Abstract
Objective:
We describe long-term functional, neurodiagnostic, and psychosocial outcomes of a cohort of 12 children from Colorado diagnosed with acute flaccid myelitis (AFM) in 2014.
Methods:
Children were assessed every 3 months for 1 year or until clinical resolution. Assessments included neurologic examination, MRI, EMG/nerve conduction studies (NCS), functional measures (Assisting Hand Assessment, Hammersmith Functional Motor Scale), and Patient-Reported Outcomes Measurement Information System questionnaires.
Results:
Eight of 12 children completed the study. Six of 8 had persistent motor deficits at 1 year; 2 demonstrated full recovery. Four were not enrolled, 2 of whom reported full recovery. The 6 affected were weakest in proximal muscles, showing minimal to no improvement and significant atrophy at 1 year. All patients improved in distal muscle groups. Cranial nerve dysfunction resolved in 2 of 5 and improved in all. Four of 5 showed progressive functional improvement at 6 and 12 months. Two of 8 reported pain at 1 year. Three of 8 reported depressive symptoms. Repeat MRI was performed in 7 of 8 children a median of 7 months after onset and showed significant improvement or normalization in all but one child. Repeat EMG/NCS was performed on 4 children a median of 8 months after onset and showed ongoing denervation and chronic reinnervation in 3 children with persistent deficits.
Conclusions:
At 1 year, children with AFM demonstrated functional gains but weakness persisted. EMG changes correlated with persistent deficits better than imaging. Despite improvements, AFM had substantial long-term functional effects on affected children.
Poliovirus and other nonpolio enteroviruses, including enterovirus (EV) A71 and D70, cause acute flaccid paralysis with variable, often incomplete, recovery and long-term neurologic sequelae.1–5 In 2014, we described a cluster of 12 children with acute flaccid myelitis (AFM) who presented to Children's Hospital Colorado (CHCO) in Aurora.6 Nationwide surveillance through the Centers for Disease Control and Prevention (CDC) detected 120 cases of AFM in 34 states.7 This correlated with a widespread outbreak of severe respiratory illness due to EV-D68 across the United States, suggesting a causal relationship.7,8 While the initial clinical presentation, neuroimaging, and electrophysiologic studies in these children were similar to acute flaccid paralysis due to other EVs, the clinical course and long-term outcomes of these recent cases of AFM is unknown.
The primary aim of this study was to characterize the short- and long-term neurologic outcomes of this cluster of children with AFM by measuring motor strength, cranial nerve function, objective functional assessments, quality of life, and neurodiagnostic outcomes.
METHODS
From August 1, 2014, to October 31, 2014, we identified 12 children with AFM, defined by acute onset limb weakness or cranial nerve dysfunction with imaging changes predominantly in the gray matter of the spinal cord or the brainstem.6 All 12 families were approached for participation in the clinical research study. Children were followed every 3 months for 1 year following presentation or until complete clinical resolution of deficits. Follow-up was provided in a multidisciplinary clinic that included providers in child neurology, physical medicine and rehabilitation, psychology, physical therapy, and occupational therapy. An infectious disease specialist and research collaborator was instrumental in study design and data collection.
At each visit, patients underwent physical examinations by both a neurologist and physiatrist, assessing neurologic and functional status, respectively. A consistent provider assessed muscle strength using the Medical Research Council (MRC) scale (0/5 to 5/5) for each muscle group. Reflexes were assessed on a scale of 0 to 4. Cranial nerve function was assessed clinically.
Objective functional outcomes of the upper extremities were assessed by a registered occupational therapist using the Assisting Hand Assessment (AHA). The AHA is a validated performance measure of hand function, for children ages 18 months–12 years, measuring how well children can use the affected hand collaboratively with the nonaffected hand in bimanual play. A change of greater than 5 points on the AHA between assessments or anything less than a full score is considered clinically significant.9,10 Objective functional outcomes of lower extremities were measured by a licensed physical therapist using the Hammersmith Functional Motor Scale. This scale provides assessment of motor ability and clinical progression. This scale has been validated as a reliable tool for assessing functional impact of strength in patients with spinal muscular atrophy, a motor neuron disease, but has also been used in a variety of conditions causing weakness.11 Any score below a full score of 66 is considered clinically abnormal.
Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires were used to assess self-reported functional and psychosocial outcomes. PROMIS was developed by the NIH to evaluate outcomes across physical, social, and mental health domains. Questionnaires are designed for pediatric self-report (ages 8–17 years) or parents serving as proxy reporters for children (ages 5–17 years).12 A change of >1 SD was considered clinically significant. Functional outcomes were assessed with PROMIS questionnaires for upper extremity and mobility function. Psychological outcomes were measured with PROMIS questionnaires for fatigue, pain, depression, and stigma. In addition, patients and their caregivers were given the opportunity to meet with a pediatric psychologist at each clinic visit. Clinical assessment focused on psychosocial domains affected by medical illness, including adjustment and coping with the diagnosis of AFM, social and academic development, and overall emotional/behavioral health.
Patient-reported outcomes measurement system.
Patients underwent MRI of the brain or entire spine at either 1.5T or 3.0T with and without contrast within 1 month of initial presentation. MRI was repeated as clinically indicated within a year of symptom onset. Patients underwent EMG and nerve conduction studies (NCS) according to Kimura standard techniques, with a Teca Synergy N-EP system (CareFusion, San Diego, CA). Sensory and motor NCS were analyzed based on age-matched reference values.13 Initial EMG/NCS was performed within 2 months of presentation. EMG/NCS was repeated within a year of symptom onset, in patients with limited improvement, to assess for persistent electrophysiologic abnormalities and prognosis.
Standard protocol approvals, registrations, and patient consents.
The Colorado Multiple Institution Review Board approved this study. Participants of standardized study visits provided informed consent. Photograph consent was obtained for all images. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado School of Medicine.14 The study was supported by NIH/NCATS Colorado CTSA grant UL1 TR001082.
Statistical analysis was conducted with SAS (SAS Institute, Cary, NC) software, version 9.4. A Kaplan-Meier time-to-event analysis of the entire 12-patient cohort was conducted with complete clinical resolution of deficits as the endpoint and patients lost to follow-up censored at the time of last documented contact. The remainder of the outcome analysis was conducted with the 8 patients who participated in standardized follow-up. Spaghetti plots were generated for outcome scales to display change over time by participant, and change in outcome scales from initial measurement was also analyzed.
RESULTS
Study population.
Of the 12 cases of AFM in the CHCO cluster, 8 consented for standardized follow-up studies and reached the 1-year study endpoint or had complete clinical resolution of deficits prior to 1 year (figure 1). Of the 4 children not consented, 1 was lost to follow-up, 1 was unable to travel to CHCO, and 2 reported complete resolution of deficits within 2 months of symptom onset (via telephone or clinic encounter). A Kaplan-Meier plot reflecting time to complete recovery of the entire 12-patient cohort is presented in figure 2.
Figure 1. Study inclusion chart.
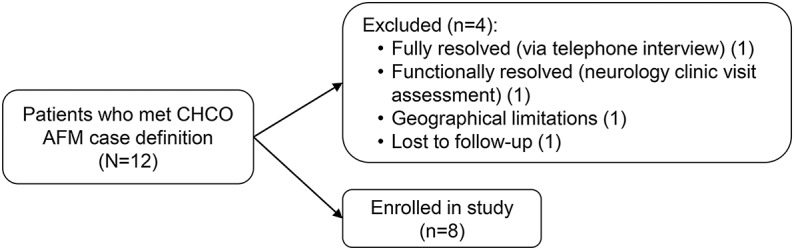
AFM = acute flaccid myelitis; CHCO = Children's Hospital Colorado.
Figure 2. Kaplan-Meier plot of time to neurologic recovery.

All 12 patients with acute flaccid myelitis in the Children's Hospital Colorado cluster are included in this analysis. Two patients were lost to follow-up after initial presentation and were censored at time 0. One patient who was not enrolled in the study reported full recovery via telephone interview at 1 month and another patient who was not enrolled in the study was noted to have full functional recovery at follow-up neurologic clinic visit at 2 months.
Motor outcomes.
Six of 8 (75%) study patients had persistent motor or functional deficits at 12 months. Visually apparent muscle atrophy was observed in 5 of the patients with persistent deficits (figure 3). One patient had complete clinical resolution of deficits by 6 months and another by 9 months.
