Abstract
Hepatitis B reactivation associated with immune suppressive and biological therapies is emerging to be an important cause of morbidity and mortality in patients with current or prior exposure to hepatitis B virus infection. The population at risk for HBV reactivation includes those who are either currently infected with HBV or have past exposure to HBV. Since curative and eradicative therapy for HBV is not currently available, there is a large reservoir of individuals at risk for HBV reactivation in the general population. HBV reactivation with its potential consequences is particularly a concern when these people are exposed to either cancer chemotherapy, immunosuppressive or biologic therapies for the management of rheumatologic conditions, malignancies, inflammatory bowel disease, dermatologic conditions, or solid organ or bone marrow transplantation. With the advent of newer and emerging forms of targeted biologic therapies, it has become important to understand the mechanisms whereby certain therapies are more prone to HBV reactivation. The aim of this review is to provide a comprehensive update on the current concepts, risk factors, molecular mechanisms, prevention and management of hepatitis B reactivation. In addition, we provide recommendations for future research in this area.
Keywords: liver failure, cirrhosis, liver disease, mortality, fulminant hepatic failure, viral hepatitis, chronic hepatitis B, guidelines
Introduction
It is estimated that approximately one in every third individual in this world may have been exposed to hepatitis B virus infection (HBV)1, 2. Furthermore, HBV is one of the leading causes of chronic liver disease and hepatocellular carcinoma worldwide. Based upon recent estimates, approximately 350 million people worldwide suffer from chronic hepatitis B infection (CHB). In the United States, as many as 2.2 million Americans are estimated to have CHB2. However, only a minority of these individuals know that they have CHB and receive medical care and treatment for CHB. The majority of infected patients are either unaware that they have chronic HBV infection, have been exposed to HBV or have risk factors for acquiring HBV infection. Therefore, the risk and consequences of hepatitis B reactivation is significantly increased when these HBV-infected individuals who are exposed to either immunosuppressive therapy or cancer chemotherapy.
The population at risk for HBV reactivation includes those who are either currently infected with HBV or have past exposure to HBV3. Since curative and eradicative therapy for HBV is not currently available, there is a large reservoir of individuals at risk for HBV reactivation in the general population. HBV reactivation with its potential consequences is particularly a concern when these people are exposed to either cancer chemotherapy, immunosuppressive or biologic therapies for the management of rheumatologic conditions, malignancies, inflammatory bowel disease, dermatologic conditions, or solid organ or bone marrow transplantation4. With the advent of newer and emerging forms of targeted biologic therapies, it has become important to understand the mechanisms that make certain therapies more prone to HBV reactivation5, 6.
In this review, we will discuss the epidemiology, virology and management of HBV reactivation in the setting of immune suppressive and biological modifier therapy. Due to space constraints, we will not be covering the risk of HBV reactivation after bone marrow transplant or solid organ transplant and refer the readers to other reviews on the topic3, 6–10.
Epidemiology
In the United States, HBV reactivation related acute liver failure is being increasingly recognized and has emerged to be an important and preventable cause of acute liver failure4. HBV reactivation is defined as a sudden and rapid increase in HBV DNA level by at least a 100-fold in those with previously detectable HBV DNA or reappearance of HBV DNA viremia in individuals who did not have viremia prior to the initiation of immune suppressive or biological modifier therapy or cancer chemotherapy.
The HBV reactivation may be classified into two broad categories based upon baseline virologic profile: 1) HBV reactivation in those who are positive for hepatitis B surface antigen (HBsAg) in the serum with or without detectable HBV DNA viremia in the blood.
2) Reverse seroconversion is defined as reappearance of HBsAg and HBV DNA in individuals who are initially negative for HBsAg and HBV DNA in the serum prior to immunosuppression and then become positive after exposure to immunosuppressive therapies.
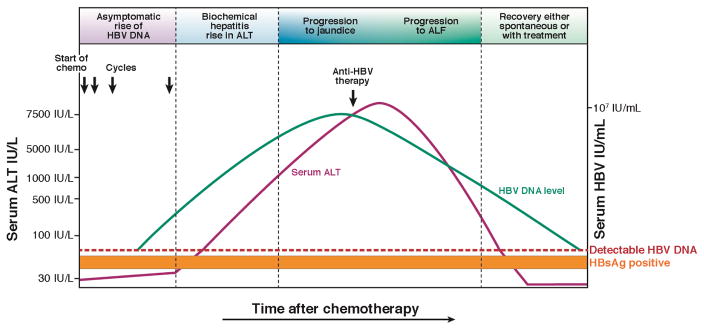
The natural history of HBV reactivation may be classified into the following stages (Figure 1.)
Figure 1. Course of HBV reactivation after receiving immunosuppressive therapy.
The course of HBV reactivation is depicted above when a patient at risk is exposed to cancer chemotherapy (as an example). All patients may not follow these phases in this sequence but it underscores the point that there is an asymptomatic phase early on in HBV reactivation that provides a window of opportunity to initiate treatment. In HBsAg positive patients, this asymptomatic phase is characterized by a rapid rise in HBV DNA, which is followed by a rapid rise in serum ALT levels. In HBsAg-negative patients, this asymptomatic phase is characterized by first reappearance of HBsAg and then sudden rise in HBV DNA, followed by an increase in serum ALT. Within a few weeks, after rapid HBV replication and increase in serum ALT, the bilirubin starts increasing and once it is above 3 mg/dl scleral icterus becomes apparent, and then some patients may progress to acute liver failure characterized by an increase in prothrombin time, development of ascites, and hepatic encephalopathy. The risk of mortality is significantly increased in those who develop ALF. Once patients are started on anti-HBV therapy the HBV DNA as well as serum ALT decrease rapidly, and this can happen spontaneously in some patients (however, spontaneous improvement after ALF is rare). This figure illustrates the natural history of HBV reactivation.
Increased in viral replication from baseline: After initial exposure to immunosuppressive therapies, viral replication may abruptly increase and continue to rise. Early into this phase the patient may still be asymptomatic. Many patients may not go on to develop HBV reactivation related hepatitis, which is described below and defined as an increase in ALT or AST to ≥3 X baseline values.
Increase in serum ALT and AST: Approximately within a few weeks (or in some cases days) of a rise in HBV DNA levels, serum alanine and aspartate aminotransferases start rising. This stage is also classified as HBV-reactivation related hepatitis or a hepatic flare. Typically, serum ALT and AST may rise between 5–10 times ULN or baseline levels. The majority of patients may remain asymptomatic but a small number of patients experiencing a more severe flare of hepatitis may experience constitutional symptoms, right upper quadrant tenderness and jaundice.
Spontaneous or on-treatment improvement/resolution: The next phase in the natural history in some patients is spontaneous improvement in the flare of serum ALT and AST in most cases due to completion of the course of the immunosuppressive therapy or cycle of cancer chemotherapy. In some cases, HBV reactivation is recognized and start of anti-viral therapy may also lead to resolution of the flare of hepatitis and then reduction in serum HBV DNA levels.
