Abstract
Elevated keratinocyte carcinoma (KC) risk is present with several immune-related conditions, e.g., solid organ transplantation and non-Hodgkin lymphoma. Because many immune-related conditions are rare, their relationships with KC have not been studied. We used Medicare claims to identify cutaneous squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) cases in 2012, and controls matched on sex and age. All subjects were aged 65–95 years, of white race, and had attended ≥1 dermatologist visit in 2010–2011. Immune-related conditions were identified during 1999–2011 using Medicare claims. Associations were estimated with logistic regression, with statistical significance determined after Bonferroni correction for multiple comparisons. We included 258,683 SCC and 304,903 BCC cases. Of 47 immune-related conditions, 21 and 9 were associated with increased SCC and BCC risk, respectively. We identified strongly elevated KC risk with solid organ transplantation (SCC odds ratio [OR]=5.35; BCC OR=1.94) and non-Hodgkin lymphoma (SCC OR=1.62; BCC OR=1.25). We identified associations with common conditions, e.g., rheumatoid arthritis (SCC OR=1.06, 95% confidence interval [95%CI]=1.04–1.09) and Crohn’s disease (SCC OR=1.33, 95%CI=1.27–1.39; BCC OR=1.10, 95%CI=1.05–1.15), and rare or poorly characterized conditions, e.g., granulomatosis with polyangiitis (SCC OR=1.88, 95%CI=1.61–2.19), autoimmune hepatitis (SCC OR=1.81, 95%CI=1.52–2.16), and deficiency of humoral immunity (SCC OR=1.51, 95%CI=1.41–1.61; BCC OR=1.22, 95%CI=1.14–1.31). Most conditions were more positively associated with SCC than BCC. Associations were generally consistent regardless of prior KC history. Many immune-related conditions are associated with elevated KC risk, and appear more tightly linked to SCC. Immunosuppression or immunosuppressive treatment may increase KC risk, particularly SCC.
Keywords: keratinocyte cancer, cutaneous squamous cell carcinoma, basal cell carcinoma, immunosuppression, autoimmune disease
Introduction
Approximately 5.4 million keratinocyte carcinomas (KCs, also known as non-melanoma skin cancers) are diagnosed in the United States annually (1). Squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) are the primary KC types, and risk for both increases with ultraviolet radiation (UVR) exposure, fair skin pigmentation, and older age (2). Risk is also increased among people with certain medical conditions affecting the immune system. Most notably, solid organ transplant recipients have markedly increased risk of cutaneous SCC and BCC compared to the general population (3–5). Elevated risk of cutaneous SCC and BCC has also been identified in human immunodeficiency virus (HIV)-infected people and non-Hodgkin lymphoma patients (6, 7), and there is evidence of high skin cancer risk among bone marrow transplant recipients (8).
Increased risk may result from effects of immune-related disease processes or treatments (e.g., immunosuppressive or immunomodulatory medications). Among transplant recipients, elevated KC risk may be driven by specific immunosuppressant medications, with particularly high risk observed among people taking azathioprine or cyclosporine (9–11). Immunomodulatory therapy has also been linked to KC incidence among people with rheumatoid arthritis or inflammatory bowel disease, two common autoimmune conditions (12).
Many other rare immune-related conditions have not been studied, but further research could provide insights into the relationship between immune function and KC risk. Clinical populations with substantially elevated risk may benefit from screening, as currently suggested in guidelines for transplant recipients (13–15). In the current study, we utilize Medicare claims data to assess associations between a wide spectrum of immune-related conditions and incidence of cutaneous SCC and BCC in the U.S. elderly population.
Materials and Methods
We conducted a case-control study among people aged 65–95 years in the U.S. Medicare population in 2012. Medicare is the U.S. federal health insurance program for people age 65 and older, and Medicare claims provide information on clinical visits, medical diagnoses, and procedures. We limited the population to non-Hispanic whites, as only small proportions of KCs were diagnosed outside this group. The first year during which International Classification of Diseases (version 9, ICD-9) diagnosis codes differentiated between SCC and BCC was 2012 (16). We included people with non-health maintenance organization (HMO) Medicare coverage (because claims for individual diagnoses and procedures are not submitted by HMOs) that began before 2012 and ended after January 1, 2012, and who had at least 1 Medicare claim prior to 2012. Use of Medicare claims for this study was exempt from institutional review board review.
For our study, we identified Medicare patients with SCC or BCC in 2012, based on a physician claim with both an ICD-9 diagnosis code of 173.x2 (for SCC cases) or 173.x1 (for BCC cases) and a Healthcare Common Procedure Coding System (HCPCS) code indicating a skin cancer treatment: 11600–11606, 11620–11626, 11640–11646, 17260–17266, 17270–17276, 17280–17286, 17304, 17311, 17313, as described previously (1). For people with multiple diagnoses in 2012, only the first diagnosis was included. If a person was diagnosed with BCC and SCC on the same day, one diagnosis was chosen at random to categorize the case. Controls were selected from people enrolled in Medicare without SCC or BCC diagnoses in 2012. Controls were selected with a 10:1 ratio, frequency matched to cases on sex and age (in 5-year categories).
For cases and controls, we used Medicare claims during 1/1/1999–12/31/2011 to identify 47 different immune-related conditions, including 3 primary immunodeficiency conditions, 3 transplant-related conditions, 32 autoimmune diseases, 6 hematologic malignancies and related conditions, and 3 allergic diseases (listed in Table 1). The included conditions were identified based on review of prior articles that assessed numerous immune-related conditions (17, 18) and through consultation with physician experts. The prevalence of the immune-related conditions ranged widely, from allergic rhinitis (diagnosed in 18.1% of initially selected controls, Table 1) and rheumatoid arthritis (3.9%) to Behçet’s disease (<0.1%) and deficiency of cell-mediated immunity (<0.1%). Ten conditions that are not immune-related and lack known associations with skin cancer (‘control conditions’) were also assessed for comparison. Each condition was identified based on the presence of one hospital claim, or two outpatient/provider claims more than 30 days apart (codes listed in Table 1). Procedure and diagnosis-related group codes were used in addition to diagnosis codes to identify solid organ transplantation and bone marrow transplantation.
Table 1.
