Abstract
Objective:
Amyotrophic lateral sclerosis (ALS) progresses at different rates between patients, making clinical trial design difficult and dependent on large cohorts of patients. Currently, there are few data showing whether the left and right limbs progress at the same or different rates. This study addresses rates of decline in specific muscle groups of patients with ALS and assesses whether there is a relationship between left and right muscles in the same patient, regardless of overall progression.
Methods:
A large cohort of patients was used to assess decline in muscle strength in right and left limbs over time using 2 different methods: The Tufts Quantitative Neuromuscular Exam and Accurate Test of Limb Isometric Strength protocol. Then advanced linear regression statistical methods were applied to assess progression rates in each limb.
Results:
This report shows that linearized progression models can predict general slopes of decline with good accuracy. Critically, the data demonstrate that while overall decline is variable, there is a high degree of correlation between left and right muscle decline in ALS. This implies that irrespective of which muscle starts declining soonest or latest, their rates of decline following onset are more consistent.
Conclusions:
First, this study demonstrates a high degree of power when using unilateral treatment approaches to detect a slowing in disease progression in smaller groups of patients, thus allowing for paired statistical tests. These findings will be useful in transplantation trials that use muscle decline to track disease progression in ALS. Second, these findings discuss methods, such as tactical selection of muscle groups, which can improve the power efficiency of all ALS clinical trials.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder in which a loss of motor neurons results in muscle weakness, paralysis, and death within 3–5 years of diagnosis. There is no successful treatment and the fact that progression rates are different in each patient makes clinical trial design challenging even when incorporating many patients with ALS into open source anonymized databases.1,2
One promising cell therapy approach involves the transplantation of stem/progenitor cells into localized regions of the CNS, permitting new clinical trials where only discreet neuronal groups are treated.3 Unilateral treatments are expected to affect the side of transplantation and results can be compared to the nontreated side. This trial design permits the use of more powerful pairwise statistics, in which measurements are taken side by side at the same time, potentially increasing the power of the clinical trial and alleviating the need for a placebo-controlled cohort. Critically, this clinical design requires understanding left and right limb progression rates. Currently, however, there are little data showing whether the left and right limbs progress at the same or different rates in the same patient.
The current study analyzed disease progression in patients with ALS by comparing the right to left side of muscle pairs and by comparing different muscle groups. To do this, we assessed a large dataset of patients who were followed over time for muscle strength via fixed dynamometry using the Tufts Quantitative Neuromuscular Exam (TQNE).4,5 In addition, we assessed a set of patients who were followed using the more recently developed Accurate Test of Limb Isometric Strength (ATLIS).6 This report shows that linearized progression models can predict general slopes of decline with good accuracy. Critically, the data from patients demonstrate that while overall decline is variable, there is a high degree of correlation between left and right muscle decline in ALS. This study clearly shows that specific muscle groups may be better to follow in patients over time due to their consistency in decline rate, and that most muscles show a very similar rate of decline between the left and right side of individual patients with ALS.
METHODS
Standard protocol approvals, registrations, and patient consents.
For the TQNE and ATLIS measurements, all participants provided written informed consent as approved by each site's institutional review board (IRB) (Massachusetts General Hospital: IRB approval 2013P000702/MGH; Tufts: Developing a database of the natural history of neuromuscular diseases—IRB approval dates 1978–1995 [no number available]; Cedars-Sinai: IRB approval 38868).
Data collection.
A longitudinal, historical database from Tufts Medical Center consisting of TQNE measurements for 6 muscle groups (hand grip, biceps, triceps, quadriceps, hamstrings, and anterior tibialis) for each side from 846 patients was analyzed.4 The data were collected between 1978 and 1995, with individual participants being typically followed for between 1 and 4 years. However, patients who did not include at least 3 months of data for each muscle were excluded; therefore, only 644 patients were included in all analyses. A cutoff of 1,500 days tracking was applied, as few participants remained present in the dataset by this point and these participants were outliers. Additional data for the same muscles were collected using the newer ATLIS protocol for a smaller cohort of 99 participants. ATLIS data used El Escorial criteria of definite or probable ALS diagnosis.
Generalized linear mixed modeling to assess symmetry.
Generalized linear mixed models (GLMM) were used to assess the trends of decline in each muscle comparing the left and right side, thus answering the hypothesis of how symmetrical the decline was for each muscle. See the e-Methods at Neurology.org for details.