Acute liver failure/persistent liver injury: A small minority of patients may continue to have a progressive decline in the synthetic function of the liver leading to worsening serum bilirubin levels, prolongation of the prothrombin time and may develop acute liver failure with other features of hepatic decompensation such as ascites, altered sensorium, and sequelae of portal hypertension. Some of these individuals may need a liver transplant if they are candidates despite initiation of anti-viral therapy. If unrecognized or untreated, these individuals have a high risk of death from liver failure.
Resolution with immune recovery: The majority of individuals will recover from HBV reactivation with the initiation of anti-viral therapy or with the cessation of immunosuppressive therapy that led to the HBV reactivation.
These stages do not necessarily follow each other as outlined above. Many individuals may only develop transient increased HBV viremia with or without ALT elevation, but do not exhibit any clinical consequences. The mechanism by which individuals exhibit varying severity of HBV reactivation is unclear and this variability in severity of HBV reactivation probably relates to both host and viral factors as described below.
Onset of HBV Reactivation
The timing of onset of HBV reactivation can be variable depending upon the host status, underlying disease and the type of immunosuppressive therapies. It may occur as early as within the first 2 weeks of onset of chemotherapy or up to a year after the cessation of immunosuppression. Understanding the risk factors and mechanisms that cause HBV reactivation help understand and quantify the magnitude of the risk of HBV reactivation and its consequences.
Risk factors of HBV Reactivation
The key risk factors for reactivation can be broadly classified into three categories: 1) host factors, 2) virologic factors and 3) type and degree of immunosuppression. Host factors include male sex, older age, presence of cirrhosis, type of disease needing immunosuppression e.g. lymphoma11, 12. The virologic factors associated with increased risk of reactivation include high baseline HBV DNA, HBeAg positivity, and chronic hepatitis B13–15. HBV genotype has been increasingly linked to treatment response, disease severity and progression16, 17 While its association with HBV reactivation is unknown, a few small studies have suggested infection with non-A genotype may be more prone to reactivation17–19. The prevalence of HBV genotypes has a variable and divergent worldwide distribution. Thus the association of HBV genotypes with HBV reactivation will be an important question to address. Co-infection of HBV with HCV, hepatitis D virus (HDV) or human immunodeficiency virus infection presents an unusual setting for potential HBV reactivation. Treatment of co-infected patients with antivirals directed at the virus, such as direct-acting antivirals for HCV, lonafarnib for HDV, and non-B antiretroviral therapy for HIV can result in HBV reactivation 20–22. These host and virologic factors are important considerations that may further increase the likelihood of HBV reactivation. Therefore, the assessment of host as well as virologic risk factors should be important caveats to help decide whether to initiate prophylactic therapy before initiating immunosuppression.
The risk of reactivation can be broadly divided into high risk (if the rate of HBV reactivation is ≥10 %), moderate risk (if the risk of reactivation is between 1–10%), and low risk (if the risk of reactivation is < 1%) based upon type of immunosuppressive therapy stratified by presence of HBsAg or absence of HBsAg but positive for anti-hepatitis B core antibody (HBsAg-negative and anti-HBc-positive with or without anti-HBs).
Routine HBV screening is recommended by HBsAg and anti-HBC testing among all patients who are at risk of HBV reactivation16. Prophylactic therapy with potent oral anti-HBV therapies is strongly recommended for patients at a high or medium risk of reactivation (see below in the section on the regimen for prophylaxis). For patients at a low risk of reactivation, either preemptive therapy or watchful monitoring is recommended. Table 1 provides a list of therapies stratified by their risk of reactivation. Among those who are HBsAg-negative and anti-HBc positive patients, the evidence for risk of HBV reactivation and preemptive therapy is considerably controversial in many situations. In general, this risk for HBV reactivation is much lower in the HBsAg-negative and anti-HBc positive patients than the HBsAg-positive patients. The greatest risk of reactivation that mandates preemptive therapy is the use of B cell depleting therapies, or in the setting of bone marrow transplant or solid organ transplantation. In most other scenarios in patients who are HBsAg-negative and anti-HBc positive, watchful monitoring may be a reasonable choice.
Table 1.
Risk of hepatitis B reactivation associated with immunosuppressive therapies
| Risk of reactivation in HBsAg positive patients | Immunosuppressive therapies |
|---|---|
| High risk of reactivation | B-cell depleting agents including rituximab, ofatumumab, ustekinumab, natalizumab, alemtuzumab, ibritumomab, High-dose corticosteroids Anthracyclines including doxorubicin, epirubicin, More potent TNF-α inhibitors including infliximab, adalimumab, certolizumab, golimumab, Local therapy for HCC including TACE |
| Moderate risk of reactivation | Systemic chemotherapy Less potent TNF-α inhibitors including etanercept Cytokine-based therapies including abatacept, ustekinumab, mogamulizumab, natalizumab, vedolizumab Immunophilin inhibitors including cyclosporine Tyrosine-kinase inhibitors including imantnib, nilotinib, Proteasome inhibitors such as bortezomib Histone deacetylase inhibitors (HDIs) Moderate-dose corticosteroids |
| Low risk of reactivation | Antimetabolites, azathioprine, 6-mercaptopurine, methotrexate Short-term low dose corticosteroids Intra-articular steroid injections (extremely low risk) |
| Risk of reactivation in HBsAg-negative and anti-HBc positive patients* | Immunosuppressive therapies |
| High risk of reactivation | B-cell depleting agents including Rituximab, ofatumumab, ustekinumab, natalizumab, alemtuzumab, ibritumomab, |
| Moderate risk of reactivation | High-dose corticosteroids Anthracyclines including doxorubicin, epirubicin, More potent TNF-α inhibitors including infliximab, adalimumab, certolizumab, golimumab, Systemic cancer chemotherapy including HCC Cytokine-based therapies including abatacept, ustekinumab, mogamulizumab, natalizumab, vedolizumab, Immunophilin inhibitors including cyclosporine tyrosine-kinase inhibitors including imantnib, nilotinib, Proteasome inhibitors such as bortezomib Histone deacetylase inhibitors (HDIs), |
| Low risk of reactivation | Moderate and low dose prednisone Antimetabolites, azathioprine, 6-mercaptopurine, methotrexate, |
The risk of HBV reactivation in those who are HBsAg-negative and anti-HBc positive individuals receiving B cell depleting therapies is the highest. For the moderate and low risk groups, the evidence for risk of HBV reactivation is considerably controversial.
Among patients who are HBsAg-positive, the following therapies are at a high risk of reactivation (incidence rate of HBV reactivation of 10% or higher).
B-cell depleting therapies such as rituximab and ofatumumab have significantly increased risk of reactivation in both HBsAg-positive as well as in HBsAg-negative and anti-HBc positive patients23, 24. This class of drugs are most notorious for causing severe HBV reactivation, and can lead to increased risk of HBV-reactivation related liver failure and liver related mortality if HBV reactivation is not promptly recognized and treated24. Patients with Non-Hodgkin Lymphoma are routinely prescribed rituximab and have extremely high rates of reactivation due to host as well as immunosuppression related factors. Rituximab is also being used for the treatment of several rheumatologic conditions such as rheumatoid arthritis, and vasculitides. Food and Drug Adminstration has recently placed a black-box warning for rituximab to increase awareness regarding HBV reactivation in patients exposed to rituximab5.