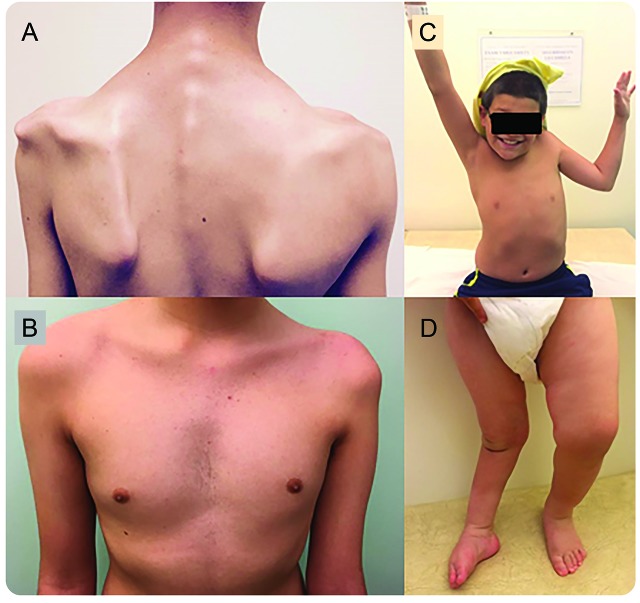
Figure 3. Muscle atrophy of affected limbs in children with acute flaccid myelitis (AFM).
(A) Patient 5, 8 months after onset of symptoms with atrophy of the left proximal upper extremity and shoulder girdle. (B) Patient 11, 11 months after onset of symptoms with atrophy of the proximal left arm and chest. (C) Patient 1, 6 months after onset of symptoms with inability to raise the affected left arm. (D) Two-year-old patient with AFM 1.5 years after onset of symptoms in 2013 with persistent asymmetric atrophy of the affected right leg and inability to bear weight.
Individual motor and functional outcomes are summarized in table 1 and figure 4. Seven of 8 study patients (88%) initially had upper extremity weakness. All were more affected