International Classification of Diseases, version 9 (ICD-9) codes used to identify immune-related and control conditions and frequency of conditions in source population for controls
| ICD-9 codes | N(%) in control source population* | |
|---|---|---|
| Immunosuppressive conditions | ||
| Deficiency of humoral immunity | 279.0 | 20,256 (0.2) |
| HIV infection | V08, 42.X-44.X | 5,464 (0.1) |
| Deficiency of cell-mediated immunity | 279.1 | 749 (<0.1) |
| Transplant conditions | ||
| Solid organ transplant | V42.0-1, V42.6-7, V42.83-84, 996.81-84, 996.86-87 | 16,444 (0.2) |
| Bone marrow transplant | V42.81-V42.82, 996.85, 279.5 | 4,866 (<0.1) |
| Graft vs. host disease | 279.5 | 436 (<0.1) |
| Autoimmune conditions | ||
| Rheumatoid arthritis | 714.0 | 414,564 (3.9) |
| Pernicious anemia | 281.0 | 320,960 (3.0) |
| Psoriasis | 696.0, 696.1 | 258,266 (2.4) |
| Polymyalgia rheumatica | 725.X | 200,019 (1.9) |
| Ulcerative colitis | 556.X | 104,833 (1.0) |
| Crohn’s disease | 555.X | 61,599 (0.6) |
| Graves’ disease | 242.0 | 48,866 (0.5) |
| Scleroderma | 701.0, 710.1 | 51,178 (0.5) |
| Giant cell arteritis | 446.5 | 48,866 (0.5) |
| Hashimoto’s thyroiditis | 245.2 | 47,529 (0.4) |
| Sjögren’s syndrome | 710.2 | 47,529 (0.4) |
| Systemic lupus erythematosus | 710.0 | 45,675 (0.4) |
| Addison’s disease | 255.41 | 25,810 (0.2) |
| Uveitis | 364.3 | 24,550 (0.2) |
| Celiac disease | 579.0 | 24,716 (0.2) |
| Immune thrombocytopenic purpura | 287.31 | 24,716 (0.2) |
| Multiple sclerosis | 340.X | 25,202 (0.2) |
| Myasthenia gravis | 358.0 | 22,018 (0.2) |
| Sarcoidosis | 135.X | 19,481 (0.2) |
| Ankylosing spondylitis | 720.0 | 18,455 (0.2) |
| Scleritis | 379.0 | 18,057 (0.2) |
| Discoid lupus erythematosus | 695.4 | 16,422 (0.2) |
| Vitiligo | 374.53, 709.01 | 14,850 (0.1) |
| Polymyositis/dermatomyositis | 710.3, 710.4 | 13,278 (0.1) |
| Guillain-Barré syndrome | 357.0 | 11,946 (0.1) |
| Autoimmune hemolytic anemia | 283.0 | 8,635 (0.1) |
| Primary biliary cirrhosis | 571.6 | 7,734 (0.1) |
| Granulomatosis with polyangiitis | 446.4 | 4,478 (<0.1) |
| Autoimmune hepatitis | 571.42 | 3,450 (<0.1) |
| Polyarteritis nodosa | 446.0 | 2,886 (<0.1) |
| Reactive arthritis | 99.3 | 1,534 (<0.1) |
| Behçet’s disease | 136.1 | 375 (<0.1) |
| Hematologic malignancies and related conditions | ||
| Non-Hodgkin lymphoma | 200.X, 202.X, 204.1 | 171,963 (1.6) |
| Aplastic anemia | 284.1, 284.8, 284.9 | 120,871 (1.1) |
| Paraproteinemias/related disorders | 273.0–273.3 | 89,029 (0.8) |
| Multiple myeloma | 203.0, 203.1 | 35,022 (0.3) |
| Leukemia | 204.0, 204.2, 204.8, 205.X-207.X | 22,537 (0.2) |
| Hodgkin lymphoma | 201.X | 12,789 (0.1) |
| Allergic condition | ||
| Allergic Rhinitis | 477.X | 1,929,244 (18.1) |
| Asthma | 493.X | 1,163,028 (10.9) |
| Atopic dermatitis/eczema | 691.8 | 164,642 (1.5) |
| Control conditions | ||
| Esophageal reflux | 530.81 | 3,386,297 (31.8) |
| Glaucoma | 365.X | 2,084,295 (19.6) |
| Acute sinusitis | 461.X | 1,553,964 (14.6) |
| Accidental fall | E880–E888 | 787,340 (7.4) |
| Calculus of kidney | 592.0 | 607,205 (5.7) |
| Dysthymic disorder | 300.4 | 463,889 (4.4) |
| Tinea pedis | 110.4 | 301,930 (2.8) |
| Cholecystitis | 574.1 | 199,896 (1.9) |
| Acute appendicitis | 540.X | 66,179 (0.6) |
| Motor vehicle traffic accident | E810–819 | 27,919 (0.3) |
Conditions are sorted within categories by frequency.
The source population for controls includes white, non-Hispanic individuals in the U.S. Medicare population who were frequency matched by age and sex to the initial 467,948 SCC and 597,882 BCC cases in 2012 (N=10,658,300). This population only included individuals with non-HMO coverage who had at least 1 Medicare claim in 2012.
Solid organ transplants were also identified if any of the following claims were present: HCPCS codes 44136, 47135, 47136, 48160, 50360, 50365, 32851–32854, 33935, or 33945; DRG codes 302, 480, 495, 512, or 513 before Jan. 1, 2007; or DRG codes 005–008, 010, or 652 after Dec. 31, 2007.
Bone marrow transplants were also identified if any of the following claims were present: HCPCS codes of 38240 or 38241; DRG code 481 before Jan. 1, 2007; or DRG code 009 after Dec. 31, 2007.
Conditions characterized by skin manifestations
HIV=human immunodeficiency virus
We also assessed factors that could influence skin cancer risk or ascertainment. Frequencies of dermatologist and non-dermatologist physician visits were calculated for 2010–2011 as measures of medical engagement before cancer diagnosis. Average annual ambient UVR was determined by linking the zip code of residence in 2012 to daily estimates of cloud-adjusted noon-time ultraviolet B radiation from a national database (19).
Associations of immune-related conditions with SCC and BCC were estimated using multivariable logistic regression. In preliminary analyses stratified by the number of dermatologist visits in 2010–2011 (not shown), many associations with immune-related conditions differed in direction and/or magnitude across strata, with stronger positive associations among people with no dermatologist visits, particularly for skin conditions. This could indicate differential surveillance of the skin during non-dermatologist medical visits based on the presence or absence of an immune-related condition involving the skin. By contrast, individuals seen by a dermatologist would be expected to have some type of skin examination, and as a result more uniform ascertainment of skin cancers, regardless of whether an immune-related condition was present. To reduce the possibility of surveillance bias, we limited the population to people with at least one dermatologist visit in 2010–2011. In this population, we estimated associations adjusting for age, sex, UVR decile category, frequency of dermatologist visits (continuous variable), and frequency of non-dermatologist medical visits (continuous variable). Associations were also estimated separately among people with and without a history of KC diagnosis in Medicare before 2012.
Statistical significance was determined after Bonferroni correction for multiple comparisons. As 57 conditions (47 immune-related and 10 control conditions) were assessed with 2 outcomes (BCC and SCC), the Bonferroni-adjusted alpha level was 0.05/114=0.000439. All tests were two-sided.