Anthracycline derivatives such as doxorubicin and epirubicin are also associated with a high risk of reactivation25, 26. Patients with hepatocellular carcinoma and hepatitis B undergoing transarterial chemoembolization (TACE) therapy are a particular concern.
Chronic prednisone therapy either medium dose (10–20 mg orally daily) or high dose (> 20 mg orally daily) for more than 4 week duration increases the likelihood of HBV reactivation into a high risk of reactivation9.
Patients receiving cancer chemotherapy for lymphomas, acute myeloid leukemias, and chemotherapy for breast cancer, pancreatic cancer, lung cancer, may end up receiving either above therapies or high-dose pulse steroids and should be considered at a high risk of reactivation and received screening and anti-viral prophylaxis for the prevention of HBV reactivation11, 25.
Tumor necrosis factor-alpha (TNF-α) inhibitors such as infliximab, adalimumab, certolizumab, have a high risk (ranges between 12%–39%) of HBV reactivation in HBsAg-positive patients27, 28. The risk is higher with infliximab (a more potent TNF-α blocker) than etanercept (much lower risk, approximately 1–5%). These therapies are commonly utilized in the treatment of inflammatory bowel disease (IBD), rheumatologic conditions such as rheumatoid arthritis.
Treatment with a moderate risk of reactivation (incidence rate of HBV reactivation of 1–10%):
Systemic chemotherapy other than the situation described above.
Less potent TNF-α inhibitors such as etanercept have a moderate risk (approximately 1–5%) of HBV reactivation in HBsAg-positive patients and even lower in HBsAg-negative and anti-HBc-positive patients28–33.
Cytokine or integrin inhibitors such as abatacept, ustekinumab, mogamulizumab, natalizumab, vedolizumab have been associated with moderate risk of reactivation in patients who are HBsAg-positive34, 35. These therapies are being commonly utilized in the management of IBD and rheumatologic as well as dermatologic conditions.
Tyrosine kinase inhibitors such as imatinib, nilotinib, have been associated with moderate risk of HBV reactivation in both HBsAg positive as well as in HBsAg-negative and anti-HBc positive patients36–38. These therapies are commonly utilized in the treatment of chronic myeloid leukemia, and gastrointestinal stromal tumors among others.
Bortezomib is commonly used for the treatment of multiple myeloma and has been linked to an increased risk of HBV reactivation39, 40.
Histone deacetylase inhibitors (HDIs) such as romidepsin are used in the treatment of T-cell lymphomas and have been associated with reactivation of DNA viruses including HBV41. The risk of reactivation appears to be moderate with this class of agents.
Low-dose corticosteroid therapies such as prednisone 10 mg orally daily over 4 weeks may increase the risk of reactivation up to 10% in HBsAg-positive individuals10. Rarely, patients receiving steroids for Bell’s palsy may also be at risk of HBV reactivation. Therefore, these individuals require careful monitoring.
Medium-dose corticosteroids such as prednisone 10–20 mg orally daily may increase the risk of reverse seroconversion in HBsAg-negative and anti-HBc-positive individuals. Therefore, these individuals require careful monitoring.
Anthracycline inhibitors such as doxorubicin and epirubicin may moderately increase the risk of reactivation in HBsAg-negative and anti-HBc-positive individuals but the overall risk appears to be probably lower than what has been previously reported14, 42.
There is evidence that immunophilin inhibitors such as cyclosporine, tacrolimus may also increase the risk of HBV reactivation43. Based upon their immunosuppressive potential, we expect that it would amount to a moderate risk of reactivation for HBV and therefore, anti-HBV prophylaxis may be considered in this patient population until more definitive evidence becomes available.
Treatment with a low risk of reactivation (incidence rate of HBV reactivation of <1%): individuals undergoing these treatments could be monitored without a need for prophylaxis as the risk of reactivation is low.
Patients receiving methotrexate, azathioprine or 6-mercaptopurine based therapies are at a low risk of HBV reactivation9.
Patients receiving intra-articular steroid injections or those receiving a low dose of prednisone < 10 mg orally daily9.
Immune checkpoint inhibitors such as anti-PD-L1 (e.g. nivolumab) and anti-CTLA4 (e.g. ipilimumab) have been used increasingly in treating various cancers 44. The question regarding whether this category of biologics may predispose to HBV reactivation has been raised. Based on their mechanism of action – activating immune response, HBV reactivation is unlikely and has not been reported. On the other hand, because of the concern about immune activation leading to severe exacerbation of hepatitis B, anti-HBV prophylaxis has been recommended for HBV-infected patients undergoing this type of therapy if they are not on treatment already.
Among patients who are HBsAg-negative and anti-HBc positive, we have tried to stratify the immunosuppressive therapies by the risk of reactivation (Table 1.). The greatest risk of HBV reactivation in these settings lie with B-cell depleting therapies such as rituximab, ofatumumab, ustekinumab, natalizumab, alemtuzumab, and ibritumomab (Table 1), We recommend pre-emptive therapy for prevention of HBV reactivation in this patient population. For these patients who are exposed to therapies that have a medium risk of reactivation, the data are sparse to exactly quantify the risk of reactivation. Therefore, monitoring with HBV DNA or HBsAg and ALT may be considered rather than routine pre-emptive therapy on a case-by-case basis depending upon the co-morbid conditions, the prevalence of anti-HBc positivity in the population, and resources available to health care system (Table 1.). In patients receiving immunosuppressive therapies with a low risk of reactivation, anti-HBV therapy is not needed, and monitoring is also not mandatory.
Mechanisms of HBV Reactivation
The molecular biology of HBV, its replication and mechanisms of immune control have been studied in great detail and the readers are referred to several recent reviews for a more comprehensive discussion of the topics 2, 45. In brief, HBV can efficiently infect a host and leads to acute infection in a large majority of exposed individuals. The virus gains entry into hepatocytes by interacting with a series of host factors, with the sodium-taurocholate co-transporter (NTCP) being the key liver-specific receptor 46. After entry, the released nucleocapsid containing the partially double-stranded viral genome is then imported into the nucleus, where it is then repaired into a full-length, circular DNA (cccDNA) by the viral polymerase (P). The cccDNA complexes with various host histones, histone-related enzymes and other proteins to form a mini-chromosome as the template for viral transcription 47, which is regulated by epigenetic modifications and various transcriptional factors 48. Viral proteins such as core and X proteins have been shown to be part of the mini-chromosome complex and probably play an important role in the functions and metabolism of the cccDNA 49. The level of cccDNA is amplified and replenished by the replicating HBV DNA via nuclear recycling of nucleocapsid from the cytoplasm 50. The cccDNA is quite stable in infected cells and can persist in a latent state as a reservoir for HBV reactivation. Previous studies have shown that HBV DNA, presumably cccDNA and/or replicating HBV DNA, can persist in the liver of patient decades after apparent recovery from HBV infection 51. This persistence occurs in spite of active anti-HBV immune response. In addition, clinical studies have demonstrated that therapy with nucleoside analog can potently suppress of HBV DNA, but the reduction of cccDNA was modest after a year of treatment 52, 53. Similar findings have been reported for the extraordinary stability of cccDNA in the animal models 54. All these observations support the concept that HBV infection is rather difficult to eradicate and its persistence, albeit at a low level, explains the potential of HBV reactivation in any individuals who have been infected with the virus.
