Abstract
The present study examines the interaction between a polygenic score and an elementary-school based universal preventive intervention trial and its effects on a discrete time survival analysis of time to first smoking marijuana. Research has suggested that initiation of substances is both genetically and environmentally driven (Rhee et al., 2003; Verweij et al., 2010). Previous work has found a significant interaction between the polygenic score and the same elementary-school based intervention with for tobacco smoking (Musci et al., in press). The polygenic score reflects the contribution of multiple genes and has been shown in prior research to be predictive of smoking cessation, tobacco use, and marijuana use (Uhl et al., 2014). Using data from a longitudinal preventive intervention study (N=678), we examined age of first marijuana use from 6th grade to age 18. Genetic data were collected during emerging adulthood and were genotyped using the Affymetrix 6.0 microarray (N=545). The polygenic score was computed using these data. Discrete time survival analysis was employed to test for intervention main and interaction effects with the polygenic score. We found main effect of the polygenic score approaching significance, with the participants with higher polygenic scores reporting their first smoking marijuana at an age significantly later than controls (p=.050). We also found a significant intervention × polygenic score interaction effect at p=.003, with participants at the higher end of the polygenic score benefiting the most from the intervention in terms of delayed age of first use. These results suggest that genetics may play an important role in the age of first use of marijuana and that differences in genetics may account for the differential effectiveness of classroom-based interventions in delaying substance use experimentation.
Increased commercialization of marijuana for medicinal and non-medicinal (i.e., ‘recreational’) purposes currently underway in the United States, Netherlands, Uruguay, and elsewhere is likely to increase the drug’s availability and opportunities to use, including among adolescents (MacCoun & Reuter, 1997; Pacula, 2010). Marijuana smoking initiated earlier in adolescence may pose a greater risk of disruption to brain and cognitive development, poorer educational and life outcomes, and development of problematic cannabis use (Volkow, 2014; Hall, 2015). Therefore, school-based interventions in childhood targeting possible antecedents of adolescent substance use, such as aggressive or coercive behaviors, are a potential means of preventing or delaying marijuana onset, and thus reducing the risk and added health care costs related to these later adverse outcomes. The effectiveness of these interventions could differ to the degree an individual’s genetic variability modifies direct or indirect pathways leading to the first cannabis experience. Our group previously found a gene by classroom intervention interaction on the timing of tobacco onset and in trajectories of tobacco and marijuana use in adolescence among a longitudinal sample of urban youth (Musci et al., 2015a; Musci et al., 2015b). In this study, we extend our investigation to possible gene by intervention modification in the timing of marijuana onset.
The Tailoring of Interventions Based on One’s Genome
There is a growing body of scientific literature on the personalization of treatments for physical diseases such as cancer based on one’s genome (see Guan et al., 2012). Although this work has received a great deal of attention in the print and electronic media, there is also a nascent scientific literature on the use of the genome in tailoring treatments for addiction to commonly used substances such as tobacco (e.g., Uhl et al., 2010a). Indeed, Uhl and colleagues (Uhl et al., 2010a; Uhl et al., 2010b) have reported in a series of the studies on the use of a polygenic score to predict cessation success among adult tobacco smokers enrolled in smoking cessation treatment trials. This polygenic score was derived from genome-wide association studies of the adult participants from 3 prior smoking cessation trials and was found to successfully predict quit success (Uhl et al., 2010a). The use of a genome-wide derived polygenic scores is consistent with the belief that complex human behavior is likely a product of a multiple genes working in concert (Duncan et al. 2014; Plomin et al., 2009) as illustrated in Musci et al. (2015a), wherein a polygenic score was used to predict the course of internalizing symptoms across the adolescence along with early environmental risk.
Although Uhl and colleagues’ work has largely focused on smoking cessation treatment, Uhl et al. (2014) demonstrated that their quit success score could be also used to predict the initial and continuing course of the frequency of use of commonly addictive substances (tobacco, marijuana, and alcohol) in the aggregate from adolescence through young adulthood. Moreover, in an extension of Uhl et al. (2014), Musci and colleagues (2015b) examined the relationship between Uhl et al. (2014)’s quit success score and tobacco and marijuana trajectories, separately, in adolescence. Moreover, Musci et al. (2015b) examined the interaction between the score and a parallel trajectory of environmental risk/protective factors (parent monitoring and friends’ tobacco and marijuana use, respectively). Significant interactions were found between the score and the risk/protective factors trajectory for both marijuana and tobacco, separately.
The Theoretical Basis for the Present Study
We rely on several theoretical frameworks for the present study. One of which, the differential susceptibility hypothesis/framework (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pleuss, 2009), is the guiding framework for our analysis of Gene by Environment interactions. This framework has an emphasis on the plasticity of response to positive and negative environmental variation as opposed to the diathesis-stress framework and its exclusive focus on risk in terms of the characteristics of the individual and the environment. More specifically, the diathesis framework is focused solely on the untoward outcome in the presence of both the diathesis (genetic/biological propensity) and the environmental risk. In contrast, consistent with the concept of “for better or worse” (Belsky et al., 2007; Belsky & Pleus, 2009), the differential susceptibility framework expands the focus to include plasticity in response to enriched as well as distressed environments.
