HER2 positive breast cancer cell lines SKBR3 and BT474, and two HER2 negative cancer cell lines MDA-MB231 and UM-SCC1, which harbour activated Src were used to test the role of HER2 and Src in LDHA phosphorylation and cell invasion (Figure 3c). We explored the effect of targeting HER2 or Src on LDHA phosphorylation, LDH activity and cell invasion using specific inhibitors lapatinib or saracatinib, respectively (Supplementary Figures S4f and g). Treatment with the HER2 inhibitor lapatinib decreased phospho-Y10 LDHA level, LDH activity, and invasive potential in HER2 positive SKBR3 and BT474 cells (Figures 3d–f), while Src inhibitor saracatinib decreased LDHA phosphorylation and activation as well as invasive potential in HER2 negative MDA-MB231 and UM-SCC1 cells (Figure 3g–i). LDHA knockdown cells were resistant to lapatinib or saracatinib treatment in terms of cell invasion suggesting that HER2 or Src confers invasive potential by signalling through LDHA (Figure 3j and k). Similar results were obtained by genetically targeting HER2 or Src using specific shRNA (Supplementary Figures S4h–m).
Figure 3.
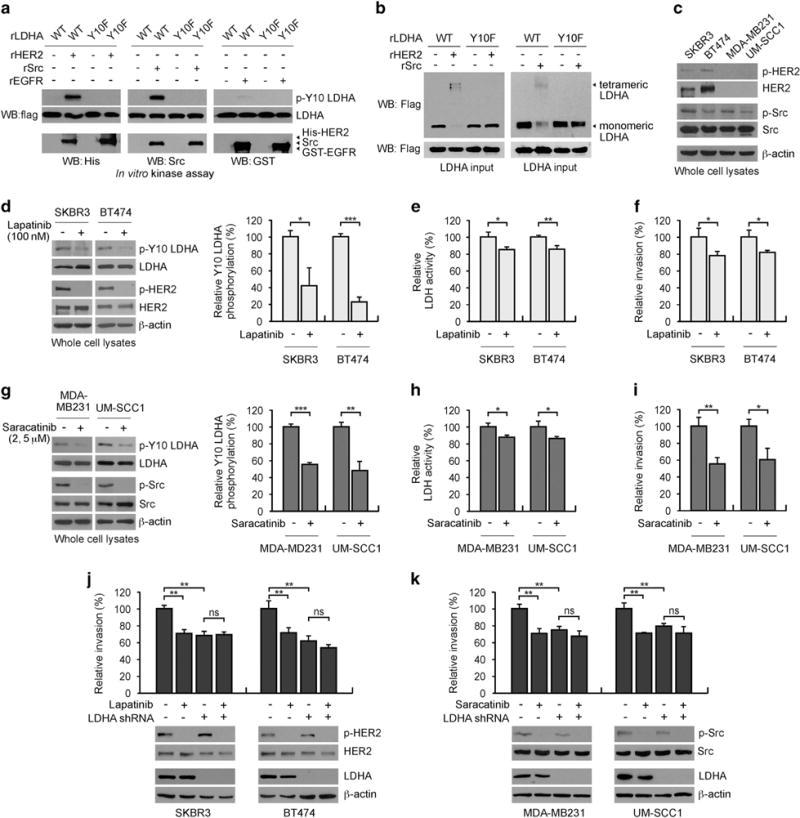
HER2 and Src directly phosphorylate LDHA at Y10 to activate LDHA and promote cancer cell invasion in breast cancer and SCCHN cells. (a) HER2 and Src, but not EGFR, directly phosphorylate LDHA at Y10. Purified recombinant LDHA (rLDHA) WT and Y10F were incubated with recombinant active HER2, Src or EGFR. Phosphorylation of LDHA at Y10 was detected by western blot using specific antibody against phospho-LDHA Y10. (b) HER2 or Src promotes tetramer formation of WT but not Y10F mutant of LDHA in vitro. rLDHA WT and Y10F proteins were incubated with recombinant active HER2 or Src in the in vitro kinase assay followed by crosslinking assay. (c) Expression and activation levels of HER2 and Src. HER2 and Src activation was assessed by phosphorylation levels at Y1248 HER2 and Y418 Src, respectively. (d–f) Inhibition of HER2 by lapatinib (100 nM) decreases LDHA Y10 phosphorylation (d), LDH activity (e), and cell invasion (f) in SKBR3 and BT474 cells. (g–i) Inhibition of Src by saracatinib (2 μM: MDA-MB231, 5 μM: UM-SCC1) decreases LDHA Y10 phosphorylation (g), LDH activity (h) and cell invasion (i) in MDA-MB231 and UM-SCC1 cells. (j–k) Cells with or without LDHA knockdown were treated with 100 nM Lapatinib (j) or 2 or 5 μM saracatinib (k) and invasion assay was performed for 24 h after mitomycin C treatment. Densitometry analyses of three independent biological replicates of phospho-Y10 LDHA blots are shown for panels (d) and (g). The error bars shown in all except (a–d) and (g) represent the mean values ± s.d. from three technical replicates of each sample. P values were determined using two-tailed Student’s t-test (ns: not significant, *0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001).