The outcome of HBV infection is determined by adaptive T and B cells responses of the host 55. Recovery from HBV infection is mediated by an effective immune control of HBV replication via these two arms of immunity. A robust, polyclonal, multi-specific CD4+ and CD8+ T cell response with associated B cell response and production of neutralizing anti-HBs antibodies is associated with viral clearance. The HBV-specific T cells either directly target infected cells for elimination via cytopathic mechanisms or suppress viral replication via non-cytopathic cytokine (predominately interferons)-mediated pathways 56. Neutralizing antibodies produced by the activated B cells clear the circulating viruses and further limit the spread of HBV infection. The role of innate immunity, while not extensively studied, probably plays a previously unrecognized role in controlling HBV infection as well 57. Although these immune mechanisms are sufficient to control active HBV replication, they are probably not potent enough to eradiate all the niches of infected cells harboring either “latent” HBV cccDNA or low-level replicating HBV that escape targeting by the HBV-specific immune cells. These cells thus constitute a reservoir of persisting HBV. While the size and nature of this reservoir in individuals with serological evidence of HBV recovery is unknown, it is clearly a source of HBV reactivation once the immune control mechanisms are perturbed or suppressed.
HBV reactivation can occur in a variety of settings and is typically associated with medical treatments targeting at certain aspects of host functions (Figure 2). Immunosuppressive therapies are the most commonly reported causative agents. Many of these agents have a general mechanism of action that suppresses many immune functions across the board. For example, anthracycline derivatives, alkylating agents and antimetabolites are cytotoxic and diminish lymphocyte proliferation. Steroid suppresses cell-mediated immunity by inhibiting production of interleukins important for T and B cell proliferation 58. Immunophilin inhibitors, such as cyclosporine, suppress T lymphocyte functions by binding to immunophilins and inhibiting interleukin production 59. It is thus not surprising that these general immunosuppressive effects lead to broad immune dysfunctions and potential HBV reactivation. As mentioned above, molecularly targeted therapies acting on specific host pathways with the aim to alter disease process have been increasingly associated with HBV reactivations. The targets whereby these agents act, however, render an unexpected and unique insight into the mechanisms of immune control and viral clearance in HBV infection. A few examples of such agents are highlighted below.
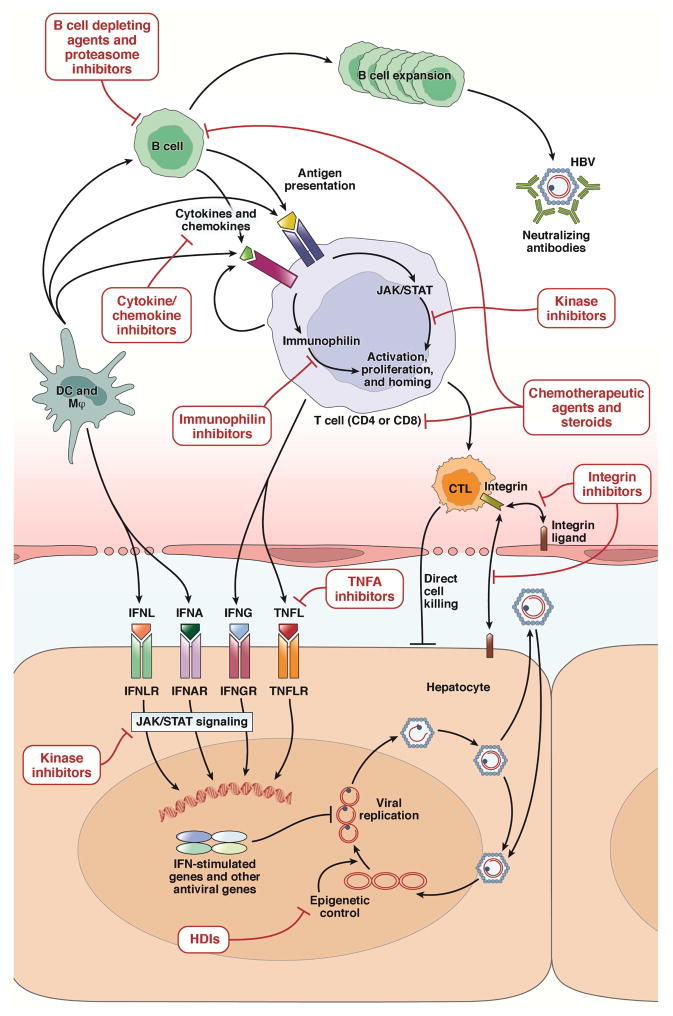
Figure 2.
HBV life cycle and mechanisms associated with hepatitis B reactivation linked to immunosuppressive therapies. HBV replication and propagation is controlled by various innate and adaptive immune mechanisms as shown in the figure. The epigenetic regulation of HBV transcription can be altered by the HDIs. The innate immune control mechanisms such as IFNA and TNF-like molecules (TNFL) and their signaling pathways can be blocked by various immunosuppressive therapies such as TNFA and kinase inhibitors. The adaptive immunity controlling HBV including antigen-presenting cells (dendritic cells and macrophages), T and B cells can be inhibited by various immunosuppressive therapies targeting at different steps of immune response. See text for more explanation.
TNF-α inhibitors
TNF-α and related cytokines are well known as proinflammatory agents. Drugs or biologics blocking their pathways have been used extensively in various inflammatory and autoimmune conditions 60, 61. Its widespread use has been associated with HBV reactivation. Initially it was not clear why these agents should be associated with HBV reactivation. It was thought that these cytokines, other than causing nonspecific inflammation, may exert subtle regulation of the adaptive immune system responsible for HBV immune control. However the mechanism of action remains largely unknown. Recent advances in understanding HBV replication and cccDNA regulation renders a intriguing explanation as to how TNF-α and related cytokines may play a crucial role in regulating the functions of cccDNA. In particular, TNF-α, like IFN-α/γ, can activate a unique host antiviral pathway, the APOBEC proteins, that cause the degradation of cccDNA in HBV-infected cells 62. Thus blocking this endogenous antiviral pathway may lead to a higher HBV replication state and HBV reactivation.
Rituximab and other B cell depleting agents
Rituximab is a monoclonal antibody against CD20, which is primarily expressed on the surface of the B lymphocytes. It targets and destroys B cells and is used to treat hematological cancers with this B cell marker and inflammatory rheumatic diseases 63, 64. As discussed above, B cells, by producing neutralizing antibodies, contribute to HBV clearance by preventing viral spread and eliminating circulating viruses. It was thought that T lymphocytes are the predominant mechanism in suppressing HBV replication 56. The compelling evidence of suppression of the B cell immunity leading to HBV reactivation highlights that the B cells probably play a previously unappreciated role in HBV immune control. It is conceivable that the B lymphocytes, besides producing neutralizing antibodies, may exert additional functions in suppressing HBV replication.