Both Uhl et al. (2014) and Musci et al. (2015b) utilized data from a randomized trial of an early elementary school-based preventive intervention, whose proximal (1st grade) and distal targets (adolescence) included aggressive/coercive behaviors and substance use, respectively (Ialongo et al., 1999). The link between aggressive/coercive behaviors in childhood and adolescent substance use has both an empirical (Elkins et al., 2007; Kellam et al., 2008; 2014) and theoretical basis (Patterson, Reid, & Dishion, 1992). Regarding the latter, Patterson and colleagues theorize that aggressive/coercive behaviors in childhood result in rejection by teachers, parents, and mainstream peers, which subsequently precipitates drift into a deviant peer group, where a wide range of antisocial behavior is encouraged and reinforced including drug use. Prompted by the finding that the Uhl and colleagues’ quit success score interacted with environmental risk/protective factors in predicting the course of adolescent tobacco and marijuana use (Musci et al., 2015b) based on the data drawn from the Ialongo et al. (1999) prevention intervention trial, Musci et al (2015c) tested whether the score would also moderate the impact of the intervention on survival to first cigarette smoked. Musci et al., (2015a) did in fact find a significant intervention × quit success score interaction. More specifically, the effect of the intervention on age of first cigarette smoked was greatest among those 1 SD above the mean on the polygenic score.
The explanation we offered in Musci et al. (2015a) with regard to the relationship found between Uhl’s polygenic quit success score and age of first cigarette smoked drew on 4 lines of evidence. The first centered on nicotine’s role in activating the brain’s reward system (Corrigal, Coen, & Adamson, 1994; Laviolette & Van de Kooy, 2004; Tapper, Nashmi, & Lester, 2006). The second is the finding that prolonged exposure to cigarette smoke among non-smokers may increase the likelihood of inhaling secondary smoke and experiencing the reinforcing effects of nicotine (Okoli, Kelly and Hahn, 2007). The third is that the age of 1st cigarette smoked is associated with the number of one’s friends who smoke tobacco cigarettes (Wang et al., 2009; 2012). The fourth is Wang et al. (2012)’s finding that the effect of our early elementary classroom-based preventive intervention on age of first cigarette smoked was via its effect on delaying the reported age a cigarette was first offered by a peer. Synthesizing these 4 lines of evidence, we contended that the quit success score may be an index of one’s sensitivity to the reinforcing effects of substances and that that frequent and prolonged exposure to cigarette smoke within one’s peer group may increase the likelihood of inhaling secondary smoke and experiencing the reinforcing effects of a variety of substances. This, in turn, would then make it more likely that the non-smoker would accept an offer to try any substance from his/her friends. Finally, the effect of early elementary school interventions on age of first use might vary as a function of one’s sensitivity to reinforcing effects of nicotine within the context of interactions with friends who smoke cigarettes.
Consequently, we would expect the Uhl quit success score to influence age of first marijuana use, since first, like tobacco, marijuana activates the brain’s reward system (Covey, Wenzel, & Cheer, 2015; Vlachou & Panagis, 2014; Lupica, Riegel, & Hoffman, 2004). More specifically, it is thought that marijuana’s psychoactive constituent, Δ-THC, may activate CB1 cannabinoid receptors in the brain’s reward circuitry. Second, we found evidence that the quit success score was related to the course of early use of marijuana in adolescence (Musci et al., 2015b). Third, we also found evidence that the greater the number of friend’s who used marijuana, the greater the frequency of marijuana use over the course of adolescence (Musci et al., 2015b).
Overview of the Present Study
The above hypothesis with respect to the quit success score’s action mechanism and Musci et al. (2015b)’s finding of a score × environment interaction in terms of the course of marijuana use in adolescence formed the basis for the current paper. More specifically, using the Ialongo et al. (1999) preventive intervention trial data, we tested whether Uhl et al. (2010)’s quit success score would moderate the potential effect of the intervention on the age at which marijuana was first smoked. We hypothesized that like with tobacco, that a higher quit success score would be associated with greater intervention impact indexed as delaying the initial use of marijuana. We reasoned that the quit success score may be an indicator of sensation seeking and novelty preference, thereby influencing an individual’s propensity for substance use initiation.
Method
Participants
The primary data from this study come from a longitudinal randomized controlled trial (RCT) testing the impact of the Classroom-Centered (CC) Intervention and Family School Partnership (FSP) intervention relative to a control condition. A detailed description of the participants and design is provided elsewhere (Ialongo et al., 1999). Data collection began in 1993 with 678 first graders and their caregivers. The evaluation battery consisted of structured teacher, parent, and child interviews. A randomized block design was employed with schools serving as the blocking factor. Children and teachers were randomly assigned to classroom and then classrooms were randomly assigned to intervention condition with each of the three conditions being represented in each of the 9 participating schools. The interventions were provided over the first grade year only, following a pretest in the early fall.
