Abstract
Objective:
To identify the significance of baseline motor features to the lifelong prognostic motor subtypes in a Parkinson disease (PD) cohort.
Methods:
In a previous study of 166 PD cases, we observed different prognosis in tremor-dominant, akinetic-rigid, and mixed subtypes. This study includes the same cases, but we excluded 10 cases with symptoms of ≥15 years duration at baseline. Relative severity of tremor, bradykinesia/akinesia, and rigidity at baseline were evaluated as predictors of the motor subtypes, which are known to have different prognosis.
Results:
The most common motor subtype was mixed, followed by akinetic-rigid and then the tremor-dominant. Seventy cases were not receiving antiparkinsonian drugs at baseline. The prognostic subtypes could be predicted at baseline in 85% of all and in 91% of the treatment-naive cases. Sensitivity, specificity, and positive predictive values were strong for the mixed and the akinetic-rigid but weak for the tremor-dominant subtype.
Conclusions:
Our data show that motor profile at baseline can predict prognosis in most PD cases. These findings can be incorporated into clinical practice.
Parkinson disease (PD), the most common degenerative Parkinson syndrome (PS) variant,1–3 is characterized by substantia nigra (SN) neuronal loss and Lewy body (LB) inclusions. Clinical diagnosis of PD is based on 2 of 3 of bradykinesia/akinesia, rigidity, and rest tremor.2,4–6 Freezing of gait and dementia are rare at PD onset.3,7–9 Prior to recognition of multiple system atrophy and progressive supranuclear palsy (PSP),1 clinical distinction between different degenerative variants of PS was not possible and most cases were diagnosed as PD. Accuracy of PD clinical diagnosis improves with follow-up,2 but definite diagnosis requires pathologic findings.2,10–12
When PD is first diagnosed, patients need information on nature of disease, treatment options, and prognosis.13
Several clinical subtypes have been proposed to predict prognosis, but the methodology and classification criteria are not uniform.6,7,14–20 Most studies are based on one assessment, but the subtypes change with time.6,15,19,20 We reported on PD subtypes considering the entire clinical course of disease.7 As observed by others, tremor-dominant (TD) cases had better outcome than mixed (MX) and akinetic-rigid (AR) subtypes.3,6,7 In addition, brain biochemical abnormality is the most pronounced in AR cases.21
The objective of this study was to determine if patients evolving into these 3 prognostic subtypes7,21 and hence the course of PD could be identified at first neurologic assessment.
We hypothesize that predominant tremor at baseline would evolve into TD, predominant akinesia/bradykinesia and rigidity into AR, and equal severity of tremor and bradykinesia/rigidity into MX subtypes.
METHODS
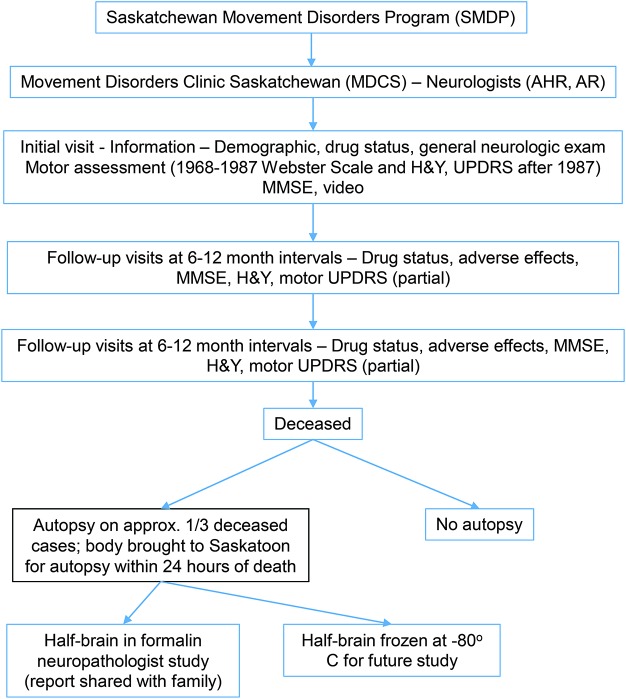
This study was conducted at Saskatchewan Movement Disorders Program. The figure is a flow diagram of that program. Patients included in this study are the same as reported previously.7
Figure. Flow diagram of Movement Disorders Clinic Saskatchewan.