Histone deacetylase inhibitors (HDIs)
This class of compounds target histone deacetylase, a histone-modifying enzyme that is important for epigenetic regulation of gene expression 65. HDIs can inhibit tumor proliferation by activating expression of tumor suppressor genes and have been used as anti-cancer agents 66. As mentioned above, the cccDNA mini-chromosome complexes with various histones and histone modifying enzymes, which epigenetically regulate HBV transcription. It has been shown that acetylation of certain histones on the mini-chromosome leads to active gene expression 48, 67. Conversely, deacetylated histones are associated with transcriptionally silent mini-chromosome, which is probably the state of HBV genome in individuals with inactive HBV disease. Thus HDIs can also reverse the deacetylation status of the silent mini-chromosome and result in active HBV transcription and then HBV reactivation.
Chemokine or integrin inhibitors
These drugs have been developed to inhibit local inflammatory response associated with immune-mediated diseases by blocking the localization and traffic of activated lymphocytes68. It is known that liver is an active immune organ with active influx and efflux or immune cells69. These agents may therefore reduce the local immune control of HBV replication in the liver and predispose the treated individuals to HBV reactivation.
Kinase inhibitors
Activation of various kinase signaling pathways is essential for immune activation and proliferation of lymphocytes as well as other cell types70. Many of these kinase inhibitors have been developed to target these critical pathways in order to treat hematological or other malignancies70. Given the importance of HBV-specific lymphocytes in immune control of HBV replication, it is not unexpected that these kinase inhibitors may suppress these immune control mediators resulting in HBV reactivation.
Proteasome inhibitors
Bortezomib, used for treatment of multiple myeloma, targets cellular pathways that are important for proliferation of malignant plasma cells. It may also interfere with the functions of healthy B and plasma cells that as mentioned above, are important in HBV immune control.
Screening for Hepatitis B prior to immunosuppressive therapies
There is considerable heterogeneity in the approaches that various professional medical societies have taken to address the issue of screening for hepatitis B prior to starting immunosuppressive therapies4, 10, 16, 71–74. This review reflects our expert opinion based on our interpretation of the currently available data. All patients who are receiving therapies that are either high or moderate risk of reactivation or have recently been diagnosed with a cancer should be screened with at least HBsAg, anti-HBc, and anti-HBs. Screening of anti-HBc in highly endemic areas, especially in resource-limited countries, may not be a cost-effective strategy and requires further studies. All those who are negative for HBsAg, anti-HBc and anti-HBS should be vaccinated against HBV as per published guidelines. A decision analysis has shown that lamivudine prophylaxis prior to initiation of high-risk therapies is cost-effective and should be considered prior to initiation of lymphoma therapy75.
Management and Prophylaxis of Hepatitis B Reactivation
The management of HBV reactivation is centered on the likelihood of the risk of reactivation based upon risk factor profile of an individual patient as described above. All patients who are either high or moderate risk of HBV reactivation as described above should be considered candidates for prophylactic anti-HBV therapy. Usually we recommended starting anti-HBV therapy prior to starting immune suppressive therapy and a baseline complete metabolic profile, complete blood count, prothrombin time, and serum HBV DNA levels are recommended. It is important to evaluate if the patient has chronic hepatitis B and should be a candidate for treatment of CHB based upon serum ALT, AST, albumin, platelet count, and other laboratory parameters and physical examination. In endemic areas, if a patient presents with elevated ALT in the setting of immunosuppressive therapies it is prudent to consider checking for serum HBV DNA. We also recommend routine monitoring with above tests every 3 months while on anti-HBV therapy. Consideration of referral to either a hepatology or infectious disease specialist prior to cancer chemotherapy in those at risk of hepatitis B reactivation is recommended.
Type of Anti-HBV Regimen for Prophylaxis
Previous meta-analyses have shown that prophylactic therapy with lamivudine significantly reduced the risk of HBV reactivation, HBV-related hepatitis and HBV-related acute liver failure and HBV-related mortality in patients receiving cancer chemotherapy 11, 76. Preemptive treatment prior to starting cancer chemotherapy has also been shown to reduce the risk of interruption of cancer chemotherapy. With the reduced likelihood of elevations in liver enzymes and serum bilirubin levels, the likelihood of receiving the complete course of chemotherapy is significantly higher in those receiving preemptive therapy than those who experience HBV reactivation. These data led to the 2008 guidelines by the Centers for the Disease Control to recommend routine HBV screening prior to initiation of cancer chemotherapy in the United States.
Although lamivudine is effective and may be utilized in resource limited setting for the prevention of HBV reactivation, it is not considered the agent of choice as it has a low barrier for development of drug resistance11, 16. The rates of lamivudine resistance at 1 year and 2 year are 20% and 30%, and increase exponentially with continued use16. Therefore, lamivudine is not favored especially if therapy would be needed beyond one year. Development of resistance to lamivudine increases the risk of resistance related hepatic flares and also reduces the likelihood of future response to other therapies such as entecavir, or telbivudine16. Therefore, entecavir or tenofovir are recommended as therapies for the prevention of HBV reactivation as these anti-HBV therapies have a high barrier to resistance10, 16, 77–79.
Huang et al. conducted a randomized controlled trial comparing the efficacy of entecavir 0.5 mg orally daily versus lamivudine 100 mg orally daily (initiated 1 week prior to the start of chemotherapy and continued until six months after chemotherapy) in preventing HBV reactivation in HBsAg-positive Chinese patients with untreated diffuse large B-cell lymphoma receiving chemotherapy treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). They reported that entecavir was better than lamivudine in reducing the risk of HBV-related hepatitis (0% versus 13.3%), HBV-reactivation (6.6% versus 30%), and the risk of chemotherapy disruption (1.6% versus 18.3%)77. Chen et al. reported retrospective data on the superiority of entecavir over lamivudine in reducing the risk of HBV reactivation in HBsAg-positive patients undergoing cancer chemotherapy for solid-tumors. Furthermore, they showed that tenofovir was efficacious in the management of reactivation events in either entecavir or lamivudine treated patients79. Other therapies that may also be utilized for the prevention of HBV reactivation including telbivudine or adefovir but these have lower barrier to resistance than entecavir and tenofovir16, 80, 81. Interferon-based therapies are not used for prophylaxis. A recent network meta-analysis has shown that tenofovir and entecavir may be the most efficacious therapies for the prevention of HBV reactivation82. Decision analyses have shown that it is cost-effective to screen for HBsAg and anti-HBc in patients undergoing lymphoma chemotherapy or early stage breast cancer83, 84. Most experts recommend routine screening prior to cancer chemotherapy8, 85.
In resource limited countries, the use of therapies that are less potent and have a lower barrier towards resistance such as lamivudine, adefovir or telbivudine may be considered, especially in individuals who are HBsAg-positive but have either undetectable or very low levels of HBV DNA in the blood.