Results
Study population
We identified 467,948 SCC and 597,882 BCC cases among elderly Medicare beneficiaries in 2012. After limiting to people with at least 1 dermatologist visit in 2010–2011, 258,683 SCC cases and 304,903 BCC cases remained (Table 2, BCC:SCC ratio of 1.2:1). Among SCC cases, 61.7% were male and 33.2% were younger than 75 years of age. Among BCC cases, 63.1% were male and 38.7% were younger than 75 years of age. Among the controls frequency matched to SCC and BCC cases, 1,012,520 and 1,284,902, respectively had at least 1 dermatologist visit in 2010–2011 and were included in analyses. The age and sex distributions of controls were similar to the distributions for cases (Table 2). In 1999, the first year of claims data evaluated for immune-related conditions, 50.6% of SCC cases and 50.1% of SCC controls were enrolled in Medicare, while 45.2% of BCC cases and 45.1% of BCC controls were in Medicare. About a third of SCC and BCC cases resided in areas with the highest quintile of annual UVR exposure, while only about a quarter of controls lived in these areas. Cases more frequently visited a dermatologist, with 73.1% of SCC cases and 69.5% of BCC cases having more than one dermatologist visit during 2010–2011, compared to 59.0% of SCC controls and 58.6% of BCC controls. Cases also had slightly more frequent non-dermatologist physician visits (Table 2).
Table 2.
Characteristics of squamous cell carcinoma cases, basal cell carcinoma cases and corresponding controls in the Medicare population
| Squamous cell carcinoma (SCC) | Basal cell carcinoma (BCC) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases (N=258,683) | Controls (N=1,012,520) | Cases (N=304,903) | Controls (N=1,284,902) | |||||
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 159,715 | 61.7 | 640,174 | 63.2 | 192,354 | 63.1 | 813,954 | 63.4 |
| Female | 98,968 | 38.3 | 372,346 | 36.8 | 112,549 | 36.9 | 470,948 | 36.7 |
| Age on January 1, 2012, in years | ||||||||
| 65 – 69 | 33,741 | 13.0 | 121,145 | 12.0 | 50,046 | 16.4 | 195,051 | 15.2 |
| 70 – 74 | 52,206 | 20.2 | 206,047 | 20.4 | 68,030 | 22.3 | 289,745 | 22.6 |
| 75 – 79 | 57,778 | 22.3 | 233,710 | 23.1 | 68,921 | 22.6 | 299,601 | 23.3 |
| 80 – 84 | 58,452 | 22.6 | 236,169 | 23.3 | 64,065 | 21.0 | 278,012 | 21.6 |
| 85 – 89 | 40,402 | 15.6 | 156,078 | 15.4 | 39,687 | 13.0 | 165,579 | 12.9 |
| 90 – 94 | 16,104 | 6.2 | 59,371 | 5.9 | 14,154 | 4.6 | 56,914 | 4.4 |
| First year of available Medicare claims | ||||||||
| 1999 | 130,827 | 50.6 | 506,968 | 50.1 | 137,875 | 45.2 | 580,053 | 45.1 |
| 2000–2001 | 28,549 | 11.0 | 121,486 | 12.0 | 33,798 | 11.1 | 151,445 | 11.8 |
| 2002–2003 | 27,290 | 10.6 | 100,976 | 10.0 | 33,329 | 10.9 | 135,208 | 10.5 |
| 2004–2005 | 21,671 | 8.4 | 88,592 | 8.8 | 27,968 | 9.2 | 122,996 | 9.6 |
| 2006–2007 | 20,705 | 8.0 | 82,424 | 8.1 | 28,447 | 9.3 | 119,727 | 9.3 |
| 2008–2009 | 18,436 | 7.1 | 69,339 | 6.9 | 26,671 | 8.8 | 108,486 | 8.4 |
| 2010–2011 | 11,205 | 4.3 | 42,735 | 4.2 | 16,815 | 5.5 | 66,987 | 5.2 |
| Ambient ultraviolet radiation, mW/m2* | ||||||||
| ≤24.77 | 30,594 | 11.8 | 166,659 | 16.5 | 43,605 | 14.3 | 209,845 | 16.3 |
| 24.78–27.92 | 40,306 | 15.6 | 198,456 | 19.6 | 50,301 | 16.5 | 249,967 | 19.5 |
| 27.93–34.73 | 40,813 | 15.8 | 177,549 | 17.5 | 50,979 | 16.7 | 226,237 | 17.6 |
| 34.74–43.64 | 53,034 | 20.5 | 212,608 | 21.0 | 63,943 | 21.0 | 271,764 | 21.2 |
| 43.65+ | 93,936 | 36.3 | 257,248 | 25.4 | 96,075 | 31.5 | 327,089 | 25.5 |
| Dermatologist visits† | ||||||||
| 1 | 69,500 | 26.9 | 415,052 | 41.0 | 92,968 | 30.5 | 532,382 | 41.4 |
| 2+ | 189,183 | 73.1 | 597,468 | 59.0 | 211,935 | 69.5 | 752,520 | 58.6 |
| Non-dermatologist physician visits† | ||||||||
| ≤3 | 28,079 | 10.9 | 124,579 | 12.3 | 39,509 | 13.0 | 167,164 | 13.0 |
| 4–7 | 52,679 | 20.4 | 225,610 | 22.3 | 68,922 | 22.6 | 293,277 | 22.8 |
| 8–11 | 48,564 | 18.8 | 202,580 | 20.0 | 59,747 | 19.6 | 257,314 | 20.0 |
| 12–19 | 56,916 | 22.0 | 222,327 | 22.0 | 65,937 | 21.6 | 277,824 | 21.6 |
| 20+ | 72,445 | 28.0 | 237,424 | 23.5 | 70,788 | 23.2 | 289,323 | 22.5 |
Ambient ultraviolet radiation is based on annual daily average noon-time ultraviolet B irradiance as measured by the National Aeronautics and Space Administration’s Total Ozone Mapping Spectrometer.
Physician visits were counted during time covered by Medicare during 2010–2011.