proximally with a median of 2/5 strength at onset of symptoms. Deficits persisted, with a median of 2/5 strength at 6 months and 2/5 strength at 1 year, with 6 of 7 (86%) still affected. Distal upper extremities were involved in 6 of 7 (86%) patients initially, with a median of 4+/5 strength at onset. Only one patient with distal upper extremity weakness had persistent distal weakness, 4/5 finger flexion, at 12 months follow-up.
Table 1.
Motor and functional outcomes of children with acute flaccid myelitis over 1 year
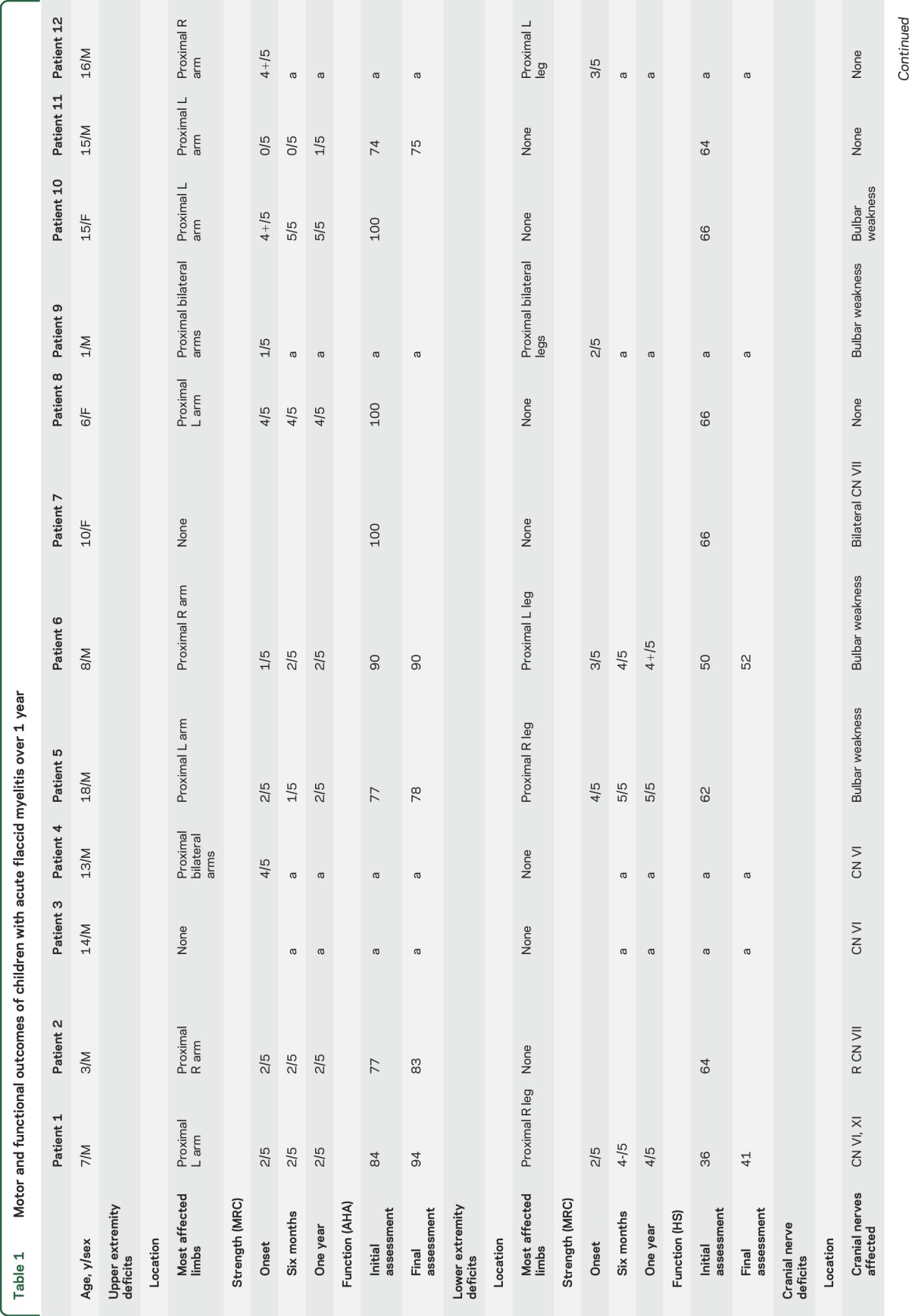
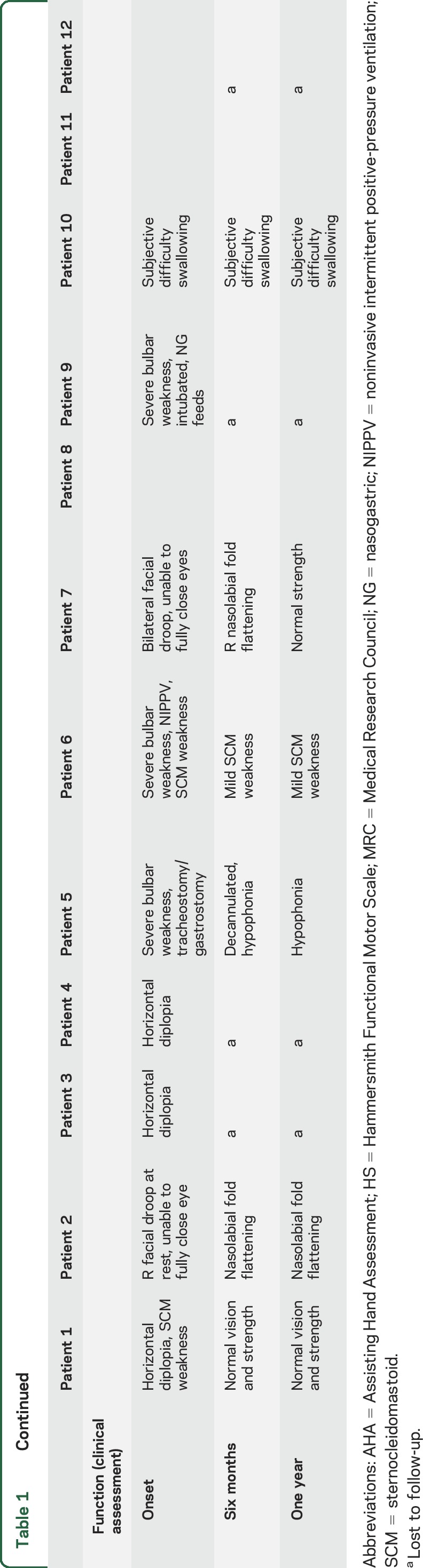
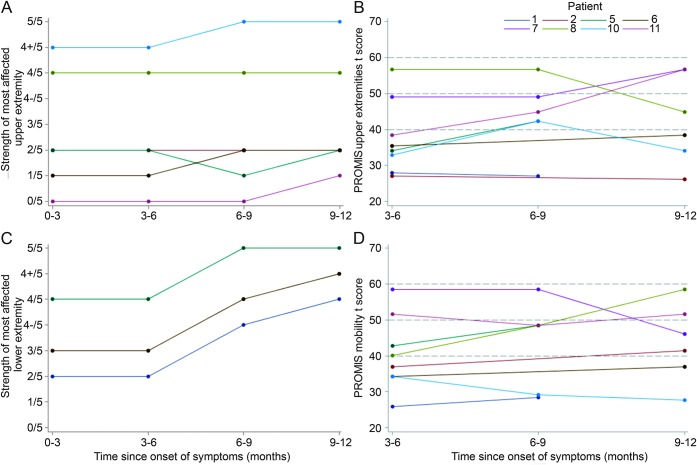
Figure 4. Strength and self-reported function of most affected upper and lower extremity in children with acute flaccid myelitis over 1 year.