Duration of Antiviral Prophylaxis
Although the duration of antiviral prophylaxis has not yet been systematically studied in randomized controlled trials. The data derived from the onset of risk of reactivation suggests that anti-viral therapy should be continued for at least 6 month after the last dose of immunosuppressive or cancer chemotherapy9. However, in the case of B-cell depleting therapies (e.g. rituximab), it is recommended that anti-viral prophylaxis should be continued until 12 months after the last dose of rituximab9. The rationale for longer continuation of anti-viral prophylaxis is that immune recovery may be delayed and risk of reactivation with rituximab has been seen up to an year (rarely even 2 years) after the last dose of rituximab86. It is also suggested that after anti-viral therapy is stopped, patients should undergo routine testing for HBV DNA and serum ALT and AST 3–6 months after discontinuation to monitor for rise in HBV DNA suggesting HBV reactivation post-withdrawal of anti-viral therapy87.
In summary, universal screening with serological tests for hepatitis B with HBsAg, anti-HBs and anti-HBc should be done prior to initiation of cancer chemotherapy or above mentioned immunosuppressive therapies (see Figure 3)8. Patients with chronic hepatitis B as defined by presence of HBsAg in the serum, serum HBV DNA ≥ 2000 IU/ml, and an elevated ALT should initiate antiviral therapy based upon published guidelines. Inactive HBV carriers, as defined by presence of HBsAg, HBV DNA <2000 IU/ml, and normal ALT and AST, when exposed to high and moderate risk immunosuppressive therapy should undergo prophylaxis against HBV reactivation. Prophylaxis should ideally be started 2 to 4 weeks before the initiation of immunosuppressive therapy and maintained for at least 6 months after the last dose of immunosuppressive therapy. It is recommended to use either entecavir or tenofovir as first line antiviral agents82. Among those who are inactive HBsAg carriers who may be exposed to low-risk immune suppressive therapy and patients with HBsAg negative/anti-HBc positive (HBV infection in the past), the strategy should be monitoring of viral reactivation with aminotransferases and HBV DNA determination in every 3 months. In the case of rituximab containing regimens, we recommend routine prophylaxis in patients who are HBsAg-negative and anti-HBc positive to reduce the risk of reactivation. Furthermore, the risk of reactivation remains high even after several months or beyond one year from the last dose of rituximab. Therefore, HBV DNA monitoring (or HBsAg monitoring in the case of HBsAg-negative and anti-HBc positive patients) may be continued up to 2 years after the last dose of rituximab.
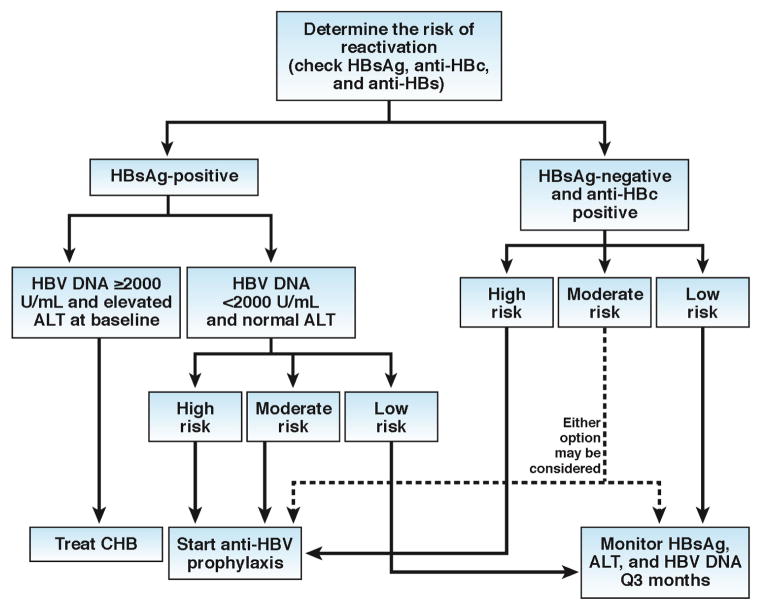
Figure 3. Algorithm for management of hepatitis B reactivation.
Screening with serological tests for hepatitis B with HBsAg, anti-HBs and anti-HBc should be done prior to initiation of cancer chemotherapy or above mentioned immunosuppressive therapies. Patients with chronic hepatitis B as defined by the presence of HBsAg in the serum, serum HBV DNA ≥ 2000 IU/ml, and an elevated ALT should initiate antiviral therapy based upon published AASLD guidelines. Inactive HBV carriers, as defined by presence of HBsAg, HBV DNA <2000 IU/mlL and normal ALT and AST, when exposed to high and moderate risk immunosuppressive therapy should undergo prophylaxis against HBV reactivation. Prophylaxis should ideally be started 2 to 4 weeks before the initiation of immunosuppressive therapy and maintained for at least 6 to 12 months after the last dose of immunosuppressive therapy. Among those who are inactive HBsAg carriers and exposed to low-risk immunosuppressive therapy and patients with HBsAg negative/anti-HBc positive (HBV infection in the past), monitoring with serum ALT and HBsAg (and HBV DNA in those who are HBsAg-positive) is recommended. In patients who are exposed to rituximab-containing or other high-risk regimens, we recommend routine prophylaxis in patients who are HBsAg-negative and anti-HBc positive to reduce the risk of reactivation. Watchful monitoring may also be a reasonable choice in most other scenarios in patients who are HBsAg-negative and anti-HBc positive. Those who have a moderate risk of reactivation but HBsAg negative/anti-HBc positive (HBV infection in the past), anti-HBV prophylaxis should be considered or they could also be monitored with serum ALT and HBsAg every 3 months until 6 months after the last dose of immunosuppressive therapy. However, the HBV reactivation may occur up to 1–2 years after the last dose of rituximab therapy. Therefore, patients exposed to rituximab the anti-HBV prophylaxis may be continued up to 2 years after the last dose of rituximab.
Future Directions and Research Priorities
Despite our success in developing effective vaccines and therapies, HBV has proven to be a wily foe. As the medical community continues to explore uncharted territories targeting the immune system to treat various diseases, HBV reactivation will remain a vexing and persistent problem. Considering the vast number of patients infected or previously exposed to the virus in the world, such a problem poses a major public health burden in terms of global morbidity and possibly mortality. At present there are no reliable markers in predicting the risk of HBV reactivation in patients, nor are there any validated tests to determine whether a particular drug or biologic can be associated with HBV reactivation. The latter point was often determined by our empirical experience in a clinical setting. While decades of these experiences have helped us identify important classes of drugs and thus manage these situations appropriately, we still cannot accurately assess the risk of a new class of drugs prior to its clinical application. In addition, we don’t have a publicly available reporting system to accurately and thoroughly track cases of HBV reactivation that are potentially associated with these drugs until these cases are being reported in the literature. In light of these concerns, we are proposing a set of actions to address these unmet needs in both research setting and clinical practice.
Set research priorities to comprehensively elucidate the mechanisms of HBV reactivation associated with various drugs and biologics.
Elevate funding to support basic, translational and clinical research on the fundamental mechanisms of immune control and viral clearance in HBV infection.
Develop advanced tools and technologies to pursue research at the cutting-edge of HBV research.
Establish better or improve existing animal models to study HBV immune control and thus HBV reactivation in more biologically meaningful settings.
Invest in a public database that will allow comprehensive and timely reporting of all drugs, either new or old, in association with HBV reactivation.