H&Y = Hoehn & Yahr scale; MSE = Mini-Mental State Examination; UPDRS = Unified Parkinson’s Disease Rating Scale.
Standard protocol approvals, registrations, and patient consents.
All patients with PD or their families provided written consent for autopsy and use of brain for research. The study was approved by the University of Saskatchewan/Saskatoon Health Region.
Patient selection.
Patients were assessed at Movement Disorders Clinic Saskatchewan (MDCS) and autopsied between 1968 and 2006. All Saskatchewan residents carry general tax-funded health insurance and have equal access to MDCS.22 Most patients seen at the MDCS are referred by family physicians. All cases of autopsy-confirmed PD12 were considered for inclusion. Final diagnosis was made by the treating neurologist considering all the clinical and pathology information. Excluded were cases with additional disorders that may modify motor symptoms, e.g., essential tremor, PSP, corticobasal degeneration, ablative surgery, and drug-induced parkinsonism.7 No patient was excluded because of other comorbidity. Further excluded from the previous study of 166 cases7 were 10 cases that had 15 years or longer duration of symptoms at baseline visit.
Clinical data.
Age, sex, age at onset, and first motor symptom are recorded at initial evaluation. As a rule, patients are followed at 6- to 12-month intervals. The severity of bradykinesia, rigidity and tremor,23,24 Hoehn & Yahr (H&Y) stage,3,24 antiparkinsonian drugs, response to treatment, motor response fluctuations, and dyskinesia are recorded at each visit. Each patient is examined by one or both movement disorders neurologists (A.H.R., A.R.) at every clinic visit. Patients have free telephone access to the treating neurologist between clinic visits. From 1968 to 1987, resting tremor, bradykinesia, and rigidity in the upper limbs were measured by the Webster Scale.23 That scale did not include lower limb motor severity assessment. Global disability was measured by the H&Y scale3 and subsequently by the Unified Parkinson's Disease Rating Scale (UPDRS).24 For uniformity in the entire cohort,7,25 UPDRS–H&Y stage 1.5 was converted to original stage 1 and the modified UPDRS–H&Y stage 2.524 to the original stage 3 H&Y3 where needed. Since UPDRS motor scores in the lower limbs were not available for the entire study interval, we used only the upper limb measurements. Patients were evaluated as they came to the MDCS and not specifically during “on” or “off” state. Dementia was diagnosed when there was substantial cognitive impairment considering the age and education of the patient.7
For this study, the most pronounced rigidity at any upper limb joint, the most pronounced bradykinesia with any maneuver (pronation/supination, finger tapping, fist opening and closing), and the most severe resting tremor in either upper limb recorded during assessment were regarded as representative of the particular symptom at that point in time.
Prognostic subtyping.
The motor subtyping classification was based on all the clinical observations made at the MDCS. Patients who had 1 grade higher resting tremor score by either Webster23 or UPDRS scale24 compared to both the bradykinesia and the rigidity on ≥75% of assessments were classified as TD, those with 1 grade higher bradykinesia or rigidity compared to resting tremor on ≥75% of the assessments were classified as AR, and those who did not meet either of those 2 criteria were classified as MX subtype.7
Baseline motor classification.
The classification of baseline motor profile was based on the relative severity of the upper limb resting tremor, bradykinesia, and rigidity at the first MDCS evaluation.23,24 Patients with 1 grade higher resting tremor score compared to each the bradykinesia and rigidity were classified as baseline tremor predominant (BTR). One grade higher bradykinesia or rigidity compared to resting tremor at baseline was classified as baseline akinetic-rigid predominant (BAR). Those who did not fall in either of those 2 groups were classified as baseline mixed (BMX).
Pathology study.
Autopsy procedure is outlined in the flow chart (figure). All anatomic sites of known significance to PS are examined.7 Standard contemporary protocol is followed. All the informative staining techniques including silver stain, ubiquitin, α-synuclein, and tau that were commercially available at our institution at the time of autopsy were used.7 Pathologic diagnosis of PD is based on marked substantia nigra neuronal loss and LB inclusions.1,2 Standard pathology at our institution does not include Consortium to Establish a Registry for Alzheimer’s Disease criteria for diagnosis of Alzheimer disease.