(A) Spaghetti plot of Medical Research Council (MRC) strength score of patients' most affected upper extremity muscle at onset and 3, 6, and 12 months follow-up, revealing little to no change in strength in the most affected extremity at 1 year. (B) Spaghetti plot shows Patient-Reported Outcomes Measurement Information System (PROMIS) scores for upper extremity function at 3, 6, and 12 months follow-up. Mean score is 50 with 1 SD = 10 points. Initially, 6 patients were greater than 1 SD below the mean. This improved to 3 patients >1 SD below the mean at 1 year. (C) Spaghetti plot shows MRC strength score of patients' most affected lower extremity muscle at onset and 3, 6, and 12 months follow-up, showing improvement in strength in all 3 patients with lower extremity weakness. (D) Spaghetti plot shows PROIMS scores for mobility function at 3, 6, and 12 months follow-up. Mean score is 50 with 1 SD = 10 points.
Three of 8 (37%) patients initially had lower extremity weakness. Two of the 3 patients (67%) had persistent deficits at 1 year. Both had maximal weakness of 2/5 and 3/5 strength in the proximal lower extremities on hip flexion and extension, which improved to 4/5 and 4+/5 strength at 1 year, respectively.
Six of 8 (75%) patients had cranial nerve dysfunction at onset, which included diplopia, facial weakness, and bulbar weakness. All 6 patients showed improvement and the 2 patients in the original cohort with cranial nerve deficits had full resolution by report.
Functional outcomes.
Initial objective functional assessments of the upper extremities were performed on 6 of 8 patients at 3 months, and 2 patients had their first assessment at the 6-month visit. Five of 8 (63%) had abnormal function of the upper extremities on initial assessment; 2 showed significant improvement at 1 year with AHA score changes of 10 and 6 points. The remaining 3 patients (38%) showed minimal to no functional improvement across assessments (≤5 AHA points).
Objective functional assessments of the lower extremity were performed on all 8 patients within 6 months after onset of symptoms using the Hammersmith Scale. Two of 8 patients (25%) had abnormal initial assessments with lower extremity functional deficits that persisted out to 1 year. One patient remained wheelchair-dependent due to asymmetric flaccid weakness in the proximal lower extremities, while the other patient had notable gait impairment with ankle eversion and foot drop on the affected side.
Patient-reported outcomes.
Six of 8 patients (75%) self-reported upper extremity functional deficits at 3 months. Of those, 4 reported no improvement at 1 year.
Three patients reported functional mobility impairment at 1 year. Two had objective lower extremity weakness and functional deficits, while one had persistent complaints of pain limiting mobility but normal neurologic examinations and functional assessments.
One of 8 (12%) patients reported symptoms of fatigue at 3 months, which persisted at 1 year. Although 8 of the initial 12 patients (75%) reported symptoms of pain at the onset of neurologic symptoms, this was persistent in only 1 of 8 (12%) patients at 3 months, and persisted in this same patient at 1 year.
Three of 8 patients (37%) reported depressive symptoms at 3 months with concern for decreased psychosocial functioning. This persisted in 1 patient at 1 year. Substantial social stigma was not reported by any patient. Psychosocial challenges reported included anxiety and depression, behavioral outbursts, difficulty coping with the diagnosis, school avoidance, and difficulty functioning at school.
Neuroimaging outcomes.
Seven of 8 patients (88%) had follow-up brain and spine MRI a median of 7.5 months following onset of symptoms. Hyperintensities on T2-weighted imaging were noted in all patients in the spinal cord gray matter or brainstem on initial imaging. On last follow-up imaging, ranging 5–9 months after presentation, 6 patients (75%) had complete (n = 1) or near complete (n = 5) resolution of T2 hyperintensities in the brainstem or spinal cord. One patient (14%) had no change in imaging at 8 months after onset of symptoms. Nerve root enhancement was present in 4 of 8 (50%) patients on subacute imaging (1 week to 1 month after onset of symptoms). On follow-up, no new lesions or volume loss were noted and all areas of enhancement had resolved.