Linear modeling of individual participants.
A large matrix of general linear models, using the same transformations as applied in linear mixed models previously,7,8 were constructed for each set of muscles including side of the body, time, and interactions for each participant. This was used solely to generate estimates of the rate of decline for each muscle, side, and participant for use as measures in principal component (PC) analyses.
PC analyses.
PC analysis was performed on the predicted rates of muscle decline for each participant determined by a matrix of GLMM against each muscle on each side for every participant. PCs that contributed less than 9% of the total variance were excluded. The percentage of variance for each PC was separated into the contribution to it by each muscle and plotted. Biplots were used to assess not only the distribution of participants in each component but also the contribution of each muscle to that separation. A matrix of vectors was generated representing the contribution of each muscle by PC, considered variance maps. These variance maps were then compared using sum of squares methodology and plotted as a heatmap of strength of similarity with dendograms. These comparisons were then divided by descriptive terms and compared.
RESULTS
Generalized linear mixed modeling can study trends in the decline of each pair of muscles.
For the TQNE analysis, the strength of 6 muscle groups, 3 upper (hand grip, biceps, and triceps) and 3 lower (quadriceps, hamstrings, and anterior tibialis), was assessed on each side of the body for 644 participants with ALS. The decline in each muscle's strength was assessed over time for each participant.
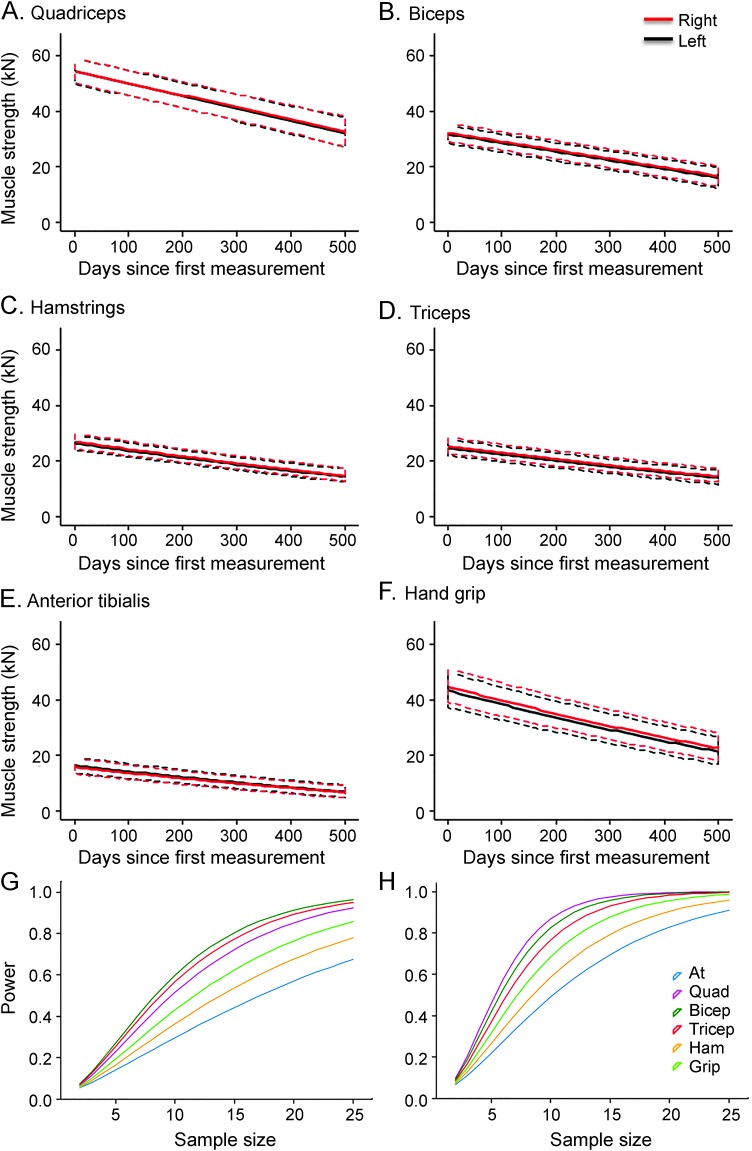
Six GLMMs were produced in order to assess each pair of muscles and predictions were generated for each (figure 1, A–F). Measuring predicted muscle strength score (kN) interestingly showed that the biceps and hand grip were the only muscles that had a bias comparing left with right, which may be explained by right hand dominance. All 4 other pairs of muscles showed very similar rates of decline comparing left vs right sides (table e-1). We also observed that each muscle did on average reach effective paralysis (kN score of 0) at different relative rates, with paralysis being earlier in the anterior tibialis (figure 1E) and later in the quadriceps and triceps (figure 1, A and D). Even in a very rapidly declining patient and a relatively slowly declining patient, the similarity between left and right quadriceps decline within individuals is evident (figure 1, G and H).
