Abstract
Slurry packing capillary columns for ultrahigh pressure liquid chromatography is complicated by many interdependent experimental variables. Previous results have suggested that combination of high slurry concentration and sonication during packing would create homogeneous bed microstructures and yield highly efficient capillary columns. Herein, the effect of sonication while packing very high slurry concentrations is presented. A series of six, 1 m × 75 μm internal diameter columns were packed with 200 mg/mL slurries of 2.02 μm bridged-ethyl hybrid silica particles. Three of the columns underwent sonication during packing and yielded highly efficient separations with reduced plate heights as low as 1.05.
Keywords: UHPLC, Packed capillary columns, High slurry concentrations, Sonication, High efficiency
1. Introduction
The benefits of sub-2 μm chromatographic supports have largely focused separation technologies towards ultrahigh pressure liquid chromatography (UHPLC) [1]. Theoretically, sorbents on this scale provide higher separation efficiencies and shorter separation times. True benefit from these materials is dependent, however, on homogeneous packing of the sorbent into a column. This is complicated by the smaller particle’s requirement of significantly increased packing pressure and presents a major challenge in the creation of a uniform bed structure. As packing material continues in the direction of smaller particles, dispersion due to transcolumn heterogeneity becomes significantly more important. This type of dispersion is estimated to account for up to 70% of the total dispersion for small molecules in UHPLC columns [2].
Packing that results in a well performing column requires the formation of a homogeneous bed structure across all scales, from transchannel to transcolumn, within the column [3]. Study of “optimal” packing conditions has lead to more detailed understanding of the physical process [4–14]. Unfortunately this process is dynamic and highly influenced by many interdependent parameters. The results of these studies have yielded many opinions on the “art” of column packing as opposed to the science. More recently, collaboration between our groups has examined certain packing parameters including the effects of particle properties, capillary column diameter and slurry concentration [15–17]. Most importantly these studies have been informed by three-dimensional reconstructions via confocal laser scanning microscopy (CLSM) of the packing microstructure. These renderings have allowed for detailed expositions of morphological features corresponding to specific packing conditions, which are then related to explain the column’s kinetic performance.
Morphological heterogeneity between a column’s wall region and bulk packing is often the main contributor to poor column performance [18–23]. Previous studies have indicated that the differences in these regions are dependent on slurry concentration [16,17]. Detailed understanding of the packing microstructure has guided empirical packing studies to obtain well performing columns. For example, our initial results suggest that there is a specific “intermediate” slurry concentration capable of balancing the antagonizing effects associated with a low or high slurry concentration for each particle diameter [16]. Further study of this proposition confirmed balancing of packing defects and demonstrated that increasing slurry concentration suppresses wall effects and reduces transcolumn bed heterogeneities through prevention of both locally high porosity and particle size segregation [17].
We further noticed that even though slurry concentrations higher than “optimal” continue to suppress wall effects, the columns perform poorly [17]. The benefits of high slurry concentrations eventually begin to diminish as the number and size of packing voids in the bed structure increases. Giddings suggested voids would impact eddy dispersion negatively and contribute significantly to chromatographic band broadening [3]. Voids increase velocity extremes and eddy dispersion on both transchannel and short-range interchannel scales. The detriment of incorporated voids was also illustrated with dispersion simulations that resulted in stating the column’s overall performance is more dependent on reduction of large voids than obtaining high packing densities [24].
The conclusions to our most recent study propose that even higher efficiency UHPLC columns may result from formation of a homogenous bed structure across the entire column through the combination of high slurry concentration and sonication to prevent the formation of larger voids [17]. To date sonication has been used in column packing, but only to limited effect and not in association with very high slurry concentrations [25–29]. Reduction of the total number of voids should allow for realization of more homogeneous and highly efficient columns.
To test this a set of 6 capillary columns, all 1 m in length × 75 μm internal diameter (i.d.), were packed with 200mg/mL slurries. Three of the columns were packed with the application of sonication and three were not. Columns packed with sonication yielded performance with reduced plate heights approaching 1 and a realized (instead of extrapolated as often reported) 470,000 plates/m.