We further explored whether Src serves as a downstream target of HER2 to phosphorylate LDHA and contribute to cancer cell invasion. Both genetic and pharmacological inhibition of Src attenuated LDHA phosphorylation, LDH activity and cell invasion in HER2 positive cells, suggesting that Src also regulates LDHA in HER2 positive cells (Figures 4a–f). HER2 positive cells with Src knockdown were less sensitive to lapatinib treatment compared to cells expressing Src (Figure 4g–i). However, inhibition of both HER2 and Src further attenuated LDHA phosphorylation/activation and cell invasion, suggesting that although HER2 partially signals through Src to phosphorylate LDHA and contribute to cancer cell invasion, Src also mediates LDHA activation and promotes cancer cell invasion in HER2-independent manner. These data together suggest that HER2 and Src phosphorylate LDHA at Y10 leading to LDHA activation and cell invasion in breast cancer and SCCHN cells.
Figure 4.
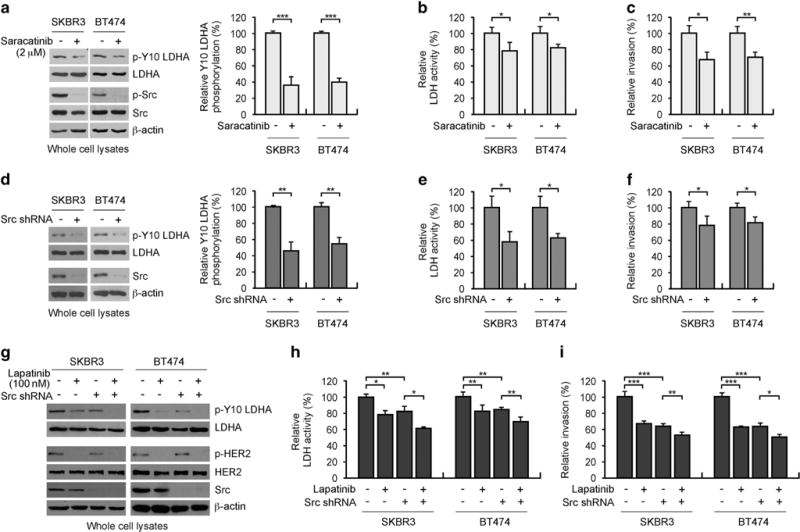
HER2 partially signals through Src to activate LDHA and promote cancer cell invasion in HER2 positive breast cancer cells. (a–c) Inhibition of Src by saracatinib (2 μM) decreases LDHA Y10 phosphorylation (a), LDH activity (b) and cell invasion (c) in SKBR3 and BT474 cells. (d–f) Stable knockdown of Src decreases LDHA Y10 phosphorylation (d), LDH activity (e) and cell invasion (f) in SKBR3 and BT474 cells. (g–i) Cells with or without Src knockdown (g) were treated with lapatinib (100 nM) followed by LDH activity assay (h) and invasion assay (i) after mitomycin C treatment. Densitometry analyses of three independent biological replicates of phospho-Y10 LDHA blots are shown for (a) and (d). The error bars shown in all except (a), (d) and (g) represent the mean values ± s.d. from three technical replicates of each sample. P values were determined using two-tailed Student’s t-test (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001).
The catalytically less active LDHA Y10F mutant expression leads to decreased cancer cell invasion, anoikis resistance
To elucidate the function of LDHA Y10 phosphorylation in cell invasion and tumour metastasis, we used invasive human breast cancer MDA-MB231 cells to stably knockdown endogenous human LDHA and rescue express flag-tagged shRNA-resistant form of human LDHA WT or phospho-deficient Y10F mutant (Figure 5a). Rescue expression of Y10F mutant showed decreased lactate level compared to LDHA WT cells (Figure 5b). Silencing LDHA using LDHA shRNA significantly attenuated the invasive ability of MDA-MB231 cells and sensitized cells to anoikis induction, whereas expression of WT LDHA, but not the Y10F mutant that is phosphorylation deficient at Y10, significantly rescued the decreased cell invasion induced by LDHA knockdown. This was measured by various assays including matrigel invasion assay in presence of mitomycin C, matrix metalloproteinase activity assay, scratch wound assay and spheroid invasion assay (Figure 5c and Supplementary Figures S5a–d). LDHA WT but not Y10F mutant rescued the increased anoikis sensitivity induced by LDHA knockdown (Figure 5d and Supplementary Figure S5e). To determine whether LDHA enzymatic activity mediated by Y10 phosphorylation is critical for cancer cell invasion and anoikis resistance, we tested whether restoration of decreased intracellular lactate, the product of LDHA, can reverse the attenuated cell invasion and increased anoikis sensitivity in LDHA knockdown cells or cells with LDHA Y10F expression. Indeed, lactate significantly rescued the decreased invasive potential (Figure 5e) and increased anoikis sensitivity in cells with LDHA knockdown or Y10F LDHA expression (Figure 5f and Supplementary Figure S5f). In support, we obtained similar results when the parental cancer line is incubated with culture media from the four cell lines expressing LDHA variants secreting different levels of lactate. Lactate level, cell invasion and anoikis resistance were decreased when MDA-MB231 cells were cultured in the media collected from LDHA knockdown cells or LDHA Y10F expressing cells, compared to media from parental or LDHA WT expressing cells (Figure 5g and Supplementary Figures S5g–h). Since LDHA is implicated in redox regulation, we examined whether LDHA phosphorylation controls intracellular ROS levels to promote cell invasion and anoikis resistance. Knockdown or rescue expression of LDHA Y10F resulted in intracellular ROS level increase, attenuated cell invasion and anoikis resistance, while antioxidant N-acetylcysteine treatment in these cells rescued the increased ROS (Figure 5h and Supplementary Figure S5i), reduced cell invasion (Figure 5i) and anoikis resistance (Figure 5j and Supplementary Figure S5j). These data suggest that LDHA phosphorylation at Y10 promotes its enzymatic activity and controls ROS levels, which enhances invasive and anoikis resistance potential in cancer cells.