Electrophysiologic outcomes.
Initial EMG/NCS were performed on 7 study patients a mean of 1.3 months after onset of symptoms (table e-1 at Neurology.org). All had normal sensory NCS. Six of 7 (86%) had normal median and ulnar responses, while 1 patient with arm weakness had reduction in amplitude of motor responses. Of the 4 patients with lower extremity weakness who underwent initial EMG/NCS (1 lost to follow-up), 2 had reduced amplitudes of tibial motor responses, and 1 had reduced amplitudes of peroneal motor responses. Of the 6 patients with grade 4+ or lower MRC strength, 5 had fibrillation potentials in the affected muscles, and all 6 had reduced recruitment of motor unit action potentials (MUAP; table e-2). Fibrillation potentials were seen in clinically unaffected muscles of 2 patients with normal MUAP and recruitment. This corresponded to the spinal cord level of lesions seen on MRI.
Follow-up EMG/NCS were performed on 4 patients a mean of 10.5 months after onset of symptoms. Sensory and motor NCS were normal in all patients, including normalization of reduced tibial motor response amplitude in the 1 patient with an abnormal initial study. One patient had normalization of neurogenic EMG changes correlating with normalization of muscle strength at follow-up. In the remaining 3 patients with moderate persistent weakness, ongoing denervation with fibrillation potentials and chronic neurogenic changes with reduced recruitment were seen. No voluntary MUAP were seen in 2 patients with muscles with MRC grades of 0 and giant MUAP were seen in 1 patient.
DISCUSSION
We report motor, functional, neurodiagnostic, and psychosocial outcomes of children with AFM at 1 year. Though functional improvement was seen in all patients, recovery was incomplete, with persistent motor and functional deficits in most.
Motor deficits primarily localized to the proximal upper extremity, specifically C5–C7 myotomes. The most affected muscles at onset remained severely affected on neurologic examination and functional testing. Distal extremity deficits were less severe at onset and more likely to recover completely. Cranial nerve deficits were more likely to show recovery, though mild deficits persisted in most patients at 1 year. Despite functional compensation for some deficits, motor deficits on formal testing were persistent in most patients at 1 year.
In addition to the 12 patients in this cohort, one additional patient was identified from the fall of 2013 that fit our case definition for AFM and had EV-D68 identified in a nasopharyngeal specimen. At onset, he had flaccid weakness (MRC 0/5) in his proximal and distal right lower extremity. At 18 months following onset of symptoms, he had persistent motor and functional deficits with 3/5 strength in the proximal lower extremity and significant atrophy, and was ambulating with a 2-wheeled walker (figure 3D). Follow-up MRI at 19 months showed faint residual T2 hyperintensities in the anterior horns from T11 through the conus medullaris and resolved nerve root enhancement. Repeat EMG/NCS 2 years after onset showed a lack of motor unit potentials in the affected extremity without ongoing denervation. This case demonstrates the possibility of subtle functional improvements up to 2.5 years after AFM onset as well as the permanence of motor deficits.
