Abstract
Joubert syndrome (JS) is a recessive neurodevelopmental disorder characterized by hypotonia, ataxia, abnormal eye movements, and variable cognitive impairment. It is defined by a distinctive brain malformation known as the “molar tooth sign” on axial MRI. Subsets of affected individuals have malformations such as coloboma, polydactyly, and encephalocele, as well as progressive retinal dystrophy, fibrocystic kidney disease, and liver fibrosis. More than 35 genes have been associated with JS, but in a subset of families the genetic cause remains unknown. All of the gene products localize in and around the primary cilium, making JS a canonical ciliopathy. Ciliopathies are unified by their overlapping clinical features and underlying mechanisms involving ciliary dysfunction. In this work, we identify biallelic rare, predicted-deleterious ARMC9 variants (stop-gain, missense, splice-site, and single-exon deletion) in 11 individuals with JS from 8 families, accounting for approximately 1% of the disorder. The associated phenotypes range from isolated neurological involvement to JS with retinal dystrophy, additional brain abnormalities (e.g., heterotopia, Dandy-Walker malformation), pituitary insufficiency, and/or synpolydactyly. We show that ARMC9 localizes to the basal body of the cilium and is upregulated during ciliogenesis. Typical ciliopathy phenotypes (curved body shape, retinal dystrophy, coloboma, and decreased cilia) in a CRISPR/Cas9-engineered zebrafish mutant model provide additional support for ARMC9 as a ciliopathy-associated gene. Identifying ARMC9 mutations as a cause of JS takes us one step closer to a full genetic understanding of this important disorder and enables future functional work to define the central biological mechanisms underlying JS and other ciliopathies.
Keywords: Joubert syndrome, ciliopathy, zebrafish, primary cilium, cilia, basal body, ARMC9
Introduction
Joubert syndrome (JS [MIM: P213330]) is a recessive neurodevelopmental disorder characterized by motor and cognitive impairments and a distinctive hindbrain malformation giving the appearance of the “molar tooth sign” (MTS) on axial MRI. In addition to the obligate neurological features, subsets of individuals with JS have progressive retinal dystrophy, fibrocystic kidney disease, and liver fibrosis, as well as malformations such as chorioretinal coloboma and polydactyly. Despite this distinctive clinical presentation, mutations in more than 35 genes cause JS, highlighting its marked genetic heterogeneity.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 All of the genes to date encode proteins that function in or around the primary cilium, rendering JS a canonical ciliopathy; ciliopathies are disorders grouped by their overlapping clinical features and molecular disease mechanisms involving cilium dysfunction.24, 25, 26, 27 The primary cilium is a nearly ubiquitous microtubule-based organelle sheathed in a specialized membrane that projects from the cellular surface and functions like an antenna, detecting light, mechanical, and chemical cues, as well as regulating key signaling pathways such as Hedgehog28, 29, 30, 31, 32, 33, 34 and PDGF.35, 36 Significant advances have been made in recent years on the complex genetics underlying JS, and multiple cellular and developmental defects have been associated with loss of function for JS-associated genes in model systems.11, 22, 31, 37, 38, 39, 40, 41, 42, 43, 44, 45 Despite this remarkable progress identifying candidate mechanisms, the common cellular dysfunction across genetic causes of JS is elusive. Therefore it is essential to identify the complete set of genetic defects that underlie JS to pinpoint the unifying molecular mechanism. In this work, we present evidence for mutations in armadillo repeat containing 9 (ARMC9) as a cause of JS, based on human genetic, protein localization, and zebrafish model data.
Material and Methods
Subject Ascertainment and Phenotypic Data
Informed consent was obtained for all participants, who were enrolled under approved human subjects research protocols at the University of Washington (UW), Seattle Children’s Hospital, or King Faisal Specialist Hospital and Research Centre (KFSHRC). All participants have clinical findings of JS (intellectual impairment, hypotonia, ataxia, and/or oculomotor apraxia) and diagnostic or supportive brain imaging findings (MTS or cerebellar vermis hypoplasia). Clinical data were obtained by direct examination of participants, review of medical records, and structured questionnaires.
Variant Identification
Samples from individuals affected by JS were previously screened using a molecular inversion probes (MIPs) targeted capture46 for AHI1 (MIM: 608894), ARL13B (MIM: 608922), B9D1 (MIM: 614144), B9D2 (MIM: 611951), C2CD3 (MIM: 615944), C5ORF42 (MIM: 614571), CC2D2A (MIM: 612013), CEP290 (MIM: 61042), CEP41 (MIM: 610523), CSPP1 (MIM: 611654), IFT172 (MIM: 607386), INPP5E (MIM: 613037), KIF7 (MIM: 611254), MKS1 (MIM: 609883), NPHP1 (MIM: 607100), OFD1 (MIM: 300170), RPGRIP1L (MIM: 610937), TCTN1 (MIM: 609863), TCTN2 (MIM: 613846), TCTN3 (MIM: 613847), TMEM138 (MIM: 614459), TMEM216 (MIM: 613277), TMEM231 (MIM: 614949), TMEM237 (MIM: 614423), TMEM67 (MIM: 609884), TTC21B (MIM: 612014), and ZNF423 (MIM: 604557).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 47 In samples without causal variants, exome sequencing was performed as previously described48 using Roche Nimblegen SeqCap EZ Human Exome Library v2.0 capture probes (36.5 Mb of coding exons) and paired-end 50 base pair reads on an Illumina HiSeq sequencer. In accordance with the Genome Analysis ToolKit’s (GATK) best practices, we mapped sequence reads to the human reference genome (hg19) using the Burrows-Wheeler Aligner (v.0.6.2), removed duplicate reads (PicardMarkDuplicates v.1.70), and performed indel realignment (GATK IndelRealigner v.1.6) and base-quality recalibration (GATK TableRecalibration v.1.6). We called variants using the GATK UnifiedGenotyper and flagged with VariantFiltration to mark potential false positives that did not pass the following filters: Heterozygous Allele Balance (ABHet) > 0.75, Quality by Depth > 5.0, Quality (QUAL) _ 50.0, Homopolymer Run (Hrun) < 4.0, and low depth (< 6x). We used SeattleSeq for variant annotation and the Combined Annotation Dependent Depletion (CADD) score to determine deleteriousness of identified missense variants.49 Based on CADD score data for causal variants in other JS-associated genes, we used a CADD score cutoff of 15 to define deleterious variants.47
LR09-023 was ascertained as part of a larger study of genetic causes for Dandy-Walker malformation (DWM). Exome sequencing of this individual and his parents was performed by Beckman Coulter genomics as follows: genomic DNA isolated from peripheral blood was captured using the Agilent SureSelect V5 enrichment kit and sequenced on an Illumina HiSeq 2000 as 150-bp paired-end runs. Reads were aligned with BWA; variants were called with GATK and freebayes. Variants called by both GATK and freebayes were annotated using Gemini,50 including datasets from the ExAC Exome,51 NHLBI 6500 Exome,52 and 1000 Genomes projects for variant frequencies, and amino-acid-change functional predictions from CADD scores.49 Variants were filtered for de novo, recessive (compound heterozygous or homozygous), or X-linked inheritance, <0.001 frequency in public databases, predicted to be deleterious by CADD > 10, and expressed in human fetal (BrainSpan53) or adult (GTex54) cerebellum.
For the two families in the Saudi Arabian cohort, DNA from the affected individuals as well as their unaffected siblings and parents were genotyped using the Axiom SNP Chip platform to determine the candidate autozygome.55, 56 The previously described “Mendeliome” targeted sequencing assay was then performed on DNA from affected members to search for likely causal variants in the known JS genes followed by whole-exome sequencing in samples without causal variants.57 WES was performed using TruSeq Exome Enrichment kit (Illumina) following the manufacturer’s protocol. Samples were prepared as an Illumina sequencing library, and in the second step, the sequencing libraries were enriched for the desired target using the Illumina Exome Enrichment protocol. The captured libraries were sequenced using an Illumina HiSeq 2000 Sequencer. The reads were mapped against UCSC hg19 by BWA. SNPs and indels were detected by SAMTOOLS. Homozygous rare, predicted-deleterious, and coding/splicing variants within the autozygome of the affected individual were considered as likely causal. We defined rare variants as those with frequency of <0.1% in publicly available variant databases (1000 Genomes, Exome Variant Server, and gnomAD) as well as a database of 2,379 in-house ethnically matched exomes, and defined deleterious if predicted to be pathogenic by PolyPhen, SIFT, and CADD (score > 15).
