Abstract
We report an isolated sulfite oxidase deficiency in the first child boy of a non-consanguineous Caucasian family. He's a compound heterozygote for the sulfite oxidase gene, presenting low cystine, undetectable homocysteine and normal uric acid blood concentrations and undetectable sulfite oxidase activity in his cultured fibroblasts. Both mutations are not reported yet. The clinical presentation was typical and severe, with generalized status epilepticus and premature death.
Abbreviations: SUOX, sulfite oxidase
Keywords: Compound heterozygote, Point mutation, Missense mutation, Transition, Transversion, Fibroblasts, Sulfite oxidase activity
1. Case report
A term infant boy was born to a primipara and primigesta mother from France and a father of Moroccan origin. No significant familial antecedents were reported.
A caesarean was performed at 37 weeks and 2 days because of non-progression of delivery and abnormalities of foetal heart rate. The baby's measurements were 2220 g of weight (5%), 45 cm of size (5%) and 33 cm of cranial perimeter 40%) Apgar score at 1 and 5 min recorded normal values (10/10).
Three weeks later the child presents hyperextension of the trunk and tremor after meal. Some days later he's hospitalized with 38.5 °C body temperature and generalized status epilepticus. A nystagmus followed by the fixity of the regard and a noisy respiration complete the clinical picture.
Physicians suspected meningitis or meningoencephalitis or an inborn metabolic disease and prescribed a broad-spectrum antibiotherapy with cefotaxime, amoxicillin, amikacin and acyclovir. A lumbar puncture and metabolic screening tests were performed. The child received Phenobarbital and Midazolam in order to stop seizures.
The initial cerebral tomography scan analysis revealed no pericerebral collection and no obvious focal lesion but a hypodense aspect of the cortical and sub-cortical layers of the parietal brain. Periventricular frontal hyperdensities and lacunar lesions in the caudate were bilaterally identified. The brain magnetic resonance imaging (MRI) detected no vascular lesion but some anomalies diffusing bilaterally in the cortical and subcortical layers of the fronto-parieto-occipital region. All these findings were consistent with a viral meningoencephalitis, but the immunological explorations of the cerebrospinal fluid have yielded rapid negative results.
2. Biochemical studies
Urinalysis with a sulfitest dipstick (Merckoquant Sulfite Test Strips, Merck KgaA, Darmstadt, Germany) at the same time with the onset of the neurological problems revealed the presence of sulfite 60 mg/l (normal < 15 mg/l). Subsequently, chromatography of urinary amino acids detected an abnormal elevated sulfocysteine 200 mmol/mol creatinine (normal < 2.7 mmol/mol creatinine) and taurine 617 mmol/mol creatinine (normal 6 to 99 mmol/mol creatinine). Conversely, plasma free cystine dropped to 1 μmol/l (normal 32–51 μmol/l) and total plasma homocysteine was undetectable (normal 32–51 μmol/l). These biochemical findings coupled with a normal level of uric acid in two blood samples 156 and 185 μmol/l (normal 120–320 μmol/l) were consistent with the diagnosis of isolated sulfite oxidase deficiency.
Indeed, sulfite oxidase activity in the cultured fibroblasts of the patient and the control was very different, 1.5 absorbance units/min/g protein and 12.5 absorbance units/min/g respectively.
3. Clinical evolution
The clinical picture deteriorated progressively. In few months, the child presented refractory epilepsy with severe encephalopathy, pyramidal tetraparesia, dystonia, microcephalia and failure to thrive.