Figure 1. Generalized linear mixed modeling of Tufts Quantitative Neuromuscular Exam data, comparing left vs right muscle decline across 6 muscle groups.
(A–F) Predictions generated from generalized linear models for muscle decline of each muscle pair (A, quadriceps; B, hamstrings; C, anterior tibialis; D, biceps; E, triceps; F, hand grip) were plotted (muscle strength against time since first assessment, ±95% confidence interval). ***Significant differences between left and right (p < 0.001) in the rate of decline. (G, H) Exemplar plots show examples of extremely rapid (G) and slow (H) declining muscle strength from quadriceps of individual patients, showing a typically high level of symmetry.
Rate of muscle strength loss is mirrored between the same muscles in both sides of the body.
Generalized linear models (GLM) were used to generate estimated rates of decline for all patients in the study for each muscle on each side. These rates of decline for all 6 muscle groups on both sides across participants were used as individual patient measures for PC analysis (rate of decline for each muscle on each side), thus allowing analysis of which differences between muscles best distinguish participants by variance in the relative decline of each muscle (figure 2A). This analysis showed that all of the muscles contributed significantly to the first component, implying that the first component best represents an overall rate of decline in the participant. This may be due to differences in slope or the point in disease progression in which the participant entered the study. To further support this, we plotted the magnitude and direction of the contribution of each muscle for each of the first 5 PCs (figure 2B). This plot showed that all of the muscles were monotonic and similarly contributed to PC-1, but in contrast the muscles were not monotonic or similar with respect to PC-2 through 5. This suggests that PC-1 separates participants by how quickly each declined over all muscles, where PC-2 through PC-5 concerned differences in how individual muscles were declining relative to each other. Interestingly, all of the muscles showed similar contribution in the same direction when comparing left vs right for the same muscle in each PC.
Figure 2. Principal component (PC) analysis (PCA) of the variability in muscle decline across all participants.
The rates of decline in each muscle from each patient were analyzed using PCs. (A) A graph shows the percentage of the total variance explained by the first 5 PCs (those that represented more than 9% individually). In addition, the bars on this graph have been subdivided by the modulus of the normalized contributions of each muscle to each PC. (B) A bar plot represents both the magnitude and direction of each muscle's loading in the first 5 PCs, for both sides. (C) A scatterplot of all participants and their coordinate in each of the first 5 PCs. (D) A symmetric heatmap represents the sum of squares for comparisons of the variance plots between every pair of muscles with a dendogram representing the hierarchical levels of similarity between every muscle. Lower body muscles are labeled in red text, upper body muscles in blue text. (E) Box and whisker plots compare meta-groups of muscle comparisons, including same.body, muscles compared that are in the same broad region of the body (upper or lower); same.muscle, the same muscle compared left vs right; same.side, muscles compared that are on the same side (left or right) of the body; same.side.body, where the muscle is on the same side (left or right) and same broad region of the body (upper or lower); and those that do not fit any of these categories, none. (F) Box and whisker diagrams compare different meta-groups of muscle comparisons, defined by whether they are upper or lower body muscles. This includes where an upper muscle is compared with a lower muscle, a lower muscle is compared with another lower muscle, and an upper muscle is compared with another upper muscle. Asterisks indicate statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001) by Bonferroni corrected t test. Muscles abbreviated as follows: at = anterior tibialis; grip = hand grip; ham = hamstrings; quad = quadriceps.