Array CGH
To assess copy-number variation, we performed array comparative genomic hybridization using a custom 8x60K oligonucleotide array (Agilent Technologies)58 targeting AHI1, ARL13B, ARMC9, B9D1, B9D2, C2CD3, C5ORF42, CBY1 (MIM: 607757), CC2D2A, CEP104 (MIM: 616690), CEP120 (MIM: 613446), CEP290, CEP41 (MIM: 610523), CEP83 (MIM:615847), CSPP1, DDX59 (MIM: 615464), EXOC8 (MIM: 615283), HYLS1 (MIM:610693), IFT172, INPP5E, KCTD10 (MIM: 613421), KIAA0556 (MIM: 616650), KIAA0586 (MIM: 610178), KIAA0753 (MIM: 617112), KIF7, MKS1, NPHP1, NPHP3 (MIM: 608002), NPHP4 (MIM: 607215), OFD1, PDE6D, PIBF1 (MIM: 607532), POC1B (MIM: 614784), RPGRIP1L, TBC1D32 (MIM: 615867), TCTN1, TCTN2, TCTN3, TMEM107 (MIM: 616183), TMEM138, TMEM17 (MIM: 614950), TMEM216, TMEM218, TMEM231, TMEM237, TMEM67, TTC21B, and ZNF423. Probe spacing was a median of 11 bp in the exons, and a median of 315 bp throughout the intronic regions and 100 kb on either side of each gene. Data were generated on an Agilent Technologies DNA Microarray Scanner with Surescan High-Resolution Technology using Agilent Scan Control software and were processed and analyzed using Agilent Feature Extraction and Agilent Cytogenomics software.
RNA Isolation and Quantitative PCR
We cultured human fibroblasts from healthy controls in DMEM + 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin under standard conditions.59, 60, 61 To induce ciliogenesis, we cultured in DMEM without FBS once the cells reached ∼70% confluency. After treatment with 0.05% trypsin, cycling cells and serum-starved cells were harvested and RNA extracted using the Aurum Total RNA Mini Kit (Bio-rad). We generated cDNA from 2 μg of total RNA using the iScript Reverse Transcription Supermix for RT-qPCR (Biorad). The expression of ARL13B and ARMC9 mRNAs was determined using qPCR. Each cDNA sample was amplified using Power SYBR Green PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific) on the C1000 Thermal Cycler CFX (Bio-rad). After an initial denaturation of 10 min at 95°C, each cycle (x39) consisted of denaturation at 95°C for 15 s and anneal/extend at 60°C for 1 min with a plate read. The primers for ARL13B and ARMC9 are listed in Table S1. GAPDH was used as an endogenous control to normalize each sample. The experiment was performed in triplicate.
Cell Lines, Antibodies, and Microscopy
Human telomerase-immortalized retinal pigment epithelium (hTERT-RPE1) cells were grown in DMEM (PAA) supplemented with F12 in a 1:1 ratio with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were plated on glass coverslips for immunofluorescence imaging. At 24 hr after plating, cells were serum starved for 48 hr in 0.2% FBS medium to induce cell cycle arrest and ciliogenesis. Cells were rinsed once with 1× PBS at room temperature and then fixed in 2% paraformaldehyde for 20 min and permeabilized with 1% Triton-X for 5 min. Cells were blocked in freshly prepared 2% bovine serum albumin for 45 min and then incubated with the following antibodies for 1 hr: rabbit anti-ARMC9 (Atlas Antibodies cat# HPA019041, RRID: AB_1233489; 1:200), guinea pig anti-RPGRIP1L (SNC040, 1:300), a monoclonal anti-acetylated tubulin (clone 6-11-B1, Sigma-Aldrich, T6793; 1:1,000), and a mouse monoclonal anti-γ-tubulin (Sigma T5326, 1:500). Anti-ARMC9 recognizes an epitope at the N-terminal portion of the protein. Cells were stained with secondary antibodies for 45 min. The following secondary antibodies were used (all from Life Technologies/Thermo Fisher Scientific; all diluted 1:500 in 2% BSA): anti-guinea pig IgG Alexa Fluor 647, anti-rabbit IgG Alexa Fluor 488, and anti-mouse IgG Alexa Fluor 568. DAPI (4′,6-diamidino-2-phenylindole) stained the nucleus. Confocal imaging was done with the Zeiss LSM 880 Laser scanning microscope equipped with Airyscan technology.
Antibody Validation with siRNA Knock Down
Reverse transfections on hTERT-RPE1 cells were performed with pre-designed Silencer Select siRNA to ARMC9 (Ambion from Life Technologies), as well as a positive control to knock down GAPDH (Ambion from Life Technologies) and a scrambled non-targeting negative control (Ambion from Life Technologies). Lyophilized siRNAs were re-constituted with RNase-free water (Thermo Scientific) to a final working concentration of 50 nM. Lipofectamine RNAiMax (LifeTechnologies) and Opti-MEM (LifeTechnologies) were used for siRNA transfection and done in accordance with the manufacturer’s protocol. Cells were harvested and lysed with RIPA lysis buffer supplemented with Complete Protease Inhibitor Cocktail Tablets (Roche) 72 hr after transfection and western blotting was performed. Primary antibodies mouse monoclonal GAPDH (Thermo Scientific) and rabbit polyclonal ARMC9 (Human Protein Atlas Sigma Aldrich) were used at 1:1,000 and 1:500 dilutions, respectively, and incubated overnight at 4°C. Secondary antibodies goat anti-rabbit IRDye800 (LI-COR Biosciences) and goat anti-mouse IRDye680 (LI-COR Biosciences) were used at a dilution of 1:10,000 and incubated with blots for 1 hr at room temperature. Imaging was done with the Odyssey CLx imaging system (LI-COR Biosciences). Protein quantification was performed using Image Studio Lite software (LI-COR Biosciences).
Zebrafish In Situs, CRISPR, Mutation Assay, and Histology
Zebrafish were maintained at 28°C with a 14 hr/10 hr light/dark cycle as previously described.62 All zebrafish protocols were in compliance with internationally recognized guidelines for the use of zebrafish in biomedical research, and the experiments were approved by local authorities (Veterinäramt Zürich TV4206).
In situ hybridization was performed following standard protocols with a probe spanning over 800 bp at the 3′ end of armc9 generated using the primers 5′-AGCTCAACTCAGCGACCATC-3′ and 3′-TGCTGTTACAGGAAGCTGGA-5′. sgRNAs for CRISPR/Cas9 mutagenesis were designed using the CHOPCHOP website: 3′-GGGATTGGGCACAAATGGCA-5′ and 3′-GGAACAGCTCCTGAAAGGAC-5′ for exon 4 and 5′-GGTCAAAGAGCTGAATGACT-3′ and 3′-GGAGCTCCGTCAGGACTTTC-5′ for exon 14/15. Pairs of sgRNAs for each target site were mixed with Cas9 protein (gift from Darren Gilmour) and injected into 1-cell stage embryos obtained through natural matings. Individual larvae were lysed for assessment of mutagenesis efficiency. Amplification of the target regions for genotyping was performed using primer pairs 5′-CAGCCAAACCACTGAGTTCC-3′ and 3′-TGACCCTGTAGTGTGTGCGTA-5′ for exon 4 and 5′-CACGCCTTGCACCACACT-3′ and 3′-CACTCCCCTGTTTGAGCAAT-5′ for exons 14/15. The PCR products were analyzed on gel electrophoresis and bands were cut out and subcloned before sequencing. The remaining F0 fish were raised. Brains of four F0 fish with curved bodies were dissected out at 5 months of age, halved, and fixed in 2.5% Glutaraldehyde in 0.1 M Cacodylate buffer and prepared for scanning electron microscopy (SEM) following standard protocols. SEM was performed on a ZEISS Supra VP 50 microscope. Cryosections were performed according to standard protocols and IHC was performed as previously described,37, 62 using the zpr1 antibody.63 Vybrant DiO (ThermoFisherScientific) and DAPI were used for counterstaining. Images were acquired on a Leica HCS LSI confocal microscope. Histological sections using Technovit were performed as previously described.62 Images were acquired on an Olympus BX61 microscope.
Results
Exome Sequencing Reveals ARMC9 Mutations as a Cause for JS
To identify novel genetic causes of JS, we performed whole-exome sequencing on a cohort of 53 individuals (51 families) with a clinical diagnosis of JS enrolled in the University of Washington (UW) Joubert Syndrome Research Program. Inclusion criteria comprised the presence of clinical findings of JS (developmental delays, hypotonia, ataxia, and/or oculomotor apraxia), diagnostic brain imaging findings, and lack of mutations in 28 JS-associated genes (NPHP1, AHI1, CEP290, RPGRIP1L, TMEM67, CC2D2A, ARL13B, INPP5E, OFD1, TMEM216, CEP41, TMEM237, TCTN2, KIF7, TCTN1, TMEM138, MKS1, C5ORF42, TMEM231, TCTN3, CSPP1, PDE6D, IFT172, ZNF423, TTC21B, B9D1, B9D2, and C2CD3) based on targeted sequencing.46, 47 Variants from the exome-sequencing data that were rare (minor allele frequency < 1% in the exome variant server [EVS] database) and predicted to be deleterious (stop-gain, frameshift, canonical splice variants, and variants with CADD score > 15) were retained for further analysis.
We identified pairs of siblings in two families that shared two rare, predicted-deleterious variants (RDVs) in ARMC9 (GenBank: NM_025139.4): UW132-3 and -4 carry c.205G>A (p.Gly69Arg) and c.1336C>T (Arg446Cys) and UW335-3 and -4 carry c.259C>T (p.Arg87∗) and c.1027C>A (p.Arg343Ser) (Table 1 and Figure 1).
Table 1.