2. Materials and methods
2.1. Chemicals and materials
75 μm i.d. cylindrical fused-silica tubing was purchased from Polymicro Technologies (Phoenix, AZ). The capillaries were packed with C18-modified bridged-ethyl hybrid (BEH) silica particles provided by Waters Corporation (Milford, MA) with a Sauter diameter of 2.02 μm. The Sauter diameter was calculated from a scanning electron microscope (SEM) based particle size distribution obtained from the measurement of ~1200 C18-modified 1.9 μm BEH particles from the same batch using a JSM-7500F SEM (Joel, München, Germany). HPLC grade acetonitrile, acetone (reagent grade), tri-fluoroacetic acid (TFA), and the test analytes for chromatographic characterization (L-ascorbic acid, hydroquinone, resorcinol, catechol, 4-methyl catechol) were obtained from Fisher Scientific (St. Louis, MO). Kasil frits for the packed capillaries were prepared with potassium silicate from PQ Corporation (Valley Forge, PA) and formamide from Sigma–Aldrich (St. Louis, MO). HPLC grade water for chromatographic experiments was obtained from a Millipore NANOpure water system (Billerica, MA).
2.2. Preparation of capillary UHPLC columns
Preparation of the capillary UHPLC columns has been described previously in detail [8–14]. Modifications to the procedure will be highlighted here. Column blanks (160 cm × 75 μm i.d.) were fritted using the Kasil method [30]. The extra 60 cm was needed to over pack slightly to allow for bed compression (~10 cm) as well as to reach from the packing vessel to the sonication bath (~50 cm). In order to prepare outlet frits, the ends of capillaries were depressed onto a glass microfiber filter (Reeve Angel, Clifton, NJ) wetted with 50/50 (v/v) potassium silicate/formamide. The column blanks were then dried overnight at 50 °C and the resulting frits were ~125 μm in length. Slurries were prepared by mixing a known mass of the particles in a known volume of acetone (to achieve 200 mg/mL) and suspended with a 10 min sonication cycle using a Cole Parmer Ultrasonic Cleaner 8891 (Vernon Hills, IL).
Prior to packing, the inlet to the column blank was fixed within a UHPLC fitting. The outlet was threaded through the top of a piece of shipping foam padding that was cut to fit snugly within the sonication bath’s included basket. The portion of capillary blank to be packed (in this case ~108 cm) was pulled through the top of the foam entirely. This portion of the column blank was then coiled and taped to the bottom of the foam padding to keep it in place. To ensure the created outlet frit did not loose integrity due to sonication, it was threaded back through the shipping foam padding (from the bottom side, in which the majority of the capillary was taped) until the frit and 2 cm of outlet end of the column blank protruded from the top of the foam padding. This arrangement corresponded to the outlet of column blank being 2 cm above the water line in the sonication bath. The slurry was then placed into a packing reservoir and the inlet of the column blank was secured to the reservoir using the already affixed UHPLC fitting. The foam supporting the coiled capillary was placed into the sonication bath, ensuring that the desired final length (already coiled and secured to the bottom of the foam) remained submerged under water and that the 2 cm of the blanks outlet, including the installed frit, remained above the water line. Sonication during packing was conducted with an Elmasonic P 60 H (Elma Schmidbauer GmbH, Singen, Germany) sonication bath. The sonication bath was set to sweep mode at 80 kHz. Packing was initiated using acetone as a pushing solvent at 150 bar from a DSHF-300 Haskel pump (Burbank, CA). The packing pressure was immediately increased to 2070 bar when the 2 visible cm of bed had been packed. The maximum packing pressure was chosen to maintain consistency between these experiments and previously reported packing studies [15–17]. The column was allowed to pack until the formed bed was visible outside the packing foam, which meant the 108 cm of bed had been packed. The temperature of the bath was kept at 30° C by adding a small amount of ice as necessary and measured using the sonication bath’s temperature readout on the display. After the desired length was reached, the packing pressure was slowly released to atmospheric pressure. The column was then connected to a DSXHF-903 Haskel pump (Burbank, CA) using an UHPLC injection apparatus. Each column was flushed for 1 h in 50/50 (v/v) water/acetonitrile with 0.1% TFA at 3500 bar, after which the pressure was gradually released and reinitiated at 700 bar to form a temporary inlet frit with a heated wire stripper from Teledyne Interconnect Devices (San Diego, CA). Columns were then clipped to a 100 cm bed length and an inlet frit was installed using the Kasil method.