One of the considered outcomes of this approach is that participants may have been roughly categorized by discreet groups by differential patterns of decline rate by muscle. Indeed this notion could be considered consistent with the evidence that patients with ALS can be loosely categorized by onset in specific muscle groups. However, studying the positions of all participants on each PC demonstrated approximately normal distributions for participants in each PC, and thus showed that no clusters were forming, thereby suggesting that distinct populations of participants did not exist (figure 2C), at least as could be separated by these measures. However, different muscles contribute differently to this linear scale of variability. Ultimately, it is likely that participants vary significantly with regard to each muscle, but do so randomly without clear subdivision into particulate clusters.
To compare how the relative rates of decline of each muscle separated participants, a variance against PC map was produced for each muscle. The maps of each right and left muscle were compared using a sum of squares methodology, and each comparison was plotted on a heat map (figure 2D; upper body shown in blue, lower body shown in red). Once again, specific muscles showed closer relationships than others; the positioning of these muscles on both axes of the heat map is based on similarity according to a dendogram showing the hierarchy of similarities. In most cases, this results in left and right muscles sitting within the lowest and most similar clade of the dendogram, and adjacent to each other. This demonstrates that the same muscle on the opposite side was generally most similar to each. One exception was that the biceps and triceps on the same side exhibited closer similarity than left compared to right. However, in general, the same muscle on opposing sides showed remarkable consistency, especially in the case of the quadriceps, triceps, and biceps.
In order to understand which comparisons are most important, each comparison was categorized into 5 groups: (1) muscles on the same side of the body, (2) muscles both of the upper or lower body, (3) the same muscle on the opposite side of the body, (4) muscles that are both of the upper or lower body and on the same side, or (5) none. These categories were compared by using the sum of the squares, with lower numbers signifying greater similarity. Results demonstrated that the same muscle on the opposite side consistently showed the highest level of similarity, and muscles of the same region showed some significance (figure 2E). In addition, the upper body muscles were significantly closely related, unlike the lower body muscles (figure 2F).
Finally, comparing the levels of left vs right similarity for the 6 muscles showed that quadriceps had a unique level of similarity (0.26 sum of squares, compared with average 0.78 ± 0.083 [SD]) (figure e-1). Interestingly, the biceps and triceps had greater similarity with each other on opposite sides than when comparing the same muscle left vs right (left bicep vs tricep 0.12, right bicep vs tricep 0.6, tricep left vs right 0.7, and bicep left vs right 0.86).
Different muscles show different relationships between variability and time.
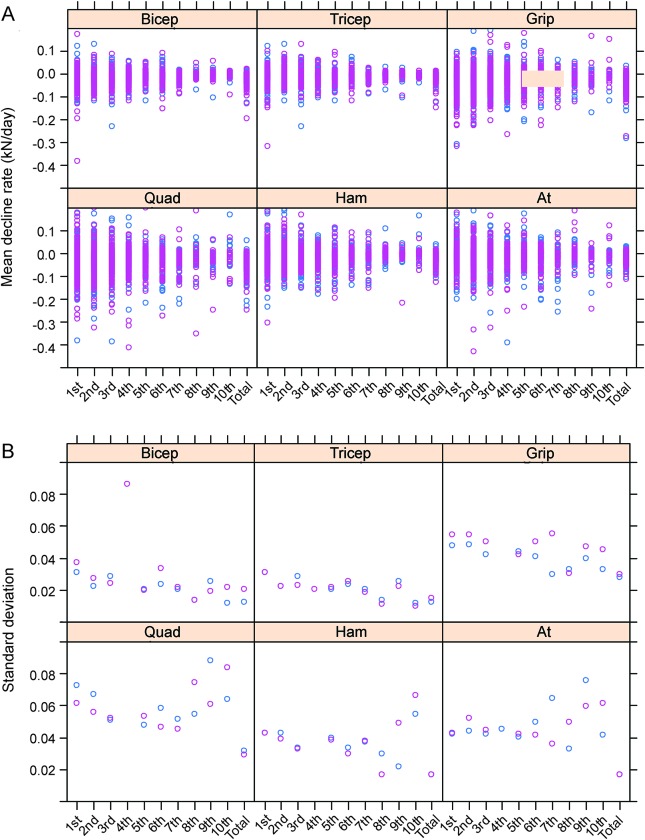
A matrix of GLM was generated for each patient and each muscle, but separated into time periods of 100 days in order to assess the rates of decline in the left and right side and to determine how consistent this decline was over time. However, when studying the 100-day time periods independently, we found that the rates of decline become more consistent as the disease progresses in the biceps and triceps (figure 3A). Further, different muscles showed different levels of variability over time, with the biceps and triceps showing lower variability than the quadriceps or hand grips. The coefficient of variance for each time group was next quantified in order to assess variability between participants at each time point, allowing for changing sample size. The triceps and biceps body show a relatively stable variance across the time ranges, and to the last time points (figure 3B). In contrast, the lower body and grip muscles show a sudden rise in variation in the <700 time ranges. Collectively, these data are an important consideration for clinical trial design, especially a unilateral and sequential clinical trial, as large increases in variability over time during patient decline would reduce the power of the experiment.
