Abstract
Yellow fever virus (YFV) causing a deadly viral disease is transmitted by the bite of infected mosquitoes. In Brazil, YFV is restricted to a forest cycle maintained between non-human primates and forest-canopy mosquitoes, where humans can be tangentially infected. Since late 2016, a growing number of human cases have been reported in Southeastern Brazil at the gates of the most populated areas of South America, the Atlantic coast, with Rio de Janeiro state hosting nearly 16 million people. We showed that the anthropophilic mosquitoes Aedes aegypti and Aedes albopictus as well as the YFV-enzootic mosquitoes Haemagogus leucocelaenus and Sabethes albiprivus from the YFV-free region of the Atlantic coast were highly susceptible to American and African YFV strains. Therefore, the risk of reemergence of urban YFV epidemics in South America is major with a virus introduced either from a forest cycle or by a traveler returning from the YFV-endemic region of Africa.
Introduction
Yellow fever (YF) is an arboviral disease endemic to tropical regions of Africa and South America that may cause hemorrhagic fever in humans. The etiologic agent is the yellow fever virus (YFV), the prototype member of the genus Flavivirus (family Flaviviridae). It is a single-stranded, positive sense RNA virus with a genome of approximately 11 kb. Until now, seven lineages have been identified: five in Africa, with two in West Africa (West Africa I and II) and three in East and Central Africa (East Africa, East/Central Africa, and Angola), and two in the Americas (South America I and II)1–3. The YFV strains circulating in the Americas derived from a Western African lineage ancestor, and most isolates from Brazil belong to the South American genotype I4, 5. These findings are in line with the hypothesis that YFV emerged in Africa and was imported into the American East coast from West Africa during the slave trade which has undoubtedly favored the introduction of the African YF mosquito Aedes (Stegomyia) aegypti (Linnaeus)2, 4.
Despite the availability of effective vaccines, YF remains an important public health issue in Africa and South America, with an annual incidence of around 200,000 cases and 30,000 deaths6. The 2015–2016 YFV epidemic in Angola exemplifies the threat posed by the reemergence of this virus; a six-month urban outbreak in Angola reported 4,000 suspected cases and ~600 deaths. The epidemic reached the Democratic Republic of Congo, and viremic people dispersed to densely populated and Aedes-infected zones, in and outside Africa (e.g. China)7.
Yellow fever is a zoonotic disease with two main epidemiological transmission cycles: the forest and the urban cycles. Traditionally, the YFV is transmitted within a forest cycle between non-human primates (NHPs) and canopy-breeding mosquitoes. During epizootics of YF, humans can be contaminated if they live close to the forest fringe or when entering into the wild. While YFV in Africa circulates within a forest cycle before emergence in an urban cycle involving humans and Ae. aegypti, it has remained an enzootic virus restricted to rainforests and savannah-like forests in the Americas since the 1940s3, 8.
In the Americas, the disease was a major human health issue from the 18th to the early 20th century with devastating urban epidemics. However, the development of two attenuated vaccines in the 1930s as well as a continental eradication program of Ae. aegypti led to a clearance of urban YF3. Brazil was certified free of Ae. aegypti in 19579. Over the ensuing decades, human YFV infections have been acquired only in the forest cycle, despite the reinvasion of Ae. aegypti in Brazil from the late 1960s onwards10. Today, the forest YFV cycle is still very active in Brazil, and generates outbreaks every 6–10 years in the Southern, Southeast and Central-West regions, and every 14 years, in the Amazon8, 11. Humans are contaminated by the bite of forest canopy-dwelling mosquitoes of the genera Haemagogus (primary vectors) and Sabethes (secondary vectors)4.
Since the late 1990s, YFV has extended its traditional range of distribution reaching southern and southeastern regions in Brazil, approaching the most densely populated and highly Aedes-infested cities having low vaccination coverage12–14. Thus, the YFV lineage belonging to the South America genotype I (named 1D) responsible for epizootics from 1998 to 20014 has been replaced by a new viral lineage (1E) causing massive deaths of howler monkeys (Alouatta caraya) and human cases (Fig. 1)15. Since late 2016, a severe YFV epidemic has been reported in southeastern Brazil, causing 79 laboratory confirmed deaths. Most alarmingly, the ongoing epidemic has been progressively spreading toward the Atlantic coast, causing deaths of NHPs and humans in a zone free of YF for more than 70 years but highly infested by Ae. aegypti and Ae. albopictus (Brazilian Ministry of Health, “Ministério da Saúde divulga novos dados de febre amarela”, 2017; http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/27482-ministerio-da-saude-divulga-novos-dados-de-febre-amarela). If the re-establishment of Ae. aegypti posed a threat by itself16, the invasion and spread of Ae. albopictus since the late 1980s have stepped up the risk of urban YFV outbreaks in Brazil (Fig. 1)13. Moreover, some Brazilian populations of both Ae. aegypti and Ae. albopictus were susceptible to YFV17–19.
Figure 1.
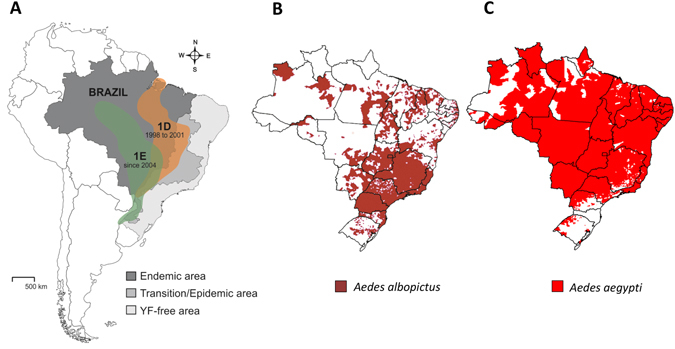
Geographic distribution of YFV strains (71016-1D and 4408-1E) (A), the mosquitoes Ae. aegypti (B) and Ae. albopictus (C) according to ref. 13. The map was created using software the CorelDraw X5 software (http://www.coreldraw.com/br/).
The mosquito Ae. albopictus is a very opportunistic species able to colonize a wide range of habitats besides being willing to feed on different mammals. It may also move from the forest to peri-urban sites and vice-versa20, 21. Coincidentally, the highest infestation indexes for Ae. albopictus in Brazil are reported in the Southeastern and Southern regions where YFV is now circulating13. We then hypothesize that YFV-competent Ae. albopictus may play the role of “bridge vector” linking the forest cycle to the urban YFV cycle. To address the question of the potential role of Ae. albopictus in the urban expansion of zoonotic YFV, we compare the vector competence for three YFV isolates of Brazilian and African populations of Ae. albopictus as well as of co-occurring populations of Ae. aegypti, and Neotropical sylvatic vectors from genera Haemagogus and Sabethes.