The neuropathologist report is shared with the family and are offered consultation with neurologists.
Statistics.
SPSS version 17 (SPSS Inc., Chicago, IL) was used for all statistical analyses. The statistical tests were considered as significant at α ≤ 0.05. The patient baseline motor profiles were compared with the 3 prognostic motor subtypes reported previously7 using χ2 for categorical variables and analysis of variance for continuous variables. Kappa statistics were calculated to evaluate the accuracy of the predictive hypothesis. The sensitivity and specificity for predominant motor symptoms at baseline were evaluated by dichotomous categories—AR vs not AR, TD vs not TD, and MX vs not MX subtypes.7 Patients who were not receiving antiparkinsonian drugs at baseline were also analyzed separately.
RESULTS
A total of 187 patients followed at MDCS between 1968 and 2006 had pathology-verified PD. Twenty-one patients were excluded due to comorbidity7 that modifies parkinsonian motor manifestations. Four had ablative surgery, 4 had additional PSP pathology, and 2 manifested PD only after neuroleptic drug use. Eight patients who had long history of essential tremor before PD onset were also excluded. Three patients were excluded due to insufficient data. Ten more patients who at baseline had 15 years or longer duration of symptoms were excluded. A total of 156 cases included in this study are the same as in a previous study dealing with prognosis.7
Table 1 is a summary of all 156 cases and the subgroup of 70 cases not receiving antiparkinsonian drugs at baseline. The most common subtype was MX. H&Y stage could not be determined accurately at baseline in 11 cases. The drug therapy profile was similar in the 3 subtypes. Of the 91 (58%) patients not receiving levodopa at baseline, 21 were on another antiparkinsonian drug, but 70 (45%) were on no antiparkinsonian drug.
Table 1.
Patient characteristics and symptoms in lifelong motor subtypes at initial Movement Disorders Clinic Saskatchewan assessment
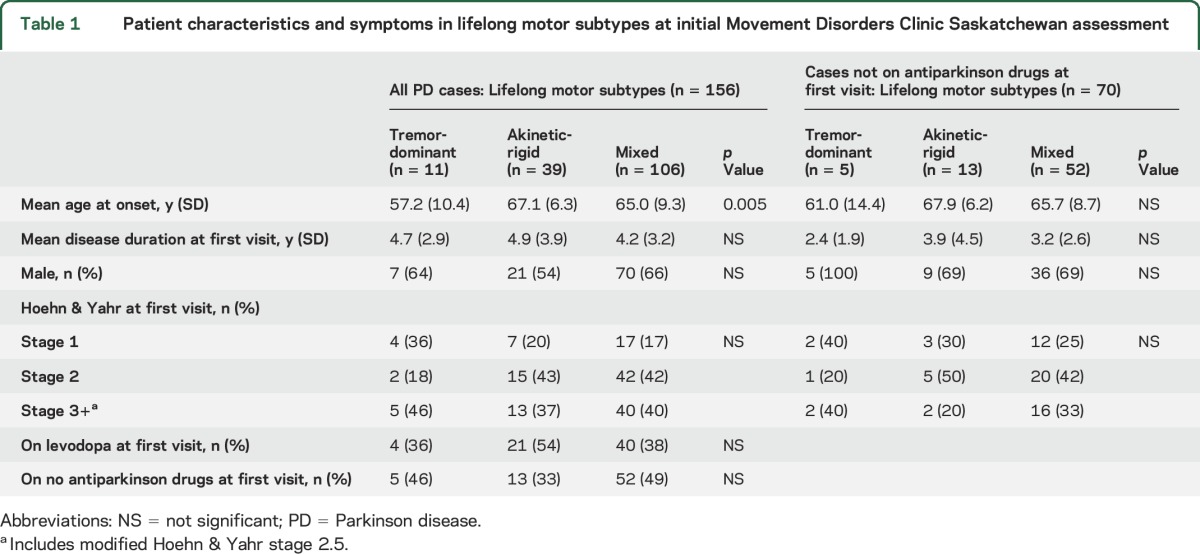
Table 2 shows the distribution of baseline motor profile and prognostic subtypes in all 156 cases. The figures on sensitivity, specificity, and positive predictors are robust for the AR and MX but not for TD subtype. Regardless of treatment status, the baseline data accurately predicted subtypes in 85% cases. The majority of all cases (93%) had either AR or MX subtype. These 2 subtypes could be predicted in 127 (88%) of those cases.