Associations of immune-related conditions with SCC and BCC
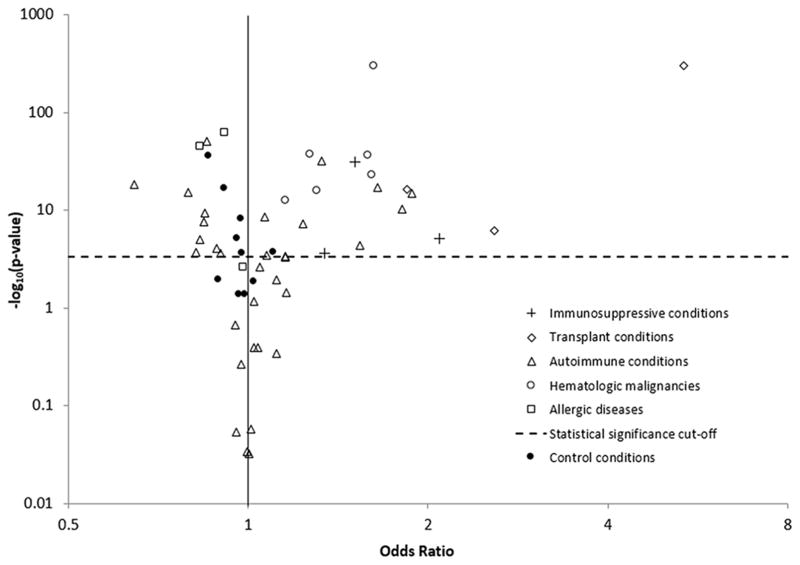
After adjustment for age, sex, dermatologist and non-dermatologist physician visit frequency, and annual UVR exposure, 21 (45%) conditions were significantly associated with elevated SCC risk, including all primary immunodeficiency conditions, transplant conditions, and hematologic malignancies (Table 3, Figure 1). Only 28% of autoimmune conditions and no allergic conditions were associated with elevated risk. The conditions associated with the largest increases in SCC risk were solid organ transplantation (odds ratio [OR]=5.35, 95%CI=5.07–5.65; Table 3), graft vs. host disease (OR=2.58, 95%CI=1.77–3.76), deficiency of cell-mediated immunity (OR=2.09, 95%CI=1.52–2.88), granulomatosis with polyangiitis (also known as Wegener’s granulomatosis, OR=1.88, 95%CI=1.61–2.19), bone marrow transplant (OR=1.84, 95%CI=1.60–2.13), and autoimmune hepatitis (OR=1.81, 95%CI=1.52–2.16). The most prevalent conditions associated with increased SCC risk included rheumatoid arthritis (OR=1.06, 95%CI=1.04–1.09), non-Hodgkin lymphoma (OR=1.62, 95%CI=1.58–1.66), and aplastic anemia (OR=1.27, 95%CI=1.22–1.31). Two of the three allergic conditions and 28% of autoimmune conditions (N=9) were associated with lower risk (Figure 1). Of the 7 autoimmune conditions characterized by skin manifestations (marked with an asterisk in Table 1), 5 were associated with lower SCC risk. Only one control condition (10%), acute appendicitis, was associated with elevated risk, and the elevation was smaller than for most of the immune-related conditions (OR=1.10, Figure 1, Table 3). Half of the control conditions were associated with lower SCC risk (Figure 1).
Table 3.
Associations of immune-related conditions and control conditions with cutaneous squamous cell and basal cell carcinoma
| Squamous cell carcinoma (SCC) | Basal cell carcinoma (BCC) | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Immunosuppressive conditions | ||||
| Deficiency of humoral immunity | 1.51 (1.41–1.61) | 2.93E-32* | 1.22 (1.14–1.31) | 7.34E-09* |
| HIV infection | 1.34 (1.15–1.57) | 2.28E-04* | 1.31 (1.14–1.51) | 2.10E-04* |
| Deficiency of cell-mediated immunity | 2.09 (1.52–2.88) | 6.72E-06* | 1.25 (0.89–1.75) | 1.95E-01 |
| Transplant conditions | ||||
| Solid organ transplant | 5.35 (5.07–5.65) | <1.0E-100* | 1.94 (1.82–2.06) | 2.16E-99* |
| Bone marrow transplant | 1.84 (1.60–2.13) | 5.88E-17* | 1.38 (1.20–1.58) | 5.25E-06* |
| Graft vs. host disease | 2.58 (1.77–3.76) | 7.41E-07* | 1.44 (0.97–2.14) | 6.76E-02 |
| Autoimmune conditions | ||||
| Rheumatoid arthritis | 1.06 (1.04–1.09) | 3.50E-09* | 0.99 (0.97–1.01) | 2.40E-01 |
| Pernicious anemia | 1.02 (1.00–1.05) | 6.70E-02 | 0.95(0.92–0.97) | 4.62E-06* |
| Psoriasis | 0.85 (0.84–0.87) | 5.48E-51* | 0.72 (0.71–0.74) | <1.0E-100* |
| Polymyalgia rheumatica | 1.04 (1.02–1.07) | 2.45E-03 | 1.03 (1.00–1.06) | 5.08E-02 |
| Ulcerative colitis | 1.07 (1.03–1.11) | 3.37E-04* | 1.03 (0.99–1.07) | 1.06E-01 |
| Crohn’s disease | 1.33 (1.27–1.39) | 1.19E-32* | 1.10 (1.05–1.15) | 5.47E-05* |
| Graves’ disease | 0.84 (0.79–0.90) | 2.38E-08* | 0.87 (0.83–0.92) | 1.55E-06* |
| Scleroderma | 0.84 (0.80–0.89) | 4.84E-10* | 0.78 (0.74–0.82) | 1.26E-21* |
| Giant cell arteritis | 1.02 (0.97–1.08) | 4.07E-01 | 1.02 (0.96–1.07) | 5.38E-01 |
| Hashimoto’s thyroiditis | 0.79 (0.75–0.84) | 4.60E-16* | 0.86 (0.82–0.91) | 6.62E-09* |
| Sjögren’s syndrome | 0.90 (0.85–0.95) | 2.30E-04* | 0.86 (0.81–0.90) | 1.91E-08* |
| Systemic lupus erythematosus | 0.89 (0.83–0.94) | 8.00E-05* | 0.77 (0.73–0.82) | 1.61E-17* |
| Addison’s disease | 1.16 (1.07–1.25) | 4.09E-04* | 0.96 (0.88–1.04) | 2.65E-01 |
| Uveitis | 1.00 (0.92–1.09) | 9.28E-01 | 0.95 (0.88–1.03) | 1.87E-01 |
| Celiac disease | 0.95 (0.88–1.03) | 2.17E-01 | 0.98 (0.91–1.06) | 6.32E-01 |
| Immune thrombocytopenic purpura | 1.23 (1.14–1.33) | 5.01E-08* | 1.01 (0.93–1.09) | 8.86E-01 |