This cohort presented during the 2014 outbreak of EV-D68 respiratory disease in Colorado, with the virus identified in the nasopharynx of 5 of 11 (45%) children tested.6,15 Case-control analysis and CDC nationwide epidemiologic data support an association between the increase in AFM cases and EV-D68.7,16 EV-D68-associated AFM has similarities with and differences from acute flaccid paralysis secondary to other known viral etiologies, such as poliovirus, EV-A71, EV-D70, and West Nile virus. These viruses demonstrate a tropism for motor neurons in the spinal cord and brainstem motor nuclei consistent with a virally mediated active infectious neuroinvasive process.1–5,17–20 Unlike EV-A71, cardiopulmonary failure due to brainstem encephalitis was not seen, and unlike West Nile virus, supratentorial lesions and encephalitis were absent in our cohort.19,21 The high percentage of patients with long-term motor and functional deficits with profound muscle atrophy in our cohort mirrors the permanent deficits of other infectious causes of poliomyelitis.2–4,18,19 Similar to West Nile virus acute flaccid paralysis, the patients and muscle groups with less profound initial weakness showed the greatest strength gains.18 No worsening of motor or functional outcomes was seen after the subacute period in this cohort, but longer term assessment is needed to evaluate for a postpoliomyelitis syndrome.22,23
Following identification of this cohort, the CDC convened a multidisciplinary group of experts to provide interim recommendations for management of AFM. The group found insufficient evidence to recommend corticosteroids, IV immunoglobulin, or plasmapheresis.24 These treatments were variably administered among our cohort of patients, which does not allow systematic assessment of response to treatment. There was no clear correlation with short- or long-term outcomes with any of these interventions. The lack of observed response to treatment was also noted in the short-term follow-up of 10 children reported from Salt Lake City, Utah, when followed to a median of 6 months, as well as in a series of 59 patients in California followed out to a median of 9 months.25,26
This study provides important insights into the ability of diagnostic tools to evaluate recovery in AFM. Though diagnostic at onset, MRIs were incongruous with clinical findings in subsequent months, failing to correlate with physical or functional outcomes. Normalized imaging may lead to underdiagnosis of AFM in those who present in the subacute and chronic phase of illness. Conversely, EMG/NCS in affected patients showed denervation potentials with severity of neurogenic changes accurately reflecting the degree of ongoing weakness. Of note, follow-up EMG/NCS were performed primarily in those with persistent severe symptoms, so it is unknown if the same abnormalities would be noted in patients who reported resolution or significant improvement. In contrast to MRI, EMG/NCS may be a useful tool to evaluate recovery of affected muscles and warrants further evaluation as a diagnostic tool in late presentations of illness.
AFM had a substantial psychosocial effect in some patients, particularly in the 3 months following onset of symptoms. Prior to the onset of illness, 1 patient had a history of depression, while 4 patients reported no prior symptoms of depression or anxiety. Information was unavailable for the remaining 3. While our psychologist did identify emotional, coping, and behavioral difficulties on initial assessments, outcomes improved over time. At 1 year, depressive symptoms were minimal, all of our patients had returned to school, and most declined to meet with our psychologist due to lack of ongoing challenges. The children and families in our study demonstrated a high degree of resilience and recovery, despite their debilitating illness. We recommend screening for psychosocial difficulties following AFM diagnosis to enable effective support and intervention to promote positive coping and psychosocial functioning.
This study was limited by cohort size and lack of follow-up in 4 of 12 original patients. As more severely and persistently affected patients are more apt to follow-up, this report likely represents the more severe end of the spectrum of this disease. Consistent with this limitation, 2 of the patients who did not follow-up reported complete functional recovery. Although trends and associations between functional and patient-reported outcomes and diagnostic testing were observed, statistical analysis was limited by small sample size. Though validated for similar conditions, the functional testing measures utilized have not been previously studied in AFM. The lack of standardized schedule for follow-up imaging and EMG/NCS limits conclusions that can be drawn about their utility in AFM and warrants further study.
In our cohort of patients with AFM, the physical and functional deficits were severe and are likely permanent. Future studies should investigate tools to accurately predict the course of this disease and identify effective treatments to prevent the severe long-term outcomes observed in this cohort.
GLOSSARY
- AFM
acute flaccid myelitis
- AHA
Assisting Hand Assessment
- CDC
Centers for Disease Control and Prevention
- CHCO
Children's Hospital Colorado
- EV
enterovirus
- MRC
Medical Research Council
- MUAP
motor unit action potentials
- NCS
nerve conduction studies
- PROMIS
Patient-Reported Outcomes Measurement Information System
Footnotes
Supplemental data at Neurology.org
Editorial, page 112
AUTHOR CONTRIBUTIONS
J.A. Martin is a coinvestigator of the clinical research study to follow-up patients with AFM, collected neurologic clinical data and patient questionnaires, created figures for the manuscript, and wrote the first draft of the manuscript. K.M. is the principal investigator of the clinical research study to follow-up patients with AFM, consented and enrolled patients, collected biologic and clinical data, and assisted in writing, reviewing, and revising the manuscript. M.L.Y. collected neurophysiologic clinical data, wrote the neurophysiologic portions of the manuscript, and reviewed and revised the manuscript. J.A. Maloney collected neuroradiologic clinical data and reviewed and revised the manuscript. J.L. collected psychosocial clinical data, wrote the psychosocial portion of the manuscript, and reviewed and revised the manuscript. T.C. and P.K. collected objective functional outcomes clinical data and reviewed and revised the manuscript. S.H.S. provided statistical analysis of clinical data collected and created figures for the manuscript. J.O. collected functional clinical data and reviewed and revised the manuscript. K.L.T. participated in patient evaluations and examinations and reviewed and revised the manuscript. S.R.D. is a coinvestigator of the clinical research study to follow-up patients with AFM, created figures for the manuscript, and reviewed and revised the manuscript. T.L.S. is a coinvestigator of the clinical research study to follow-up patients with AFM, collected neurologic clinical data, and reviewed and revised the manuscript.