Table 2.
Baseline predominant motor profile used to predict lifelong subtype in all PD cases
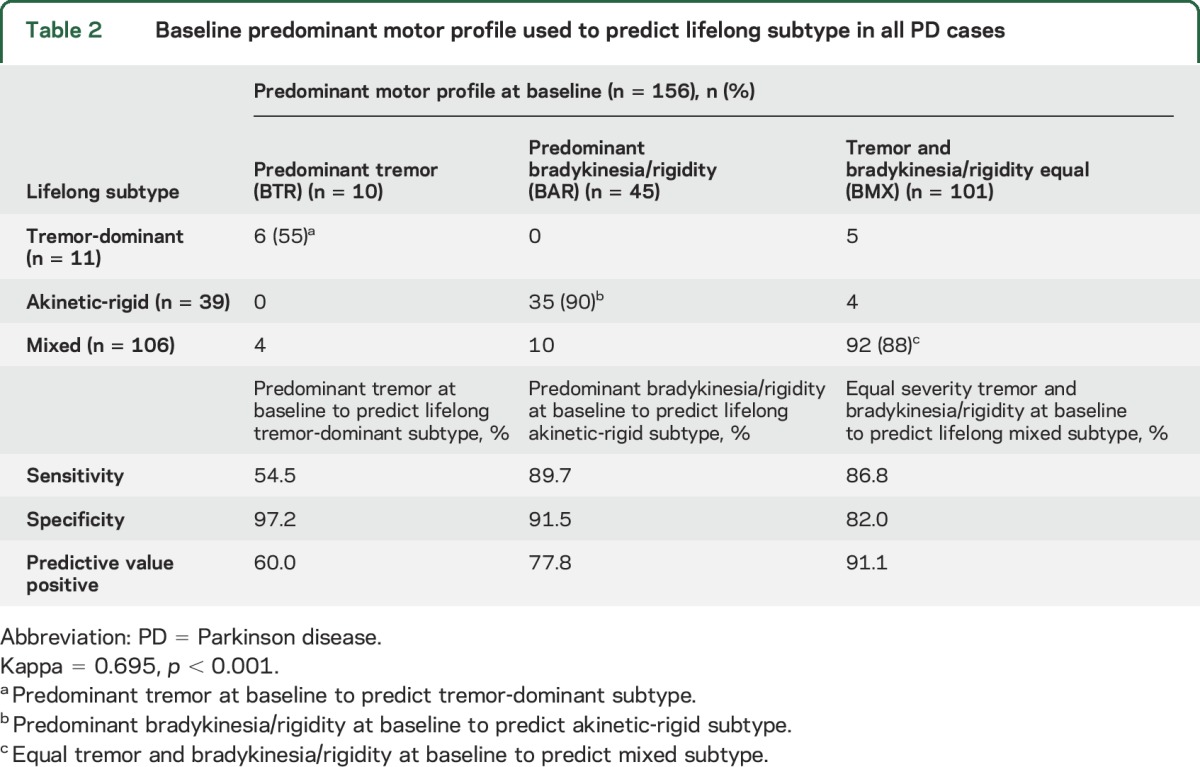
Table 3 shows the baseline motor profile and prognostic subtypes in the 70 untreated PD cases. Baseline data accurately predicted subtypes in 64 (91%) cases. Sensitivity, specificity, and positive predictive values were strong for AR and MX but not for TD. Tables 2 and 3 show that no patient with baseline tremor predominant profile evolved into AR subtype and no patient with baseline bradykinesia/rigidity profile evolved into TD subtype.
Table 3.