2.3. Chromatographic analysis
Column efficiency was tested under isocratic elution conditions using a 200 μM test mixture (L-ascorbic acid, dead-time marker; hydroquinone, resorcinol, catechol, and 4-methyl catechol) and an UHPLC injection apparatus [9]. The mobile phase used for evaluation was 50/50 (v/v) water/acetonitrile with 0.1% TFA. Analytes were detected amperometrically. Electrochemical detection was conducted at a 8 μm × 300 μm carbon fiber microelectrode held at +1.1 V vs. Ag/AgCl reference electrode [31]. This electrode was placed at the outlet of the UHPLC column. Current-to-voltage conversion was conducted using an SR750 current amplifier (Stanford Research Systems, Sunnyvale, CA) with a 109 V/A gain and a 3 Hz, 3 dB low-pass bandwidth filter. An Intel Core 2 Duo desktop computer with a 16-bit A/D converter was used to acquire data at 21 Hz. Data were collected with a custom-written LabView 6.0 program (National Instruments, Austin, TX).
Columns were analyzed over a range of mobile phase velocities to create plots of the plate height H vs. the average mobile phase velocity uav for each analyte in the test mixture. Reduced plate height curves h = H/dp vs. v = uavdp/Dm were calculated using the particles’ Sauter diameter (dp = 2.02 μm) and Dm, the pressure-dependent diffusion coefficient of an analyte in the bulk mobile phase [32]. High frequency noise was removed from the chromatograms using a digital frequency filter and low frequency baseline drift was eliminated by background subtraction. Retention times and theoretical plate counts N were determined using an iterative statistical moments (±3σ) algorithm written in Igor Pro 6.0 (Wavemetrics, Inc., Lake Oswego, OR) [11].
3. Results and discussion
Studied here are six capillaries packed at a very high slurry concentration. Previous studies of 100 mg/mL slurries yielded relatively well performing capillary columns with minimum reduced plate height (hmin) values near 1.5 [10,16]. For this experiment a concentration of 200 mg/mL was chosen to ensure excess to an intermediate slurry concentration, enhanced suppression of radial defects and a high number of large packing voids, wherein the cumulative effects of these voids would be expected to yield relatively poor chromatographic efficiency. Three of the capillaries within this study underwent sonication and three did not. For the sake of consistency, all six were placed in the same orientation within the sonication bath during packing, whether sonication was applied or not.
Plotted in Fig. 1 is an example chromatogram showing the performance of one of the three columns packed with sonication. Inset in Fig. 1 is an enlargement of the hydroquinone peak, overlaid with a Gaussian fit and residuals. Iterative statistical moments (±3σ) were used for plate counts of all reported data. These plate counts are more conservative than those calculated by full width at half height and Gaussian fit methods. For example the inset hydroquinone peak would have plate counts of 558,000 using full width at half height and 556,000 using a Gaussian fit. Reduced plate height h for these plate determination methods would be 0.88. Fig. 2 plots the reduced van Deemter fits (h = a + b/v + cv) for hydroquinone for each of the six columns. Most notably the six columns fall into two distinct groups, those that underwent the application of sonication and those that did not. The consistency of the columns that underwent sonication is very high. Overall efficiency of these columns approaches a reduced plate height of 1.05. Columns that were not exposed to sonication did not exhibit similarly high reproducibility in reduced parameters and showed poorer performance with hmin between 1.8 and 2.2.
Fig. 1.