Results
Aedes mosquitoes were efficient to disseminate and transmit from day 14 post-infection
To define the days after infection for examining viral dissemination and transmission in Ae. aegypti and Ae. albopictus, we infected Ae. aegypti AE-GOI and Ae. albopictus AL-GOI collected in Goiânia in the state of Goiás, Central Brazil, in the YFV-epidemic/epizootic region with three YFV strains (two from Brazil (74018-1D and 4408-1E) and one from Senegal (S-79)). When examining AE-GOI, mosquitoes were infected and disseminate YFV from 7 days post-infection (dpi), and viral particles were only detected in mosquito saliva at 14 dpi (Fig. 2). For AL-GOI, infection and dissemination started from 7 dpi and detection of virus in saliva from 14 dpi (Fig. 2).
Figure 2.
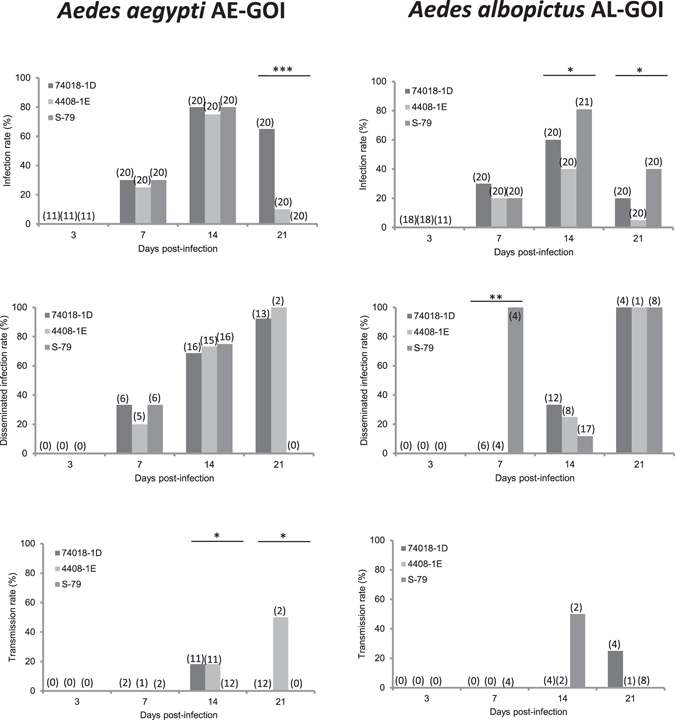
Infection, Dissemination and Transmission of YFV by Aedes aegypti AA-GOI and Aedes albopictus AL-GOI from the epizootic/epidemic region of Goiânia. Mosquitoes were exposed to blood meals at a titer of 106 PFU/mL. Engorged females were maintained in laboratory conditions until examination at 3, 7, 14, 21 days post-infection. Mosquito thorax and abdomen were processed individually to determine the infection rate (IR, proportion of mosquitoes with infected body among the engorged mosquitoes). The mosquito head was used to define the disseminated infection rate (DIR, proportion of mosquitoes with infected head among infected mosquitoes) and the saliva collected from individual females to determine the transmission rate (TR, proportion of mosquitoes with infectious saliva among mosquitoes with disseminated infection). Asterisks refer to a significant difference (*p < 0.05, **p < 10−2, ***p < 10−3). In brackets, the number of mosquitoes tested.
Interestingly, out of 447 female mosquitoes studied at 3 dpi, no mosquitoes showed infection. Therefore, specimens examined at 3 dpi were not considered in further analysis. Moreover, considering all Ae. aegypti populations on the one hand, and all Ae. albopictus populations on the other hand, logistic regression models showed similar rates of infection at 14 and 21 dpi (p = 0.10 and p = 0.73, respectively). Therefore, all further analyses were conducted considering 14 and 21 dpi together.
Brazilian Aedes aegypti were susceptible to American as well as African YFV genotypes
To determine if Ae. aegypti populations from Brazil were competent vectors of YFV, three populations (AE-MAN, AE-GOI, AE-RIO collected in enzootic-, epizootic/epidemic- and free-YFV areas, respectively; Fig. 3) were experimentally infected with three YFV strains (74018-1D, 4408-1E and S-79). When analyzing viral infection, AE-MAN, AE-GOI and AE-RIO presented significant differences of IR for the three YFV strains (p < 0.05, Fig. 3) with values ranging from 30% (AE-MAN infected with S-79) to 85% (AE-RIO infected with 4408-1E). After controlling for population, virus and dpi, level of infection was higher with the strain 74018-1D in AE-GOI, was higher with the strain 4408-1E and 74018-1D in AE-MAN, while no difference was observed in AE-RIO (Table 1).
Figure 3.
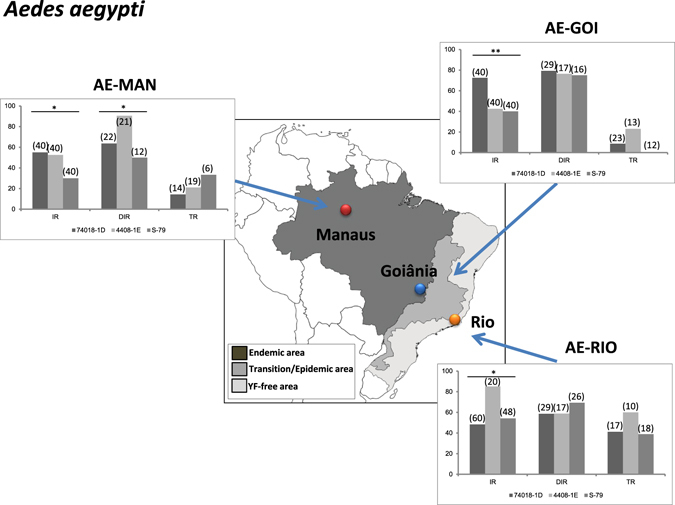
Vector competence of three Aedes aegypti populations (AA-MAN, AA-GOI and AA-RIO) for three YFV strains (74018-1D, 4408-1E and S-79). Mosquitoes were exposed to blood meals at a titer of 106 PFU/mL. Engorged females were maintained in laboratory conditions until days 14–21 post-infection. Mosquitoes were processed as previously described to determine the infection rate (IR), the disseminated infection rate (DIR) and the transmission rate (TR). Asterisks refer to a significant difference (*p < 0.05, **p < 10−2). In brackets, the number of mosquitoes tested. The map was created using software the CorelDraw X5 software (http://www.coreldraw.com/br/).