Phenotypic Features of Individuals with ARMC9-Related Joubert Syndrome with Mutations
| ID# | UW132-3 | UW132-4 | UW348-3 | UW116-3 | UW335-3 | UW335-4 | UW349-3 | LR09-023 | SA1-3 | SA2-3 | SA2-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 | ARMC9 |
| Mutation 1 | c.205G>A (p.Gly69Arg) | c.205G>A (p.Gly69Arg) | c.51+5G>T (splice) | c.1027C>T (p.Arg343Cys) | c.259C>T (p.Arg87∗) | c.259C>T (p.Arg87∗) | c.1474+1G>C (splice) | c.1474G>A (p.Gly492Arg) | c.1027C>T (p.Arg343Cys) | c.1559C>T (p.Pro520Leu) | c.1559C>T (p.Pro520Leu) |
| MAF | 0.000025 (7/277212) | 0.000025 (7/277212) | ND | ND | 0.000018 (5/277214) | 0.000018 (5/277214) | ND | ND | 0.000053 (13/246244) | 0.0000041 (1/246192) | 0.0000041 (1/246192) |
| CADD v1.3 | 33 | 33 | 14.54 | N/A | 35 | 35 | 23.5 | 26 | 34 | 34 | 34 |
| Parent | paternal | paternal | unable | maternal | paternal | paternal | maternal | maternal | maternal | maternal | maternal |
| Mutation 2 | c.1336C>T (p.Arg446Cys) | c.1336C>T (p.Arg446Cys) | c.51+5G>T (splice) | c.1211_1334del (p.Arg405Alafs∗7) | c.1027C>A (p.Arg343Ser) | c.1027C>A (p.Arg343Ser) | c.1027C>T (p.Arg343Cys) | c.1027C>T (p.Arg343Cys) | c.1027C>T (p.Arg343Cys) | c.1559C>T (p.Pro520Leu) | c.1559C>T (p.Pro520Leu) |
| AF | 0.0000041 (1/246076) | 0.0000041 (1/246076) | ND | 0.000053 (13/246244) | 0.0000041 (1/246244) | 0.0000041 (1/246244) | 0.000053 (13/246244) | 0.000053 (13/246244) | 0.000053 (13/246244) | 0.0000041 (1/246192) | 0.0000041 (1/246192) |
| CADD v1.3 | 34 | 34 | 14.54 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 |
| Parent | maternal | maternal | unable | paternal | maternal | maternal | paternal | paternal | paternal | paternal | paternal |
| Ethnicity/country | white/US | white/US | white/Israel | white/US | white/Australia | white/Australia | >1 race/US | white/US | Arab/Saudi | Arab/Saudi | Arab/Saudi |
| Gender | F | M | F | F | F | M | M | M | M | F | M |
| Age (years) | 33 | 29 | 10 | 8 | 5 | 7 | 2 | 8 | 7 | 4 | 5 |
| Dev. disability | y | y | y | y | y | y | y | y | y | y | y |
| Apnea/tachyp | n | n | unk | both | both | n | A | T | y∗ | n | y∗ |
| Abnl eye mvts | y | y | y | y | y | y | y | unk | y | y | n |
| Retinal dystrophy | n | n | y | n | n | n | n | unk | unk | y | n |
| Kidney | n | n | unk | unk | n | n | n | unk | n | n | n |
| Liver | n | n | unk | unk | n | n | n | unk | n | n | n |
| Polydactyly | n | y | unk | n | n | n | n | n | n | y | n |
| Coloboma | n | n | unk | n | n | n | n | unk | unk | n | n |
| Ptosis | n | y | unk | y | n | n | y | y | y | y | y |
| Seizures | y | n | n | y | n | n | n | n | n | n | n |
| Other | hysterectomy 2016 (heavy bleeding); worsening visual acuity; seizures | lithium-induced hypo-thyroidism; L foot postaxial polydactyly | abnormal ERG | G-tube | – | single heterotopia (left occipital horn) | micrognathia; high palate; bifid uvula; bilateral optic nerve hypoplasia; GH deficiency; micropenis; eyelid implants; possible hearing loss; borderline HSM | Dandy Walker malformation; ventriculo- and cysto-peritoneal shunts; non-ambulatory; non-verbal at age 8 years | – | broad nasal bridge; thin upper lip; Y-shaped 2/3 toe syndactyly | – |
Abbreviations are as follows: A, apnea; AF, allele frequency based on gnomAD;51 ERG, electroretinogram; F, female; GH, growth hormone; HSM, hepatosplenomegaly; ID, identification; M, male; n, no; ND, not documented; T, tachypnea; unk, unknown; y, yes. Asterisk (∗) indicates transient neonatal.
Figure 1.
ARMC9 Mutations Cause JS
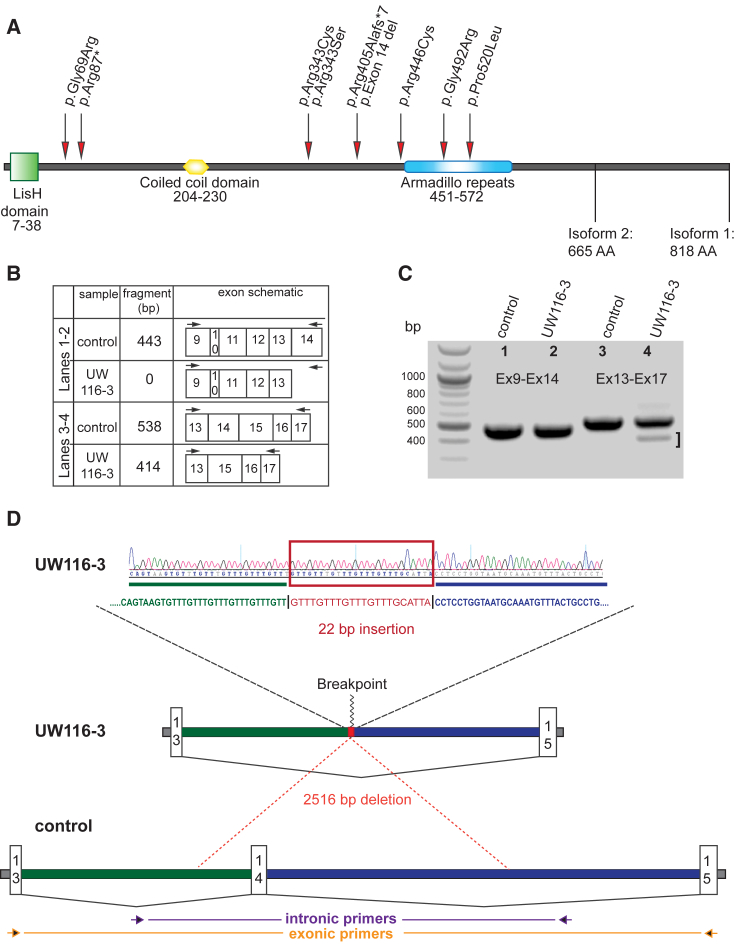
(A) The ARMC9 gene encodes a protein with an N-terminal LisH domain (green square), a coiled coil domain (yellow polygon), and a series of armadillo repeats (blue oval). Patient mutations are indicated by red arrows.
(B–D) Confirmation of ARMC9 exon 14 deletion in UW116-3. No difference in the size or number of PCR products is observed between cDNA isolated from UW116-3 and two unaffected control cell lines using primers in exons 9 and 14 (C and D). Primers in exons 13 and 17 amplify a full-length product and a shorter product (bracket) in UW116-3, but only the full-length product in the two control lines. Sequencing genomic DNA amplified by primers flanking exon 14 reveals a 2,516 bp deletion with a 22 bp insertion (D).
We then performed targeted sequencing of ARMC9 using the Molecular Inversion Probe (MIPs) capture method followed by next generation sequencing on samples from 534 individuals in 456 families with and without known causes. Three additional individuals in three families have ARMC9 RDVs: UW348-3 has a homozygous c.51+5G>T, predicted splice variant, UW349-3 has two RDVs (predicted splice c.1474+1G>C and missense c.1027C>T [p.Arg343Cys]), and UW116-3 has a single heterozygous c.1027C>T (p.Arg343Cys). Based on decreased sequence coverage for two consecutive MIPs covering exon 14 in UW116-3, we suspected a deletion in UW116-3. We performed comparative genomic hybridization using a custom array targeting the JS genes58, 64, 65 and identified a 2.5 kb deletion encompassing exon 14 in UW116-3 (Figures 1B–1D). Exon 14 is 124 bp long, so its loss is predicted to result in a frameshift and truncation of the protein or nonsense-mediated decay of the transcript.