Figure 3. Variability of muscle over time in participants with amyotrophic lateral sclerosis (ALS).
(A) A matrix of plots shows the mean rates of decline for each muscle in participants with ALS for both left and right (blue and purple, respectively), within 100 days time ranges (post first analysis), and followed by the mean rate of decline for the participants. The rates of decline were plotted in 10 time periods of 100 days over a 1,000-day period, and the average rate of decline was plotted fitted across the full 1,000 days. (B) A matrix plot shows coefficients of variance for muscle decline for each muscle, both left and right (blue and purple, respectively), within 100 days time ranges (post first analysis), and followed by the overall SD of decline for the participants. Muscles abbreviated as follows: at = anterior tibialis; grip = hand grip; ham = hamstrings; quad = quadriceps.
Finally, the strength and significance of the correlation between rates of decline and time were assessed in order to determine whether the rates of decline slow or accelerate over the course of disease progression. Correlation tests were subsequently performed in order to determine the strength of monotonic relationships (Spearman rank test) and the strength of linear relationships (Pearson product-moment test). Only the biceps and hand grip showed a weak but significant correlation between time and rate of decline; however, this was likely due to the large dataset (table e-2). Assessing the correlation between variability in decline and time showed that there was a significant negative relationship between variability and time in all 3 upper body muscles, but not in the lower body muscles (table e-3), which is consistent with figure 4. Curiously, the upper body muscles also showed a slightly higher correlation between rate of decline and time (table e-3).
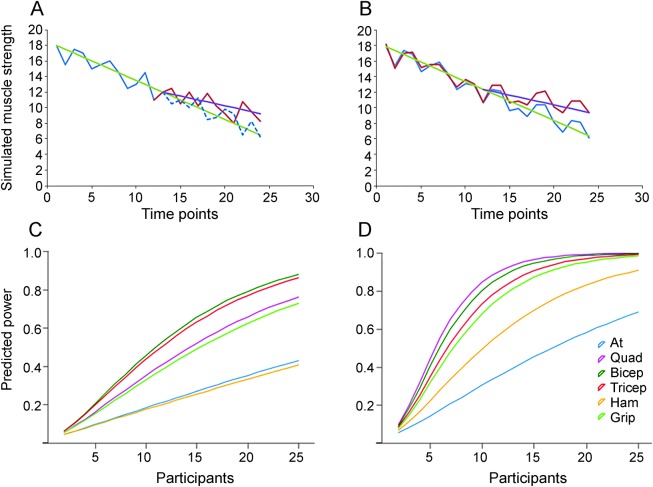
Figure 4. A comparison of the relationship between power and number of participants for both experimental designs and for each muscle.
(A) A demonstration of a participant where participants were followed for 6 months without treatment and then followed for 6 months with treatment, assuming a 50% change in decline with treatment. The green line indicates the mean rate of decline, the purple line the expected mean decline given a 50% reduction in rate, the solid blue line mock muscle recordings before treatment, the red line mock muscle recordings after treatment, and the dashed blue line mock muscle recordings assuming the treatment is ineffective. (B) A demonstration of a participant where participants were followed for 6 months without treatment and then followed for 6 months with unilateral treatment, assuming a 50% change in decline with treatment. The green line indicates the mean rate of decline, the purple line the expected mean decline given a 50% reduction in rate, the solid blue line mock muscle recordings for the untreated side, and the red line mock muscle recordings for the treated side. (C) Power vs number of participants (per group) where participants were followed for 6 months without treatment and then followed for 6 months with treatment, assuming a 50% change in decline with treatment. (D) Power vs number of participants (per group) where treatment was unilateral and the participant followed for 6 months, assuming a 50% change in decline with treatment. Muscles abbreviated as follows: at = anterior tibialis; grip = hand grip; ham = hamstrings; quad = quadriceps.