Table 1.
Comparison of infection rates (logistic regression model*).
| Population | Virus | Day post-infection | Species | |
|---|---|---|---|---|
| Aedes aegypti (AE) | Aedes albopictus (AL) | |||
| Goiânia (GOI) | 4408-1E | 7 | 1 | 0.46 (0.26–0.83) |
| 14–21 | 2.58 (1.58–4.22) | 1.19 (0.56–2.52) | ||
| 74018-1D | 7 | 2.10 (1.13–3.91) | 1.20 (0.59–2.42) | |
| 14–21 | 5.43 (2.41–12.23) | 3.10 (1.30–7.37) | ||
| S-79 | 7 | 1.19 (0.64–2.21) | 1.34 (0.66–2.70) | |
| 14–21 | 3.08 (1.39–6.85) | 3.45 (1.46–8.17) | ||
| Manaus (MAN) | 4408-1E | 7 | 2.87 (1.26–6.50) | 0.32 (0.12–0.88) |
| 14–21 | 3.85 (1.79–8.24) | 0.43 (0.17–1.08) | ||
| 74018-1D | 7 | 2.75 (1.16–6.54) | 0.38 (0.15–0.97) | |
| 14–21 | 3.68 (1.67–8.09) | 0.51 (0.23–1.12) | ||
| S-79 | 7 | 1.41 (0.58–3.33) | 0.38 (0.15–0.98) | |
| 14–21 | 1.89 (0.84–4.22) | 0.51 (0.22–1.19) | ||
| Rio de Janeiro (RIO) | 4408-1E | 7 | 5.90 (2.49–13.99) | 0.28 (0.10–0.78) |
| 14–21 | 7.47 (3.20–17.44) | 0.36 (0.13–0.95) | ||
| 74018-1D | 7 | 3.28 (1.41–7.63) | 0.19 (0.07–0.53) | |
| 14–21 | 4.15 (1.96–8.79) | 0.29 (0.10–0.61) | ||
| S-79 | 7 | 3.64 (1.56–8.49) | 0.42 (0.17–1.08) | |
| 14–21 | 4.61 (2.13–9.98) | 0.53 (0.23–1.26) | ||
| Congo (CON) | 4408-1E | 7 | 2.82 (1.27–6.25) | 1.57 (0.67–3.82) |
| 14–21 | 2.44 (1.18–5.06) | 1.39 (0.61–3.17) | ||
| 74018-1D | 7 | 1.36 (0.55–3.39) | 0.96 (0.41–2.23) | |
| 14–21 | 1.18 (0.51–2.71) | 0.83 (0.38–1.84) | ||
| S-79 | 7 | 1.59 (0.65–3.91) | 2.20 (0.99–4.88) | |
| 14–21 | 1.38 (0.61–3.12) | 1.91 (0.91–2.98) | ||
*Model with interaction between species and population, between species and virus, between population and virus, and also between population and day post-infection.
After midgut infection, the virus must propagate inside the mosquito hemocele to allow detecting a positive viral dissemination. Dissemination as determined by the presence of virus in mosquito heads, were similar regardless of the YFV strain except for AE-MAN which presented higher viral dissemination of YFV 4408-1E (90.47%) (Fig. 3, Table 2).
Table 2.
Comparison of disseminated infection rates (logistic regression model*).
| Population | Virus | Species | |
|---|---|---|---|
| Aedes aegypti (AE) | Aedes albopictus (AL) | ||
| Adj. OR [95% CI] | Adj. OR [95% CI] | ||
| Goiânia (GOI) | 4408-1E | 1 | 0.28 (0.14–0.58) |
| 74018-1D | 1.49 (0.60–3.70) | 0.42 (0.14–1.31) | |
| S-79 | 1.64 (0.64–4.18) | 0.46 (0.16–1.33) | |
| Manaus (MAN) | 4408-1E | 3.76 (1.16–12.17) | 6.84 (1.38–33.81) |
| 74018-1D | 1.03 (0.37–2.86) | 1.88 (0.46–7.69) | |
| S-79 | 1.00 (0.31–3.22) | 1.82 (0.48–6.95) | |
| Rio de Janeiro (RIO) | 4408-1E | 1.43 (0.48–4.27) | 1.36 (0.35–5.32) |
| 74018-1D | 0.93 (0.35–2.45) | 0.88 (0.23–3.42) | |
| S-79 | 1.08 (0.41–2.86) | 1.03 (0.26–4.13) | |
| Congo (CON) | 4408-1E | 6.30 (1.85–21.46) | 5.31 (1.47–19.16) |
| 74018-1D | 3.94 (0.93–16.65) | 3.31 (0.85–12.95) | |
| S-79 | 1.12 (0.36–3.47) | 0.94 (0.33–2.66) | |
*Adjusted for time, with interaction between species and population and also between population and viral strain.
For viral transmission to occur, the virus in the hemocele must reach mosquito salivary glands and be excreted with saliva expectorated by the mosquito. TR as determined by the presence of virus in saliva, were similar for all three Brazilian Ae. aegypti populations regardless of the YFV strain (p > 0.05; Fig. 3, Table 3). These results suggest that the salivary glands behave as a more efficient barrier for the release of viruses than the midgut for dissemination. Moreover, all mosquito populations present similar ability to deliver particles of the two YFV strains in saliva: 74018-1D responsible for epizootics from 1998 to 20014 and the new viral lineage, 4408-1E, causing increasing deaths of monkeys and human cases15.
Table 3.
Comparison of transmission rates (logistic regression model*).
| Adjusted OR (95% CI) | P | |
|---|---|---|
| Species | ||
| Aedes aegypti (AE) | 1 | 0.95 |
| Aedes albopictus (AL) | 0.98 (0.52–1.83) | |
| Population | ||
| Congo (CON) | 6.97 (2.75–17.65) | <0.001 |
| Goiânia (GOI) | 1 | |
| Manaus (MAN) | 2.63 (0.97–7.10) | |
| Rio de Janeiro (RIO) | 7.93 (3.08–20.39) | |
| Virus | ||
| 4408-1E | 1 | 0.14 |
| 74018-1D | 0.70 (0.36–1.38) | |
| S-79 | 0.49 (0.24–1.00) | |
| Day post-infection | ||
| 7 | 1 | <0.001 |
| 14–21 | 13.98 (4.12–47.48) | |
*No significant interaction.