Of the original 678 participants, 53.2% were male, 86.8% were African American, and 13.2% were Caucasian. Additionally, 63.4% of the participants qualified for free or reduced lunch, a proxy for low socioeconomic status (Ensminger et al., 2000). Over half of the African American sample qualified for free or reduced lunch (70.6%) and just over half of the Caucasian sample qualified for free or reduced lunch (53.8%). As for the racial breakdown by design, 188 African Americans were in the control condition, while 201 African Americans were in the CC intervention and 196 African Americans were in the FSP intervention conditions. Additionally, 31 Caucasians were in the control condition, and 29 Caucasian and 33 Caucasian participants were in the CC intervention and FSP intervention conditions, respectively. The participants ages at the start of first grade ranged from 5.3 years to 7.7 years (mean 6.2, SD ± .34). Assessments were carried out in the fall of grade 1, with annual follow-up assessments in the spring of grades 6 through 12. Genetic samples were collected shortly after high school. Written informed consent was obtained from each participant and the Institutional Review Board approved the study. For additional information on the design of the trial, see Bradshaw and colleagues (2009); Ialongo and colleagues (1999); or Ialongo and colleagues (2001).
Chi-square tests revealed no significant differences in terms of ethnicity (χ2 =4.974, p = .083, free or reduced lunch status (χ2=2.126, p = .163), and design status (χ2 =1.145, p = .766) between the 545 who provided both phenotypic and genetic data and those who did not. T-tests revealed no differences between these groups in terms of age at entrance to first grade. However, those included in this study were more likely to be female compared to those not included in this study (χ2= 7.473, p = .007).
Intervention
The Classroom-Centered (CC) intervention was designed to reduce the early risk behaviors of poor achievement and aggressive behavior through enhancements to the curriculum, improvements in teacher instructional and classroom behavior management practices, and specific strategies for children not performing adequately (Ialongo et al., 1999). Each intervention classroom was divided into three heterogeneous groups, which provided the underlying structure for the curricular and behavioral components of the intervention. Additionally, the intervention program enhanced the Baltimore City Public School curriculum in language arts and mathematics by adding material to increase critical thinking, composition and comprehension skills (Petras, Masyn, & Ialongo, 2011). The primary behavior management component was a behaviorally-focused classroom management program called the Good Behavior Game (GBG), which in previous trials demonstrated a beneficial impact on student behavior (Barrish, Saunders, & Wolf, 1969; Kellam et al., 2008). The GBG is a whole-class strategy that aims to decrease disruptive behaviors by assigning children to teams and only allowing the teams that do not exceed a specified criterion of precisely defined off-task, disruptive, and aggressive behaviors to “win”.
The Family-School Partnership (FSP) Intervention was designed to improve achievement and reduce early aggression and concentration problems by enhancing parent-teacher communication and providing parents’ with effective teaching and child behavior management strategies. The major mechanisms for achieving those aims were (1) training for teachers and other staff members in parent-teacher communication and partnership building, (2) weekly home-school learning and communication activities, and (3) a series of nine workshops for parents led by the first-grade teacher and the school psychologist or social worker. The Parents on Your Side program (Canter & Canter, 1991) was the basis for training teachers in partnership building and parent-teacher communication. The program included a three-day seminar with follow-up supervisory visits and an explicit training manual accompanied by videotape training. The Parents and Children series developed by Webster-Stratton (1984) formed the basis for the parenting workshops center around parent discipline and child behavior management.
Measures
Genotype scores
Using Affymetrix 6.0 genotype data that passed overall quality control metrics for each participant, we assessed alleles at the 12,058 SNPs that comprised the previously described v1.0 quit success score. The SNP names, chromosomal location, abstinence-associated allele and weight used in contributing to the total v1.0 score is detailed in Supplemental Information from Uhl and colleagues (2014). SNPs included in the score displayed nominally-significant association with ability to abstain in at least one of three smoking cessation success clinical trials that enrolled largely African- and European-American smokers whose allele frequencies, taken together, were similar to those of African and European-Americans in the current sample (Uhl et al., 2010; Rose et al., 2010). Greater weights were applied to alleles associated with quit success in multiple independent samples. We reasoned that, while genome-wide multiple tests result in false positive associations in single samples, fewer false positive associations would be identified in multiple samples. Genotype scores were included in the present analysis as a continuous variable. A higher score indicated a) greater success in smoking cessation as an interaction with nicotine replacement dose in one independent study in which dose was assigned randomly.
First Marijuana Use
Participants were asked yearly “Have you ever used marijuana?” The possible responses were “yes” or “no.” The earliest age when the participant answered “yes” to this question was used to indicate the age when marijuana use was initiated, varying from age 6 to age 16. Eighty participants had not initiated marijuana by age 18.
Population Stratification
When exploring genetic associations, population stratification, or genetic differences between subpopulations, is important to identify and control for so that any significant associations found is not due to ancestry. Previous studies have emphasized the importance of this control, particularly in admixed populations (e.g., African American; Montana & Pritchard, 2004; Sankararaman, Sridhar, Kimmel, & Halperin, 2008). The process through which we created the variables to control for population stratification was multidimensional scaling (MDS), completed in PLINK. This process extracts, from genome wide SNP data, clusters of individuals based on their estimated identity by descent. Subjects are assigned a score on each of these clusters representing their membership in a given population cluster. To reduce the computational intensity, we selected a set of one million SNPs randomly across the genome to test for the presence of stratification using the MDS approach. Although these were not a priori identified ancestry information markers, it has been shown that “randomly” selected SNPs perform equally well (Pritchard & Rosenberg, 1999). The results of the MDS allowed for the use of one factor, which accounted for a majority of the variance in population stratification.