In parallel, exome sequencing in two other cohorts identified biallelic RDVs in ARMC9: three individuals (SA1-3, SA2-3, and SA2-4) from two families in a cohort of 47 Saudi Arabian families66 affected by JS had homozygous ARMC9 missense RDVs (c.1027C>T [p.Arg343Cys] and c.1559C>T [p.Pro520Leu]) and a single individual with JS (LR09-023) from a mixed cohort of 100 individuals with Dandy-Walker malformation and cerebellar hypoplasia had compound heterozygous ARMC9 RDVs (c.1474G>A [p.Gly492Arg] and c.1027C>T [p.Arg343Cys]). In total, we identified 10 different ARMC9 RDVs (1 stop-gain, 2 splice, 6 missense, and 1 single exon deletion) in 11 individuals from 8 families. All variants were validated by Sanger sequencing, and for ten individuals from seven families their segregation with the disease was confirmed in parents and siblings; segregation was not performed in the remaining family because DNA was not available from parents (Table 1). c.1027C>T (p.Arg343Cys) appears to be a recurrent mutation rather than a founder variant, since it is present in families of diverse ethnicities, and a second variant (c.1027C>A [p.Arg343Ser]) affects the same position. ARMC9 is predicted to have a Lissencephaly type-1-like homology (LisH) motif, a coiled-coil domain, and armadillo repeats (Figure 1A). Two of the missense RDVs are in the armadillo repeats, and the other four missense RDVs are not located in known domains.
ARMC9-Related JS Is Indistinguishable from JS due to Other Genetic Causes
All of the affected individuals have typical features of JS including hypotonia and developmental disability, most severely affecting motor and speech function (Table 1). Ages range from 2 to 33 years. Most of the individuals have isolated neurodevelopmental issues, including two with seizures (UW132-3 and UW116-3). Two individuals (UW132-4 and SA2-3) have postaxial polydactyly, and SA2-3 also has syndactyly. Two individuals (UW348-3 and SA2-3) also have retinal dystrophy, but none have kidney or liver involvement. UW349-3 has a more complex presentation with hypopituitarism, bilateral optic nerve hypoplasia, bifid uvula, and an abnormal brainstem (see below).
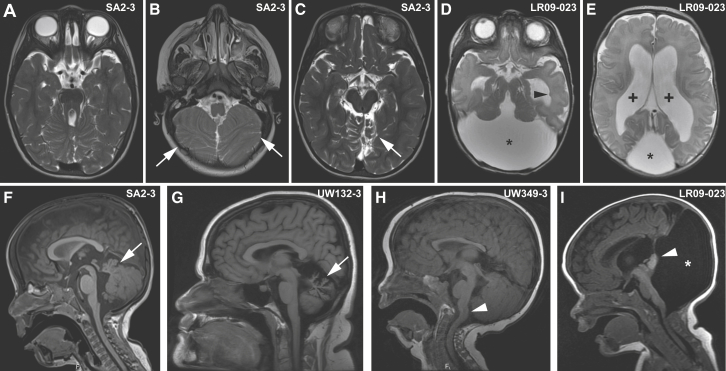
Based on direct review of the brain MRIs, all of the affected individuals have the “molar tooth sign” as well as dysplasia of the superior cerebellar folia (Figures 2A, 2B, 2F, and Table S2). Three individuals (UW335-4, LR09-023, and SA2-3) have cerebellar hemisphere dysplasia, seen in up to 1/3 of individuals with JS (Figure 2C).67 In addition to the MTS, LR09-023 has a large posterior fossa with a rotated cerebellar vermis consistent with Dandy-Walker malformation (Figures 2D, 2E, and 2I). LR09-023 also has a single periventricular heterotopia (Figure 2D), as do UW116-3 and UW335-4. The two oldest individuals (UW132-3 and -4) have an atrophic appearance to their cerebellum, more severely affecting the vermis than the hemispheres (Figure 2G). UW349-3 has a kinked brainstem and cervicomedullary junction heterotopia (Figure 2H) seen in a small subset of individuals with JS,67, 68, 69, 70, 71 and UW349-3 and SA2-3 have an absent posterior pituitary bright spot, but only UW349-3 has known pituitary insufficiency.
Figure 2.
Brain Imaging Findings in Individuals with ARMC9-Related Joubert Syndrome
(A–C) MTS (A), inferior cerebellar dysplasia (white arrows in B), and superior cerebellar dysplasia (white arrow in C) in SA2-3.
(D and E) MTS (D), posterior fossa cyst (asterisks in D and E), and ventriculomegaly (plus signs in E) in LR09-023. Note the single periventricular nodular heterotopia (black arrowhead in D).
(F) Vermis hypoplasia and elevated roof of the 4th ventricle in SA2-3 (white arrow).
(G) Cerebellar vermis hypoplasia and atrophy in UW132-3 (white arrow).
(H) Kinked brainstem and cervicomedullary heterotopia in UW349-3 (white arrowhead).
(I) Enlarged posterior fossa fluid collection (white asterisk) and rotated vermis (white arrowhead) in LR09-032.
Axial T2-weighted images in (A)–(E); sagittal T1-weighted images in (F)–(I).
ARMC9 Localizes to the Basal Bodies of Primary Cilia
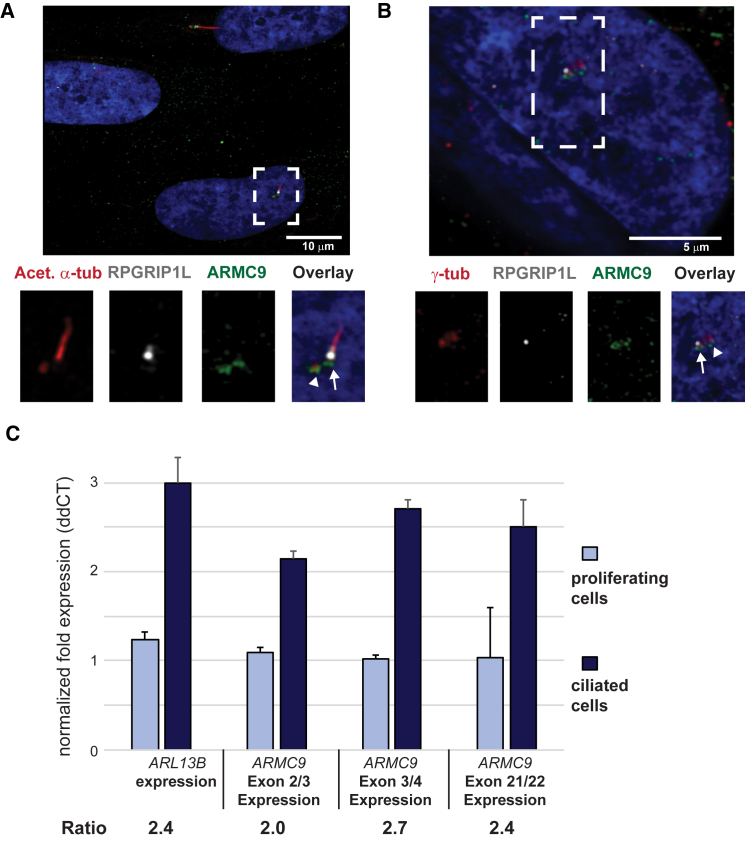
JS-associated proteins have been shown to localize in and around primary cilia;40, 45, 72, 73, 74 therefore, we used a commercially available ARMC9 antibody to evaluate endogenous ARMC9 localization in ciliated hTERT-RPE1 cells. ARMC9 localized to the ciliary basal body (Figure 3A, white arrowhead), basal to but not overlapping with the transition zone marker RPGRIP1L, as well as to the daughter centriole (Figure 3A, white arrow) marked by acetylated α-tubulin antibody (Figure 3A). ARMC9 co-localizes with γ-tubulin at the basal body (Figure 3B, white arrow), proximal to RPGRIP1L, and at the daughter centriole (Figure 3B, white arrowhead).
Figure 3.
ARMC9 Localization and ARMC9 Expression in Ciliated and Proliferating Cells
(A) ARMC9 (green) localizes at the basal body (white arrow) and at the daughter centriole (white arrowhead) of the primary cilium in serum-starved hTERT RPE1 cells. The ciliary marker anti-RPGRIP1L (white) marks the ciliary transition zone and anti-acetylated α-tubulin (red) marks the ciliary axoneme.
(B) ARMC9 (green) co-localizes with γ-tubulin (red) at the ciliary basal body in serum-starved hTERT RPE1 cells. Anti-RPGRIP1L marks distal to the basal body (white) and DAPI (blue) stains the nuclei.
(C) ARMC9 expression in control human fibroblasts grown with serum (proliferating cells) and without serum (ciliated cells), assessed using qPCR with GAPDH as a reference gene. ARL13B is used as a positive control for a gene upregulated in ciliated cells.
Error bars indicate standard deviation.
ARMC9 Expression Is Upregulated in Ciliated Cells
Based on data from model systems and humans, many genes involved in cilium function are upregulated in ciliated cells.75, 76, 77, 78, 79 We evaluated ARMC9 expression by quantitative PCR in control human fibroblasts with and without serum in the medium. In the presence of serum, fibroblasts actively divide and few have cilia, but in response to serum starvation, 80%–90% drop out of the cell cycle and make cilia, similar to other published results.80 ARMC9 expression was 2.0- to 2.7-fold higher in serum-starved cells than in cells grown with serum (Figure 3C). For comparison, expression of another JS-associated gene, ARL13B, was 2.4-fold higher in serum-starved cells.