Baseline predominant motor profile used to predict lifelong subtype in 70 patients who were not using any antiparkinson drug at initial Movement Disorders Clinic Saskatchewan visit
DISCUSSION
The objective of our study was to determine if the motor profile at initial MDCS evaluation can predict the 3 subtypes—TD, MX, and AR—that determine prognosis in PD.7 Our data show that prognosis linked subtypes and thus the prognosis could be predicted at baseline in the majority (85%) of all PD cases, regardless of treatment status. The prediction rate was 91% in the treatment-naive cases. The prediction rate was the lowest in TD but that subgroup accounted for only 7% of cases. Other studies with long-term clinical follow-up also had low TD prevalence rates of 3%6 and 8%.20
The scientific foundation of this study is our 2 previous studies.7,21 We showed that AR cases had more pronounced and more widespread brain biochemical abnormalities than the MX and TD subtypes.21 A second study looked at the disease course in these 3 subtypes.7 The progression from onset to reaching stage 4.0 H&Y3 (severely disabled) was accelerated in AR compared to MX and in MX compared to TD.7 A larger proportion of MX and TD patients improved on levodopa vs AR patients. The cumulative incidence of dementia was the highest in AR, intermediate in MX, and lowest in TD. Survival was the shortest in AR followed by MX and then TD. Thus the prognosis was the worst in AR, the best in TD, and intermediate in MX subtypes.7
As the motor subtypes can change with duration of disease,19,20 we used the composite motor profile based on the entire clinical follow-up for the subtyping. The follow-up in our cases ranged from none to 24 years and the number of clinical evaluations varied from 1 to 54.7 All cases had autopsy study to confirm the diagnosis of PD, thereby excluding other entities that have different prognosis.2,10,11
Our study has limitations. It is based on patients seen at a movement disorders clinic, which is subject to referral bias; as well, there could be autopsy consent bias. The majority (56%) of our cases had mild disease (stage 1 or 2 H&Y3) at first visit. At other locations, that ratio may be different. Clinical data were collected over 4 decades. During that time, new motor measurement scales were developed.24 The difference between the older3,23 and new scale, however, is small.25 Moreover, we did not use absolute scores but the difference in the motor severity scores regardless of the scale. We did not use the full UPDRS24 as that scale was not available for the first 20 years of study. The full UPDRS scale is long and is not ideal for clinical practice. We used the H&Y scale3,24 to measure disease progression. This scale has been in use for nearly 5 decades and is easy to use. It has been used in several other studies that evaluated progression of PD.16,26–31
Dementia was not assessed in more detail. The Mini-Mental State Examination scale became available after we started data collection. The scores change with age and education of the patient. Some of our patients were unable to read and write. Therefore we used functional assessment, considering age and education for dementia diagnosis,7 as has been done in another longitudinal clinical/pathology study.32
We restricted the assessment of parkinsonian findings to upper limb motor severity because lower limb motor assessment was not part of the Webster Scale,23 which we used for the first 20 years of this study. Motor onset in PD is most common as upper limb tremor.4,8,9 Although not ideal, upper limb motor assessment has also been used for PD severity by others.33–35
Our observations would be useful in predicting PD prognosis at first visit in real practice. A possible limitation is that a substantial proportion (42%) of our patients (table 1) at baseline were on levodopa, which is known to modify symptoms and staging of PD. Tables 1 and 3 show that the predictive values in those not receiving antiparkinsonian drug at baseline assessment and the entire cohort are remarkably similar. The clinicians are not aware of the final PD diagnosis at the time of first evaluation.2,10–12 As such, the results of our study, which is based on clinical and pathologic findings, are not completely extrapolatable to every PS case seen in clinical practice.
We did not evaluate patients during “on” or “off” state. That is not possible in clinical practice, as reported by others.8,17
Our study has several strengths. The subtyping is based on sound scientific basis.7,21 All patients had definite PD.12 Three is a small number of subtypes and practical for clinical use.18 Upper limb motor assessment is reasonably easy to perform.
Information on prognosis of PD during the early stage of disease remains a major unmet need in the care of these patients.13 Predicting the outcome for patients with PD when first evaluated is a question all practicing neurologists face. Observations of our study can be used in the office practice of neurology.