Measure of Pre-Intervention Aggression
The Teacher Observation of Classroom Adaptation-Revised (TOCA-R) was used to assess the participants’ aggressive behaviors at baseline (i.e., fall of grade 1 prior to randomization; Werthamer-Larsson, Kellam & Wheeler, 1991). This measure included items such as, harms or hurts others physically, starts fights with classmates to assess aggression. Teachers rated student behavior on a six-point Likert scale from “almost never” to “always”. Previous research on the TOCA-R has demonstrated a high level of predictive validity (Petras et al., 2004; Petras et al., 2011). See Petras and colleagues (2011) for additional information on the reliability and validity of these measures.
Analysis
A discrete time survival analysis (DTSA) using Mplus Version 7.4 (Muthén & Muthén, 1998–2013) was performed to explore longitudinal risk of marijuana initial use (Muthén & Masyn, 2005). Discrete time survival analysis is a specific type of survival analysis that models the timing of events, specifically when events are measured in discrete-time or grouped-time intervals (Masyn, 2014). This model specification allows for the inclusion of time-varying and time-invariant predictors. The event of interest for this particular analysis is defined for each participant as marijuana use initiation, and the survival time is defined as the time elapsed from age to the first marijuana use. The time scale was recorded in discrete-time intervals – age – so although the time-to-event process may actually be more continuous in nature, the data limitations required the process be modeled using DTSA. Fall of first grade aggression and the population stratification variable were grand mean centered to ease in the interpretation of the interaction. In order to explore the moderation of one covariate effect by another, an interaction term is included as a predictor in the model. The interaction effect can be then decomposed by displaying hazard curves for differing levels of the covariates included in the interaction (Masyn, 2014). School/grade/section was included to account for cluster of students in classrooms. This technique, using the clustering command in Mplus, uses a sandwhich procedure to calculate robust standard errors (Muthén & Muthén, 1998–2012).
Missing Data
Using full information maximum likelihood estimation, Mplus (Muthén & Muthén, 1998–2012) assumes that the data were missing at random. This technique adjusts the estimates of the parameters for attrition. Full information maximum likelihood is considered the appropriate method for handing data missing at random (Muthén & Sheden, 1999; Shafer & Graham, 2002).
Results
Descriptive and Univariate Statistics
Proportions of first use at each age can be seen in Table 1. The proportion of individuals initiating began at only 1% of the population at the first time point. Subsequently, there was an increase in first use rates across the time period, peaking at age 13 and age 14, with around 20% of the remaining sample initiating at each age respectively. At the last time point, 15% of the remaining sample reported their first use. Mean scores for fall teacher rated aggression and the polygenic score can also be seen in Table 1.
Table 1.
Participant Characteristics
| Characteristics | Count | Proportion |
|---|---|---|
| African American | 585 | 86.3% |
| Male | 362 | 53.4% |
| Female | 316 | 46.6% |
| Classroom Intervention | 230 | 33.9% |
| Family-School Partnership | 229 | 33.8% |
| Control Group | 219 | 32.3% |
| Pre-Intervention Aggression Mean | 1.62 | |
| Polygenic Score Mean | 38.8* |
| First marijuana Use | Control Count | CC Count | FSP Count |
|---|---|---|---|
| Initiation at age 6–9 | 5 | 2 | 3 |
| Initiation at age 10 | 4 | 9 | 6 |
| Initiation at age 11 | 8 | 9 | 9 |
| Initiation at age 12 | 15 | 16 | 20 |
| Initiation at age 13 | 28 | 21 | 28 |
| Initiation at age 14 | 21 | 19 | 29 |
| Initiation at age 15 | 24 | 19 | 25 |
| Initiation at age 16 | 18 | 13 | 18 |
| Initiation at age 17 | 6 | 6 | 3 |
| Initiation at age 18 | 0 | 2 | 1 |
Indicates pre-standardization mean
Survival Analysis for Marijuana First Use
A discrete time survival analysis model was ran using Mplus Version 7.1 (Muthén and Muthén, 1998–2013). The proportional hazard assumption was not violated for the polygenic score (−2LL=7.42, df=9, p=.59), which suggest that the relationship between the polygenic score and first marijuana use remained constant across grades. Similarly, the proportional hazard assumption was not violated for the population stratification variable (−2LL=8.46, df=9, p=.49). Intervention was not a significant predictor of survival to first marijuana use (est.= −.191, S.E.=0.168, p=.257). The polygenic score was a marginally significant predictor of risk of first marijuana use (est.=−0.159, S.E.=.081, p=.050).
With respect to the covariates included in the model, the relationship between pre-intervention aggression levels and age of first use was not statistically significant (est.=.0.083, S.E.=.073, p=.256). Additionally, gender was not a significant predictor (est.=−0.134, S.E.=0.161, p=.404). The population stratification variable was a significant predictor of age of first marijuana use (est.=3.652, S.E.=1.473, p=.013). For regression estimates of all covariates, please see Table 2.
Table 2.