Optimizing the experimental design of clinical trials in ALS.
When designing a clinical trial, it is important to consider the method of assessment and its potential for statistical power. In this study, we considered 2 experimental designs that have different purposes for clinical trials. The first consideration was systemic or bilateral studies, in which it is typical to monitor the participant's rate of decline in a given muscle for 3–6 months, and then follow the participant for at least an equal length of time after treatment using the same muscle (figure 4A). The second experimental design was unilateral treatments; for example, transplantation into one side of the spinal column or brain (figure 4B). Both experimental designs allow for paired statistical tests, which abrogate certain aspects of participant variability. The unilateral test has the further advantage of allowing for a paired test over time, which further abrogates time-specific participant variability. In the bilateral experimental design, the variance was restricted to the variance in decline from individual participants.
Using the SDs predicted by GLM, power tests were performed iteratively for between 2 and 25 participants, assuming a 50% change in decline when treated and a significance level of 0.05. The difference in power against number of participants is striking when comparing the 2 methodologies (figure 4, C vs D). The unilateral approach shows that significantly fewer participants were required per group in order to achieve 80% power at 0.05% CI compared with bilateral treatment (table e-4A). In the unilateral experimental design, it is not necessary to observe the participant prior to treatment to determine a baseline, unless there is an intention to assess both potential unilateral and bilateral effects of the treatment.
Another interesting observation is the significant difference in the number of participants needed to achieve sufficient power in different muscles. The triceps and biceps performed well with both experimental designs, requiring 21 and 20 participants per group in the sequential experimental design, respectively, and 11 and 10 participants per group in the unilateral experimental design (table e-4A). Interestingly, the quadriceps also performed well under the unilateral approach (9 participants per group to reach 80% power), but performed relatively poorly under the sequential approach (27 participants per group to reach 80% power). This is likely because, although the quadriceps display a relatively high level of consistency in decline by side, they show higher levels of variability over time.
To next compare TQNE data with data collected using a more easily performed method, the ATLIS system assessed the same 6 muscles longitudinally in 99 participants, with some for over a maximum period of 600 days. GLMM were used to assess differences between left and right as well as variability in patient decline (figure 5, A–F). In contrast to the TQNE data, no difference was observed when comparing left vs right. However, this is likely due to the smaller sample size being unable to detect relatively small differences and the fact that participants were followed over a shorter period of time.
Figure 5. Generalized linear mixed modeling of Accurate Test of Limb Isometric Strength chair data, comparing left vs right muscle decline across 6 muscle groups, and power curves for speculative clinical trials.
(A–F) Predictions generated from GLM models for muscle decline of each muscle pair (A, quadriceps; B, hamstrings; C, anterior tibialis; D, biceps; E, triceps; and F, hand grip) were plotted (muscle strength against time since first assessment, ±95% confidence interval). ***Significant differences between left and right (p < 0.001) in the rate of decline. (G, H) Predicted power against sample size plots for bilateral and unilateral clinical trials with a 50% efficacy.
In order to study the power achievable using the ATLIS system for clinical trial, we assessed both unilateral and bilateral experimental paradigms, assuming a treatment efficacy of a 50% reduction in decline rate. Consistent with the TQNE results, ATLIS results showed that a greater power could be achieved using the unilateral treatment option due to the simultaneous internal control offered by the other side of the body (figure 5, G vs H). While the ATLIS data are predicted to perform slightly better than the TQNE data using the sequential experimental paradigm, both measurements performed similarly using the unilateral experimental design (table e-4B).
DISCUSSION
In this study, we examined the different aspects of variability in muscle decline across participants with ALS. We found that for most of the muscles analyzed, the left and right sides on average decline at the same rate. This result is in agreement with a recent study that assessed Amyotrophic Lateral Sclerosis Functional Rating Scale–revised scores.9 Unlike this study, however, we assessed quantitative measures of left and right muscle strength within individual patients based on TQNE and ATLIS and, importantly, we used additional analysis including linear mixed regression modeling and PC analysis. We observed highly similar rates of decline comparing left and right, but increased starting strength in both the biceps and grip strength on the right side, consistent with the idea that participants were more likely to be right-handed than left-handed. One of the principal advantages of a longitudinal study is the ability to solely measure decline rate, despite the effects of handiness on starting strength. Shorter-term or cross-sectional studies would be highly susceptible to this effect; however, we found that several muscles were significantly less affected as candidates. The new knowledge that muscle decline in general is a very accurate measure of disease progression and very similar between limbs in the same patient will benefit future clinical trials using unilateral treatment approaches.