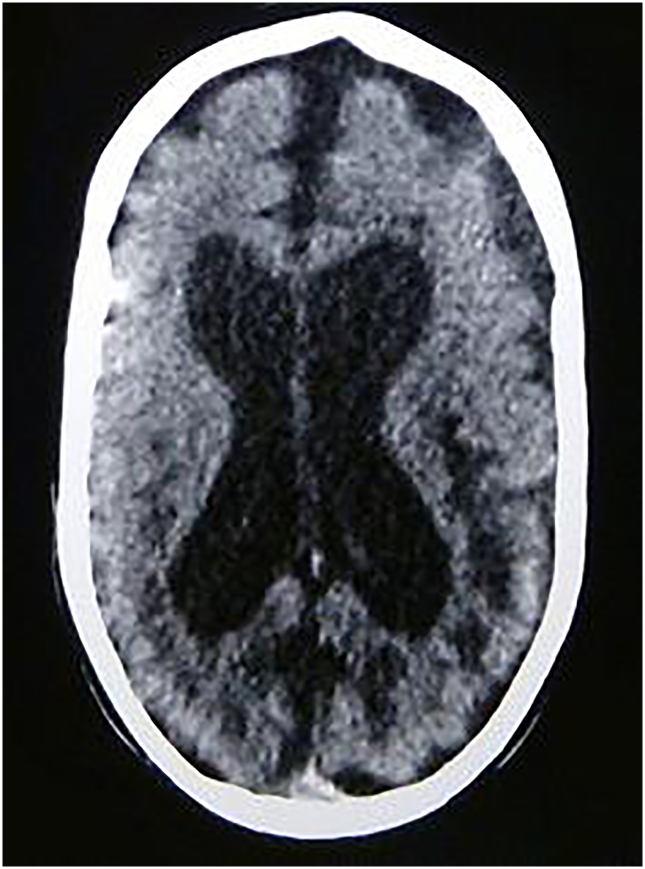
At the age of 20 months, the second cerebral scan of the child revealed an important expansion of the lateral ventricles and an enlargement of the peri-cerebral spaces and of the sylvian fissure. Some hypodense lesions appeared in the periventricular white matter, surrounding the occipital horns of the lateral ventricles (Fig. 1) The child died 1 year later.
Fig. 1.
Cerebral scan at the age of 20 months.
4. Molecular analysis
Genomic DNA of the affected child and of his mother was extracted from peripheral blood samples using the QIAamp Blood Kit (Qiagen) according to manufacturer's instructions. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2005. Informed consent was obtained from the mother for SUOX genetic testing for herself and her affected child. DNA was not available from the father.
The extracted genomic DNA was amplified in a hot-start-Taq polymerase chain reaction (PCR), using exon-flanking intronic primers and overlapping exonic primers for the two coding exons of SUOX gene.
PCR products were purified using Microcon-PCR filter unit (Millipore). Both DNA strands were sequenced using forward and reverse amplification primers as sequencing primers and BigDye Terminator V1.1 Cycle Sequencing Kit reagents according to manufacturer's instructions (Applied Biosystems). EDTA-ethanol purified sequencing fragments were separated by capillary electrophoresis and detected via laser-induced fluorescence on an ABI Prism 3130xl Genetic Analyzer (Applied Biosystems). Sequences obtained from patient samples were compared with the SUOX GenBank reference sequence (accession number AY056018) using SeqScape software V2.5 (Applied Biosystems).
5. Mutational study of SUOX gene
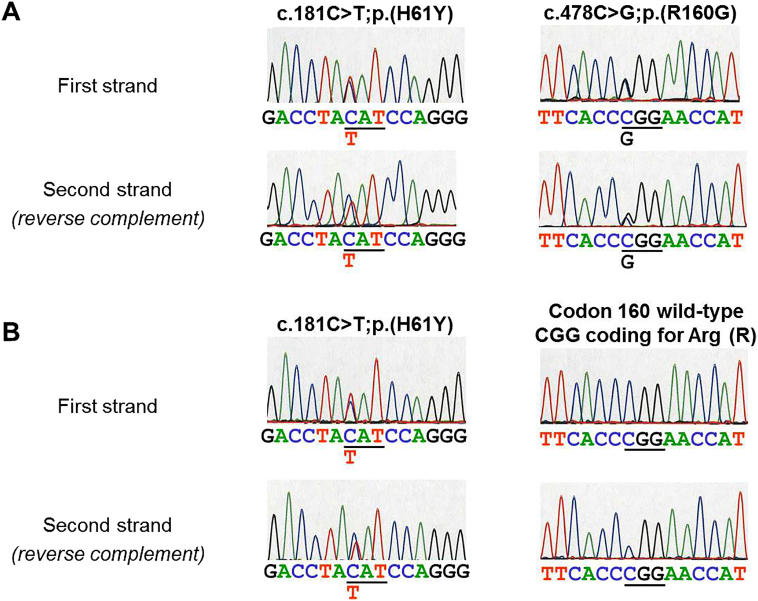
Sequence analysis showed that the affected child was a compound heterozygote for two novel SUOX gene mutations. A cytosine to thymine transition present in the heterozygous state was discovered at nucleotide 181, resulting in the single amino acid substitution from histidine to tyrosine at position 61 in the sulfite oxidase protein. This mutation was associated to a cytosine to guanine transversion present in the heterozygous state at nucleotide 478, resulting in the single amino acid substitution from arginine to glycine at position 160 in the protein (Fig. 2) The unaffected mother was found heterozygous for the c.181C > T;p.(H61Y) mutation and did not carry the c.478C > G;p.(R160G) mutation, indicating that both mutations identified in the affected child are located on separate alleles (Fig. 2).