Model results for the final survival analysis model (LL=563.323, number of parameters = 16)
| Covariate | Est. | SE | p |
|---|---|---|---|
| CC Intervention | −0.191 | 0.168 | 0.257 |
| Polygenic Score | −0.159 | 0.081 | 0.050 |
| Gender | −0.134 | 0.161 | 0.404 |
| Aggression | 0.083 | 0.073 | 0.256 |
| Pop. Stratification | 3.652 | 1.473 | 0.013 |
| CC Intervention × Polygenic Score | −0.400 | 0.133 | 0.003 |
| Thresholds | Est. | SE | p |
| Age 6–9 | 4.897 | 0.661 | <.01 |
| Age 10 | 3.358 | 0.346 | <.01 |
| Age 11 | 3.023 | 0.211 | <.01 |
| Age 12 | 2.379 | 0.216 | <.01 |
| Age 13 | 1.637 | 0.156 | <.01 |
| Age 14 | 1.646 | 0.172 | <.01 |
| Age 15 | 1.445 | 0.204 | <.01 |
| Age 16 | 1.482 | 0.216 | <.01 |
| Age 17 | 2.377 | 0.337 | <.01 |
| Age 18 | 2.843 | 0.694 | <.01 |
In the moderation model, an interaction term was created to measure the interaction between the polygenic score and intervention. This interaction term was a significant predictor of risk of first marijuana use (est.=−.400, S.E.=0.133, p=.003), suggesting that those individuals who received the classroom-centered intervention and who had a high polygenic score had the lowest risk of marijuana use from age 6 to age 18 (see Figure 1). In the presence of an interaction, we took the following steps to decompose the interaction to further facilitate its interpretation: hazard probabilities were plotted for the control and intervention groups at the mean level of the polygenic score (est.=−.198, p=.236, 95% CI[−.472, .077]), one standard deviation above the mean of the polygenic score (est.= −.598, p=.003, 95% CI[−.924, −.272]), and one standard deviation below the mean of the polygenic score (est.=.202, p=.374, 95% CI[−.172, .577]). As can be seen in Figure 1, hazard probabilities were lowest across waves for individuals in the intervention condition and one standard deviation below the mean for the polygenic score.
Figure 1.
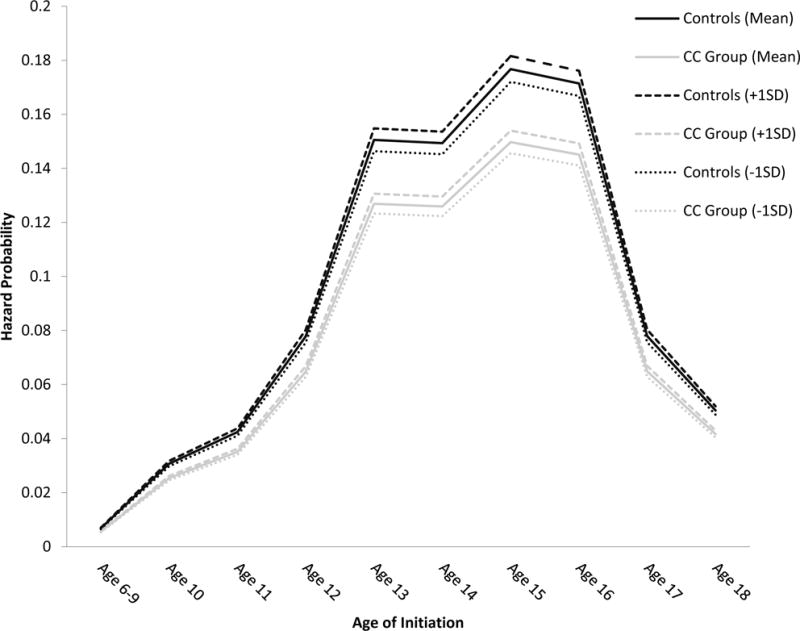
Hazard probability of marijuana initiation.
An identical model was run to explore the impact of the FSP intervention of age of marijuana initiation. With respect to covariates in the model, neither teacher reported aggression in the fall of first grade (est.=0.092, p=0.420) nor gender (est.=−0.164, p=0.378) were significant predictors of age of marijuana initiation. Similarly, the polygenic score was not a significant predictor (est.= −0.031, p=0.613). The FSP intervention was also not a significant predictor of age of marijuana initiation (est.=0.137, p=0.355). The interaction between the polygenic score and FSM was also non-significant (est.= −0.122, p=.249).
Discussion
The last two decades have witnessed a dramatic increase in the number of studies of the role of gene × environment interactions in the development and course of substance and mental disorders. More recently, prevention researchers have begun to extend the gene × environment interaction paradigm to include gene × intervention interactions. Brody and colleagues have been pioneers in this area (Brody et al., 2009; Beach et al., 2010; Brody, Chen et al., 2012). The promise of this line of research is two-fold. First, in line with the concept of precision medicine, the knowledge gleaned from gene × intervention interaction studies may serve to improve the efficiency in which limited prevention resources are allocated. More specifically, an individual’s genetic profile might be used to determine the extent they would benefit from a particular preventive intervention. A second promise that gene × intervention research holds out is the potential for informing theories of etiology of substance abuse and mental disorders as discussed below within the context of the findings of the present study.
Like in Musci et al (2016a), wherein the focus was on age of first tobacco use, we found evidence of enhanced effects of our Classroom-Centered intervention on age of 1st use of marijuana for those individuals at the upper end Uhl el al. (2010)’s smoking quit success score. The higher the score, the greater the delay in age of first use of marijuana. The significance of this enhanced effect is highlighted by the literature suggesting that the earlier the age of first use of most substances, the greater likelihood of escalating and prolonged use over development.