| Multiple sclerosis | 0.82 (0.74–0.91) | 1.77E-04* | 0.80 (0.73–0.88) | 3.03E-06* |
| Myasthenia gravis | 1.16 (1.07–1.25) | 4.91E-04 | 1.00 (0.92–1.08) | 9.63E-01 |
| Sarcoidosis | 1.12 (1.02–1.21) | 1.14E-02 | 1.02 (0.94–1.10) | 7.17E-01 |
| Ankylosing spondylitis | 1.04 (0.95–1.14) | 4.02E-01 | 1.04 (0.96–1.13) | 3.25E-01 |
| Scleritis | 0.97 (0.89–1.06) | 5.46E-01 | 1.00 (0.92–1.08) | 9.96E-01 |
| Discoid lupus erythematosus | 0.64 (0.58–0.71) | 7.42E-19* | 0.63 (0.57–0.69) | 2.52E-22* |
| Vitiligo | 0.83 (0.77–0.90) | 8.84E-06* | 0.66 (0.60–0.71) | 6.30E-23* |
| Polymyositis/dermatomyositis | 1.00 (0.90–1.10) | 9.25E-01 | 0.95 (0.86–1.05) | 3.40E-01 |
| Guillain-Barré syndrome | 1.01 (0.90–1.14) | 8.75E-01 | 0.90 (0.80–1.01) | 7.26E-02 |
| Autoimmune hemolytic anemia | 1.64 (1.47–1.84) | 5.34E-18* | 1.18 (1.04–1.33) | 9.63E-03 |
| Primary biliary cirrhosis | 1.16 (1.01–1.33) | 3.55E-02 | 0.86 (0.74–0.99) | 3.35E-02 |
| Granulomatosis with polyangiitis | 1.88 (1.61–2.19) | 9.13E-16* | 1.30 (1.11–1.53) | 1.14E-03 |
| Autoimmune hepatitis | 1.81 (1.52–2.16) | 4.77E-11* | 1.08 (0.88–1.32) | 4.51E-01 |
| Polyarteritis nodosa | 1.54 (1.25–1.89) | 4.50E-05* | 1.22 (0.99–1.49) | 5.71E-02 |
| Reactive arthritis | 1.11 (0.84–1.48) | 4.55E-01 | 1.21 (0.93–1.58) | 1.47E-01 |
| Behçet’s disease | 0.95 (0.50–1.82) | 8.85E-01 | 0.86 (0.47–1.57) | 6.16E-01 |
| Hematologic malignancies and related conditions | ||||
| Non-Hodgkin lymphoma | 1.62 (1.58–1.66) | <1.0E-100* | 1.25 (1.22–1.28) | 2.62E-69* |
| Aplastic anemia | 1.27 (1.22–1.31) | 6.67E-38* | 1.02 (0.98–1.06) | 3.25E-01 |
| Paraproteinemias/related disorders | 1.15 (1.11–1.20) | 1.72E-13* | 1.11 (1.07–1.15) | 4.95E-08* |
| Multiple myeloma | 1.30 (1.22–1.38) | 8.30E-17* | 1.04 (0.98–1.11) | 2.08E-01 |
| Leukemia | 1.59 (1.48–1.70) | 3.16E-37* | 1.15 (1.06–1.23) | 2.94E-04* |
| Hodgkin lymphoma | 1.61 (1.47–1.76) | 5.41E-24* | 1.43 (1.31–1.57) | 2.11E-15* |
| Allergic condition | ||||
| Allergic Rhinitis | 0.91 (0.90–0.92) | 3.31E-63* | 0.93 (0.92–0.94) | 1.41E-42* |
| Asthma | 0.98 (0.97–0.99) | 2.36E-03 | 0.92 (0.91–0.93) | 8.54E-38* |
| Atopic dermatitis/eczema | 0.83 (0.81–0.85) | 6.87E-46* | 0.73 (0.72–0.75) | <1.0E-100* |
| Control conditions | ||||
| Esophageal reflux | 0.97 (0.96–0.98) | 6.89E-09* | 0.94 (0.93–0.95) | 2.73E-45* |
| Glaucoma | 0.99 (0.98–1.00) | 4.38E-02 | 0.99 (0.98–1.00) | 3.97E-02 |
| Acute sinusitis | 0.98 (0.97–0.99) | 2.50E-04* | 0.97 (0.96–0.98) | 1.56E-07* |
| Accidental fall | 0.96 (0.94–0.98) | 8.02E-06* | 0.89 (0.88–0.91) | 1.43E-36* |
| Calculus of kidney | 1.02 (1.00–1.04) | 1.44E-02 | 1.04 (1.02–1.05) | 4.50E-06* |
| Dysthymic disorder | 0.86 (0.84–0.88) | 2.56E-36* | 0.85 (0.83–0.87) | 8.87E-46* |
| Tinea pedis | 0.91 (0.89–0.93) | 1.41E-17* | 0.87 (0.85–0.88) | 2.95E-44* |
| Cholecystitis | 0.97 (0.94–1.00) | 4.37E-02 | 0.98 (0.95–1.01) | 2.26E-01 |
| Acute appendicitis | 1.10 (1.05–1.16) | 1.89E-04* | 1.05 (1.00–1.10) | 6.63E-02 |
| Motor vehicle traffic accident | 0.89 (0.82–0.97) | 1.12E-02 | 1.00 (0.92–1.08) | 9.73E-01 |
The model is restricted to subjects with 1+ dermatologist visits in 2010–2011, and is adjusted for age, sex, ultraviolet B decile, dermatologist visits, and non-dermatologist physician visits.
Statistically significant, p-value<0.000439
Figure 1.
Associations of immune-related conditions and controls conditions with squamous cell carcinoma of the skin
Each point represents the association between a condition and squamous cell carcinoma risk among subjects with at least 1 dermatology visit, adjusted for age, sex, dermatologist visits, non-dermatologist physician visits, and ultraviolet B exposure based on residence. The dotted horizontal line represents p=0.000439, the Bonferroni threshold for statistical significance. As the y-axis shows p-values plotted on the -log10 scale, associations appearing above the dotted line are statistically significant.