Zebrafish armc9 Mutants Display Typical Ciliopathy Phenotypes
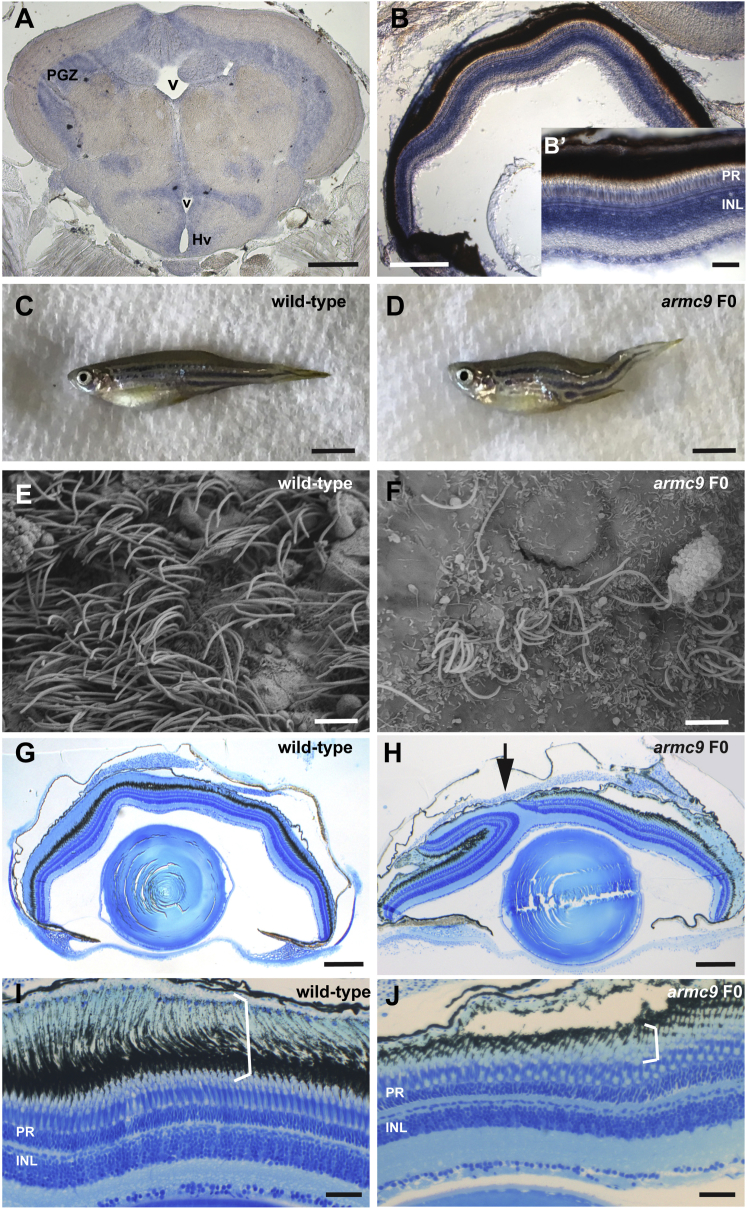
To investigate the function of ARMC9 in vivo, we turned to the zebrafish model. Zebrafish have a single ARMC9 ortholog that has 58% identity and 72% similarity with the human protein. Based on database predictions and manual curation, both the LisH domain and the armadillo-fold domain are conserved in zebrafish at similar positions to the human protein (amino acids 7–39 and 375–600, respectively) (Figure S1). In adult zebrafish, armc9 is expressed in multiple CNS regions based on in situ hybridization, including the cerebellum (Figure S1), all periventricular regions (Figure 4A), and all layers of the retina (Figure 4B). To explore whether loss of armc9 function results in ciliopathy phenotypes, we engineered frameshift mutations in zebrafish using CRISPR/Cas9 (Figure S2). Co-injecting pairs of small guide RNAs targeting either exon 4 or exons 14–15 (the latter corresponding to the middle of the armadillo-fold domain), we generated mutations with very high efficiency (91% of sequenced clones from individual F0 larvae carried indels, the majority of which were out-of-frame; Figure S2). Of approximately 140 surviving F0 fish raised, 10 developed a curved body axis around 6 weeks of age (Figures 4C and 4D), including both exon 4- and exon 14-15-targeted animals. The body curvature phenotype correlated well with the presence of indels affecting the targeted exons; genotyping of 49 F0 fish by gel electrophoresis demonstrated various armc9 indels in 11 fish, only 1 of which did not have a curved body shape. Moreover, such body curvature was never observed in hundreds of raised F0 fish injected with sgRNA targeting non-ciliary genes (20 different sgRNAs). A recent study suggested body curvature in zebrafish is caused by deficient ependymal cilia-generated cerebrospinal fluid (CSF) flow.81 Indeed, using SEM we observed a substantial reduction of cilia numbers on the ventricular surface of adult zebrafish harboring armc9 mutations (Figures 4E and 4F). In addition, a subset of F0 fish with body curvature also displayed a retinal coloboma and had shortened photoreceptor outer segments, typical phenotypes observed with ciliary dysfunction (Figures 4G–4J and S2).38 Taken together, these results confirm that armc9 loss of function in zebrafish causes typical ciliopathy phenotypes and strongly support a role for armc9 in ciliary function.
Figure 4.
armc9 Loss of Function in Zebrafish Leads to Typical Ciliopathy Phenotypes
(A and B) Expression of armc9 in zebrafish adult brain (A) and retina (B) by in situ hybridization. Note the expression along ventricular surfaces in (A) and in all retinal layers, including the photoreceptor (PR) and the inner nuclear layer (INL) (B, B′).
(C and D) Adult zebrafish harboring armc9 mutations display a curved body shape (D) compared to wild-type controls (C).
(E and F) Scanning electron microscopy image of the ventricular surface demonstrates bundles of cilia in wild-type (E) but substantial reduction of cilia numbers in F0 armc9 fish (F).
(G and H) Histological sections through adult zebrafish eyes of wild-type (G) and F0 armc9 mutants (H) showing a coloboma (arrow).
(I and J) Higher-magnification images show the different retinal layers in wild-type fish (I) including the PRs and their long outer segments (OS, bracket) which represent highly specialized ciliary compartments. (J) Note the shortened OS in F0 armc9 mutants (bracket).
Scale bars are 200 μm in (A) and (B), 50 μm in (B′), 5 mm in (C) and (D), 3 μm in (E) and (F), 250 μm in (G) and (H), and 50 μm in (I) and (J). Abbreviations are as follows: v, ventricle; PGZ, periventricular gray zone of optic tectum; Hv, ventral zone of periventricular hypothalamus; PR, photoreceptors; INL, inner nuclear layer.
Discussion
Mutations in more than 35 genes have been identified in individuals with JS, explaining the genetic cause in 62% to 97% of case subjects, depending on the study.47, 66, 82 In addition to these known causes, we now identify ARMC9 mutations as an additional cause of JS accounting for almost 1% of families in our cohort of >500. Substantial functional evidence supports ARMC9 as a ciliopathy-associated gene.
ARMC9 Localizes to the Basal Body
We provide evidence that ARMC9 localizes to the ciliary basal body, similar to other JS-associated proteins (Figures 3A and 3B). The basal body originates from the mother centriole that docks at the cell membrane during interphase to nucleate the ciliary axoneme. The daughter centriole remains tethered to the basal body by an interconnecting fiber, and both structures often appear as juxtaposed puncta on immunofluorescence images. Basal bodies are composed of nine short triplet microtubules arranged in a circle, and two of the three microtubules in each basal body triplet extend as axonemal microtubules. Several other JS-associated proteins (OFD1, KIAA0586, and C2CD3) also localize to the basal body.40, 45, 72, 73, 74, 83 Strikingly, ARMC9 and OFD1 both have an N-terminal LisH motif that is known to bind to microtubules (Figure 1A).72, 84, 85 Two published proteomic studies in murine inner medullary collecting duct (IMCD3) cells also provide support for ARMC9 as a cilium-associated protein: (1) ARMC9 was detected a total of 31 times across ciliary fractions via MudPIT mass spectrometry of isolated cilia86 and (2) proximity labeling using a promiscuous biotinylating enzyme conjugated to the transition zone protein NPHP3 detected ARMC9-specific peptides (6 spectral counts), thus providing direct evidence for their adjacency.87
ARMC9 Is Present in Ciliated Organisms and Upregulated in Ciliated Cells
The use of comparative genomics to compile genes exclusively present in ciliated organisms versus non-ciliated organisms is a common technique in identifying ciliary genes.88, 89 ARMC9 homologs are present in ciliated eukaryotes from humans to unicellular flagellates such as Trochaic trifallax but absent in plants, fungi, bacteria, viruses, and Archaea.90 Expression of the ARMC9 C. elegans homolog, F59G1.4, is restricted to sensory neurons, the only ciliated cells in this organism.91, 92, 93 Ciliary gene induction was first linked to ciliogenesis in classical experiments in unicellular flagellates, notably Chlamydomonas, a model system particularly well suited to study ciliary biology. These experiments demonstrated that new protein synthesis was required for full cilia regeneration via use of protein synthesis inhibitors or enucleating cells.75, 76 Later experiments show that ciliary genes are widely induced during ciliogenesis and maintained at high levels in ciliated cells.77, 78, 79 Ciliary genes are similarly upregulated in higher organisms.36, 94, 95 Similar to other ciliary genes, we demonstrate that ARMC9 expression is upregulated in human ciliated fibroblasts, as compared to cycling cells (Figure 3C).