As noted in the introduction, our rationale for examining Uhl et al. (2010)’s quit success score in relation to age of first marijuana use was largely driven by our theory of the interplay between the CC intervention and Uhl et al.’s score in delaying onset of first tobacco use (Musci et al., 2015a). As we know from Haberstick, Zieger, et al. (2011), there are likely common and specific, or unique, genetic influences on substance use. In Musci et al. (2015a), we posited a unique genetic influence might best explain the gene × intervention interaction that we found with respect to age of first tobacco use. More specifically, we posit that the quit success score may serve in part as measure of shared genetic liability for substance use as well as the tendency to experience the reinforcing effects of nicotine and that the effect of the classroom-centered intervention on age of first cigarette smoked may have varied as a function of this genetic propensity. The fundamental nature of polygenic scores are such that they do not explicitly test specific biological mechanisms; but offer an explanation to the genetic liability of a particular behavior (Maher, 2015). One could posit that biological pathways related to novelty seeking and reward circuitry may play a large role in the common genetic liability of substances; with substance specific pathways also playing a role. The exact mechanism through which the polygenic score is impacting substance use behavior is unknown, and likely we would see associations between this polygenic score and a number of behaviors related to novelty or sensation seeking. Ultimately proof of our theory of the mechanism underlying the polygenic score × intervention interaction found in the present study and Musci et al. (2015a) will require a thorough investigation of the biological functions of each of the SNPs making up the quit success score.
Like in Wang et al. (2012), where no main effect of the family-school partnership intervention was found on age of first tobacco cigarette smoked, we did not find a main or interaction effect for the family-school partnership intervention in terms of first marijuana use. Consistent with the explanation we offered in Musci et al. (2015a), the effects of the family-school partnership intervention as reported in prior studies have been less broad and somewhat smaller in magnitude than the classroom-centered intervention effects. One potential explanation for this is the difference in the dose of training and mentoring received by teachers and parents. By design and necessity–given the universal nature of the intervention—parents were only offered 9, 2-hour training workshops focused on parenting, whereas teachers received 24 hours of training and coaching/mentoring in classroom behavior management and instruction throughout the school year. Moreover, only about a 1/3 of the parents attended more than half of the parent training workshops. We are aware of the substantial benefits reported in the literature with respect to family-based substance abuse preventive interventions. However, what is often overlooked in these reports is that the control group only includes those parents who were willing to participate in the intervention if assigned to it. In our study, parents only had to agree to their child being assessed and not to their participation in the interventions. As a consequence, the control group in our study includes a much larger proportion of the population denominator (that is, those who were eligible to participate) than what is seen in the typical family-based preventive intervention trials.
We suggested above that one of the promises of gene × intervention interaction studies was the potential for the use of genetic information to inform who would be best served by a particular preventive intervention. Although this may be the case in the context of selective or indicated preventive interventions, it is less applicable to universal preventive intervention trials like ours where the focus is on improving the family and school environment for all children. Moreover, genetic screening on a universal level would prove logistically and economically infeasible. It would also raise ethical issues as their potential for the use of one’s genetic profile to deny them intervention or treatment.
Further, in any analysis of gene by environment interactions, gene environment correlation is important to address. Previous research has found gene environment correlation in relation to substance use (Cleveland, Wiebe, & Rowe, 2005; Fowler et al., 2007; Rutter & Silberg, 2002), which occurs when genetics affect the likelihood of experiencing particular environmental factors. This becomes an issue when the environmental variable of interest is not randomly assigned. However, because intervention status, in this case, was randomly assigned, gene environment correlation is not an issue.
With respect to limitations and future directions, the present study’s sample small for studies of gene × intervention interactions, and thus we see relatively small effect sizes. Indeed, too small to study gender differences, despite well-documented gender differences in the amount and type of substance use. The study sample was also largely African-American and socioeconomically disadvantaged. Future studies will require considerably larger and more ethnically and economically diverse samples. This may be best achieved by multi-site studies.
Acknowledgments
This work used data from the Center for Prevention and Early Intervention at the Johns Hopkins Bloomberg School of Public Health. We are grateful for the collaboration of the Baltimore City Public Schools, teachers, parents, and students who participated in the study.
Funding
This research was supported by grants to Nicholas Ialongo from the National Institute of Mental Health (MH57005, T32 MH18834), and the National Institute on Drug Abuse (R37DA11796, R01DA036525).
Footnotes
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health approved the study.