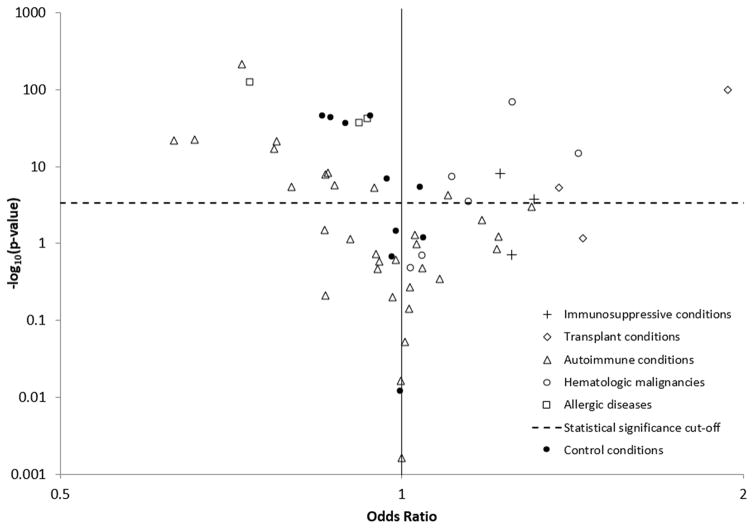
In adjusted models, 9 (19%) conditions were associated with higher BCC risk, including 2 (67%) primary immunodeficiency conditions, 2 (67%) transplant conditions, 4 (67%) hematologic malignancies, and 1 (3%) autoimmune conditions (Figure 2). Conditions associated with the largest increases in BCC risk were solid organ transplantation (OR=1.94, 95%CI=1.82–2.06), Hodgkin lymphoma (OR=1.43, 95%CI=1.31–1.57), bone marrow transplant (OR=1.38, 95%CI=1.20–1.58), HIV (OR=1.31, 95%CI=1.14–1.51), and non-Hodgkin lymphoma (OR=1.25, 95%CI=1.22–1.28) (Table 3). The most prevalent conditions associated with increased BCC risk included non-Hodgkin lymphoma, paraproteinemias/related disorders (OR=1.11, 95%CI=1.07–1.15), and Crohn’s disease (OR=1.10, 95%CI=1.05–1.15). All allergic conditions and 31% of autoimmune conditions were significantly associated with lower risk (Figure 2). Of the 7 autoimmune conditions largely characterized by skin manifestations, 5 were associated with lower BCC risk. One control condition (10%), calculus of the kidney, was associated with elevated risk, but the elevation was smaller than for many immune-related conditions (OR=1.04, Table 3). Half of control conditions were associated with lower BCC risk (Figure 2).
Figure 2.
Associations of immune-related conditions and controls conditions with basal cell carcinoma of the skin
Each point represents the association between a condition and basal cell carcinoma risk among subjects with at least 1 dermatology visit, adjusted for age, sex, dermatologist visits, non-dermatologist physician visits, and ultraviolet B exposure based on residence. The dotted horizontal line represents p=0.000439, the Bonferroni threshold for statistical significance. As the y-axis is plotted on the -log10 scale, associations appearing above this dotted line are statistically significant.
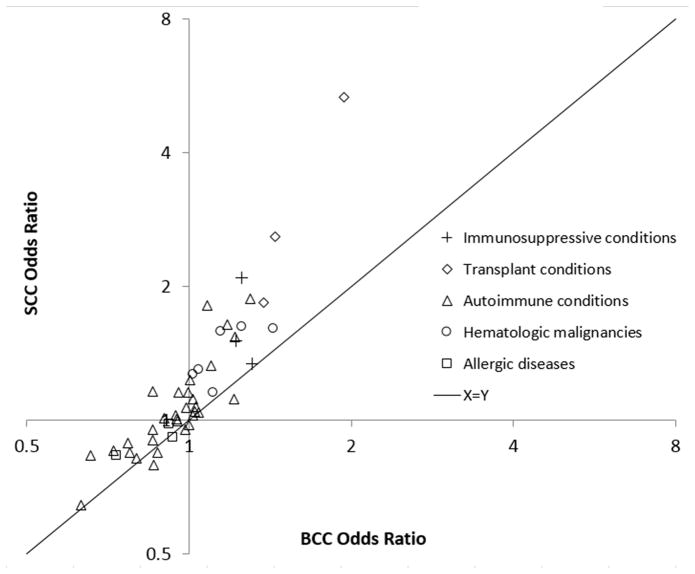
Overall, 40 immune-related conditions (85%) manifested associations with SCC and BCC in the same direction, though associations with SCC were frequently more positive (Figure 3). Among 24 immune-related conditions positively associated with BCC (ORs greater than 1.00), 22 (92%) had stronger associations with SCC. Among the 23 immune-related conditions negatively associated with BCC (ORs less than 1.00), 18 (78%) had associations with SCC that were more positive, i.e., closer to the null or ORs greater than 1.00.
Figure 3.
Comparison of odds ratios for associations of immune-related conditions with squamous cell carcinoma and basal cell carcinoma
Each point represents a condition and its associations with SCC and BCC risk based on models for people with at least one dermatology visit in 2010–2011, adjusted for age, sex, dermatologist visits, non-dermatologist physician visits, and ultraviolet B exposure based on residence. Odds ratios are depicted on logarithmic scales. The solid diagonal line represents where conditions would be if associations with SCC and BCC were equal. Conditions above and to the left of the solid line have a more positive association with SCC than BCC. Conditions below and to the right of the solid line have a more positive relationship with BCC than SCC. SCC=squamous cell carcinoma of the skin, BCC=basal cell carcinoma of the skin
Associations among people with and without a personal history of KC
When we stratified analyses by history of a prior KC diagnosis, most associations were similar in magnitude (Supplementary Table 1). Of the 21 immune-related conditions associated with elevated SCC risk and the 9 immune-related conditions associated with elevated BCC risk in the primary analysis, 20 (95%) and 8 (89%) of these conditions, respectively, had ORs greater than 1.00 among people with and without a prior KC diagnosis, though many were not statistically significant in both strata. Associations of ulcerative colitis with SCC and paraproteinemia with BCC were observed only among people with a prior KC.
Discussion
In our study, we found that many immune-related conditions were associated with elevated risk of KC, especially SCC. Transplant conditions, primary immunodeficiency conditions, and hematologic malignancies were most consistently associated with higher risks. Most immune-related conditions were associated with greater elevations in SCC than BCC risk, suggesting that immune status may be especially important in the etiology of SCC. Moreover, associations with immune-related conditions were stronger and more frequent than those for control conditions, pointing to unique contributions of immune system dysfunction in KC etiology. Mechanisms underlying associations with immune-related conditions could include effects of disease processes, specific treatments, behavioral changes in response to disease, or common risk factors.
Solid organ transplant recipients are the most well-known example of an immunosuppressed population with high skin cancer risk, with prior studies estimating SCC risk 40–120 times higher and BCC risk 6–10 times higher than in the general population (3–5). These elevations are larger than we observed, but these studies did not account for differences in medical visit attendance and included younger individuals who have low KC risk in the absence of transplantation. Transplant recipients use immunosuppressant medications to prevent organ rejection (3, 5). Some immunosuppressants have documented photosensitizing properties, and so elevations in KC risk may not be solely due to immunosuppression (9, 10, 20, 21).
Non-Hodgkin lymphoma (including chronic lymphocytic leukemia) has been associated with KC risk in prior studies (7, 22, 23), and KC diagnosis is also associated with subsequent non-Hodgkin lymphoma risk (23, 24). This reciprocal pattern suggests a common underlying cause for both cancer types, such as a shared genetic predisposition or underlying immune dysfunction. We observed strong increases in KC risk for bone marrow transplant recipients and specifically recipients with graft vs. host disease, an immunologic complication of bone marrow transplantation. Importantly, some immunosuppressants used in solid organ transplantation are also used to treat graft vs. host disease as well as autoimmune conditions (25–29). As a result, effects of these medications, either through increases in immune dysfunction or photosensitization, could explain the elevated KC risk associated with a number of different conditions.