We recommend the following baseline evaluation procedure. Determine the severity of upper limb resting tremor by UPDRS motor scale with the patient lying supine on the examination table with arms fully supported or the patient seated with the arms fully resting on the armrests of a chair. The most severe tremor on either side is noted. Rigidity is measured by UPDRS scale at the wrist, the shoulder (by internal/external rotation), or the elbow. The most pronounced rigidity on either side with any of these maneuvers is noted. Bradykinesia is measured by UPDRS scale with pronation/supination, rapid finger tapping (both sides together), and fist opening/closing. Whichever of those measurements elicits the most pronounced bradykinesia in either upper limb is used to classify baseline profile. The severity of rest tremor is compared with the severity of rigidity or bradykinesia. If the tremor score is 1 grade higher than both the bradykinesia and rigidity, the patient has BTR profile. If a patient has 1 grade higher score for either bradykinesia or rigidity than the tremor, the patient has BAR profile. If the patient does not fall in either of the above 2 categories, the patient has BMX profile. This baseline classification is used to predict the prognostic subtypes.7
This portion of the motor UPDRS scale can be carried as a small plasticized card or cell phone app. Such evaluation needs to be done only at the initial assessment and as such would not require additional time in the clinic, considering that the discussion on the prognosis would be shortened.
ACKNOWLEDGMENT
The authors thank L. Beatty for support in preparation of the manuscript.
GLOSSARY
- AR
akinetic-rigid
- BAR
baseline akinetic-rigid predominant
- BMX
baseline mixed
- BTR
baseline tremor predominant
- H&Y
Hoehn & Yahr
- LB
Lewy body
- MDCS
Movement Disorders Clinic Saskatchewan
- MX
mixed
- PD
Parkinson disease
- PS
Parkinson syndrome
- PSP
progressive supranuclear palsy
- TD
tremor-dominant
- UPDRS
Unified Parkinson's Disease Rating Scale
AUTHOR CONTRIBUTIONS
Ali Rajput: study concept and design, acquisition of data, manuscript preparation. Michele Rajput: statistical analysis, manuscript preparation. Leslie Ferguson: manuscript preparation. Alex Rajput: study concept and design, acquisition of data, manuscript preparation.
STUDY FUNDING
Financial support was provided by Greystone Management and by the Royal University Hospital Foundation unrestricted grants.
DISCLOSURE
A.H. Rajput, M.L. Rajput, and L. Ferguson report no disclosures relevant to the manuscript. A. Rajput received unrestricted research support from the Regina Curling Classic, Greystone Classic for Parkinson's, Inc. and the Dr. Ali Rajput Endowment for Parkinson's Disease and Movement Disorders, and received honoraria from Teva Pharmaceuticals and Parkinson Society Canada for speaking engagements. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Jellinger K. The pathology of parkinsonism. In: Marsden CD, Fahn S, eds. Movement Disorders 2. London: Butterworths; 1987:124–165. [Google Scholar]
- 2.Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism: a prospective study. Can J Neurol Sci 1991;18:275–278. [DOI] [PubMed] [Google Scholar]
- 3.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 4.Pagano G, Ferrara N, Brooks DJ, et al. Age at onset and Parkinson disease phenotype. Neurology 2016;86:1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1599. [DOI] [PubMed] [Google Scholar]
- 6.Reinoso G, Allen JC, Au WL, et al. Clinical evolution of Parkinson's disease and prognostic factors affecting motor progression: 9-year follow-up study. Eur J Neurol 2015;22:457–463. [DOI] [PubMed] [Google Scholar]
- 7.Rajput AH, Voll A, Rajput ML, et al. Course in Parkinson's disease subtypes: a 39-year clinicopathological study. Neurology 2009;73:206–212. [DOI] [PubMed] [Google Scholar]
- 8.Uitti RJ, Baba Y, Wszolek ZK, et al. Defining the Parkinson's disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat Disord 2005;11:139–145. [DOI] [PubMed] [Google Scholar]