Zebrafish Harboring armc9 Mutations Display Typical Ciliopathy Phenotypes
In a vertebrate model, zebrafish harboring CRISPR-engineered armc9 mutations display phenotypes similar to those seen with loss of function for zebrafish orthologs of other ciliopathy-associated genes (Figure 4).38, 96, 97 The retinal dystrophy and/or curved body axis are also seen in loss-of-function models for the cc2d2a, ahi1, and ift genes.38, 97, 98, 99, 100 Furthermore, we show a strong reduction of cilia in the brain ventricles of zebrafish harboring armc9 mutations, suggesting that the gene product may participate in early stages of ciliogenesis or be required for ciliary maintenance. The mosaic state of the animals analyzed in this work does not allow for discrimination between these two possibilities. Indeed, the presence of residual cilia on some ventricular cells may be explained by lack of armc9 mutations in those cells or by incomplete penetrance of the phenotype. Future analysis of stable lines will help address these possibilities. The identical phenotypes observed in multiple animals generated using guides targeting different armc9 regions, combined with the lack of these phenotypes with CRISPR/Cas9-generated deletions in 20 different non-ciliary genes, strongly supports the specificity of the observed phenotypes and argues against non-specific or off-target effects of CRISPR/Cas9.
In conclusion, we demonstrate that mutations in ARMC9 cause JS and show that ARMC9 localizes to the basal body. Given the LisH domain, ARMC9 likely binds microtubules there, but the details of its function remain to be elucidated. Delineating all of the genes involved in JS will enable future work to determine how proteins that localize to the basal body, transition zone, cilium proper, and cilium tip all contribute to the molecular mechanism(s) underlying JS. Understanding the molecular mechanisms of JS will lead to more specific treatments in the future and further our understanding of basic ciliary biology in health and disease.
Acknowledgments
We deeply thank the families who participated in this study. We also thank the Joubert syndrome and Related Disorder Foundation for referring families, Heather Mefford and her laboratory for help with arrays, the Center for Microscopy and Image Analysis, University of Zurich (ZMB), for their assistance with Scanning Electron Microscopy, in particular Gery Barmettler, the Genotyping and Sequencing Core Facilities at King Faisal Specialist Hospital & Research Centre for their technical help, and Darren Gilmour for the Cas9 protein. This work was supported by the following: NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development U54HD083091 (Genetics Core and Sub-project 6849) to D.D., NIH National Institute of Neurological Diseases and Stroke (R01NS064077 to D.D. and R01NS050375 to W.B.D.), private donations from families to D.D., the Swiss National Science Foundation (PZ00P3_163979) to R.B.-G., the Netherlands Organization for Scientific Research (NWO Vici-865.12.005) to R.R., the King Salman Center for Disability Research to F.S.A., Dandy-Walker Alliance to W.B.D., and NIH National Human Genome Research Institute (U54HG006493) to M.B. and D.N. We acknowledge the support of the Saudi Human Genome Program (F.S.A.).
Published: June 15, 2017
Footnotes
Supplemental Data include three figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2017.05.010.
Contributor Information
Ruxandra Bachmann-Gagescu, Email: ruxandra.bachmann@imls.uzh.ch.
Dan Doherty, Email: ddoher@uw.edu.
Accession Numbers
The disease-associated variants described in this paper have been uploaded to ClinVar under accession numbers SCV000579394–SCV000579403, available after June 26, 2017.
Web Resources
Burrows-Wheeler Aligner, http://bio-bwa.sourceforge.net/
CHOPCHOP, http://chopchop.cbu.uib.no/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
samtools, https://github.com/samtools/
SeattleSeq Annotation 137, http://snp.gs.washington.edu/SeattleSeqAnnotation137/
UCSC Genome Browser, http://genome.ucsc.edu
Supplemental Data
References
- 1.Valente E.M., Logan C.V., Mougou-Zerelli S., Lee J.H., Silhavy J.L., Brancati F., Iannicelli M., Travaglini L., Romani S., Illi B. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat. Genet. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuz K., Bachmann-Gagescu R., O’Day D.R., Hua K., Isabella C.R., Phelps I.G., Stolarski A.E., O’Roak B.J., Dempsey J.C., Lourenco C. Mutations in CSPP1 cause primary cilia abnormalities and Joubert syndrome with or without Jeune asphyxiating thoracic dystrophy. Am. J. Hum. Genet. 2014;94:62–72. doi: 10.1016/j.ajhg.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas S., Legendre M., Saunier S., Bessières B., Alby C., Bonnière M., Toutain A., Loeuillet L., Szymanska K., Jossic F. TCTN3 mutations cause Mohr-Majewski syndrome. Am. J. Hum. Genet. 2012;91:372–378. doi: 10.1016/j.ajhg.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srour M., Schwartzentruber J., Hamdan F.F., Ospina L.H., Patry L., Labuda D., Massicotte C., Dobrzeniecka S., Capo-Chichi J.M., Papillon-Cavanagh S., FORGE Canada Consortium Mutations in C5ORF42 cause Joubert syndrome in the French Canadian population. Am. J. Hum. Genet. 2012;90:693–700. doi: 10.1016/j.ajhg.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srour M., Hamdan F.F., Schwartzentruber J.A., Patry L., Ospina L.H., Shevell M.I., Désilets V., Dobrzeniecka S., Mathonnet G., Lemyre E., FORGE Canada Consortium Mutations in TMEM231 cause Joubert syndrome in French Canadians. J. Med. Genet. 2012;49:636–641. doi: 10.1136/jmedgenet-2012-101132. [DOI] [PubMed] [Google Scholar]
- 6.Sayer J.A., Otto E.A., O’Toole J.F., Nurnberg G., Kennedy M.A., Becker C., Hennies H.C., Helou J., Attanasio M., Fausett B.V. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 7.Sang L., Miller J.J., Corbit K.C., Giles R.H., Brauer M.J., Otto E.A., Baye L.M., Wen X., Scales S.J., Kwong M. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romani M., Micalizzi A., Kraoua I., Dotti M.T., Cavallin M., Sztriha L., Ruta R., Mancini F., Mazza T., Castellana S. Mutations in B9D1 and MKS1 cause mild Joubert syndrome: expanding the genetic overlap with the lethal ciliopathy Meckel syndrome. Orphanet J. Rare Dis. 2014;9:72. doi: 10.1186/1750-1172-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisi M.A., Bennett C.L., Eckert M.L., Dobyns W.B., Gleeson J.G., Shaw D.W., McDonald R., Eddy A., Chance P.F., Glass I.A. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am. J. Hum. Genet. 2004;75:82–91. doi: 10.1086/421846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.H., Silhavy J.L., Lee J.E., Al-Gazali L., Thomas S., Davis E.E., Bielas S.L., Hill K.J., Iannicelli M., Brancati F. Evolutionarily assembled cis-regulatory module at a human ciliopathy locus. Science. 2012;335:966–969. doi: 10.1126/science.1213506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.E., Silhavy J.L., Zaki M.S., Schroth J., Bielas S.L., Marsh S.E., Olvera J., Brancati F., Iannicelli M., Ikegami K. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat. Genet. 2012;44:193–199. doi: 10.1038/ng.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L., Szymanska K., Jensen V.L., Janecke A.R., Innes A.M., Davis E.E., Frosk P., Li C., Willer J.R., Chodirker B.N. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am. J. Hum. Genet. 2011;89:713–730. doi: 10.1016/j.ajhg.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halbritter J., Bizet A.A., Schmidts M., Porath J.D., Braun D.A., Gee H.Y., McInerney-Leo A.M., Krug P., Filhol E., Davis E.E., UK10K Consortium Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am. J. Hum. Genet. 2013;93:915–925. doi: 10.1016/j.ajhg.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorden N.T., Arts H.H., Parisi M.A., Coene K.L., Letteboer S.J., van Beersum S.E., Mans D.A., Hikida A., Eckert M., Knutzen D. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am. J. Hum. Genet. 2008;83:559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Gonzalo F.R., Corbit K.C., Sirerol-Piquer M.S., Ramaswami G., Otto E.A., Noriega T.R., Seol A.D., Robinson J.F., Bennett C.L., Josifova D.J. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferland R.J., Eyaid W., Collura R.V., Tully L.D., Hill R.S., Al-Nouri D., Al-Rumayyan A., Topcu M., Gascon G., Bodell A. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 17.Edvardson S., Shaag A., Zenvirt S., Erlich Y., Hannon G.J., Shanske A.L., Gomori J.M., Ekstein J., Elpeleg O. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am. J. Hum. Genet. 2010;86:93–97. doi: 10.1016/j.ajhg.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis E.E., Zhang Q., Liu Q., Diplas B.H., Davey L.M., Hartley J., Stoetzel C., Szymanska K., Ramaswami G., Logan C.V., NISC Comparative Sequencing Program TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet. 2011;43:189–196. doi: 10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dafinger C., Liebau M.C., Elsayed S.M., Hellenbroich Y., Boltshauser E., Korenke G.C., Fabretti F., Janecke A.R., Ebermann I., Nürnberg G. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. J. Clin. Invest. 