Identify and validate predictive markers, including viral markers (HBV genotypes), biomarkers or genetic traits, of HBV reactivation.
Implement a process to test and predict the risk of HBV reactivation of drugs and biologics in the pipeline prior to clinical approve and wide use. As we garner more knowledge and develop better tools to study HBV reactivation, a set of criteria can be selectively and judiciously applied to the clinical approval process of these new drugs and biologics.
Undoubtedly, these actions will require a partnership between the public and private sectors. As what have been accomplished often in combatting the global scourges of viral hepatitis over the last 5 decades, the collective efforts among the government agencies, public foundations, advocacy groups, patients and pharmaceutical industry will once again be needed to successfully overcome this daunting challenge.
Acknowledgments
Funding support: TJL is supported by the intramural research program of the NIDDK, NIH. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award and grant K23-DK090303 and R01-DK106419-02.
We would like to acknowledge Dr. Jordan Feld for his thoughtful comments and input on the manuscript.
Footnotes
Author contributions: Rohit Loomba: Initially drafted the manuscript and content and approved the final version Jake Liang: Conceptualized the manuscript, and drafted the manuscript and approved the final version
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: From discovery to cure. Hepatology. 2015;62:1893–908. doi: 10.1002/hep.28025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–65. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 4.Doo EC, Hoofnagle JH, Rodgers GP. NIH consensus development conference: management of Hepatitis B. Introduction Hepatology. 2009;49:S1–3. doi: 10.1002/hep.22993. [DOI] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Lok AS, Martin P, et al. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703–11. doi: 10.1002/hep.27609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok AS, Ward JW, Perrillo RP, et al. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743–5. doi: 10.1059/0003-4819-156-10-201205150-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagington J. Reactivation of hepatitis b after transplantation operations. Lancet. 1977;1:558–60. doi: 10.1016/s0140-6736(77)91995-x. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JP, Vierling JM, Zelenetz AD, et al. Hepatitis B virus management to prevent reactivation after chemotherapy: a review. Support Care Cancer. 2012;20:2999–3008. doi: 10.1007/s00520-012-1576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–244. e3. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–9. doi: 10.1053/j.gastro.2014.10.039. quiz e16–7. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Rowley A, Wesley R, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–28. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Yeo W, Chan PK, Ho WM, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927–34. doi: 10.1200/JCO.2004.05.161. [DOI] [PubMed] [Google Scholar]
- 14.Yeo W, Chan PK, Hui P, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553–61. doi: 10.1002/jmv.10430. [DOI] [PubMed] [Google Scholar]
- 15.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–20. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 16.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 17.Tohme RA, Bulkow L, Homan CE, et al. Rates and risk factors for hepatitis B reactivation in a cohort of persons in the inactive phase of chronic hepatitis B-Alaska, 2001–2010. J Clin Virol. 2013;58:396–400. doi: 10.1016/j.jcv.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borentain P, Colson P, Coso D, et al. Clinical and virological factors associated with hepatitis B virus reactivation in HBsAg-negative and anti-HBc antibodies-positive patients undergoing chemotherapy and/or autologous stem cell transplantation for cancer. J Viral Hepat. 2010;17:807–15. doi: 10.1111/j.1365-2893.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Ishigami M, Ishizu Y, et al. Clinical characteristics and molecular analysis of hepatitis B virus reactivation in hepatitis B surface antigen-negative patients during or after immunosuppressive or cytotoxic chemotherapy. J Gastroenterol. 2016 doi: 10.1007/s00535-016-1187-z. [DOI] [PubMed] [Google Scholar]
- 20.Puoti M, Torti C, Bruno R, et al. Natural history of chronic hepatitis B in co-infected patients. J Hepatol. 2006;44:S65–70. doi: 10.1016/j.jhep.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 21.De Monte A, Courjon J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27–30. doi: 10.1016/j.jcv.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167–74. doi: 10.1016/S1473-3099(15)00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evens AM, Jovanovic BD, Su YC, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170–80. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozessohn L, Chan KK, Feld JJ, et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat. 2015;22:842–9. doi: 10.1111/jvh.12402. [DOI] [PubMed] [Google Scholar]
- 25.Paul S, Saxena A, Terrin N, et al. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164:30–40. doi: 10.7326/M15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med. 2007;22:237–43. doi: 10.3904/kjim.2007.22.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteve M, Saro C, Gonzalez-Huix F, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363–5. doi: 10.1136/gut.2004.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan JL, Chen YM, Hsieh TY, et al. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 2011;70:1719–25. doi: 10.1136/ard.2010.148783. [DOI] [PubMed] [Google Scholar]
- 29.Chung SJ, Kim JK, Park MC, et al. Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol. 2009;36:2416–20. doi: 10.3899/jrheum.081324. [DOI] [PubMed] [Google Scholar]
- 30.Fanouriakis A, Vassilopoulos D, Repa A, et al. Hepatitis B reactivation following treatment with abatacept in a patient with past hepatitis B virus infection. Rheumatology (Oxford) 2014;53:195–6. doi: 10.1093/rheumatology/ket221. [DOI] [PubMed] [Google Scholar]
- 31.Kato M, Atsumi T, Kurita T, et al. Hepatitis B virus reactivation by immunosuppressive therapy in patients with autoimmune diseases: risk analysis in Hepatitis B surface antigen-negative cases. J Rheumatol. 2011;38:2209–14. doi: 10.3899/jrheum.110289. [DOI] [PubMed] [Google Scholar]
- 32.Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2013;31:118–21. [PubMed] [Google Scholar]
- 33.Perez-Alvarez R, Diaz-Lagares C, Garcia-Hernandez F, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011;90:359–71. doi: 10.1097/MD.0b013e3182380a76. [DOI] [PubMed] [Google Scholar]
- 34.Koskinas J, Tampaki M, Doumba PP, et al. Hepatitis B virus reactivation during therapy with ustekinumab for psoriasis in a hepatitis B surface-antigen-negative anti-HBs-positive patient. Br J Dermatol. 2013;168:679–80. doi: 10.1111/bjd.12120. [DOI] [PubMed] [Google Scholar]
- 35.Nakano N, Kusumoto S, Tanaka Y, et al. Reactivation of hepatitis B virus in a patient with adult T-cell leukemia-lymphoma receiving the anti-CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res. 2014;44:354–7. doi: 10.1111/hepr.12117. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda K, Shiga Y, Takahashi A, et al. Fatal hepatitis B virus reactivation in a chronic myeloid leukemia patient during imatinib mesylate treatment. Leuk Lymphoma. 2006;47:155–7. doi: 10.1080/14639230500236818. [DOI] [PubMed] [Google Scholar]