Aedes albopictus in Rio de Janeiro were very efficient to deliver particles of YFV from their saliva
To determine if Ae. albopictus populations from Brazil were as competent vectors as Ae. aegypti for YFV, Ae. albopictus from Manaus (AL-MAN), Goiânia (AL-GOI) and Rio (AL-RIO) were experimentally infected with three YFV strains (two from Brazil and one from Senegal). When comparing viral infection, AL-MAN and AL-RIO presented low (ranging from 10% for AL-RIO infected with S-79 to 21.42% for AL-MAN infected with S-79; p > 0.05; Fig. 4), and comparable IR values whatever the viral strain (p = 0.71 and p = 0.19, respectively; Table 1). On the other hand, AL-GOI showed significant differences (p < 0.05) with a higher IR value for S-79 (60.97%) (Table 1). It must also be noted that the rate of infection was significantly lower in Ae. albopictus than in Ae. aegypti for all viral strains, except in females from Goiânia when infected with S79.
Figure 4.
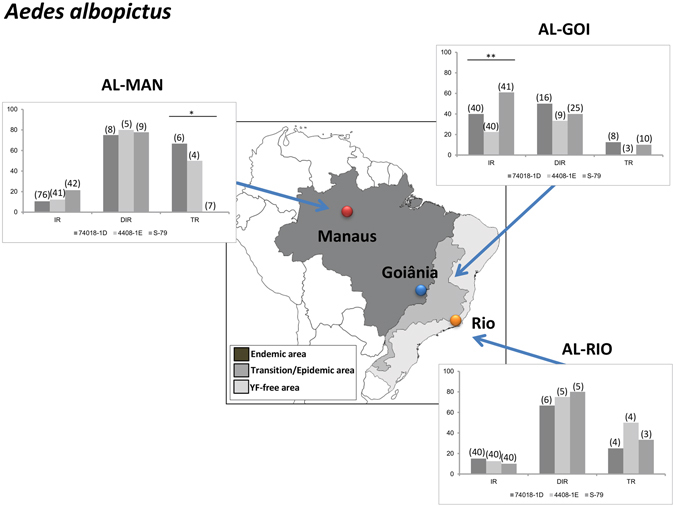
Vector competence of three Aedes albopictus populations (MAA, GOA and PMNI) for three YFV strains (74018-1D, 4408-1E and S-79). Mosquitoes were exposed to blood meals at a titer of 106 PFU/mL. Engorged females were maintained in laboratory conditions until days 14–21 post-infection. Mosquitoes were processed as previously described to calculate the infection rate (IR), the disseminated infection rate (DIR) and the transmission rate (TR). Asterisks refer to a significant difference (*p < 0.05, **p < 10−2). In brackets, the number of mosquitoes tested. The map was created using software the CorelDraw X5 software (http://www.coreldraw.com/br/).
When analyzing viral dissemination represented by DIR, dissemination was lower in AL-GOI, then higher in AL-RIO and then even higher in AL-MAN (Fig. 4, Table 2). All three Ae. albopictus populations showed similar values (p > 0.05) for the three YFV, although for AL-MAN, dissemination tended to be higher with viral strain 1E (Table 2). The highest DIR values were obtained for AL-MAN and AL-RIO populations. Interestingly, dissemination was similar in AL and in AE from Manaus and from Rio; while in female mosquitoes from GOI, the dissemination was significantly lower in AL than in AE (Table 2).
When considering viral transmission described by TR, AL-MAN population showed high TR values but based on low sample sizes (66.67% (6) with 74018-1D and 50% (4) with 4408-1E) and surprisingly, was not able to transmit the S-79 YFV from Africa. The population AL-GOI collected in the epizootic/epidemic region were able to excrete similarly all three YFV (p > 0.05) but was less efficient than AL-RIO (Fig. 4). These results suggest that AL-RIO and AL-MAN shared the same pattern of infection, dissemination and transmission with low IR, high DIR and high TR values suggesting the role of the midgut as the main barrier in the trajectory of the virus to the mosquito salivary glands. Interestingly, AL-GOI was less susceptible to YFV than the other two mosquito populations. Like Ae. aegypti, Ae. albopictus were similarly susceptible to the former YFV 74018-1D lineage and the new viral lineage 4408-1E (Table 3).
Aedes mosquitoes from an African YFV-endemic country were similarly susceptible to American as well as African YFV genotypes
To test whether Ae. aegypti and Ae. albopictus from Congo were competent for both Brazilian and West African YFV strains, mosquitoes were infected with the three YFV strains. The population AE-CON and AL-CON showed IR ranging between 25% (when infected with 74018-1D) and 38.6% (when infected with 4408-1E). Whatever the viral strain, the infection rate was not different between AE-CON and AL-CON (p > 0.05; Table 1)
Regarding dissemination, AE-CON and AL-CON also showed much higher viral dissemination rates than the other female mosquitoes (Fig. 5). Interestingly, American viral strains led to significantly higher dissemination than the African one both for AE-CON and for AL-CON (p < 0.05), but we did not observe any difference between AE-CON and AL-CON (p = 0.72).
Figure 5.
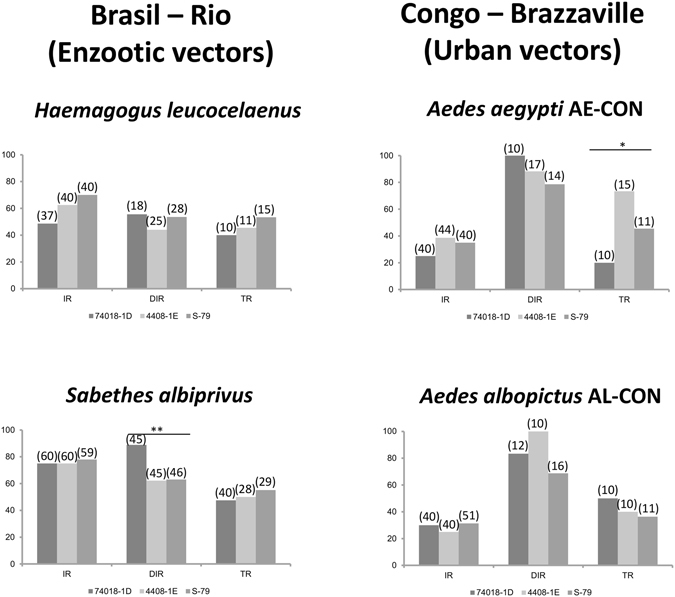
Vector competence to YFV of Brazilian enzootic vectors (Haemagogus leucocelaenus and Sabethes albiprivus) and Congolese domestic vectors (Aedes aegypti and Aedes albopictus) used as controls of YFV infection. Mosquitoes were on an infectious blood meal provided at a titer of 106 PFU/mL. Mosquitoes were processed as previously described. IR indicates to the infection rate, DIR to the disseminated infection rate and TR to the transmission rate. Asterisks refer to a significant difference (*p < 0.05, **p < 10−2). In brackets, the number of mosquitoes tested.