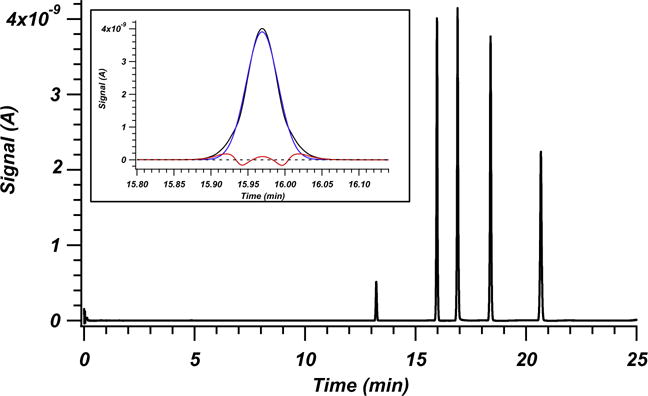
Example chromatogram for one of the three columns packed with sonication and run at 1630 bar. This column is represented by red triangles in Figs. 2 and 3. Peaks from left to right are L-ascorbic acid, hydroquinone, resorcinol, catechol and 4-methyl catechol. The inset presents an enlargement of the hydroquinone peak used for the reduced plate height curves in Fig. 2. The experimental data is plotted in black, the Gaussian fit in blue and the residuals are plotted in red. A black dashed line is overlaid at 0 signal for reference. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
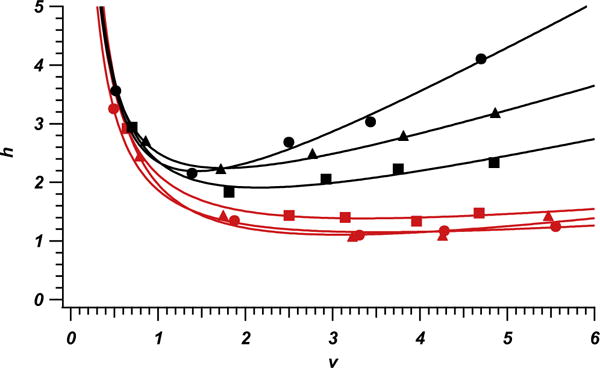
Plot of reduced van Deemter fits for hydroquinone on each of the 6, 1-m long columns. Columns packed with sonication are presented in red while columns prepared without sonication are plotted in black. Marker shapes (circles, squares and triangles) distinguish each column within the parameters represented by color. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3 plots uav vs. pressure normalized for column length. The data naturally falls into two groups separated by column packing procedure. Higher average velocities for ascorbic acid are apparent for those columns prepared with sonication. Regarding the known effects of high slurry concentrations and their tendency to incorporate very wide void size distributions [17], we can interpret these results as similar to a packing prepared with a highly poly-disperse particle size distribution (one significantly broader and more skewed than that of the packed BEH material: the utilized BEH material exhibits approximately 15% relative standard deviation for the particle size distribution). Based on Fig. 2 we know that the columns packed with sonication have a more homogeneous bed structure due to the improved efficiency. This translates into substantially narrowed distribution of sizes for local interstitial void fractions with respect to the columns that did not undergo sonication. This homogenization of the bed microstructure leads to higher observed velocity and permeability for the dead time marker through the column. Simulations yielded similar results in which a reduced width and tail in the interstitial void volume distribution improved hydraulic permeability [33]. Packing columns with very high slurry concentration and sonication boosts separation efficiency while improving mobile phase permeability. That is, a narrower width and more uniform distribution of interparticle void volumes produces more uniform and less tortuous flow paths. Whereas an ultrasound-induced homogenization of the packing microstructure over the column cross-section can explain both, higher separation efficiency and permeability, the permeability might additionally benefit from a slightly lower packing density.
Fig. 3.