References
- Barrish HH, Saunders M, Wolf MM. Good behavior game: effects of individual contingencies for group consequences on disruptive behavior in a classroom. Journal of Applied Behavior Analysis. 1969;2(2):119–124. doi: 10.1901/jaba.1969.2-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach S, Brody GH, Lei MK, Philbert R. Differential susceptibility to parenting among African American youths: testing the DRD4 hypothesis. Journal of Family Psychology. 2010;24:513–521. doi: 10.1037/a0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bradshaw CP, Zmuda JH, Kellam SG, Ialongo NS. Longitudinal impact of two universal preventive interventions in first grade on educational outcomes in high school. Journal of Educational Psychology. 2009;101(4):926. doi: 10.1037/a0016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Philibert RA, Chen Y-F, Murray VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene × environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Brody G, Chen Y, Yu T, Beach SRH, Kogan SM, Simons RL, Windle M, Philibert RA. Life stress, the dopamine receptor gene, and emerging adult drug use trajectories: a longitudinal, multilevel, mediated moderation analysis. Development & Psychopathology. 2012;24:941–951. doi: 10.1017/S0954579412000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter L, Canter M. Parents on your side: A comprehensive parent Involvement program for teachers. Santa Monica, CA: Lee Canter & Associates; 1991. [Google Scholar]
- Cleveland HH, Wiebe RP, Rowe DC. Sources of exposure to smoking and drinking friends among adolescents: a behavioral-genetic evaluation. The Journal of genetic psychology. 2005;166(2):153. [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Covey DP, Wenzel JM, Cheer JF. Cannabinoid modulation of drug reward and the implications of marijuana legalization. Brain Research. 2015;1628:233–243. doi: 10.1016/j.brainres.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Pollastri AR, Smoller JW. Mind the gap: why many geneticists and psychological scientists have discrepant views about gene-environment interaction (G X E) research. American Psychologist. 2014;69:249–268. doi: 10.1037/a0036320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono W. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Ensminger ME, Forrest CB, Riley AW, Kang M, Green BF, Starfield B. The validity of measures of socioeconomic status of adolescents. Journal of Adolescent Research. 2000;15:392–419. doi: 10.1177/0743558400153005. [DOI] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, Van Den Bree M. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102(3):413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan YF, Li GR, Wang RJ, Yi YT, Yang L, Jiang D, Peng Y. Application of next-generation sequencing in clinical oncology to advance personalized treatment of cancer. Chinese Journal of Cancer. 2012;31(10):463–470. doi: 10.5732/cjc.012.10216. http://doi.org/10.5732/cjc.012.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Zeiger JS, Corley RP, Hopfer CJ, Stallings MC, Rhee SH, et al. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011;106:215–224. doi: 10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Werthamer L, Kellam SG, Brown CH, Wang S, Lin Y. Proximal impact of two first-grade preventive interventions on the early risk behaviors for later substance abuse, depression, and antisocial behavior. American Journal of Community Psychology. 1999;27(5):599–641. doi: 10.1023/A:1022137920532. doi: [DOI] [PubMed] [Google Scholar]
- Ialongo N, Poduska J, Werthamer L, Kellam S. The distal impact of two first grade preventive interventions on conduct problems and disorder in early adolescence. Journal of Emotional and Behavioral Disorders. 2001;9:146–160. [Google Scholar]
- Kellam SG, Brown CH, Poduska JM, Ialongo N, Wang W, Toyinbo P, Petras H, Ford C, Windham A, Wilcox HC. Effects of a universal classroom behavior management program in first and second grades on young adult behavioral, psychiatric, and social outcomes. Drug Alcohol Dependance. 2008;95(Supplement 1):S5–S28. doi: 10.1016/j.drugalcdep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam SG, Wang W, Mackenzie AC, Brown CH, Ompad DC, Or F, Windham A. The impact of the Good Behavior Game, a universal classroom-based preventive intervention in first and second grades, on high-risk sexual behaviors and drug abuse and dependence disorders into young adulthood. Prevention Science. 2014;15(1):6–18. doi: 10.1007/s11121-012-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Van de Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behavior. Nature Reviews Neuroscience. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Lupica C, Riegel A, Hoffman A. Marijuana and cannabinoid regulation of brain reward circuits. British Journal of Pharmacology. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoun R, Reuter P. Interpreting Dutch cannabis policy: reasoning by analogy in the legalization debate. Science. 1997;278(5335):47–52. doi: 10.1126/science.278.5335.47. [DOI] [PubMed] [Google Scholar]
- Maher B. Polygenic scores in epidemiology: Risk prediction, etiology, and clinical utility. Current Epidemiological Reports. 2015;2:239–244. doi: 10.1007/s40471-015-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyn KE. Discrete-time survival analysis in prevention science. In: Sloboda Z, Petras H, editors. Defining Prevention Science. New York, NY: Springer Science + Business Media; 2014. pp. 513–535. Advances in Prevention Science. [DOI] [Google Scholar]
- Montana G, Pritchard JK. Statistical tests for admixture maping with case control and cases only. American Journal of Human Genetics. 2004;75:771–789. doi: 10.1086/425281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci R, Masyn K, Uhl G, Maher B, Kellam S, Ialongo N. Polygenic score × Intervention moderation: An application of discrete-time survival analysis to modeling the timing of first tobacco use among urban youth. Development & Psychopathology. 2015a;27:111–122. doi: 10.1017/S0954579414001333. [DOI] [PubMed] [Google Scholar]