- 37.Lai GM, Yan SL, Chang CS, et al. Hepatitis B reactivation in chronic myeloid leukemia patients receiving tyrosine kinase inhibitor. World J Gastroenterol. 2013;19:1318–21. doi: 10.3748/wjg.v19.i8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakhani S, Davidson L, Priebat DA, et al. Reactivation of chronic hepatitis B infection related to imatinib mesylate therapy. Hepatol Int. 2008;2:498–9. doi: 10.1007/s12072-008-9099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beysel S, Yegin ZA, Yagci M. Bortezomib-associated late hepatitis B reactivation in a case of multiple myeloma. Turk J Gastroenterol. 2010;21:197–8. doi: 10.4318/tjg.2010.0087. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Huang B, Li Y, et al. Hepatitis B virus reactivation in patients with multiple myeloma receiving bortezomib-containing regimens followed by autologous stem cell transplant. Leuk Lymphoma. 2015;56:1710–7. doi: 10.3109/10428194.2014.941833. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie D, Piekarz RL, Blombery P, et al. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica. 2009;94:1618–22. doi: 10.3324/haematol.2009.008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo W, Zee B, Zhong S, et al. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–11. doi: 10.1038/sj.bjc.6601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabrese LH, Zein NN, Vassilopoulos D. Hepatitis B virus (HBV) reactivation with immunosuppressive therapy in rheumatic diseases: assessment and preventive strategies. Ann Rheum Dis. 2006;65:983–9. doi: 10.1136/ard.2005.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudo M. Immune Checkpoint Blockade in Hepatocellular Carcinoma: 2017 Update. Liver Cancer. 2016;6:1–12. doi: 10.1159/000449342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480:672–86. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bock CT, Schwinn S, Locarnini S, et al. Structural organization of the hepatitis B virus minichromosome. J Mol Biol. 2001;307:183–96. doi: 10.1006/jmbi.2000.4481. [DOI] [PubMed] [Google Scholar]
- 48.Pollicino T, Belloni L, Raffa G, et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823–37. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Belloni L, Pollicino T, De Nicola F, et al. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc Natl Acad Sci U S A. 2009;106:19975–9. doi: 10.1073/pnas.0908365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–8. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 52.Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–8. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Sung JJ, Wong ML, Bowden S, et al. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology. 2005;128:1890–7. doi: 10.1053/j.gastro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Yamamoto T, Cullen J, et al. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311–22. doi: 10.1128/JVI.75.1.311-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–68. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 57.Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64:S60–70. doi: 10.1016/j.jhep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 58.Lowenberg M, Verhaar AP, van den Brink GR, et al. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol Med. 2007;13:158–63. doi: 10.1016/j.molmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191:5785–91. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 60.Bandzar S, Gupta S, Platt MO. Crohn’s disease: a review of treatment options and current research. Cell Immunol. 2013;286:45–52. doi: 10.1016/j.cellimm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Hwang YG, Moreland LW. Induction therapy with combination TNF inhibitor and methotrexate in early rheumatoid arthritis. Curr Rheumatol Rep. 2014;16:417. doi: 10.1007/s11926-014-0417-8. [DOI] [PubMed] [Google Scholar]
- 62.Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–8. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pers YM, Jorgensen C. Perspectives of ofatumumab as CD20 targeted therapy in rheumatoid arthritis and other autoimmune diseases. Immunotherapy. 2016;8:1091–6. doi: 10.2217/imt-2016-0003. [DOI] [PubMed] [Google Scholar]
- 64.Engelhard M. Anti-CD20 Antibody treatment of Non-Hodgkin Lymphomas. Clin Immunol. 2016 doi: 10.1016/j.clim.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–9. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 66.Chueh AC, Tse JW, Togel L, et al. Mechanisms of Histone Deacetylase Inhibitor-Regulated Gene Expression in Cancer Cells. Antioxid Redox Signal. 2015;23:66–84. doi: 10.1089/ars.2014.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tropberger P, Mercier A, Robinson M, et al. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc Natl Acad Sci U S A. 2015;112:E5715–24. doi: 10.1073/pnas.1518090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–62. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 70.Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–57. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 71.Khokhar OS, Farhadi A, McGrail L, et al. Oncologists and hepatitis B: a survey to determine current level of awareness and practice of antiviral prophylaxis to prevent reactivation. Chemotherapy. 2009;55:69–75. doi: 10.1159/000183731. [DOI] [PubMed] [Google Scholar]
- 72.European Association For The Study Of The L. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol. 2015;33:2212–20. doi: 10.1200/JCO.2015.61.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saab S, Dong MH, Joseph TA, et al. Hepatitis B prophylaxis in patients undergoing chemotherapy for lymphoma: a decision analysis model. Hepatology. 2007;46:1049–56. doi: 10.1002/hep.21783. [DOI] [PubMed] [Google Scholar]
- 76.Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006;24:1003–16. doi: 10.1111/j.1365-2036.2006.03081.x. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521–30. doi: 10.1001/jama.2014.15704. [DOI] [PubMed] [Google Scholar]
- 78.Hilgendorf I, Loebermann M, Borchert K, et al. Tenofovir for treatment of hepatitis B virus reactivation in patients with chronic GVHD. Bone Marrow Transplant. 2011;46:1274–5. doi: 10.1038/bmt.2010.290. [DOI] [PubMed] [Google Scholar]
- 79.Chen WC, Cheng JS, Chiang PH, et al. A Comparison of Entecavir and Lamivudine for the Prophylaxis of Hepatitis B Virus Reactivation in Solid Tumor Patients Undergoing Systemic Cytotoxic Chemotherapy. PLoS One. 2015;10:e0131545. doi: 10.1371/journal.pone.0131545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–72. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 81.Ho EY, Yau T, Rousseau F, et al. Preemptive adefovir versus lamivudine for prevention of hepatitis B reactivation in chronic hepatitis B patients undergoing chemotherapy. Hepatol Int. 2015;9:224–30. doi: 10.1007/s12072-015-9612-6. [DOI] [PubMed] [Google Scholar]
- 82.Zhang MY, Zhu GQ, Shi KQ, et al. Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642–58. doi: 10.18632/oncotarget.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zurawska U, Hicks LK, Woo G, et al. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol. 2012;30:3167–73. doi: 10.1200/JCO.2011.40.7510. [DOI] [PubMed] [Google Scholar]
- 84.Wong WW, Hicks LK, Tu HA, et al. Hepatitis B virus screening before adjuvant chemotherapy in patients with early-stage breast cancer: a cost-effectiveness analysis. Breast Cancer Res Treat. 2015;151:639–52. doi: 10.1007/s10549-015-3382-7. [DOI] [PubMed] [Google Scholar]
- 85.Visram A, Chan KK, McGee P, et al. Poor recognition of risk factors for hepatitis B by physicians prescribing immunosuppressive therapy: a call for universal rather than risk-based screening. PLoS One. 2015;10:e0120749. doi: 10.1371/journal.pone.0120749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ceccarelli L, Salpini R, Sarmati L, et al. Late hepatitis B virus reactivation after lamivudine prophylaxis interruption in an anti-HBs-positive and anti-HBc-negative patient treated with rituximab-containing therapy. J Infect. 2012;65:180–3. doi: 10.1016/j.jinf.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Myers RP, Swain MG, Urbanski SJ, et al. Reactivation of hepatitis B e antigen-negative chronic hepatitis B in a bone marrow transplant recipient following lamivudine withdrawal. Can J Gastroenterol. 2001;15:599–603. doi: 10.1155/2001/378980. [DOI] [PubMed] [Google Scholar]