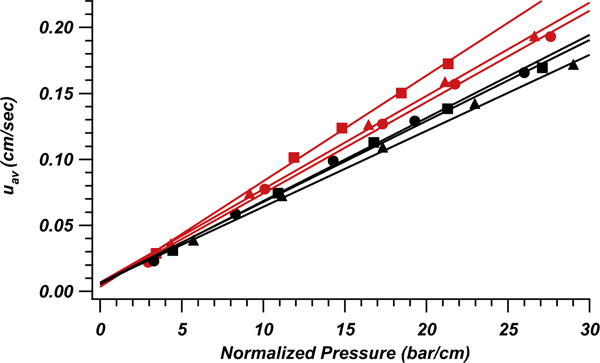
Plot of the linear velocity of the dead time marker, L-ascorbic acid, against pressure drop normalized for column length. Columns packed with sonication are presented in red while columns prepared without sonication are plotted in black. Marker shapes (circles, squares and triangles) distinguish each column within the parameters represented by color. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The performance of columns undergoing sonication far exceeds reports of other highly efficient capillary columns [10]. However, these results are not unexpected. First, the experimental setup has been optimized to limit extra-column band broadening as injection, detection and unpacked bed (frits) produce negligible extra-column band broadening. Second, it is known that reduced plate heights can approach values below 1.5 for very low aspect ratio capillaries (column-to-particle diameter ratios dc/dp < 20) [9,15]. In this case dc/dp is 37.1, nearly twice that value. No theoretical basis exists for this limitation on minimum plate height. Transcolumn exchange of an analyte molecule on the 75 μm dimension of the capillary diameter is fast and any exchange between different regions of local flow velocity is quickly terminated by transverse dispersion [34]. Third, from past CLSM studies we know that the integral porosity deviation approaches zero as slurry concentration increases (indicative of wall region in a bed that attains average packing density as the bulk region of that bed) [17]. The very high slurry concentrations utilized here should further suppress localized heterogeneities. Finally, simulations of plate height in computer-generated packing have yielded reduced plate heights below unity [22].
4. Conclusions
The results presented here highlight the beneficial effects of two packing variables: very high slurry concentration and sonication. The remarkable performance of these columns, which far exceeds previous separation efficiencies seen in our lab and elsewhere for fully porous particles packed into capillary columns, leads us to believe that we have successfully mitigated the incorporation of packing voids while suppressing radial heterogeneity previously identified at a capillary column’s wall. Packed capillary columns with performance approaching reduced plate heights of 1 offer new frontiers into the use of UHPLC columns. When packed to a meter long and producing 500,000 theoretical plates, the opportunity for fast separations at high pressure with very high peak capacities could greatly improve one-dimensional separations of very complex samples. The fundamental study of slurry packing as a function of slurry concentration has given insight into the packing process and guided studies to greatly improve slurry packing of capillary columns.
Supplementary Material
Acknowledgments
The authors would like to thank Waters Corporation (Milford, MA), the National Institute of Health (Grant #5U24DK097153) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Grant #1R01DK101473-01A1) for support of the work reported in this manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.chroma.2016.08.002.
References
- 1.Jorgenson JW. Capillary liquid chromatography at ultrahigh pressures. Annu Rev Anal Chem. 2010;3:129–150. doi: 10.1146/annurev.anchem.1.031207.113014. [DOI] [PubMed] [Google Scholar]
- 2.Gritti F, Guiochon G. Perspectives on the evolution of the column efficiency in liquid chromatography. Anal Chem. 2013;85:3017–3035. doi: 10.1021/ac3033307. [DOI] [PubMed] [Google Scholar]
- 3.Giddings JC. Part I: Principles and Theory. Marcel Dekker; New York, N.Y: 1965. Dynamics of chromatography. [Google Scholar]
- 4.Vissers JPC, Claessens HA, Laven J, Cramers CA. Colloid chemical aspects of slurry packing techniques in microcolumn liquid chromatography. Anal Chem. 1995;67:2103–2109. [Google Scholar]