Figure 5.
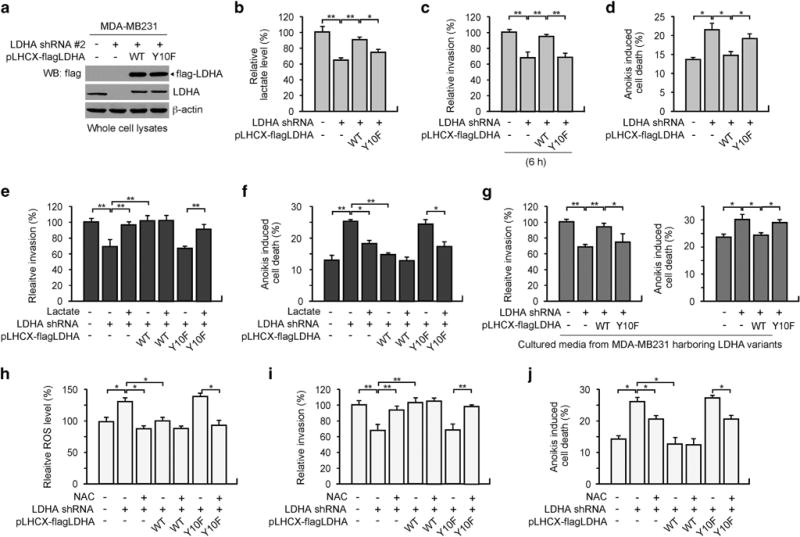
Expression of catalytically less active LDHA Y10F mutant leads to decreased cancer cell invasion and increased anoikis sensitivity. (a) MDA-MB231 cells with empty vector or stable LDHA knockdown using pLKO.1 LDHA shRNA #2 were stably transduced with retroviral vector harbouring pLHCX-flag-LDHA WT or Y10F. (b–d) Lactate level (b), invasion (c) and anoikis induction (d) were determined. Cell invasion and detachment-induced anoikis induction was tested by in vitro matrigel invasion assay and FITC-annexin V staining. (e–f) Cell invasion (e) and anoikis induction (f) were determined using cells with LDHA variants, in the presence or absence of 20 mM lactate. (g) Culture media from MDA-MB231 with LDHA variants were applied to parental MDA-MB231 for 48 h and cell invasion and anoikis induction were determined. (h–j) Intracellular ROS levels (h), cell invasion (i) and anoikis induction (j) were determined using LDHA stable cells in the presence or absence of 3 mM N-acetylcysteine. ROS levels were determined by H2DCFDA staining. All error bars shown in the figures represent the mean values ± s.d. from three technical replicates of each sample. P values were determined using two-tailed Student’s t-test (*0.01 < P < 0.05, **0.001 < P < 0.01).
Phosphorylation of LDHA at Y10 confers metastatic potential in vivo
We next tested whether LDHA phosphorylation at Y10 is needed for tumour metastasis using xenograft mouse model of experimental metastasis. MDA-MB231 cells with endogenous LDHA knockdown and LDHA WT or Y10F rescue expression were labelled with GFP and luciferase, and intravenously injected into nude mice, followed by bioluminescent imaging (Figure 6a and Supplementary Figure S6a). Mice inoculated with LDHA knockdown cells showed less metastasis when compared to the control group injected with cells harbouring empty vectors. Rescue expression of WT, but not Y10F mutant, rescued the LDHA knockdown-mediated metastatic potential decrease in the experimental xenograft nude mice (Figure 6b and Supplementary Figure S6b). These data together suggest that phosphorylation at tyrosine 10 of LDHA aids cancer cells to invade and metastasize.
Figure 6.