Viral transmission measured by TR was slightly lower except when infected with the YFV 4408-1E strain (73.33%). On the other hand, AL-CON presented roughly similar patterns than AE-CON with IR lower than 31.37% (S-79) and DIR higher than 68.75% (S-79). TR did not differ significantly (p > 0.05), varying from 36.36% (S-79) to 50% (74018-1D) strains. Thus Ae. aegypti and Ae. albopictus from Congo, city of Brazzaville presented similar vector competence indices when infected with YFV strains belonging to both the South America I and West Africa genotypes.
Wild YFV vectors Hemagogus and Sabethes from Rio de Janeiro were highly competent to transmit Brazilian and African YFV strains
To examine if enzootic mosquitoes from Brazil, Hg. leucocelaenus and Sa. albiprivus were as susceptible as Ae. aegypti and Ae. albopictus, mosquitoes were infected with the three YFV strains. Both enzootic mosquito species showed pattern of infection similar to Ae. aegypti while Ae. albopictus was significantly less often infected (as previously shown in this study). Overall, dissemination occurred in 64.3% of mosquitoes, this rate of dissemination was not different between the four species (p = 0.34), between the three viruses (p = 0.14). Overall, transmission was observed in 36.6% and was not different between the four species (p = 0.85) nor between the three viruses (p = 0.95). IRs were higher than 48%, DIRs higher than 44% and TRs higher than 40% suggesting a limited role of both barriers, midgut and salivary glands, in the virus migratory route inside the mosquito. When mosquitoes with infectious saliva were considered according to the initial number of females tested, transmission efficiencies (TE) varied from 10.81% to 20% for Hg. leucocelaenus and 23.33% to 31.66% for Sa. albiprivus (Supplementary Figure). Thus enzootic YFV vectors from Brazil were highly competent to transmit YFV from Brazil as well as from West Africa.
Discussion
Our results showed that Ae. aegypti and Ae. albopictus from YFV-free regions in Brazil were as susceptible as their counterparts in the endemic Amazon region to transmit YFV. Mosquitoes from the epizootic/epidemic region were broadly less competent suggesting that Ae. albopictus and to a lesser extent, Ae. aegypti, were unlikely to play the role of active bridge vector in the emergence zone even if it cannot definitively be excluded.
Since the main YF-vector Ae. aegypti has been eradicated from Brazil in 19579, YFV had been maintained within a forest cycle in endemic/enzootic regions where waves of epizootics coincided with the renewal of non-human primate populations, taking an interval of 6–10 years22. Consequently to the reintroduction of Ae. aegypti and the new establishment of Ae. albopictus in the 1970–80 s, YFV distribution has extended to the Central-West of Brazil (state of Mato Grosso), the Northeast (state of Bahia), Southeast (states of Minas Gerais, Espírito Santo, and São Paulo), and the Southern23. In 2008–2009, an YFV outbreak hit Rio Grande do Sul24 and São Paulo15 caused by a new lineage 1E replacing the former 1D4. We showed that Brazilian Ae. aegypti and Ae. albopictus as well as wild vectors (Haemagogus leucocelaenus and Sabethes albiprivus) were equally susceptible to the two lineages ruling out differences of vector competence as a cause of viral replacement.
Enzootic mosquitoes Hg. leucocelaenus was the most dominant species in forests close to Rio de Janeiro beside Ae. albopictus 25. We showed that Hg. leucocelaneus as well as Sa. albiprivus were highly susceptible to all three YFV strains corroborating their role as YFV-enzootic vectors26. They were able to experimentally deliver infectious viral particles in saliva after infection with the two YFV strains, the former 1D and the new viral lineage 1E, as well as the West African YFV strain isolated from Senegal in 1979. Their pattern of YFV infection, dissemination and transmission are roughly similar to those of domestic Ae. aegypti and Ae. albopictus from Brazzaville in Congo acting as potential urban vectors in Central Africa27. YFV is at the gates of the most densely populated zone in South America, the Brazilian Atlantic coast, where are located large cities like Rio de Janeiro (WHO, “Yellow fever – Brazil”, 2017; http://www.who.int/csr/don/27-january-2017-yellow-fever-brazil/en/). Ae. albopictus colonizes regions surrounding the urban environment where Ae. aegypti remains the main vector of arboviruses pathogen to humans (dengue, chikungunya, Zika). As expected, we showed that both mosquito species were able to excrete YFV from day 1428. Populations of Ae. aegypti and Ae. albopictus from -endemic, -epizootic/epidemic, and YFV-free regions were compared for their performance to be infected, disseminate and transmit YFV. We brought out that mosquitoes from the YFV-endemic (i.e. Manaus) and YFV-free regions (i.e. Rio de Janeiro) were more capable after oral infections to expectorate the two American (74018-1D and 4408-1E) and one African (S-79) YFV strains than mosquitoes from the epizootic/epidemic area (i.e. Goiânia). These two geographically distant regions are linked by an emergence region where mosquitoes are susceptible to YFV infection. Ae. albopictus as well as Ae. aegypti from Goiânia are able to deliver the three YFV strains in their saliva after experimental infections but with much less efficiency. However, a poorly competent vector may play an important role in transmission if other conditions are met, e.g. high vector densities, high human-biting rate and high daily survival rates29. Moreover, it has been shown that human population has extensively contributed to YFV dispersal2. Some environmental/ecological barriers by inhibiting movements of vectors and hosts can prevent YFV spillovers from a forest cycle. However, these barriers became more and more anecdotal in Brazil as with population growth, cities are increasingly close to YFV-enzootic forests. Besides, because of their original habitat degradation and their high propensity to explore new environments, NHPs such as capuchins and marmosets have densely invaded parks and urban areas in Rio de Janeiro and other cities in the Atlantic coast. These new urban invaders colonize a large area around cities including patches of forests30, 31 where sylvatic YFV vectors can be abundant32. Considering all these conditions, it is difficult to understand why YF is not already in the urban areas of Brazil. However, it seems that this is changing with the recent detection of human cases at less than that 100 km apart from city of Rio de Janeiro (http://portalarquivos.saude.gov.br/images/pdf/2017/abril/24/COES-FEBRE-AMARELA-INFORME-37.pdf).