- 9.Rajput AH, Pahwa R, Pahwa P, et al. Prognostic significance of the onset mode in parkinsonism. Neurology 1993;43:829–830. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: a clinicopathologic study. Neurology 2014;83:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 13.Dorsey ER, Voss TS, Shprecher DR, et al. A U.S. survey of patients with Parkinson's disease: satisfaction with medical care and support groups. Mov Disord 2010;25:2128–2135. [DOI] [PubMed] [Google Scholar]
- 14.Fereshtehnejad SM, Romenets SR, Anang JBM. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 2015;72:863–873. [DOI] [PubMed] [Google Scholar]
- 15.Velseboer DC, De Bie RMA, Wieske L, et al. Development and external validation of a prognostic model in newly diagnosed Parkinson disease. Neurology 2016;86:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selikhova M, Williams DR, Kempster PA, et al. A clinico-pathological study of subtypes in Parkinson's disease. Brain 2009;132:2947–2957. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol 2001;58:1611–1615. [DOI] [PubMed] [Google Scholar]
- 18.Marras C, Lang A. Parkinson's disease subtypes: lost in translation? J Neurol Neurosurg Psychiatry 2012;84:409–415. [DOI] [PubMed] [Google Scholar]
- 19.Simuni T, Caspell-Garcia C, Coffey C, et al. How stable are Parkinson's disease subtypes in de novo patients: analysis of the PPMI cohort? Parkinsonism Relat Disord 2016;28:62–67. [DOI] [PubMed] [Google Scholar]
- 20.Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006;21:1123–1130. [DOI] [PubMed] [Google Scholar]
- 21.Rajput AH, Sitte H, Rajput A, et al. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology 2008;70:1403–1410. [DOI] [PubMed] [Google Scholar]
- 22.Rajput AH, Rajput A. Saskatchewan movement disorders program. Can J Neurol Sci 2015;42:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster DD. Critical analysis of disability in Parkinson's disease. Mod Treat 1968;5:257–282. [PubMed] [Google Scholar]
- 24.Fahn S, Elton RL; UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, et al., eds. Recent Developments in Parkinson's Disease, 2nd ed. Florham Park: Macmillan Healthcare Information; 1987:153–305. [Google Scholar]
- 25.Rajput AH, Uitti RJ, Rajput A, et al. Timely levodopa (LD) administration prolongs survival in Parkinson's disease. Parkinsonism Relat Disord 1997;3:159–165. [DOI] [PubMed] [Google Scholar]
- 26.Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med 1989;321:1364–1371. [DOI] [PubMed] [Google Scholar]
- 27.Marttila RJ, Rinne UK. Disability and progression in Parkinson's disease. Acta Neurol Scand 1977;56:159–169. [DOI] [PubMed] [Google Scholar]
- 28.Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol 2005;62:378–382. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson LW, Rajput ML, Muhajarine N, et al. Clinical features at first visit and rapid disease progression in Parkinson's disease. Parkinsonism Relat Disord 2008;14:431–435. [DOI] [PubMed] [Google Scholar]
- 30.Goetz CG, Poewe W, Rascol O, et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19:1020–1028. [DOI] [PubMed] [Google Scholar]
- 31.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord 2008;23:790–796. [DOI] [PubMed] [Google Scholar]
- 32.Bower JH, Dickson DW, Taylor L, et al. Clinical correlates of the pathology underlying parkinsonism: a population perspective. Mov Disord 2002;17:910–916. [DOI] [PubMed] [Google Scholar]
- 33.Chan PLS, Nutt JG, Holford NHG. Pharmacokinetic and pharmacodynamic changes during the first four years of levodopa treatment in Parkinson's disease. J Pharmacokinet Pharmacodyn 2005;32:459–484. [DOI] [PubMed] [Google Scholar]
- 34.Nutt JG, Carter JH, Lea ES, et al. Evolution of the response to levodopa during the first 4 years of therapy. Ann Neurol 2002;51:686–693. [DOI] [PubMed] [Google Scholar]
- 35.Homann CN, Quehenberger F, Petrovic K, et al. Influence of age, gender, education and dexterity on upper limb motor performance in parkinsonian patients and healthy controls. J Neural Transm 2003;110:885–897. [DOI] [PubMed] [Google Scholar]