STUDY FUNDING
Supported by NIH/NCATS Colorado CTSA grant UL1 TR001082.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Horstmann DM. Clinical aspects of acute poliomyelitis. Am J Med 1949;6:592–605. [DOI] [PubMed] [Google Scholar]
- 2.Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med 1999;341:936–942. [DOI] [PubMed] [Google Scholar]
- 3.Lee HF, Chi CS. Enterovirus 71 infection-associated acute flaccid paralysis: a case series of long-term neurologic follow-up. J Child Neurol 2014;29:1283–1290. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Jiang L, Peng HL. Clinical analysis of 134 children with nervous system damage caused by enterovirus 71 infection. Pediatr Infect Dis J 2015;34:718–723. [DOI] [PubMed] [Google Scholar]
- 5.Neurovirulence of enterovirus 70. Lancet 1982;1:373–374. [PubMed] [Google Scholar]
- 6.Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015;385:1662–1671. [DOI] [PubMed] [Google Scholar]
- 7.Sejvar JJ, Lopez AS, Cortese MM, et al. Acute flaccid myelitis in the United States, August-December 2014: results of nationwide surveillance. Clin Infect Dis 2016;63:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midgley C, Watson J, Nix W, et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological evaluation. Lancet Respir Med 2015;3:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The assisting hand assessment: current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol 2007;49:259–264. [DOI] [PubMed] [Google Scholar]
- 10.Louwers A, Beelen A, Holmefur M, Krumlinde-Sundholm L. Development of the Assisting Hand Assessment for Adolescents (Ad-AHA) and validation of the AHA from 18 months to 18 years. Dev Med Child Neurol 2016;58:1303–1309. [DOI] [PubMed] [Google Scholar]
- 11.Main M, Kairon H, Mercuri E, Muntoni F. The Hammersmith Functional Motor Scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol 2003;7:155–159. [DOI] [PubMed] [Google Scholar]
- 12.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones HR, Bolton CF, Harper CM. Pediatric Clinical Electromyography. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messacar K, Hawkins SM, Baker J, et al. Resource burden during the 2014 enterovirus D68 respiratory disease outbreak at Children's Hospital Colorado: an unexpected strain. JAMA Pediatr 2016;170:294–297. [DOI] [PubMed] [Google Scholar]
- 16.Aliabadi N, Messacar K, Pastula DM, et al. Enterovirus D68 infection in children with acute flaccid myelitis, Colorado, USA, 2014. Emerging Infect Dis 2016;22:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerging Infect Dis 2005;11:1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile Virus-associated flaccid paralysis outcome. Emerging Infect Dis 2006;12:514–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis 2014;14:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sejvar JJ, Leis AA, Stokic DS, et al. Acute flaccid paralysis and West Nile virus infection. Emerging Infect Dis 2003;9:788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010;9:1097–1105. [DOI] [PubMed] [Google Scholar]
- 22.Dalakas MC. New neuromuscular symptoms in patients with old poliomyelitis: a three-year follow-up study. Eur Neurol 1986;25:381–387. [DOI] [PubMed] [Google Scholar]
- 23.Dalakas MC, Elder G, Hallett M, et al. A long-term follow-up study of patients with post-poliomyelitis neuromuscular symptoms. N Engl J Med 1986;314:959–963. [DOI] [PubMed] [Google Scholar]
- 24.Sejvar JJ, Pastula DM, Cortese MM, et al. ; for US Centers for Disease Control and Prevention. Acute flaccid myelitis: interim considerations for clinical management. Available at: cdc.gov/ncird/downloads/acute-flaccid-myelitis.pdf. Accessed November 7, 2014.
- 25.Nelson GR, Bronkowsky JL, Doll E, et al. Recognition and management of acute flaccid myelitis in children. Pediatr Neurol 2016;55:17–21. [DOI] [PubMed] [Google Scholar]
- 26.Van Haren K, Ayscue P, Waubant E, et al. Acute flaccid myelitis of unknown etiology in California, 2012–2015. JAMA 2015;314:2663–2667. [DOI] [PubMed] [Google Scholar]