The emergence in Angola in January 2016 exemplifies the threat of YFV spreading outside its historical cradle. Starting from Luanda where YFV caused nearly 600 deaths, it reached neighboring countries, the Democratic Republic of Congo (DRC) in March 2016 and Uganda in April 201632. Most cases were found in cities suggesting that transmission implicates urban vectors, mainly Ae. aegypti. Imported cases from Angola were later confirmed in China33 stressing the risk of spread outside Africa in Aedes-infested countries through non-immunized travelers. All four mosquito species, Ae. aegypti, Ae. albopictus, Hg. leucocelaenus and Sa. albiprivus from Rio de Janeiro were highly susceptible to Brazilian as well as West African YFV lineages. The city of Rio de Janeiro is an important touristic and trade center hosting nearly 6.5 million inhabitants, and where annually converge 26 millions of Brazilians/tourists in airports and 19 million via roads (http://cidades.ibge.gov.br/xtras/perfil.php?codmun=330455). If the virus is introduced from an infected traveler into Rio de Janeiro, opportunities to initiate a vectorial transmission of YFV are multiple: (i) by anthropophilic mosquitoes such as Ae. aegypti and Ae. albopictus which are highly susceptible to YFV and (ii) by YFV-enzootic mosquitoes Hg. leucocelaenus and Sa. albiprivus colonizing the forest near Rio de Janeiro. Therefore, vaccination of travelers visiting Rio de Janeiro should be highly advised to limit the risk of introducing the virus from YFV-endemic areas.
An effective YFV vaccine has been available since the 1930s. Unfortunately, incomplete coverage in regions at risk of infection is responsible for several thousands of deaths every year. In the Americas, to prevent enzootic spillovers with introduction of YFV into the urban cycle, people in contact with the jungle should be rapidly vaccinated in priority to prevent a potential urbanization of YFV.
Methods
Ethics Statements
The Institut Pasteur animal facility has received accreditation from the French Ministry of Agriculture to perform experiments on live animals in compliance with the French and European regulations on care and protection of laboratory animals. This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the Institut Pasteur and by the Institutional Ethics Committee on Animal Use (CEUA-IOC license LW-34/14) at the Instituto Oswaldo Cruz. Mosquito collections in the Atlantic forest in Rio de Janeiro were approved by local environmental authorities (PNMNI license 001/14-15; SISBIO-MMA licenses 37362-2 and 012/2016). No specific permits were required for performing mosquito collection in the urban and suburban areas in Brazil and Congo. This study did not involve endangered or protected species.
Mosquitoes
Ten American and African mosquito populations originated from three contrasting regions (enzootic, epidemic/epizootic and YFV-free areas) were challenged with YFV: four Ae. albopictus, four Ae. aegypti, one Haemagogus leucocelaenus (Dyar & Shannon) and one Sabethes albiprivus Theobald (Fig. 6, Table 4). We tested paired Ae. albopictus and Ae. aegypti populations simultaneously sampled in the same area (i.e. Brazil and Congo). Similarly, four species (Ae. albopictus, Ae. aegypti, Hg. leucocelaenus and Sa. albiprivus) collected in the Rio de Janeiro area were tested (Table 1). Populations were derived from eggs collected with ovitraps settled on the ground for sampling Ae. albopictus and Ae. aegypti in the urban and suburban sites, or suspended at the forest canopy at 5–16 m high for collecting Hg. leucocelaenus. When possible, the first generation in laboratory after collection (F1) of Ae. aegypti and Ae. albopictus was used for experimental infections. For Hg. leucocelaenus which cannot be maintained in laboratory conditions34, 35, the F0 generation derived from eggs collected fortnightly in 2015 was directly used for infections. In the case of Sa. albiprivus, mosquitoes from a colony established in the laboratory since 2013 were used.
Figure 6.
Geographical localization of tested mosquitoes in Brazil and Congo. The map was created using software the CorelDraw X5 software (http://www.coreldraw.com/br/).
Table 4.
Mosquito populations challenged with YFV.
| Mosquito Population | Collection site | Continent | Country | Epidemiological scenario | Generation used | Mosquito Specie |
|---|---|---|---|---|---|---|
| AL-MAN | Manaus, Amazônia | South America | Brazil | Endemic | F1 | Ae. albopictus |
| AE-MAN | Manaus, Amazônia | South America | Brazil | Endemic | F1 | Ae. aegypti |
| AL-GOI | Goiânia, Goiás | South America | Brazil | Epizootic/epidemic | F1 | Ae. albopictus |
| AE-GOI | Goiânia, Goiás | South America | Brazil | Epizootic/epidemic | F1 | Ae. aegypti |
| AL-RIO | Nova Iguaçu, Rio de Janeiro | South America | Brazil | YF-free area | F1 | Ae. albopictus |
| AE-RIO | Urca, Rio de Janeiro | South America | Brazil | YF- free area | F1 | Ae. aegypti |
| Haemagogus | Nova Iguaçu, Rio de Janeiro | South America | Brazil | YF-free area | F0 | Hg. leucocelaenus |
| Sabethes | Tinguá, Rio de Janeiro | South America | Brazil | YF-free area | >F10 | Sa. albiprivus |
| AL-CON | ORSTOM campus, Brazzaville | Africa | Congo | Epizootic/Endemic | >F10 | Ae. albopictus |
| AE-CON | ORSTOM campus, Brazzaville | Africa | Congo | Epizootic/Endemic | >F10 | Ae. aegypti |
Ovitraps were provided with 1 to 3 wooden paddles. Eggs were hatched by submerging paddles in dechlorinated tap water for two consecutive days. Larvae were reared in pans (25 × 25 × 10 cm) containing one liter of dechlorinated tap water, supplemented with yeast powder renewed every 2–3 days. In the case of Hg. leucocelaenus, we added senesced leaves besides yeast powder in water. We reared ~100 larvae/pan for Ae. albopictus and Ae. aegypti, and ~50 larvae/pan for Hg. leucocelaenus and Sa. albiprivus. The emerged F0 adults were morphologically identified36 maintained in insectaries (27 ± 1 °C; 80 ± 10% RH; 16 h:8 h light:dark cycle) and supplied with both 30% sucrose and/or honey solutions. Ae. albopictus, Ae. aegypti, and Sa. albiprivus females were fed three times a week on anesthetized mice to produce eggs.
Viruses
Mosquitoes were challenged with three YFV isolates: two belonging to the South America genotype I, isolated from Brazil, corresponding to two distinct lineages FIOCRUZ 74018/MG/01 (YFV-74018), isolated from a human fatal case in 2001, belonging to the lineage 1D37, and IEC-4408 (YFV-4408), isolated from a howler-monkey in 2008, from the lineage 1E (GenBank KY861728), and one strain [S79-P4 (YFV-S79)] from the West African lineage, isolated from a human case in Senegal in 197938. YFV-74018 and YFV-4408 were isolated from serum in Ae. albopictus C6/36 cell culture, and passaged four times on the same cell line, while YFV-S79 was passaged twice on newborn mice and two times on C6/36 Ae. albopictus cells. Viral stocks of all strains were produced on C6/36 Ae. albopictus cells and stored at −80 °C until used for the mosquito experimental infection assays.