2011;121:2662–2667. doi: 10.1172/JCI43639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coene K.L., Roepman R., Doherty D., Afroze B., Kroes H.Y., Letteboer S.J., Ngu L.H., Budny B., van Wijk E., Gorden N.T. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am. J. Hum. Genet. 2009;85:465–481. doi: 10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantagrel V., Silhavy J.L., Bielas S.L., Swistun D., Marsh S.E., Bertrand J.Y., Audollent S., Attié-Bitach T., Holden K.R., Dobyns W.B., International Joubert Syndrome Related Disorders Study Group Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielas S.L., Silhavy J.L., Brancati F., Kisseleva M.V., Al-Gazali L., Sztriha L., Bayoumi R.A., Zaki M.S., Abdel-Aleem A., Rosti R.O. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baala L., Romano S., Khaddour R., Saunier S., Smith U.M., Audollent S., Ozilou C., Faivre L., Laurent N., Foliguet B. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am. J. Hum. Genet. 2007;80:186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arts H.H., Doherty D., van Beersum S.E., Parisi M.A., Letteboer S.J., Gorden N.T., Peters T.A., Märker T., Voesenek K., Kartono A. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat. Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 25.Badano J.L., Mitsuma N., Beales P.L., Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 26.Dixon-Salazar T., Silhavy J.L., Marsh S.E., Louie C.M., Scott L.C., Gururaj A., Al-Gazali L., Al-Tawari A.A., Kayserili H., Sztriha L., Gleeson J.G. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am. J. Hum. Genet. 2004;75:979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin. Pediatr. Neurol. 2009;16:143–154. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chávez M., Ena S., Van Sande J., de Kerchove d’Exaerde A., Schurmans S., Schiffmann S.N. Modulation of ciliary phosphoinositide content regulates trafficking and Sonic Hedgehog signaling output. Dev. Cell. 2015;34:338–350. doi: 10.1016/j.devcel.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Dyson J.M., Conduit S.E., Feeney S.J., Hakim S., DiTommaso T., Fulcher A.J., Sriratana A., Ramm G., Horan K.A., Gurung R. INPP5E regulates phosphoinositide-dependent cilia transition zone function. J. Cell Biol. 2017;216:247–263. doi: 10.1083/jcb.201511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Gonzalo F.R., Phua S.C., Roberson E.C., Garcia G., 3rd, Abedin M., Schurmans S., Inoue T., Reiter J.F. Phosphoinositides regulate ciliary protein trafficking to modulate Hedgehog signaling. Dev. Cell. 2015;34:400–409. doi: 10.1016/j.devcel.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He M., Subramanian R., Bangs F., Omelchenko T., Liem K.F., Jr., Kapoor T.M., Anderson K.V. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat. Cell Biol. 2014;16:663–672. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko H.W., Norman R.X., Tran J., Fuller K.P., Fukuda M., Eggenschwiler J.T. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev. Cell. 2010;18:237–247. doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariani L.E., Bijlsma M.F., Ivanova A.I., Suciu S.K., Kahn R.A., Caspary T. Arl13b regulates Shh signaling from both inside and outside the cilium. Mol. Biol. Cell. 2016 doi: 10.1091/mbc.E16-03-0189. mbc.E16-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin J., Lin Y., Norman R.X., Ko H.W., Eggenschwiler J.T. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc. Natl. Acad. Sci. USA. 2011;108:1456–1461. doi: 10.1073/pnas.1011410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clement D.L., Mally S., Stock C., Lethan M., Satir P., Schwab A., Pedersen S.F., Christensen S.T. PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J. Cell Sci. 2013;126:953–965. doi: 10.1242/jcs.116426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider L., Clement C.A., Teilmann S.C., Pazour G.J., Hoffmann E.K., Satir P., Christensen S.T. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Bachmann-Gagescu R., Dona M., Hetterschijt L., Tonnaer E., Peters T., de Vrieze E., Mans D.A., van Beersum S.E., Phelps I.G., Arts H.H. The ciliopathy protein CC2D2A associates with NINL and functions in RAB8-MICAL3-regulated vesicle trafficking. PLoS Genet. 2015;11:e1005575. doi: 10.1371/journal.pgen.1005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachmann-Gagescu R., Phelps I.G., Stearns G., Link B.A., Brockerhoff S.E., Moens C.B., Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum. Mol. Genet. 2011;20:4041–4055. doi: 10.1093/hmg/ddr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craige B., Tsao C.C., Diener D.R., Hou Y., Lechtreck K.F., Rosenbaum J.L., Witman G.B. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoover A.N., Wynkoop A., Zeng H., Jia J., Niswander L.A., Liu A. C2cd3 is required for cilia formation and Hedgehog signaling in mouse. Development. 2008;135:4049–4058. doi: 10.1242/dev.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lancaster M.A., Gopal D.J., Kim J., Saleem S.N., Silhavy J.L., Louie C.M., Thacker B.E., Williams Y., Zaki M.S., Gleeson J.G. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat. Med. 2011;17:726–731. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotnikova O.V., Seo S., Cottle D.L., Conduit S., Hakim S., Dyson J.M., Mitchell C.A., Smyth I.M. INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J. Cell Sci. 2015;128:364–372. doi: 10.1242/jcs.161323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singla V., Romaguera-Ros M., Garcia-Verdugo J.M., Reiter J.F. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell. 2010;18:410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slaats G.G., Saldivar J.C., Bacal J., Zeman M.K., Kile A.C., Hynes A.M., Srivastava S., Nazmutdinova J., den Ouden K., Zagers M.S. DNA replication stress underlies renal phenotypes in CEP290-associated Joubert syndrome. J. Clin. Invest. 2015;125:3657–3666. doi: 10.1172/JCI80657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye X., Zeng H., Ning G., Reiter J.F., Liu A. C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proc. Natl. Acad. Sci. USA. 2014;111:2164–2169. doi: 10.1073/pnas.1318737111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Roak B.J., Vives L., Fu W., Egertson J.D., Stanaway I.B., Phelps I.G., Carvill G., Kumar A., Lee C., Ankenman K. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachmann-Gagescu R., Dempsey J.C., Phelps I.G., O’Roak B.J., Knutzen D.M., Rue T.C., Ishak G.E., Isabella C.R., Gorden N., Adkins J., University of Washington Center for Mendelian Genomics Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J. Med. Genet. 2015;52:514–522. doi: 10.1136/jmedgenet-2015-103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong J.X., Buckingham K.J., Jhangiani S.N., Boehm C., Sobreira N., Smith J.D., Harrell T.M., McMillin M.J., Wiszniewski W., Gambin T., Centers for Mendelian Genomics The genetic basis of Mendelian phenotypes: discoveries, challenges, and opportunities. Am. J. Hum. Genet. 2015;97:199–215. doi: 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paila U., Chapman B.A., Kirchner R., Quinlan A.R. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput. Biol. 2013;9:e1003153. doi: 10.1371/journal.pcbi.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tennessen J.A., Bigham A.W., O’Connor T.D., Fu W., Kenny E.E., Gravel S., McGee S., Do R., Liu X., Jun G., Broad GO. Seattle GO. NHLBI Exome Sequencing Project Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller J.A., Ding S.L., Sunkin S.M., Smith K.A., Ng L., Szafer A., Ebbert A., Riley Z.L., Royall J.J., Aiona K. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.GTEx Consortium The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alkuraya F.S. Autozygome decoded. Genet. Med. 2010;12:765–771. doi: 10.1097/GIM.0b013e3181fbfcc4. [DOI] [PubMed] [Google Scholar]
- 56.Alkuraya F.S. Discovery of rare homozygous mutations from studies of consanguineous pedigrees. Curr Protoc Hum Genet. 2012;Chapter 6 doi: 10.1002/0471142905.hg0612s75. Unit6 12. [DOI] [PubMed] [Google Scholar]
- 57.Saudi Mendeliome Group Comprehensive gene panels provide advantages over clinical exome sequencing for Mendelian diseases. Genome Biol. 2015;16:134. doi: 10.1186/s13059-015-0693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mefford H.C., Yendle S.C., Hsu C., Cook J., Geraghty E., McMahon J.M., Eeg-Olofsson O., Sadleir L.G., Gill D., Ben-Zeev B. Rare copy number variants are an important cause of epileptic encephalopathies. Ann. Neurol. 2011;70:974–985. doi: 10.1002/ana.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kane M.S., Davids M., Bond M.R., Adams C.J., Grout M.E., Phelps I.G., O’Day D.R., Dempsey J.C., Li X., Golas G. Abnormal glycosylation in Joubert syndrome type 10. Cilia. 2017;6:2. doi: 10.1186/s13630-017-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slaats G.G., Isabella C.R., Kroes H.Y., Dempsey J.C., Gremmels H., Monroe G.R., Phelps I.G., Duran K.J., Adkins J., Kumar S.A. MKS1 regulates ciliary INPP5E levels in Joubert syndrome. J. Med. Genet. 2016;53:62–72. doi: 10.1136/jmedgenet-2015-103250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stratigopoulos G., Martin Carli J.F., O’Day D.R., Wang L., Leduc C.A., Lanzano P., Chung W.K., Rosenbaum M., Egli D., Doherty D.A., Leibel R.L. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 2014;19:767–779. doi: 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westerfield M. M. Westerfield; Eugene, OR: 1993. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) [Google Scholar]