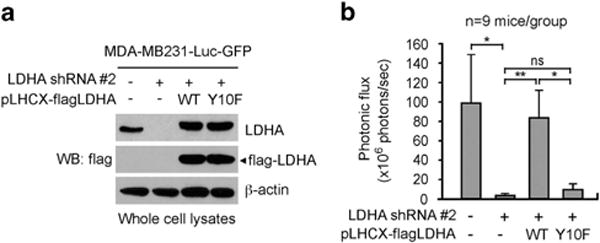
LDHA Y10 phosphorylation promotes tumour metastasis in vivo. (a) Rescue expression of LDHA WT or Y10F in MDA-MB231-luc-GFP cells with stable knockdown of endogenous LDHA used for tail vein injection. (b) Average photonic flux of each group is shown. Data represent means ± s.e.m. from nine mice for each group. P values were determined using one-tailed Student’s t-test (ns: not significant, *0.01 < P < 0.05, **0.001 < P < 0.01).
Phosphorylation levels of LDHA positively correlate with metastatic progression in clinical breast cancer patient tumour tissues
To demonstrate the clinical importance of the Y10 phosphorylation of LDHA in tumour metastasis, we tested whether LDHA phosphorylation positively correlates with metastatic progression in primary human tissues from breast cancer patients. We first evaluated the phospho-Y10 LDHA antibody using H1299 cancer cells expressing LDHA WT or Y10F with stable endogenous LDHA knockdown that were embedded in paraffin. Positive immunohistochemistry (IHC) staining of phospho-Y10 LDHA was observed in LDHA WT cells, but not in LDHA Y10F cells (Supplementary Figure S7a). In addition, we evaluated phospho-Y10 LDHA antibody by IHC using primary breast tumour tissues from cancer patients (Supplementary Figure S7b). Positive IHC staining was observed in tumour tissue stained with phospho-Y10 LDHA antibody, but not with control normal rabbit IgG. Tissue samples from 12 primary tumours and paired normal breast tissues as well as 36 primary breast tumour tissues with matched lymph node metastasized tumour tissues were used for IHC to detect phospho-Y10 LDHA and intensity of the staining was scored as 0, 1+, 2+ or 3 + (Figure 7a). The IHC studies demonstrate that the phospho-Y10 LDHA staining levels were higher in breast tumour tissues compared to the paired normal tissues of breast (Figure 7b). Moreover, the levels of LDHA Y10 phosphorylation correlated positively with progression of metastatic cancer (Figure 7c). These data implicate a functional relationship between LDHA phosphorylation and tumour metastasis in human breast cancer.
Figure 7.
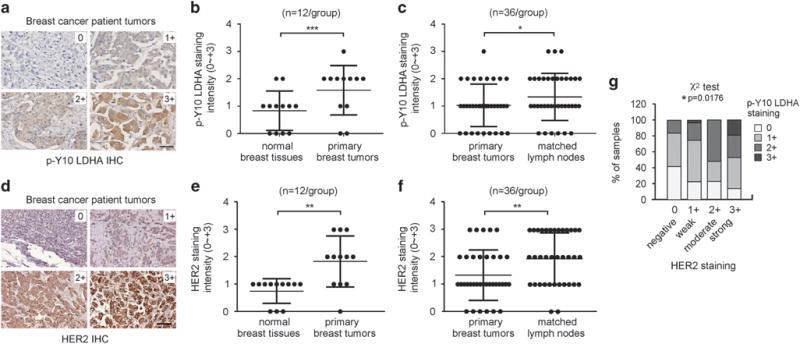
The level of phospho-Y10 LDHA correlates with metastatic cancer progression and HER2 expression in primary tumour tissues from breast cancer patients. (a) Representative tumour specimens with staining intensity of 0 (negative), 1+ (weak), 2+ (moderate) and 3+ (strong) of phospho-Y10 LDHA Y10 are shown. The representative tumour specimen images were obtained from patients with invasive/infiltrating ductal carcinoma. Scale bar represents 50 μm. (b–c) IHC analyses of phospho-Y10 LDHA levels in normal breast tissues and paired primary tumours (b), and in primary tumours and matched metastasized tumours from lymph nodes (c). (d) Representative tumour specimens with staining intensity of 0 ~ 3+ of HER2 are shown. Scale bar represents 50 μm. (e–f) Levels of HER2 expression in normal breast tissue and primary tumours (e), and primary tumour and matched tumours from lymph nodes (f). (g) The correlation between HER2 and phospho-Y10 LDHA was determined. P values were determined by two-tailed paired Student’s t-test for panels (b), (c), (e) and (f). Chi-square test was used for (g) (*0.01 < P < 0.05, **0.001 < P < 0.01, ***P < 0.001).
Consistent with LDHA Y10 phosphorylation, HER2 expression levels were higher in tumour tissues compared to normal breast tissues and higher in metastasized tumours than in primary tumours when the same set of primary tissue samples were assessed for HER2 IHC (Figure 7d–f). Furthermore, there was a significant positive correlation between levels of HER2 expression and LDHA Y10 phosphorylation (Figure 7g). This supports the functional coordination between HER2 and LDHA in breast tumour metastasis.
DISCUSSION
Our findings suggest that activation of LDHA by tyrosine phosphorylation provides anti-anoikis and pro-invasive/pro-metastatic signals in breast cancer and SCCHN cells. We demonstrate that targeting LDHA sensitizes cancer cells to anoikis induction and reduces the metastatic potential, while the WT LDHA, but not the phosphorylation deficient mutant Y10F LDHA expression, rescues the increased anoikis induction and attenuated invasive and metastatic potential induced by LDHA knockdown.