Of note, we identified a number of novel associations with elevated KC risk for conditions that had not previously been studied. For instance, granulomatosis with polyangiitis, autoimmune hepatitis, and polyarteritis nodosa were strongly associated with SCC risk. All are severe autoimmune diseases that require treatment with immunosuppressants (30–32). SCC risk was also strongly elevated in people with autoimmune hemolytic anemia, an autoimmune disorder that can require immunosuppressive treatment or splenectomy, and which can be secondary to another autoimmune disease (33). Deficiencies of cell-mediated and humoral immunity were associated with KC risk. As captured by ICD-9 codes, these conditions are somewhat vague but would presumably include inherited defects in the immune system as well as immunodeficiency secondary to another disease, such as HIV. Associations with these conditions, in which immunodeficiency is caused by the disease itself rather than by treatment, support an etiologic mechanism driven by immune dysfunction independent of photosensitizing medication effects.
For clinical populations with heightened KC risk, an increased emphasis on skin cancer screening and prevention may be appropriate. Importantly, we found consistent elevations regardless of individuals’ prior history of KC. It may be particularly important to target patients without a prior history, who might not be recognized as having a high risk for KC. For solid organ transplant recipients, skin cancer prevention and screening measures are already considered in long-term management (13–15). While elevations in KC risk for other conditions were not as high as those observed for solid organ transplantation, an evaluation of the costs and benefits of increased skin cancer screening would be appropriate for some of these other clinical populations, especially for people who might not otherwise see a dermatologist.
We also observed conditions associated with decreased KC risk, particularly autoimmune conditions with dermatologic manifestations and allergic conditions. Autoimmune and allergic diseases are characterized by an overly reactive immune system. For allergic diseases, this over-reactivity is typically not severe enough to require use of systemic immunosuppressant medications (34), and so the immune system can largely maintain normal functioning. Instead, a highly reactive immune system might help eliminate developing tumor cells (35). While research on these relationships is limited, there is some prior biologic and epidemiologic evidence indicating that allergic responses may be protective against KC development (35, 36).
Behavioral differences related to UVR exposure may affect some associations with KC risk. Many of the inverse associations were for conditions with primary skin manifestations, such as scleroderma and discoid lupus. Patients with these conditions might more frequently cover their skin to hide lesions. Other diseases, such as systemic lupus erythematosus and allergic rhinitis, might be exacerbated by sunlight exposure or time outdoors (37, 38). Multiple sclerosis, which was associated with lower KC risk, can considerably decrease mobility (39) and consequently reduce the amount of time outdoors.
Overall, many associations appeared stronger for SCC than BCC, which may indicate that mechanisms specific to SCC development are preferentially influenced by immune dysfunction. Even for conditions associated with lower risk, most associations with SCC were closer to the null. This pattern could reflect a combination of behavioral effects that reduce risk of both SCC and BCC, with immune defects that most strongly increase SCC risk.
This study has some limitations. Information was not available on factors that influence KC risk, such as sunburn history, time spent outdoors, and sunscreen use. While we accounted for differences in geographic UVR exposure based on zip codes of residence in 2012, these may not represent long-term residences and individuals may have spent substantial time in areas with different levels of UVR exposure prior to 2012. Also, we were only able to capture conditions that appeared in claims when people were 65 years of age or older, and these findings may not generalize to younger populations. Finally, many KCs go undiagnosed or untreated, and these were not captured in our study. However, specificity influences the bias observed in relative effect measures, such as ORs, more strongly than sensitivity (40). Our KC definition should provide high specificity because it would be unlikely that individuals without a KC would receive a KC diagnosis and treatment. While unlikely to bias our ORs, our use of this definition could explain the low ratio of BCCs to SCCs, as BCCs are perhaps more likely to go untreated among elderly adults. A prior study of KC incidence in the Medicare population observed a similar BCC:SCC ratio, noting that the ratio could reflect different patterns across calendar time and age groups (1).
This study also has important strengths. We included an exceptionally large population of cases and controls sampled in a representative manner from the Medicare population. Consequently, we were able to examine a wide array of conditions, including very rare conditions. The level of statistical significance for many associations was very strong (i.e., with p-values less than 10−10). We differentiated between KC types, which allowed us to demonstrate that many associations were stronger for SCC than BCC. We also accounted for the frequency of dermatologist and non-dermatologist physician visits to reduce differences in surveillance that affect KC detection. Finally, we assessed 10 control conditions and demonstrated that the strong associations with immune-related conditions did not merely reflect an artifact of our study design.
In conclusion, our findings strongly support an etiologic role for immunosuppression in KC development across a broad spectrum of immune-related conditions. Almost all conditions associated with elevated risk were either directly immunosuppressive or treated with immunosuppressive medications. Associations were consistently stronger for SCC, indicating that immunologic dysfunction is likely of particular importance for this KC type. These findings support prioritizing research focused on understanding the role of immune dysfunction in the etiology of KC. We also found a few conditions associated with lower KC risk, which could be due to protective effects of an overactive immune system or behaviors that reduce UVR exposure. For novel associations identified here, future research could help elucidate mechanisms by examining how KC risk is influenced by severity of disease, medication use, and UVR exposure. Ultimately, research along these lines will help inform skin cancer screening and prevention for high risk clinical populations.
Supplementary Material
Acknowledgments
Financial Support: E.L. Yanik, R.M. Pfeiffer, D.M. Freedman, E.K. Cahoon, and E.A. Engels were supported by the Intramural Research Program of the National Cancer Institute.
We would like to thank individuals in the Keratinocyte Carcinoma Consortium (Keracon) for useful discussions and input.
Abbreviations
- KC
keratinocyte carcinoma
- SCC
squamous cell carcinoma
- BCC
basal cell carcinoma
- UVR
ultraviolet radiation
- HIV
human immunodeficiency virus
- ICD-9
International Classification of Diseases version 9
- HMO
health maintenance organization
- HCPCS
Healthcare Common Procedure Coding System
- OR
odds ratio
Footnotes
Contributor roles: ELY and EAE conceptualized the study. ELY, EAE, RMP, and DMF developed the initial study approach and methods. MC was responsible for data curation and analysis. ELY drafted the original manuscript. All authors contributed to the review of study methods, interpretation of results, and review of the manuscript.