Mosquito experimental assays
Six- to 8-day-old females were grouped in feeding boxes (60 females/box) and starved for 24 h, except for Sa. albiprivus which were starved for 48 h. Females were fed on an infectious blood meal containing two parts of washed rabbit erythrocytes, one part of the viral suspension supplemented with a phagostimulant (ATP) at a final concentration of 5 mM maintained at 37 °C. The infectious blood-meals contained a final viral titer of 106 PFU/mL. Mosquito feeding period was limited to 1 hour. Only fully engorged females were incubated at 28 °C constant temperature, 80% RH and 16 h:8 h light:dark cycle, with daily access to 10% sucrose or honey solution. For each combination mosquito population-YFV strain, samples of 20 mosquitoes were examined at 3, 7, 14 and 21 days after virus exposure for determining vector competence indices. Infection rate (IR) refers to the proportion of mosquitoes with infected body among the engorged ones. Disseminated infection rate (DIR) corresponds to the proportion of mosquitoes with infected head among the previously detected infected mosquitoes (i.e, abdomen/thorax positive). Transmission rate (TR) represents the proportion of mosquitoes with infectious saliva among mosquitoes with disseminated infection39. Even if this is an indication, TR should not be regarded as a measure of transmission from mosquitoes to a vertebrate host.
Mosquitoes were processed as follows: abdomen and thorax (herein after referred to as body) were tested for determining infection, head for dissemination and saliva for transmission. To determine viral infection and dissemination rates, each mosquito body and head were respectively ground in 500 μL and 300 μL of Leibovitz L15 medium (Invitrogen) supplemented with 2% fetal bovine serum (FBS), centrifuged at 10,000 × g for 5 min at +4 °C and inoculated onto monolayers of Ae. albopictus C6/36 cell culture in 96-well plates. After 1 h incubation at 28 °C, 150 μL of 2.4% CMC (carboxymethyl cellulose) in Leibovitz L15 medium supplemented with 10% FBS was added per well. After 5 days incubation at 28 °C, cells were fixed with 10% formaldehyde, washed, and revealed using hyperimmune ascetic fluid specific to YFV as the primary antibody and Alexa Fluor 488 goat anti-mouse IgG as the second antibody (Life Technologies)28. To estimate viral transmission, mosquito saliva was collected in individual pipette tips containing 5 μL FBS for 30 min as previously described40. FBS containing mosquito saliva was expelled into 45 μL of Leibovitz L15 medium, inoculated on Ae. albopictus C6/36 cell culture in 96-well plates and stained as described above.
Statistical analysis
Rates (infection, disseminated infection, and transmission) were described using median and inter-quartile range (IQR). The effect of species, population, YFV strain and duration on the rates was investigated using logistic linear regression models. Two-by-two interaction between species, population, YFV strain and duration was systematically investigated. Statistical analyses were conducted using the Stata software (StataCorp LP, Texas, and USA). P-values < 0.05 were considered significant.
Electronic supplementary material
Acknowledgements
This study was funded by the Institut Pasteur, the French Government’s Investissement. d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (grant n°ANR-10-LABX-62-IBEID) and the PTR (grant n°528), the CAPES-COFECUB (grant 799-14), and the FAPERJ- Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (grant E-26/102.351/2013-CNE). We are grateful to André Yébakima as well as to Tacilaine Divina Cardoso, Paulo Leite, Marcelo Santalucia, Tayssa Pereira dos Santos, José Bento Lima, Alessandro Romano and Cristiano Fernandes for their help in collecting mosquitoes in Congo and Brazil, respectively. We also thank Thais Chouin-Carneiro and Rosilainy S. Fernandes for their assistance in the experiments, and Heloisa Dinis, Leonidas Leite and Roberta Carvalho for their help in making the maps.
Author Contributions
D.C.-L. contributed to conceive and perform the experiments. Y.M. did the statistical analysis. M.I.B., S.S.C. and M.A.M. were involved in collecting mosquito samples. F.B.S. participated in preparing viral samples. M.V. was involved in experiments on mosquitoes. P.F.C.V. provided the viral strains. R.L.O. and A.-B.F. participated in conceiving and designing the experiments, analyzing/interpreting the data and the writing of the article. All authors reviewed the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05186-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ricardo Lourenço-de-Oliveira, Email: lourenco@ioc.fiocruz.br.
Anna-Bella Failloux, Email: anna-bella.failloux@pasteur.fr.
References
- 1.Mutebi JP, Wang H, Li L, Bryant JE, Barrett AD. Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. Journal of virology. 2001;75:6999–7008. doi: 10.1128/JVI.75.15.6999-7008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS pathogens. 2007;3:e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staples JE, Monath TP. Yellow fever: 100 years of discovery. JAMA. 2008;300:960–962. doi: 10.1001/jama.300.8.960. [DOI] [PubMed] [Google Scholar]
- 4.Vasconcelos PF, et al. Genetic divergence and dispersal of yellow fever virus, Brazil. Emerging infectious diseases. 2004;10:1578–1584. doi: 10.3201/eid1009.040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes MR, et al. Genomic and phylogenetic characterization of Brazilian yellow fever virus strains. Journal of virology. 2012;86:13263–13271. doi: 10.1128/JVI.00565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasconcelos PF, Monath TP. Yellow Fever Remains a Potential Threat to Public Health. Vector borne and zoonotic diseases (Larchmont, N.Y.) 2016 doi: 10.1089/vbz.2016.2031. [DOI] [PubMed] [Google Scholar]
- 7.Woodall JP, Yuill TM. Why is the yellow fever outbreak in Angola a ‘threat to the entire world’? International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2016;48:96–97. doi: 10.1016/j.ijid.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Monath TP, Vasconcelos PF. Yellow fever. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Soper FL. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. American journal of public health and the nation’s health. 1963;53:7–16. doi: 10.2105/AJPH.53.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camara FP, Gomes AL, Carvalho LM, Castello LG. Dynamic behavior of sylvatic yellow fever in Brazil (1954–2008) Revista da Sociedade Brasileira de Medicina Tropical. 2011;44:297–299. doi: 10.1590/S0037-86822011005000024. [DOI] [PubMed] [Google Scholar]
- 11.Health, B. M. O. Ministerio da saude divulga novos dados de febre amarela. Available fom: http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/27482-ministerio-da-saude-divulga-novos-dados-de-febre-amarela (2017).