Our study for the first time demonstrates that Tyr10-phosphorylation is needed for LDHA activation to mediate resistance to anoikis, cell invasion and to form metastases in vivo. In addition, we are the first to identify LDHA as a signalling network downstream of HER2 and Src kinases. Interestingly, tyrosine kinases HER2 and Src, but not EGFR, directly phosphorylate LDHA at Y10. Approximately 90–95% of SCCHN patient tumours have a high level of EGFR expression. Around 3% of SCCHN patient tumours express HER2, whereas activation of Src has been commonly involved in epithelial–mesenchymal transition or erlotinib resistance of SCCHN.31–33 Moreover, one-fifth of breast cancer patient tumours have excessive HER2 expression, which means 80% of breast cancers do not possess dysregulated HER2. This suggests that in HER2 negative breast cancer and most cases of head and neck cancer, LDHA is not tyrosine phosphorylated by oncogenic receptor tyrosine kinases but that tyrosine phosphorylation of LDHA by kinase cascades involving cytoplasmic tyrosine kinases such as Src may mediate LDHA activation. We previously demonstrated that the LDHA Y10 phosphorylation is common in several types of human cancer cells, and the phosphorylation levels of LDHA Y10 directly correlate with activities of several oncogenic tyrosine kinases including FGFR1, JAK2, BCR/ABL and FLT3-ITD.12 Therefore, LDHA phosphorylation-mediated anti-anoikis and pro-invasive signals may not only be restricted to breast tumour and SCCHN metastasis, but may be commonly important for tumour metastasis of diverse human cancers.
It is intriguing to find that the increased anoikis sensitivity and attenuated cancer cell invasion upon LDHA knockdown or phosphorylation deprivation by phospho-deficient Y10F LDHA expression are fully rescued by expression of WT LDHA or its product, lactate. These data suggest that LDHA activity is required and it is the lactate that confers anoikis resistance and invasive potential in cancer cells. Previous work has linked the elevated lactate levels in tumour tissues with metastatic progression in human cancers.26,34,35 In addition, changes in lactate-induced signalling protein expression levels and their activation status have been studied. For instance, lactate is reported to stimulate VEGF production by endothelial cells, leading to enhanced migration.36 The TGF-β2 pathway has been implicated as a mediator of lactate-associated effects on cancer cell migration.37 Moreover, lactate is known to promote the production of hyaluronan by tumour-associated fibroblasts and provide an environment that promote the tumour cells to grow and migrate.38,39
Here we show that LDHA phosphorylation provides invasive signalling in metastatic cancer cells by regulating the redox status. As we reported, phosphorylation at Y10 activates LDHA and may generate NAD+ to sustain aerobic glycolysis.12 This may be required for cancer cells to confer pro-invasive and anti-anoikis potential. Further study is warranted to investigate the mechanisms through which lactate regulates potential downstream effectors and redox regulating enzymes to confer anoikis resistance and invasiveness in cancer cells.
Alternative post-translational modification has been implicated in LDHA regulation in human cancer. Acetylation at lysine 5 of LDHA is known to negatively regulate LDHA in pancreatic cancer.35 In addition, several additional lysine and serine/threonine residues of LDHA are acetylated and phosphorylated, respectively, in human cancer cells (www.phosphosite.org provided by Cell Signaling Technology Inc., Danvers, MA, USA). It would be interesting to further investigate any crosstalk between these modifications and enzymatic activity as well as tumour metastasis progression in human cancers.
LDHA has become an emerging anti-cancer target because of its critical role in cancer metabolism and tumour growth. Here, we evaluated a panel of breast tissue clinical specimens and showed that LDHA phosphorylation at Y10 is more abundant in breast tumour specimens compared to normal adjacent tissues. The phosphorylation status also positively correlates with metastatic tumour progression in breast cancer patients. We are the first to report that phosphorylation of LDHA contributes to tumour progression and to invasive and metastatic potential. Thus, phosphorylation at LDHA Y10 could be a promising biomarker to predict metastatic progression in human cancers. In addition, inhibitors of Src and HER2 are under clinical development for the treatment of several cancer types. Both small molecular HER2 inhibitor lapatinib and antibody-based HER2 inhibitor trastuzumab are FDA-approved for the treatment of HER2-positive breast cancer patients.40 Although Src inhibitors are being investigated in ongoing clinical trials of different phases in several cancer types including SCCHN and breast cancer, these inhibitors lack efficacy as single agents and most clinical trials are evaluating combination regimens.41,42
LDHA phosphorylation status may serve as a biomarker for assessing treatment response to Src and HER2 inhibitors. Importantly, blocking LDHA by LDHA shRNA or expression of catalytically less active LDHA can inhibit tumour growth, cell invasion and tumour metastasis. Thus, these data suggest that LDHA and its associated pathways are valuable therapeutic targets for human cancer with metastatic progression. Therefore, LDHA inhibitors such as FX-11, galloflavin and N-hydroxyindole-based agents could be useful to reduce both tumour growth and tumour metastasis in future clinical applications.