PC analyses demonstrated that rate of decline systematically represents the greatest variability between patients, rather than the variability in individual muscles. This may appear contradictory with clinical observations that the disease starts in different regions and spreads.10 However, it implies that irrespective of which muscle starts declining soonest or latest, their rates of decline following onset are more consistent. The subsequent PCs show differing contributions by each muscle, and sometimes in different directions. This demonstrates that some variability is specific to certain muscles, and some muscles are more closely related than others. A closer analysis yields an approximately normal distribution of patients across all PCs. This implies that there are no diverging subtypes of ALS with regards to rates of muscle decline, although different subtypes are observed with respect to onset in different muscles. This is consistent with the idea that ALS spreads from an initial focal point at some stage during the disease progression, but that once the disease arrives at the circuit connected to a specific muscle, it follows a fairly consistent progression and mechanism.
Unilateral treatment designs are a powerful tool already used in preclinical studies of ALS and other degenerative diseases.11,12 Indeed, a unilateral design has been used in clinical trials for the administration of growth factors or cells to patients with Parkinson disease and Huntington disease, and for the administration of cells to patients with ALS in previous and ongoing clinical trials (clinicaltrials.gov NCT02943850).13–16 Collectively, these studies indicate that a unilateral trial design could be feasible for stem cell transplantation studies. This methodology requires the ability to treat one side or part of the body (animal or human) in isolation to another side or part to be used as an internal simultaneous control. A unilateral treatment design generates a higher power with a lower sample size compared with bilateral treatment, but is obviously only suitable to specific treatments.3 A further advantage unique to the unilateral design is that following a patient for a substantial length of time to measure a pretreatment baseline is not statistically necessary. This is because pairwise statistics are comparing treated and untreated, which are measured simultaneously. However, practically speaking, it is likely necessary to track the patient for some time prior to treatment to assess suitability for the trial. One potential limitation is that it would be most susceptible to the effects of initial strength and handiness in shorter-term studies, but not longitudinal studies measuring rates of decline.
For both designs, the selection of muscles for assay makes significant differences in the expected statistical power, and consequently number of participants. When assaying quadriceps in the unilateral treatment design, it may be preferable to restrict the analyses to the earlier stages in disease progression due to the increased variability in the lower body muscles towards the end stage. Indeed, the lower body muscles showed increasing variability over time, most sharply following 700 days. This trend may be related to differential use of assistive devices such as wheelchairs that can limit the frequency and extent of lower body muscle use. Furthermore, while the biceps and triceps performed well under the unilateral experimental design, it should be considered that the right side balance in the biceps may make this muscle less suitable than the quadriceps or triceps for a unilateral trial. Studies have frequently identified grip strength as showing highly consistent rates of decline and good correlation with other ALS measures.17 In this study, we do not suggest otherwise, but we speculate that ATLIS and TQNE both provide better methods for consistently measuring strength for other muscles compared with more routinely used hand-held dynamometers.
When comparing the ATLIS system data to the larger TQNE dataset, we again observed a high degree of similarity in decline when assessing the same muscle on the left and right, and greater variability when comparing different muscles. Using the unilateral experimental design, where the untreated side of the body can serve as an internal control, no appreciable difference in the predicted power of the TQNE or ATLIS data was observed. This is consistent with the rates of decline being highly similar when comparing left and right.
These analyses demonstrate the importance of understanding variability in assays used when designing clinical trials, especially in diseases with a complex progression like ALS. Currently, it would appear that direct muscle strength assessments are the most effective assays for ALS progression, being the best compromise between ease of use and accuracy. However, choosing muscles that are least variable can further reduce the length of assessments and maintain the highest ratio of power to patients possible when using direct muscle assays. This study demonstrates that the biceps and triceps could be very effective for this purpose in both unilateral and bilateral experimental designs, with the quadriceps being only slightly better in the unilateral design. New clinical trials are required for ALS in order to develop a successful treatment, and the knowledge provided in this report will be critical for designing optimal, high power future trials.