- Musci R, Masyn K, Maher B, Benke K, Uhl G, Ialongo N. The effects of the interplay of genetics & early environmental risk on the course of internalizing symptoms from late childhood through adolescence. Development & Psychopathology. 2015b doi: 10.1017/S0954579415000401. [DOI] [PubMed] [Google Scholar]
- Musci R, Uhl G, Maher B, Ialongo N. Testing gene × environment moderation of tobacco and marijuana use trajectories in adolescence and young adulthood. Journal of Consulting & Clinical Psychology. 2015c doi: 10.1037/a0039537. [DOI] [PubMed] [Google Scholar]
- Muthen B, Asparouhov T. Growth mixture modeling: Analysis with non-Gaussian random effects. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Advances in Longitudinal Data Analysis. Chapman & Hall/CRC Press; Boca Raton, FL: 2008. pp. 143–165. [Google Scholar]
- Muthén B, Masyn K. Discrete-time survival mixture analysis. Journal Of Educational and Behavioral Statistics. 2005;30(1):27–58. [Google Scholar]
- Muthén B, Muthén L. Mplus users guide. Los Angeles: Author; 1998–2013. [Google Scholar]
- Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55(2):463–469. doi: 10.1111/j.0006-341X.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- National household survey on drug abuse: Population estimates 1999. SAMHSA, Office of Applied Studies, National Household Survey on Drug Abuse, 1999 CAI; 2001. [Google Scholar]
- Okoli CTC, Kelly T, Hahn EJ. Second hand smoke and nicotine exposure: a brief review. Addictive Behaviors. 2007;32:1977–1988. doi: 10.1016/j.addbeh.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Pacula RL. Examining the Impact of Marijuana Legalization on Marijuana Consumption 2010 [Google Scholar]
- Patterson GR, Reid J, Dishion T. A social learning approach: IV Antisocial boys. Eugene, OR: Castalia; 1992. [Google Scholar]
- Petras H, Schaeffer CM, Ialongo N, Hubbard S, Muthén B, Lambert SF, Kellam S. When the course of aggressive behavior in childhood does not predict antisocial outcomes in adolescence and young adulthood: An examination of potential explanatory variables. Development and Psychopathology. 2004;16(04):919–941. doi: 10.1017/s0954579404040076. [DOI] [PubMed] [Google Scholar]
- Petras H, Masyn K, Ialongo N. The developmental impact of two first grade preventive interventions on aggressive/disruptive behavior in childhood and adolescence: An application of latent transition growth mixture modeling. Prevention Science. 2011;12(3):300–313. doi: 10.1007/s11121-011-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Haworth C, Davis O. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. American Journal of Human Genetics. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of general psychiatry. 2003;60(12):1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm F, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: Interactions between nicotine dose, dependence and quit-success genotype score. Molecular Medicine. 2010;16(7–8):247–253. doi: 10.2119/molmed.2009.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Silberg J. Gene-environment interplay in relation to emotional and behavioral disturbance. Annual review of psychology. 2002;53(1):463–490. doi: 10.1146/annurev.psych.53.100901.135223. [DOI] [PubMed] [Google Scholar]
- Sankararaman S, Sridhar S, Kimmel G, Halperin E. Estimating local ancestry in admixed populations. American Journal of Human Genetics. 2008;82:290–303. doi: 10.1016/j.ajhg.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Tapper AR, Nashmi R, Lester HA. Neuronal nicotinic acetylcholine receptors and nicotine dependence. In: Madras BK, Colvis CM, Pollock JD, Rutter JL, Shurtleff D, von Zastrow M, editors. Cell Biology of Addiction. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 179–190. [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Ramoni M, Behm FM, Rose JE. Genome-wide association for smoking cessation success in a trial of precessation nicotine replacement. Molecular Medicine. 2010a;16(11–12):512–526. doi: 10.2119/molmed.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Walther D, David SP, Aveyard P, Murphy M, Johnstone EC, Munafo MR. Geomone-wide association for smoking cessation success: Participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics. 2010b;11(3):357–367. doi: 10.2217/pgs.09.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G, Walther D, Musci R, Fisher C, Anthony J, Storr C, Behm F, Eaton W, Ialongo N, Rose J. Smoking quit success genotype score v1.0 predicts quit success and distinct patterns of developmental involvement with common addictive substances. Molecular Psychiatry. 2014;19:50–54. doi: 10.1038/mp.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: a meta‐analysis of twin studies. Addiction. 2010;105(3):417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Panagis G. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Current Pharmaceutical Design. 2014;20:2072–2088. doi: 10.2174/13816128113199990433. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. New England Journal of Medicine. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Browne D, Petras H, Stuart E, Wagner F, Lambert S, Kellam S, Ialongo N. Depressed mood and the effect of two universal first grade preventive interventions on survival to the first tobacco cigarette smoked among urban youth. Drug and Alcohol Dependence. 2009;100:194–203. doi: 10.1016/j.drugalcdep.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Storr C, Green K, Zhu S, Stuart E, Lynne-Landsman Petras H, Kellam S, Ialongo N. The effect of two elementary school-based prevention interventions on being offered tobacco and the transition to smoking. Drug & Alcohol Dependence. 2012;120:202–208. doi: 10.1016/j.drugalcdep.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Stratton C. Randomized trial of two parent-training programs for families with conduct disordered children. Journal of Consulting and Clinical Psychology. 1984;52:666–678. doi: 10.1037//0022-006x.52.4.666. [DOI] [PubMed] [Google Scholar]
- Werthamer-Larsson L, Kellam S, Wheeler L. Effect of first-grade classroom environment on shy behavior, aggressive behavior, and concentration problems. American journal of community psychology. 1991;19(4):585–602. doi: 10.1007/BF00937993. [DOI] [PubMed] [Google Scholar]