- 63.Zou J., Lathrop K.L., Sun M., Wei X. Intact retinal pigment epithelium maintained by Nok is essential for retinal epithelial polarity and cellular patterning in zebrafish. J. Neurosci. 2008;28:13684–13695. doi: 10.1523/JNEUROSCI.4333-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hartmann C., von Spiczak S., Suls A., Weckhuysen S., Buyse G., Vilain C., Van Bogaert P., De Jonghe P., Cook J., Muhle H. Investigating the genetic basis of fever-associated syndromic epilepsies using copy number variation analysis. Epilepsia. 2015;56:e26–e32. doi: 10.1111/epi.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mefford H.C., Muhle H., Ostertag P., von Spiczak S., Buysse K., Baker C., Franke A., Malafosse A., Genton P., Thomas P. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaheen R., Szymanska K., Basu B., Patel N., Ewida N., Faqeih E., Al Hashem A., Derar N., Alsharif H., Aldahmesh M.A., Ciliopathy WorkingGroup Characterizing the morbid genome of ciliopathies. Genome Biol. 2016;17:242. doi: 10.1186/s13059-016-1099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poretti A., Huisman T.A., Scheer I., Boltshauser E. Joubert syndrome and related disorders: spectrum of neuroimaging findings in 75 patients. AJNR Am. J. Neuroradiol. 2011;32:1459–1463. doi: 10.3174/ajnr.A2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friede R.L., Boltshauser E. Uncommon syndromes of cerebellar vermis aplasia. I: Joubert syndrome. Dev. Med. Child Neurol. 1978;20:758–763. doi: 10.1111/j.1469-8749.1978.tb15307.x. [DOI] [PubMed] [Google Scholar]
- 69.Juric-Sekhar G., Adkins J., Doherty D., Hevner R.F. Joubert syndrome: brain and spinal cord malformations in genotyped cases and implications for neurodevelopmental functions of primary cilia. Acta Neuropathol. 2012;123:695–709. doi: 10.1007/s00401-012-0951-2. [DOI] [PubMed] [Google Scholar]
- 70.Poretti A., Brehmer U., Scheer I., Bernet V., Boltshauser E. Prenatal and neonatal MR imaging findings in oral-facial-digital syndrome type VI. AJNR Am. J. Neuroradiol. 2008;29:1090–1091. doi: 10.3174/ajnr.A1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yachnis A.T., Rorke L.B. Neuropathology of Joubert syndrome. J. Child Neurol. 1999;14:655–659. doi: 10.1177/088307389901401006. discussion 669–672. [DOI] [PubMed] [Google Scholar]
- 72.Romio L., Fry A.M., Winyard P.J., Malcolm S., Woolf A.S., Feather S.A. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J. Am. Soc. Nephrol. 2004;15:2556–2568. doi: 10.1097/01.ASN.0000140220.46477.5C. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi T., Kim S., Lin Y.C., Inoue T., Dynlacht B.D. The CP110-interacting proteins Talpid3 and Cep290 play overlapping and distinct roles in cilia assembly. J. Cell Biol. 2014;204:215–229. doi: 10.1083/jcb.201304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin Y., Bangs F., Paton I.R., Prescott A., James J., Davey M.G., Whitley P., Genikhovich G., Technau U., Burt D.W., Tickle C. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–664. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenbaum J.L., Moulder J.E., Ringo D.L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J. Cell Biol. 1969;41:600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamm S.L. The effect of enucleation on flagellar regeneration in the protozoon Peranema trichophorum. J. Cell Sci. 1969;4:171–178. doi: 10.1242/jcs.4.1.171. [DOI] [PubMed] [Google Scholar]
- 77.Chhin B., Pham J.T., El Zein L., Kaiser K., Merrot O., Bouvagnet P. Identification of transcripts overexpressed during airway epithelium differentiation. Eur. Respir. J. 2008;32:121–128. doi: 10.1183/09031936.00172107. [DOI] [PubMed] [Google Scholar]
- 78.Stolc V., Samanta M.P., Tongprasit W., Marshall W.F. Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc. Natl. Acad. Sci. USA. 2005;102:3703–3707. doi: 10.1073/pnas.0408358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lefebvre P.A., Nordstrom S.A., Moulder J.E., Rosenbaum J.L. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J. Cell Biol. 1978;78:8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ocbina P.J., Anderson K.V. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev. Dyn. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimes D.T., Boswell C.W., Morante N.F., Henkelman R.M., Burdine R.D., Ciruna B. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science. 2016;352:1341–1344. doi: 10.1126/science.aaf6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vilboux T., Doherty D.A., Glass I.A., Parisi M.A., Phelps I.G., Cullinane A.R., Zein W., Brooks B.P., Heller T., Soldatos A., Nisc Comparative Sequencing Program Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet. Med. 2017 doi: 10.1038/gim.2016.204. Published online January 26, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keller L.C., Romijn E.P., Zamora I., Yates J.R., 3rd, Marshall W.F. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr. Biol. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 84.Mateja A., Cierpicki T., Paduch M., Derewenda Z.S., Otlewski J. The dimerization mechanism of LIS1 and its implication for proteins containing the LisH motif. J. Mol. Biol. 2006;357:621–631. doi: 10.1016/j.jmb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Delto C.F., Heisler F.F., Kuper J., Sander B., Kneussel M., Schindelin H. The LisH motif of muskelin is crucial for oligomerization and governs intracellular localization. Structure. 2015;23:364–373. doi: 10.1016/j.str.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Ishikawa H., Thompson J., Yates J.R., 3rd, Marshall W.F. Proteomic analysis of mammalian primary cilia. Curr. Biol. 2012;22:414–419. doi: 10.1016/j.cub.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mick D.U., Rodrigues R.B., Leib R.D., Adams C.M., Chien A.S., Gygi S.P., Nachury M.V. Proteomics of primary cilia by proximity labeling. Dev. Cell. 2015;35:497–512. doi: 10.1016/j.devcel.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J.B., Gerdes J.M., Haycraft C.J., Fan Y., Teslovich T.M., May-Simera H., Li H., Blacque O.E., Li L., Leitch C.C. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 89.Avidor-Reiss T., Maer A.M., Koundakjian E., Polyanovsky A., Keil T., Subramaniam S., Zuker C.S. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 90.Coordinators N.R., NCBI Resource Coordinators Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44(D1):D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bae Y.K., Barr M.M. Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans. Front. Biosci. 2008;13:5959–5974. doi: 10.2741/3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kunitomo H., Uesugi H., Kohara Y., Iino Y. Identification of ciliated sensory neuron-expressed genes in Caenorhabditis elegans using targeted pull-down of poly(A) tails. Genome Biol. 2005;6:R17. doi: 10.1186/gb-2005-6-2-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vázquez-Manrique R.P., González-Cabo P., Ortiz-Martín I., Ros S., Baylis H.A., Palau F. The frataxin-encoding operon of Caenorhabditis elegans shows complex structure and regulation. Genomics. 2007;89:392–401. doi: 10.1016/j.ygeno.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 94.Lih C.J., Cohen S.N., Wang C., Lin-Chao S. The platelet-derived growth factor alpha-receptor is encoded by a growth-arrest-specific (gas) gene. Proc. Natl. Acad. Sci. USA. 1996;93:4617–4622. doi: 10.1073/pnas.93.10.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh S.D., Chen Y.J., Chang A.C., Ray R., She B.R., Lee W.S., Chiang H.S., Cohen S.N., Lin-Chao S. Isolation and properties of Gas8, a growth arrest-specific gene regulated during male gametogenesis to produce a protein associated with the sperm motility apparatus. J. Biol. Chem. 2002;277:6311–6317. doi: 10.1074/jbc.M106941200. [DOI] [PubMed] [Google Scholar]
- 96.Kim Y.H., Epting D., Slanchev K., Engel C., Walz G., Kramer-Zucker A. A complex of BBS1 and NPHP7 is required for cilia motility in zebrafish. PLoS ONE. 2013;8:e72549. doi: 10.1371/journal.pone.0072549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun Z., Amsterdam A., Pazour G.J., Cole D.G., Miller M.S., Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 98.Lessieur E.M., Fogerty J., Gaivin R.J., Song P., Perkins B.D. The ciliopathy gene ahi1 is required for zebrafish cone photoreceptor outer segment morphogenesis and survival. Invest. Ophthalmol. Vis. Sci. 2017;58:448–460. doi: 10.1167/iovs.16-20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krock B.L., Perkins B.D. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J. Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sukumaran S., Perkins B.D. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 intraflagellar transport mutants. Vision Res. 2009;49:479–489. doi: 10.1016/j.visres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.