MATERIALS AND METHODS
Reagents
All pLKO.1-puro lentiviral vector-based shRNA constructs for LDHA knockdown were obtained from Dharmacon (Lafayette, CO, USA). The sense strands of the LDHA shRNA were clone #1 5′-GATCTGTGATTA AAGCAGTAA-3′ (TRCN0000026537) and clone #2 5′-AAGACATCATCC TTTATTCCG-3′ (TRCN0000158441) to target the 3′ untranslated region of human LDHA mRNA and the coding region of LDHA mRNA, respectively. The sense strand of the Src shRNA is 5′-TACAAAGCCTGGATACTGACA-3′ (TRCN0000039878). pLKO.1 empty vector (RHS4080), non-targeting scrambled shRNA (RHS6848) and eGFP shRNA (RHS4459) were used as controls. LDHA gene was cloned into the pET53 or pLHCX Gateway destination vectors for expression in bacterial cells or mammalian cells, respectively, as previously described.14,43 LDHA variants that are resistant to LDHA shRNA clone #2 were generated by silently mutating the LDHA shRNA #2 target sequence using primers 5′-TGTAAAATACAGCCCGAACTGT AAATTGCTTATTGTTTCAAATC-3′ and 5′-GATTTGAAACAATAAGCAATTTACA GTTCGGGCTGTATTTTACA-3′ and QuikChange-XL site-directed mutagenesis kit (200517, Agilent Technologies, Santa Clara, CA, USA). Breast cancer cell lines, MDA-MB231, MCF-7, BT474 and SKBR-3 were from the American Type Culture Collection. SCP-28 cells have been described as a bone metastasized MDA-MB231 subline derived from in vivo selection.29 MDA-MB231 cell line with luciferase and GFP was made from MDA-MB231 cells using pLNES-HSV1-tk/GFP-cmvFLuc.44,45 SCCHN cell lines, Tu686, Tu212, 212LN, UM-SCC1 have been described previously.30,46,47 Authentication of the cell lines was carried out using short tandem repeat analysis profiling by the RADIL CellCheck™ service. Recombinant active HER2 (PV3366), Src (P3044) and EGFR (PV4803) were from Invitrogen (Carlsbad, CA, USA). Lapatinib (L-4899) and saracatinib (S-3809) were purchased from LC laboratories (Woburn, MA, USA). Mitomycin C (S8146) were from Selleck Chemicals (Houston, TX, USA). All other reagents used in the study were obtained from Sigma Aldrich (St Louis, MO, USA).
Antibodies
Antibodies against LDHA (#3582/clone C4B5), phospho-LDHA Tyr 10 (#8176), phospho-HER2 Tyr 1248 (#2247), HER2 (#4290/clone D8F12), Src (#2108) and His-Tag (#2365) were obtained from Cell Signaling Technology. Anti-β-actin (#A1978/clone AC-15), anti-flag (#F7425) and anti-glutathione-S-transferase (#G1160/clone GST-2) antibodies were obtained from Sigma Aldrich. Phospho-Src Tyr 418 (#44-660G) antibody was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture
Breast cancer cell lines including MDA-MB231, MCF-7, SCP-28, SKBR3 and BT474 cells were maintained in Dulbecco modified Eagle medium with 10% FBS. All SCCHN cell lines (Tu686, Tu212, 212LN and UM-SCC1) were cultured in Dulbecco modified Eagle medium Ham F12 50:50 mix medium with 10% FBS. Cell lines with stable LDHA knockdown and rescue expression of LDHA variants were generated using lentiviral vector pLKO.1 and retroviral vector pLHCX as previously described.14
In vitro cell invasion assay, proliferation assay and anoikis assay
Cell invasion assays were performed as described with slight modifi-cations.46,48 In brief, 1 ~ 2 × 105 cells were loaded on matrigel coated chambers (354578, BD Biosciences, Bedford, MA, USA) and incubated for 6 h for breast cancer cells and 12 h for SCCHN cells. For cell invasion assays with mitomycin C, cells were treated with 10 μg/ml of mitomycin C for 2 h to inhibit proliferation prior to seeding and incubated on the matrigel coated chamber for 24 h. The invaded cells on the lower surface of the transwell membrane were stained with 25% methanol and 0.5% crystal violet and counted. Relative invasion was obtained by normalizing the invaded cell numbers of test groups to the invaded cell numbers of corresponding control groups such as cells without target shRNA or without drug treatment. Proliferation was determined by trypan blue exclusion at 6, 12, or 24 h. For anoikis assay, approximately 5 × 105 cells were cultured on six-well tissue culture plates coated with 1% agar for 48 h. The collected cells were stained with propidium iodide solution and FITC or PE-conjugated Annexin V (556547 or 559763, BD Pharmingen, San Jose, CA, USA) and analysed by FACS for apoptotic cell population.49
In vitro kinase assay and crosslinking assay
In vitro tyrosine kinase assays were performed according to the manufacturer’s protocol as described.14 Recombinant human LDHA variants were mixed with active recombinant HER2, Src or EGFR in kinase reaction buffers (HER2: 20 mM Tris (pH 7.5), 5 mM MnCl2, 0.5 mM Na3VO4, 1 mM EGTA, 2 mM DTT, 5 mM β-glycerophosphate, 0.01% CHAPS; Src and EGFR: 50 mM HEPES (pH 7.5), 10% glycerol, 10 mM MgCl2, 0.01% Triton X-100) at 30 °C for 30 min. The in vitro kinase assays were followed by 0.025% glutaraldehyde crosslinking and the samples were separated in non-denaturing gels by electrophoresis.13
LDH activity assay, lactate production assay and intracellular ROS measurement
LDH activity assay and lactate production assay were performed as described previously.12 Briefly, activity of LDH was determined by measuring the NADH oxidation in the reaction containing 20 mM HEPES (pH 7.2), 20 μM NADH, 0.05% bovine serum albumin, and 2 mM pyruvate using a microplate reader (excitation 340 nm/emission 460 nm). Lactate production in the culture medium was assessed by using Lactate Colorimetric/Fluorometric Assay Kit (K607-100, Biovision, Milpitas, CA, USA) according to the manufacturer’s instruction. Intracellular ROS levels were determined by carboxy-H2DCFDA staining (C6827, Invitrogen).