- 5.Vissers JPC, Hoeben MA, Laven J, Claessens HA, Cramers CA. Hydrodynamic aspects of slurry packing processes in microcolumn liquid chromatography. J Chromatogr A. 2000;883:11–25. doi: 10.1016/s0021-9673(00)00276-4. [DOI] [PubMed] [Google Scholar]
- 6.Angus PDA, Demarest CW, Catalano T, Stobaugh JF. Aspects of column fabrication for packed capillary electrochromatography. J Chromatogr A. 2000;887:347–365. doi: 10.1016/s0021-9673(00)00529-x. [DOI] [PubMed] [Google Scholar]
- 7.Kirkland JJ, DeStefano JJ. The art and science of forming packed analytical high-performance liquid chromatography columns. J Chromatogr A. 2006;1126:50–57. doi: 10.1016/j.chroma.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Blue LE, Jorgenson JW. 1.1 μm superficially porous particles for liquid chromatography. Part II: column packing and chromatographic performance. J Chromatogr A. 2015;1380:71–80. doi: 10.1016/j.chroma.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 9.Treadway JW, Wyndham KD, Jorgenson JW. Highly efficient capillary columns packed with superficially porous particles via sequential column packing. J Chromatogr A. 2015;1422:345–349. doi: 10.1016/j.chroma.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Franklin EG. Ph D Dissertation. The University of North Carolina at Chapel Hill; 2012. Utilization of Long Columns Packed with Sub-2 μm Particles Operated at High Pressures and Elevated Temperatures for High-Efficiency One-Dimensional Liquid Chromatographic Separations. [Google Scholar]
- 11.Hsieh S, Jorgenson JW. Preparation and evaluation of slurry-packed liquid chromatography microcolumns with inner diameters from 12 to 33 μm. Anal Chem. 1996;68:1212–1217. doi: 10.1021/ac950682m. [DOI] [PubMed] [Google Scholar]
- 12.Mellors JS, Jorgenson JW. Use of 1.5-μm porous ethyl-bridged hybrid particles as a stationary-phase support for reversed-phase ultrahigh-pressure liquid chromatography. Anal Chem. 2004;76:5441–5450. doi: 10.1021/ac049643d. [DOI] [PubMed] [Google Scholar]
- 13.Patel KD, Jerkovich AD, Link JC, Jorgenson JW. In-depth characterization of slurry packed capillary columns with 1.0–μm nonporous particles using reversed-phase isocratic ultrahigh-pressure liquid chromatography. Anal Chem. 2004;76:5777–5786. doi: 10.1021/ac049756x. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy RT, Jorgenson JW. Preparation and evaluation of packed capillary liquid chromatography columns with inner diameters from 20 to 50 micrometers. Anal Chem. 1989;61:1128–1135. [Google Scholar]
- 15.Bruns S, Grinias JP, Blue LE, Jorgenson JW, Tallarek U. Morphology and separation efficiency of low-aspect-ratio capillary ultrahigh pressure liquid chromatography columns. Anal Chem. 2012;84:4496–4503. doi: 10.1021/ac300326k. [DOI] [PubMed] [Google Scholar]
- 16.Bruns S, Franklin EG, Grinias JP, Godinho JM, Jorgenson JW, Tallarek U. Slurry concentration effects on the bed morphology and separation efficiency of capillaries packed with sub-2 μm particles. J Chromatogr A. 2013;1318:189–197. doi: 10.1016/j.chroma.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Reising AE, Godinho JM, Hormann K, Jorgenson JW, Tallarek U. Larger voids in mechanically stable, loose packings of 1.3 μm frictional, cohesive particles: their reconstruction, statistical analysis, and impact on separation efficiency. J Chromatogr A. 2016;1436:118–132. doi: 10.1016/j.chroma.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 18.Gritti F, Martin M, Guiochon G. Influence of viscous friction heating on the efficiency of columns operated under very high pressures. Anal Chem. 2009;81:3365–3384. doi: 10.1021/ac802632x. [DOI] [PubMed] [Google Scholar]