- 12.Health, B. M. O. Reemergência da Febre Amarela Silvestre no Brasil, 2014/2015: situação epidemiológica e a importância da vacinação preventiva e da vigilância intensificada no período sazonal. Available fom: http://portalsaude.saude.gov.br/images/pdf/2015/outubro/19/2015-032–FA-ok.pdf. Boletim Epidemiológico46, 1–10 (2015).
- 13.Carvalho RG, Lourenco-de-Oliveira R, Braga IA. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Memorias do Instituto Oswaldo Cruz. 2014;109:787–796. doi: 10.1590/0074-0276140304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano AP, et al. Yellow Fever outbreaks in unvaccinated populations, Brazil, 2008-2009. PLoS neglected tropical diseases. 2014;8:e2740. doi: 10.1371/journal.pntd.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza RP, et al. Detection of a new yellow fever virus lineage within the South American genotype I in Brazil. Journal of medical virology. 2010;82:175–185. doi: 10.1002/jmv.21606. [DOI] [PubMed] [Google Scholar]
- 16.Massad E, Coutinho FA, Burattini MN, Lopez LF. The risk of yellow fever in a dengue-infested area. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95:370–374. doi: 10.1016/S0035-9203(01)90184-1. [DOI] [PubMed] [Google Scholar]
- 17.Miller BR, Ballinger ME. Aedes albopictus mosquitoes introduced into Brazil: vector competence for yellow fever and dengue viruses. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82:476–477. doi: 10.1016/0035-9203(88)90168-X. [DOI] [PubMed] [Google Scholar]
- 18.Lourenco-de-Oliveira R, Vazeille M, Bispo de Filippis AM, Failloux AB. Oral susceptibility to yellow fever virus of Aedes aegypti from Brazil. Memorias do Instituto Oswaldo Cruz. 2002;97:437–439. doi: 10.1590/S0074-02762002000300031. [DOI] [PubMed] [Google Scholar]
- 19.Lourenco de Oliveira R, Vazeille M, de Filippis AM, Failloux AB. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. The American journal of tropical medicine and hygiene. 2003;69:105–114. [PubMed] [Google Scholar]
- 20.Lourenco-de-Oliveira R, Vazeille M, de Filippis AM, Failloux AB. Aedes aegypti in Brazil: genetically differentiated populations with high susceptibility to dengue and yellow fever viruses. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2004;98:43–54. doi: 10.1016/S0035-9203(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 21.Maciel-de-Freitas R, Neto RB, Goncalves JM, Codeco CT, Lourenco-de-Oliveira R. Movement of dengue vectors between the human modified environment and an urban forest in Rio de Janeiro. Journal of medical entomology. 2006;43:1112–1120. doi: 10.1093/jmedent/43.6.1112. [DOI] [PubMed] [Google Scholar]
- 22.Vasconcelos PF. [Yellow Fever] Revista da Sociedade Brasileira de Medicina Tropical. 2003;36:275–293. doi: 10.1590/S0037-86822003000200012. [DOI] [PubMed] [Google Scholar]
- 23.Vasconcelos PF. Yellow fever in Brazil: thoughts and hypotheses on the emergence in previously free areas. Revista de saude publica. 2010;44:1144–1149. doi: 10.1590/S0034-89102010005000046. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso Jda C, et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, southern Brazil, 2008. Emerging infectious diseases. 2010;16:1918–1924. doi: 10.3201/eid1612.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alencar J, et al. Diversity of yellow fever mosquito vectors in the Atlantic Forest of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2016;49:351–356. doi: 10.1590/0037-8682-0438-2015. [DOI] [PubMed] [Google Scholar]
- 26.Goenaga S, et al. Isolation of yellow fever virus from mosquitoes in Misiones province, Argentina. Vector Borne Zoonotic Dis. 2012;12:986–993. doi: 10.1089/vbz.2011.0730. [DOI] [PubMed] [Google Scholar]
- 27.Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annual review of entomology. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 28.Vazeille M, et al. Oral receptivity of Aedes aegypti from Cape Verde for yellow fever, dengue, and chikungunya viruses. Vector borne and zoonotic diseases (Larchmont, N.Y.) 2013;13:37–40. doi: 10.1089/vbz.2012.0982. [DOI] [PubMed] [Google Scholar]
- 29.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Advances in virus research. 2003;60:187–232. doi: 10.1016/S0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira, B. et al. Good neighbours: distribution of black-tufted marmoset (Callithrix penicillata) in an urban environment. Wildlife Research42 (2015).
- 31.Oliveira LC, Grelle CEV. Introduced Primate Species of an Atlantic Forest Region in Brazil: Present and Future Implications for the Native Fauna. Tropical Conservation Science. 2012;5:112–120. doi: 10.1177/194008291200500110. [DOI] [Google Scholar]
- 32.Kraemer, M. U. et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. The Lancet. Infectious diseases, doi:10.1016/S1473-3099(16)30513-8 (2016). [DOI] [PMC free article] [PubMed]
- 33.Ling Y, et al. Yellow Fever in a Worker Returning to China from Angola, March 2016. Emerging infectious diseases. 2016;22:1317–1318. doi: 10.3201/eid2207.160469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hovanitz W. Comparisons of mating behavior, growth rate, and factors influencing egg-hatching in South American Haemogogus mosquitoes. Physiological zoology. 1946;19:35–53. doi: 10.1086/physzool.19.1.30151878. [DOI] [PubMed] [Google Scholar]
- 35.Gerber E. Manual for mosquito rearing and experimental techniques Am. Mosq. Control Assoc. 1970;5:109. [Google Scholar]
- 36.Consoli RAGB, O. R. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro. Editora FIOCRUZ, p. 228 (1994).
- 37.de Filippis AM, et al. Outbreak of jaundice and hemorrhagic fever in the Southeast of Brazil in 2001: detection and molecular characterization of yellow fever virus. Journal of medical virology. 2002;68:620–627. doi: 10.1002/jmv.10226. [DOI] [PubMed] [Google Scholar]
- 38.Rodhain F, Hannoun C, Jousset FX, Ravisse P. [Isolation of the yellow fever virus in Paris from 2 imported human cases] Bulletin de la Societe de pathologie exotique et de ses filiales. 1979;72:411–415. [PubMed] [Google Scholar]
- 39.Vega-Rua A, et al. High efficiency of temperate Aedes albopictus to transmit chikungunya and dengue viruses in the Southeast of France. PloS one. 2013;8:e59716. doi: 10.1371/journal.pone.0059716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PloS one. 2009;4:e5895. doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