References
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–6. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Gloster HM, Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55(5):741–60. doi: 10.1016/j.jaad.2005.08.063. quiz 61–4. [DOI] [PubMed] [Google Scholar]
- 3.Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sorensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol. 2010;90(5):474–9. doi: 10.2340/00015555-0919. [DOI] [PubMed] [Google Scholar]
- 4.Bannon FJ, McCaughan JA, Traynor C, O’Brien K, Gavin AT, Maxwell AP, et al. Surveillance of nonmelanoma skin cancer incidence rates in kidney transplant recipients in Ireland. Transplantation. 2014;98(6):646–52. doi: 10.1097/TP.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 5.Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer. 2013;132(6):1429–38. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP, Jr, Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105(5):350–60. doi: 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer JD, Habermann TM, Shanafelt TD. Lymphoma-associated skin cancer: incidence, natural history, and clinical management. International Journal of Dermatology. 2014;53:267–74. doi: 10.1111/ijd.12208. [DOI] [PubMed] [Google Scholar]
- 8.DePry JL, Vyas R, Lazarus HM, Caimi PF, Gerstenblith MR, Bordeaux JS. Cutaneous Malignant Neoplasms in Hematopoietic Cell Transplant Recipients: A Systematic Review. JAMA Dermatol. 2015;151(7):775–82. doi: 10.1001/jamadermatol.2015.121. [DOI] [PubMed] [Google Scholar]
- 9.Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC. Azathioprine and Risk of Skin Cancer in Organ Transplant Recipients: Systematic Review and Meta-Analysis. Am J Transplant. 2016 doi: 10.1111/ajt.13863. [DOI] [PubMed] [Google Scholar]
- 10.Yanik EL, Siddiqui K, Engels EA. Sirolimus effects on cancer incidence after kidney transplantation: a meta-analysis. Cancer medicine. 2015;4(9):1448–59. doi: 10.1002/cam4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman M, Weinstein T, Korzets A, Chagnac A, Ori Y, Zevin D, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. The Journal of laboratory and clinical medicine. 2001;137(1):14–20. doi: 10.1067/mlc.2001.111469. [DOI] [PubMed] [Google Scholar]
- 12.Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA Dermatol. 2016;152(2):164–72. doi: 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney international. 2010;77(4):299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 14.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19(1):3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. Addenda. Centers for Disease Control and Prevention; 2011. http://www.cdc.gov/nchs/icd/icd9cm_addenda_guidelines.htm. 2013[Available from: http://www.cdc.gov/nchs/icd/icd9cm_addenda_guidelines.htm. [Google Scholar]
- 17.Engels EA, Cerhan JR, Linet MS, Cozen W, Colt JS, Davis S, et al. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin’s lymphoma: a case-control study. Am J Epidemiol. 2005;162(12):1153–61. doi: 10.1093/aje/kwi341. [DOI] [PubMed] [Google Scholar]
- 18.Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103(1):112–4. doi: 10.1038/sj.bjc.6605733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Aeronautics and Space Administration; Center. GSF, editor. Total Ozone Mapping Spectrometer data product: erythemal UV exposure. Greenbelt, MD: 2004. [Google Scholar]
- 20.O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu Y-Z, Harwood CA, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309(5742):1871–4. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han W, Soltani K, Ming M, He YY. Deregulation of XPC and CypA by cyclosporin A: an immunosuppression-independent mechanism of skin carcinogenesis. Cancer prevention research. 2012;5(9):1155–62. doi: 10.1158/1940-6207.CAPR-12-0185-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi F, Randimbison L, Te V-C, La Vecchia C. Non-Hodgkin’s lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74:1847–50. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;310(6993):1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheless L, Black J, Alberg AJ. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1686–95. doi: 10.1158/1055-9965.EPI-10-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul CF, Ho VC, McGeown C, Christophers E, Schmidtmann B, Guillaume JC, et al. Risk of Malignancies in Psoriasis Patients Treated with Cyclosporine: a 5 y Cohort Study. J Invest Dermatol. 2003;120:211–6. doi: 10.1046/j.1523-1747.2003.12040.x. [DOI] [PubMed] [Google Scholar]
- 26.Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. The Lancet. 2001;358(9287):1042–5. doi: 10.1016/S0140-6736(01)06179-7. [DOI] [PubMed] [Google Scholar]
- 27.Fiorino G, Danese S. Adalimumab and Azathioprine Combination Therapy for Crohn’s Disease: A Shining Diamond? J Crohns Colitis. 2016 doi: 10.1093/ecco-jcc/jjw119. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen EG, Pottegard A, Hallas J, Friis S, Hansen K, Jensen PE, et al. Risk of non-melanoma skin cancer in myasthenia patients treated with azathioprine. Eur J Neurol. 2014;21(3):454–8. doi: 10.1111/ene.12329. [DOI] [PubMed] [Google Scholar]
- 29.Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105(10):3802–11. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuta S, Jayne D. Emerging therapies in antineutrophil cytoplasm antibody-associated vasculitis. Curr Opin Rheumatol. 2014;26(1):1–6. doi: 10.1097/BOR.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 31.Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 32.De Virgilio A, Greco A, Magliulo G, Gallo A, Ruoppolo G, Conte M, et al. Polyarteritis nodosa: A contemporary overview. Autoimmun Rev. 2016;15(6):564–70. doi: 10.1016/j.autrev.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Jaime-Perez JC, Rodriguez-Martinez M, Gomez-de-Leon A, Tarin-Arzaga L, Gomez-Almaguer D. Current approaches for the treatment of autoimmune hemolytic anemia. Arch Immunol Ther Exp (Warsz) 2013;61(5):385–95. doi: 10.1007/s00005-013-0232-3. [DOI] [PubMed] [Google Scholar]
- 34.Muraro A, Lemanske RF, Jr, Hellings PW, Akdis CA, Bieber T, Casale TB, et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137(5):1347–58. doi: 10.1016/j.jaci.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Hoste E, Cipolat S, Watt FM. Understanding allergy and cancer risk: what are the barriers? Nat Rev Cancer. 2015;15(3):131–2. doi: 10.1038/nrc3909. [DOI] [PubMed] [Google Scholar]
- 36.Hwang CY, Chen YJ, Lin MW, Chen TJ, Chu SY, Chen CC, et al. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: a nationwide cohort study in Taiwan. Int J Cancer. 2012;130(5):1160–7. doi: 10.1002/ijc.26105. [DOI] [PubMed] [Google Scholar]
- 37.National Institute of Arthritis and Musculoskeletal and Skin Diseases. Systemic Lupus Erythematosus. National Institutes of Health; 2015. http://www.niams.nih.gov/health_info/Lupus/default.asp#link_dd. [Google Scholar]
- 38.American College of Allergy, Asthma, and Immunology. Allergic Rhinitis. 2016 http://acaai.org/allergies/types/hay-fever-rhinitis2016.
- 39.Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010;26(1):109–19. doi: 10.1185/03007990903433528. [DOI] [PubMed] [Google Scholar]
- 40.Copeland KT, Checkoway H, Mcmichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488–95. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.