- 19.Guiochon G, Farkas T, Guan-Sajonz H, Koh JH, Sarker M, Stanley BJ, et al. Consolidation of particle beds and packing of chromatographic columns. J Chromatogr A. 1997;762:83–88. doi: 10.1016/s0021-9673(96)00642-5. [DOI] [PubMed] [Google Scholar]
- 20.Shalliker RA, Broyles BS, Guiochon G. Physical evidence of two wall effects in liquid chromatography. J Chromatogr A. 2000;888:1–12. doi: 10.1016/s0021-9673(00)00517-3. [DOI] [PubMed] [Google Scholar]
- 21.Khirevich S, Höltzel A, Hlushkou D, Tallarek U. Impact of conduit geometry and bed porosity on flow and dispersion in noncylindrical sphere packings. Anal Chem. 2007;79:9340–9349. doi: 10.1021/ac071428k. [DOI] [PubMed] [Google Scholar]
- 22.Khirevich S, Höltzel A, Seidel-Morgenstern A, Tallarek U. Geometrical and topological measures for hydrodynamic dispersion in confined sphere packings at low column-to-particle diameter ratios. J Chromatogr A. 2012;1262:77–91. doi: 10.1016/j.chroma.2012.08.086. [DOI] [PubMed] [Google Scholar]
- 23.Daneyko A, Khirevich S, Höltzel A, Seidel-Morgenstern A, Tallarek U. From random sphere packings to regular pillar arrays: effect of the macroscopic confinement on hydrodynamic dispersion. J Chromatogr A. 2011;1218:8231–8248. doi: 10.1016/j.chroma.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Schure MR, Maier RS. How does column packing microstructure affect column efficiency in liquid chromatography? J Chromatogr A. 2006;1126:58–69. doi: 10.1016/j.chroma.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 25.Roulin S, Dmoch R, Carney R, Bartle KD, Myers P, Euerby MR, Johnson C. Comparison of different packing methods for capillary electrochromatography columns. J Chromatogr A. 2000;887:307–312. doi: 10.1016/s0021-9673(00)00366-6. [DOI] [PubMed] [Google Scholar]
- 26.Koivisto P, Danielsson R, Markides KE. Factors affecting the preparation of packed capillary columns in supercritical carbon dioxide media. J Microcolumn. 1997 Sep 9;:97–103. [Google Scholar]
- 27.Capriotti F, Leonardis I, Cappiello A, Famiglini G, Palma P. A fast and effective method for packing nano-LC columns with solid-core nano particles based on the synergic effect of temperature slurry composition, sonication and pressure. Chromatographia. 2013;76:1079–1086. [Google Scholar]
- 28.Ehlert S, Kraiczek K, Mora JA, Dittmann M, Rozing GP, Tallarek U. Separation efficiency of particle-packed HPLC microchips. Anal Chem. 2008;80:5945–5950. doi: 10.1021/ac800576v. [DOI] [PubMed] [Google Scholar]
- 29.Wirth MJ, Ranasinghe Kodithuwakkuge S, Yerneni C, Birdsall R. WO2011127044 A2. Method of packing chromatographic columns. 2011 Oct 13;
- 30.Maiolica A, Borsotti D, Rappsilber J. Self-made frits for nanoscale columns in proteomics. Proteomics. 2005;5:3847–3850. doi: 10.1002/pmic.200402010. [DOI] [PubMed] [Google Scholar]
- 31.Knecht LA, Guthrie EJ, Jorgenson JW. On-column electrochemical detector with a single graphite fiber electrode for open-tubular liquid chromatography. Anal Chem. 1984;56:479–482. [Google Scholar]
- 32.Kaiser TJ, Thompson JW, Mellors JS, Jorgenson JW. Capillary-based instrumen for the simultaneous measurement of solution viscosity and solute diffusion coefficient at pressures up to 2000 bar and implications for ultrahigh pressure liquid chromatography. Anal Chem. 2009;81:2860–2868. doi: 10.1021/ac802467k. [DOI] [PubMed] [Google Scholar]
- 33.Vidal D, Ridgway C, Pianet G, Schoelkopf J, Roy R, Bertrand F. Effect of particle size distribution and packing compression on fluid permeability as predicted by lattice-Boltzmann simulations. Comput Chem Eng. 2009;33:256–266. [Google Scholar]
- 34.Daneyko A, Hlushkou D, Khirevich S, Tallarek U. From random sphere packings to regular pillar arrays: analysis of transverse dispersion. J Chromatogr A. 2012;1257:98–115. doi: 10.1016/j.chroma.2012.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