ACKNOWLEDGMENT
The authors thank Dr. Soshana Svendsen, Cedars-Sinai, for critical editing and review of this manuscript.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- ATLIS
Accurate Test of Limb Isometric Strength
- GLM
generalized linear model
- GLMM
generalized linear mixed model
- IRB
institutional review board
- PC
principal component
- TQNE
Tufts Quantitative Neuromuscular Exam
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
D. Rushton: study concept and design, study analysis and interpretation, statistical analysis, critical revision of the manuscript for important intellectual content. P.L. Andres: study analysis and interpretation, critical revision of the manuscript for important intellectual content. P. Allred: study analysis and interpretation, critical revision of the manuscript for important intellectual content. R.H. Baloh: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. C.N. Svendsen: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
Funding was provided to C.N.S. by the Board of Governors Regenerative Medicine Institute, Cedars-Sinai Medical Center (Los Angeles, CA), and California Institute of Regenerative Medicine (DR2A-05320).
DISCLOSURE
D. Rushton reports no disclosures relevant to the manuscript. P. Andres is inventor of ATLIS: The General Hospital Corporation and holds a patent on this device (US Patent 7,493,812 issued 2/24/09). P. Allred, R. Baloh, and C. Svendsen report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Atassi N, Berry J, Shui A, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology 2014;83:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Küffner R, Zach N, Norel R, et al. Crowdsourced analysis of clinical trial data to predict amyotrophic lateral sclerosis progression. Nat Biotechnol 2015;33:51–57. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen GM, Gowing G, Svendsen S, Svendsen CN. The past, present and future of stem cell clinical trials for ALS. Exp Neurol 2014;262:127–137. [DOI] [PubMed] [Google Scholar]
- 4.Andres PL, Hedlund W, Finison L, Conlon T, Felmus M, Munsat TL. Quantitative motor assessment in amyotrophic lateral sclerosis. Neurology 1986;36:937–941. [DOI] [PubMed] [Google Scholar]
- 5.Shields RK, Ruhland JL, Ross MA, Saehler MM, Smith KB, Heffner ML. Analysis of health-related quality of life and muscle impairment in individuals with amyotrophic lateral sclerosis using the medical outcome survey and the Tufts Quantitative Neuromuscular Exam. Arch Phys Med Rehabil 1998;79:855–862. [DOI] [PubMed] [Google Scholar]
- 6.Andres PL, Skerry LM, Munsat TL, et al. Validation of a new strength measurement device for amyotrophic lateral sclerosis clinical trials. Muscle Nerve 2012;45:81–85. [DOI] [PubMed] [Google Scholar]
- 7.Littell R, MIlliken G, Stroup W, et al. SAS for Mixed Models, 2nd ed. Cary, NC: SAS Institute Inc.; 2006. [Google Scholar]
- 8.Kizilkaya K, Tempelman R. A general approach to mixed effect modeling of residual variances in generalized mixed models. Genet Sel Evol 2005;37:31–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shefner JM, Liu D, Leitner ML, et al. Quantitative strength testing in ALS clinical trials. Neurology 2016;87:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandrioli J, Biguzzi S, Guidi C, et al. Heterogeneity in ALSFRS-R decline and survival: a population-based study in Italy. Neurol Sci 2015;36:2243–2252. [DOI] [PubMed] [Google Scholar]
- 11.Klein SM, Behrstock S, McHugh J, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther 2005;16:509–521. [DOI] [PubMed] [Google Scholar]
- 12.Gamm DM, Wang SM, Lu B, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One 2007;2:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink JS, Schumacher JM, Ellias SL, et al. Porcine xenografts in Parkinson's disease and Huntington's disease patients: preliminary results. Cell Transpl 2000;9:273–278. [DOI] [PubMed] [Google Scholar]
- 14.Mazzini L, Gelati M, Profico DC, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med 2015;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman EL, Boulis NM, Hur J, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol 2014;75:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slevin JT, Gash DM, Smith CD, et al. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg 2007;106:614–620. [DOI] [PubMed] [Google Scholar]
- 17.Richards L, Palmiter-Thomas P. Grip strength measurement: a critical review of tools, methods, and clinical utility. Crit Rev Phys Rehabil Med 1996;8:87–109. [Google Scholar]