Fig. 2.
SUOX gene DNA sequencing analysis. Panels A and B show sequencing results in the affected child and his mother, respectively. Sequences on both DNA strands are shown, the second strand being reversed complemented. The codon affected by the mutation is underlined. In each case, the normal sequence is listed on top and the mutated nucleotide is indicated below at the corresponding position. The resulting mutation is described at nucleotide and protein levels using HGVS (Human Genome Variation Society) standards.
Frequency of the c.181C > T;p.(H61Y) variant in the ExAC database is extremely low (0.00082%) and the c.478C > G;p.(R160G) variant is not described in the ExAC database nor in the latest versions of the NCBI dbSNP database (www.ncbi.nlm.nih.gov/snp) and the 1000 Genomes Project (www.1000genomes.org). This indicates that both variants don't represent polymorphisms. In addition, both mutations are predicted to be deleterious using several computational methods for missense mutation annotation (PolyPhen2, MutationTaster, MutationAssesor, SIFT, FATHMM).
6. Molecular basis of sulfite oxidase enzyme activity
Sulfite oxidase catalyzes the oxidation of sulfite to sulfate which constitutes the terminal reaction in the oxidative degradation of sulfur-containing amino acids cysteine and methionine [1], [2]. The enzyme is a homodimer of identical subunits localized to the intermembrane space of mitochondria. Each 52-kDa subunit contains a small 10-kDa N-terminal domain structurally similar to the fold of cytochrome b5 that harbors the heme group and a larger 42-kDa C-terminal domain composed of the molybdopterin-binding domain and the dimerization interface. At each active site of the enzyme, a single molybdenum atom is coordinated to the two dithiolene sulfurs of molybdopterin (also known as molybdenum cofactor) and to the sulfur of cysteine 207 of the sulfite [3].
The net reaction catalyzed by the sulfite oxidase is the transfer of one oxygen atom from water to the sulfite substrate. Coupled with substrate oxidation, two electrons are transferred from sulfite to the molybdenum center of the cofactor molybdopterin. These electrons are subsequently transferred to the heme iron atom in the cytochrome b5 domain in a two-step reaction, from where they are donated to the final electron acceptor cytochrome c [4], [5]. This mechanism firstly proposed by Hille et al. is now widely accepted [6], [7].
7. Structural insights into the physiopathology of the newly identified mutations
The heme iron atom in sulfite oxidase that transiently accepts electrons during the oxidation-reduction reaction is octahedrally coordinated by the nitrogen atoms of two histidines of sulfite oxidase protein at positions 61 and 86 and by the four pyrrole nitrogen atoms of the heme group deeply buried into the cavity of the cytochrome b5 domain [2]. Interestingly, histidines 61 and 86 are strictly conserved iron-binding residues present in sulfite oxidase of various mammals and non-mammals species (Fig. 3) as well as in cytochrome b5 of human, bovine, rat chicken and yeast [8]. By substituting the critical histidine 61 residue by a tyrosine, the novel mutation we identified could affect the proper coordination of the heme iron atom and thus impair the molybdenum-heme intramolecular electron transfer coupled to the activity of sulfite oxidase enzyme.
Fig. 3.
Alignment of the primary protein sequence of sulfite oxidase from different mammalian and non-mammalian species. Asterisks indicate the two conserved iron-binding histidine residues. The histidine and arginine residues affected by the mutation in the affected child are highlighted in black and are conserved among the different species.
Arginine 160 is also an evolutionary conserved residue in sulfite oxidase from numerous sources (Fig. 3) Arginine 160 is one of first residue identified as mutated in a patient suffering from isolated oxidase deficiency [9] and it was subsequently found mutated in other patients as well [10], [11]. Additional studies showed that the positive charge of arginine 160 is also essential for efficient intramolecular electron transfer between the heme and molybdenum centers in the human sulfite oxidase [12]. The mutation we identified in the affected child is a novel variant of arginine 160 mutation that substitutes the positively charged arginine 160 by the neutral and non-polar glycine residue.