In vivo xenograft assay and bioluminescence imaging
Animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of Emory University. Athymic nu/nu, 4–6-week-old female mice (Envigo, Huntington, UK) were injected intravenously with 2.5 × 106 of MDA-MB231-luc-GFP cells with LDHA variants. Micrometastases were monitored by bioluminescence imaging as described.44,50 Briefly, mice were administered D-luciferin (75 mg/kg) intraperitoneally for the bioluminescence imaging (122799, Perkin Elmer, Waltham, MA, USA, 15 mg in 1 ml of sterile PBS). The images were taken using a Xenogen IVIS system (Perkin Elmer). For all animal studies, animals were randomly chosen. Concealed allocation and blinding of outcome assessment were used. No statistical method was used to predetermine sample sizes.
Immunohistochemical staining (IHC)
Human specimens usage approval was given by the Institutional Review Board of Emory University. Under Health Insurance Portability and Accountability Act (HIPAA) approved protocol, the clinical tissue samples were collected with informed consent. Primary breast tumour tissues with matched lymph node metastasized tumour tissues, BR243q and BRM961, were retrieved from US Biomax (Rockville, MD, USA). Analysis of IHC was performed as previously described.51 In brief, deparaffinized human tissue sections were rehydrated. Antigen retrieval was performed using 10 mM sodium citrate buffer (pH 6.0). Anti-phospho-Y10 LDHA antibody (1:200) and anti-HER2 antibody (1:500) from Cell Signaling Technology were used for IHC staining. No staining was scored as 0, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining.
Statistical analysis
Statistical analysis was performed using Prism 6.0 (GraphPad). No statistical analysis was used to predetermine size of sample. One representative experimental data set is shown from two or three independent experiments. Data with error bars show mean ± standard deviation (s.d.) from three technical replicates, except for Figure 6b, which represents mean ± standard error of the mean (s.e.m.). Statistical analysis was based on chi-square test for Figure 7g and Student’s t test for the remaining figures. Statistical tests performed are based on a set of assumptions including normal distribution and homogeneity of variances. The variability within each group has been quantified with standard deviation, and used for statistical comparison.
Supplementary Material
Acknowledgments
We thank Dr Anthea Hammond for editing the manuscript. We acknowledge the Winship shared resources facilities. This work was supported by NIH grants R01 CA175316 (Sumin Kang), F31 CA183365 (Gina N. Alesi), ACS grant RSG-11-08101 (Sumin Kang) and Pilot Grant-2015/Winship Cancer Institute, Emory University. Gina N. Alesi is an NIH pre-doctoral fellow. Sumin Kang is an American Cancer Society Basic Research Scholar, a Robbins Scholar, and a Georgia Cancer Coalition Scholar. Grant Support: This work was supported by NIH grants R01 CA175316 (Sumin Kang), F31 CA183365 (Gina N. Alesi), and ACS grant RSG-11-08101 (Sumin Kang).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
YK, DMS, ZGC and FRK provided critical reagents. KRM conducted histopathological analysis. JF performed crosslinking assay and LDH activity assay. LJ, JC, GNA and DL conducted the other experiments. SK and LJ were responsible for the study design and wrote the paper.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 5.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 10.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Hitosugi T, Chung TW, Xie J, Ge Q, Gu TL, et al. Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells. Mol Cell Biol. 2011;31:4938–4950. doi: 10.1128/MCB.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, et al. Tyrosine phosphorylation inhibits KM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–877. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitosugi T, Zhou L, Fan J, Elf S, Zhang L, Xie J, et al. Tyr26 phosphorylation of PGAM1 provides a metabolic advantage to tumours by stabilizing the active conformation. Nat Commun. 2013;4:1790. doi: 10.1038/ncomms2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bui T, Thompson CB. Cancer’s sweet tooth. Cancer Cell. 2006;9:419–420. doi: 10.1016/j.ccr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Gao S, Tu DN, Li H, Jiang JX, Cao X, You JB, et al. Pharmacological or genetic inhibition of LDHA reverses tumor progression of pediatric osteosarcoma. Biomed Pharmacother. 2016;81:388–393. doi: 10.1016/j.biopha.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zhu S, Tong J, Hao H, Yang J, Liu Z, et al. Suppression of lactate dehydrogenase A compromises tumor progression by downregulation of the Warburg effect in glioblastoma. Neuroreport. 2016;27:110–115. doi: 10.1097/WNR.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65:904–910. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 21.Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ, Xue J, et al. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 2015;36:8093–8100. doi: 10.1007/s13277-015-3540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, et al. Targeting lactate dehydrogenase-a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizwan A, Serganova I, Khanin R, Karabeber H, Ni X, Thakur S, et al. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin Cancer Res. 2013;19:5158–5169. doi: 10.1158/1078-0432.CCR-12-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–3910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 25.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granchi C, Roy S, Giacomelli C, Macchia M, Tuccinardi T, Martinelli A, et al. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem. 2011;54:1599–1612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Deck JA, Hunsaker LA, Deck LM, Royer RE, Goldberg E, et al. Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochem Pharmacol. 2001;62:81–89. doi: 10.1016/s0006-2952(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 29.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res. 2011;17:7248–7264. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birkeland AC, Yanik M, Tillman BN, Scott MV, Foltin SK, Mann JE, et al. Identification of targetable HER2 aberrations in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:559–567. doi: 10.1001/jamaoto.2016.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandal M, Myers JN, Lippman SM, Johnson FM, Williams MD, Rayala S, et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008;112:2088–2100. doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- 33.Stabile LP, He G, Lui VW, Thomas S, Henry C, Gubish CT, et al. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clin Cancer Res. 2013;19:380–392. doi: 10.1158/1078-0432.CCR-12-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. Ketones and lactate ‘fuel’ tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464–476. doi: 10.1016/j.ccr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckert S, Farrahi F, Aslam RS, Scheuenstuhl H, Konigsrainer A, Hussain MZ, et al. Lactate stimulates endothelial cell migration. Wound Reoaur Regen. 2006;14:321–324. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 37.Baumann F, Leukel P, Doerfelt A, Beier CP, Dettmer K, Oefner PJ, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro-oncology. 2009;11:368–380. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stern R. Hyaluronidases in cancer biology. Sem Cancer Biol. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14:267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 40.de Souza JA, Davis DW, Zhang Y, Khattri A, Seiwert TY, Aktolga S, et al. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:2336–2343. doi: 10.1158/1078-0432.CCR-11-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fury MG, Baxi S, Shen R, Kelly KW, Lipson BL, Carlson D, et al. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) Anticancer Res. 2011;31:249–253. [PMC free article] [PubMed] [Google Scholar]
- 42.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011;16:566–578. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang S, Dong S, Gu TL, Guo A, Cohen MS, Lonial S, et al. FGFR3 activates RSK2 to mediate hematopoietic transformation through tyrosine phosphorylation of RSK2 and activation of the MEK/ERK pathway. Cancer Cell. 2007;12:201–214. doi: 10.1016/j.ccr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponomarev V, Doubrovin M, Serganova I, Vider J, Shavrin A, Beresten T, et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur J Nucl Med Mol Imaging. 2004;31:740–751. doi: 10.1007/s00259-003-1441-5. [DOI] [PubMed] [Google Scholar]
- 46.Kang S, Elf S, Lythgoe K, Hitosugi T, Taunton J, Zhou W, et al. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J Clin Invest. 2010;120:1165–1177. doi: 10.1172/JCI40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Liu Y, Gilcrease MZ, Yuan XH, Clayman GL, Adler-Storthz K, et al. A lymph node metastatic mouse model reveals alterations of metastasis-related gene expression in metastatic human oral carcinoma sublines selected from a poorly metastatic parental cell line. Cancer. 2002;95:1663–1672. doi: 10.1002/cncr.10837. [DOI] [PubMed] [Google Scholar]
- 48.Li D, Jin L, Alesi GN, Kim YM, Fan J, Seo JH, et al. The prometastatic ribosomal S6 kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein fascin-1 to promote tumor metastasis. J Biol Chem. 2013;288:32528–32538. doi: 10.1074/jbc.M113.500561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin L, Li D, Lee JS, Elf S, Alesi GN, Fan J, et al. p90 RSK2 mediates antianoikis signals by both transcription-dependent and -independent mechanisms. Mol Cell Biol. 2013;33:2574–2585. doi: 10.1128/MCB.01677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alesi GN, Jin L, Li D, Magliocca KR, Kang Y, Chen ZG, et al. RSK2 signals through stathmin to promote microtubule dynamics and tumor metastasis. Oncogene. 2016;35:5412–5421. doi: 10.1038/onc.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257–270. doi: 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