In conclusion, this study is the first one to identify two novel mutations of the SUOX gene that appear in a compound heterozygous state in a child with isolated sulfite oxidase deficiency. Based on structural insights, each affected residue (histidine 61 and arginine 160) can be associated to critical steps of the enzyme reaction, providing a rational explanation for the sulfite oxidase deficiency observed in the affected child.
Funding source
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Financial disclosure
Daniel Brumaru, Eric Guerin, Anne-Claire Voegeli, Didier Eyer and Michel Maitre have no financial relationships relevant to this article to disclose.
Conflicts of interest statement
Daniel Brumaru, Eric Guerin, Anne-Claire Voegeli, Didier Eyer and Michel Maitre have no conflicts of interest to disclose.
Contributors' statement
Daniel Brumaru and Michel Maitre: Drs. Brumaru and Maitre were directly implicated in the conception of the study and execution of the biochemical experiments, wrote the research report and approved the final manuscript as submitted.
Eric Guerin and Anne-Claire Voegeli: Drs. Guerin and Voegeli were directly implicated in the design and execution of the DNA experiments, reviewed and revised the research report and approved the final manuscript as submitted.
Didier Eyer: Dr. Eyer analyzed and designed the data, made a critical revision of the research report and approved the final manuscript as submitted.
References
- 1.MacLeod R.M., Farkas W., Fridovich I. Purification and properties of hepatic sulfite oxidase. J. Biol. Chem. 1961;236:1841–1846. [PubMed] [Google Scholar]
- 2.Feng C., Tollin G., Enemark J.H. Sulfite oxidizing enzymes. Biochim. Biophys. Acta. 2007;1774:527–539. doi: 10.1016/j.bbapap.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisker C., Schindelin H., Pacheco A. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell. 1997;91:973–983. doi: 10.1016/s0092-8674(00)80488-2. [DOI] [PubMed] [Google Scholar]
- 4.Cohen H.J., Betcher-Lange S., Kessler D.L. Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity. J. Biol. Chem. 1972;247:7759–7766. [PubMed] [Google Scholar]
- 5.Sullivan E.P., Jr., Hazzard J.T., Tollin G. Electron transfer in sulfite oxidase: effects of pH and anions on transient kinetics. Biochemistry. 1993;32:12465–12470. doi: 10.1021/bi00097a026. [DOI] [PubMed] [Google Scholar]
- 6.Brody M.S., Hille R. The kinetic behavior of chicken liver sulfite oxydase. Biochemistry. 1999;38:6668–6677. doi: 10.1021/bi9902539. [DOI] [PubMed] [Google Scholar]
- 7.Johnson-Winters K., Tollin G., Enemark J.H. Elucidating the catalytic mechanism of sulfite oxydizing enzymes using structural, spectroscopic and kinetic analyses. Biochemistry. 2010;49:7242–7254. doi: 10.1021/bi1008485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph M.J., Johnson J.L., Rajagopalan K.V. The 1.2 A structure of the human sulfite oxidase cytochrome b(5) domain. Acta Crystallogr. D Biol. Crystallogr. 2003;59:1183–1191. doi: 10.1107/s0907444903009934. [DOI] [PubMed] [Google Scholar]
- 9.Garrett R.M., Johnson J.L., Graf T.N. Human sulfite oxidase R160Q: identification of the mutation in a sulfite oxidase-deficient patient and expression and characterization of the mutant enzyme. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6394–6398. doi: 10.1073/pnas.95.11.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam C.W., Li C.K., Lai C.K. DNA-based diagnosis of isolated sulfite oxidase deficiency by denaturing high-performance liquid chromatography. Mol. Genet. Metab. 2002;75:91–95. doi: 10.1006/mgme.2001.3267. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.F., Mak B.S., Chi C.S. A novel mutation in neonatal isolated sulfite oxidase deficiency. Neuropediatrics. 2002;33:174–179. doi: 10.1055/s-2002-34491. [DOI] [PubMed] [Google Scholar]
- 12.Feng C., Wilson H.L., Hurley J.K. Essential role of conserved arginine 160 in intramolecular electron transfer in human sulfite oxidase. Biochemistry. 2003;42:12235–12242. doi: 10.1021/bi0350194. [DOI] [PubMed] [Google Scholar]