Abstract
BACKGROUND
Integrated chronic disease treatment models that enable patient self-care and shared treatment decision making have recently been shown to improve medication adherence and outcomes. Smartphone applications (apps) are a readily available means to enable this model, although sustained user engagement remains a challenge.
OBJECTIVE
To assess the efficacy of improving asthma control using a proactive smartphone app without required regular inputs.
METHODS
We designed a minimally intrusive smartphone app to provide individualized and timely support to patients with asthma based on the National Asthma Education and Prevention Program guidelines and Scripps management pathways. In this proof-of-concept study, we enrolled 60 adults with poorly controlled asthma to test the usability and effectiveness of this app over a 4-month period. The Asthma Control Test survey was used to assess control before, during, and after app use. As a corollary, a retrospective chart review was also used to assess changes in lung function and prescribed courses of systemic corticosteroids.
RESULTS
Our patients, with a mean age of 50 years, reported an improvement in Asthma Control Test scores from 16.6 (inadequate to poor) to 20.5 (controlled) over the study period. Concurrently, there was a 7.9% absolute increase in FEV1, while courses of systemic corticosteroids decreased from 0.5 to 0.3 courses per 6-month period. Fifty-eight of 60 patients completed the final survey, with high satisfaction reported.
CONCLUSIONS
This app improved asthma control in a cohort of patients with uncontrolled asthma (age range, 17–82 years), while minimizing burdensome inputs and proactively providing individualized teaching and treatment support. The app and treatment model are scalable to cost-effectively manage chronic disease.
Keywords: Asthma, Mobile health, Smartphone application, App, Self-care
Asthma affects 25 million Americans and is estimated to cost $3300 per patient each year in incremental medical expenses, missed work/school, and early deaths.1 Problems such as patient nonadherence to medications, failed trigger avoidance, and low patient expectations of control continue to be major barriers to improving outcomes. Studies on nonadherence have found that patients take only 30% to 70% of prescribed asthma medications.2 Reasons for poor medication adherence include mechanistic difficulties with use of inhaler devices, medication regimen complexity, and patients’ concern for adverse effects.2,3 Lack of knowledge in patients regarding the importance of adherence, avoidance of triggers, proper inhaler technique, and the reasoning behind medication recommendations contributes significantly to the problem of medication nonadherence and ultimately disease control.4 The National Asthma Education and Prevention Program recommends including patients in the development of a personalized treatment plan, increased time devoted to medication education, and positive reinforcement as ways to improve adherence and control.5 Furthermore, treatment models that include individualized treatment plans, shared decision making, patient education, and encouragement of self-care have been shown to improve patient adherence and outcomes.6–9
Although clinic visits are the primary platform in which physicians educate patients and adjust medication regimens, the time allotted to visits is relatively short compared with the time required for adequate discussion and education. Furthermore, studies show that 40% to 80% of the information provided by the physician during clinic visits is immediately forgotten, while 50% of what is remembered is remembered incorrectly.10 Meanwhile, patients have increasingly turned to the Internet, social media, and smartphone applications (apps) to answer many of their questions regarding asthma. As a reflection of this growing interest, there are roughly 200 asthma-related smartphone apps available on the iOS and Android platforms.11,12 Troubling, however, is the low quality and incorrect information found within many of these apps. A recent review found that 23 asthma apps offer guidance on management of acute symptoms, but only 4 (17%) are consistent with current guidelines. Likewise, only 3 (25%) of the 12 apps that offer guidance on proper inhaler technique are consistent with guidelines.11 Of the remaining apps, many lack personalization, put the burden on the patient to initiate usage of the app, are inconsistent with the specific recommendations of their provider, or lack patient engagement in self-care.11,12
Nonetheless, smartphone apps are a powerful tool to connect with and potentially influence the behavior of large numbers of patients. In the utilization of smartphone apps for asthma treatment, 3 highlighted target areas are self-monitoring of symptoms, medication adherence (both proper technique and avoiding missed doses), and avoidance of triggers.13 Although smartphone apps may address all these areas, there is an overemphasis on the use of medication reminders to improve adherence.14,15 A comprehensive approach targeting all 3 objectives is more likely to yield robust, long-lasting improvements in adherence and asthma outcomes. In this study, we describe a smartphone app emphasizing patient education and self-care with proactive, individualized “coaching” based on National Asthma Education and Prevention Program treatment guidelines.5
METHODS
Study design
A prospective single-arm, treatment-only, 4-month study design was used with clinical measures of asthma control compared before and after intervention. Patients with poorly controlled asthma in our large multispecialty clinic network were targeted for enrollment by query of the electronic medical record. Specifically, we identified clinic patients who had at least 2 events of urgent health care utilization over the past year (ie, unscheduled clinic or emergency department visits related to asthma). A total of 5334 letters were sent to patients via mail; of these, 160 patients responded and were directed to a Web site to review a description of the study. Patients were excluded if they did not have their own smartphone. The first 60 consenting patients were enrolled in this institutional review board—approved protocol. Those enrolled were provided with an access code and instructed to download the Scripps Asthma Coach smartphone app from the iOS or Android app store. Verbal instruction, physician encouragement, and app support were not required or provided to patients at any time during the study. Upon opening the app for the first time, patients were prompted to enter 3 pieces of information: access code, physician name, and current asthma medications. Patients then used the app for 4 consecutive months, with measurements of asthma control taken before, during, and after usage. Patients began the study period on a rolling basis from August to April, with 78% of the patients (n = 47) beginning in August or September and ending in winter. Compensation was provided for completion of 4 Asthma Control Test (ACT) surveys, but not for accessing other materials including educational and self-care content. Compensation was as follows: $50 for completing the first ACT survey, $25 for each of the second and third surveys, and $50 for the final survey.
Mobile app/intervention description
The smartphone app was designed by Scripps Clinic Division of Allergy, Asthma and Immunology using the URXmobile System platform. Patients interacted with the smartphone app when prompted by alerts and by patient-initiated actions such as information inquiries and data entry. Alerts included requests for self-assessment of asthma control, assessment of patient knowledge regarding asthma self-care, and resulting individualized coaching based on their previous entries. Self-assessment of asthma control used the ACT survey, which was completed twice during the study period. The ACT is a validated, disease-specific survey consisting of 5 questions and is recognized by the National Institutes of Health.16 The survey leverages patient-reported frequency of shortness of breath, nocturnal symptoms, use of rescue inhalers, overall feelings of disease control, and impact on ability to carry out daily activities over the last 4 weeks. Each question is scored on a scale of 1 to 5, with higher scores indicating better control. A composite score greater than 19 (green zone) indicates adequate asthma control, 16 to 19 indicates inadequate control (yellow zone), and less than 16 indicates poor control (red zone). Asthma control was assessed within the app, with patients immediately notified of their level of control as red, yellow, or green (Figure 1). Those with poor control were directed to relevant educational content based on their entries and previous interactions with the app. Separately, alerts queried patient knowledge with simple questions, such as “Do you know what triggers your asthma?”, “Do you take precautions to prevent catching a cold?”, and “Have you had an asthma flare-up and did not know why?” Similar questions were used to assess familiarity with types of asthma medications, inhaler technique, and management of acute symptoms. By these methods pertinent and timely educational materials were presented to patients.
FIGURE 1.
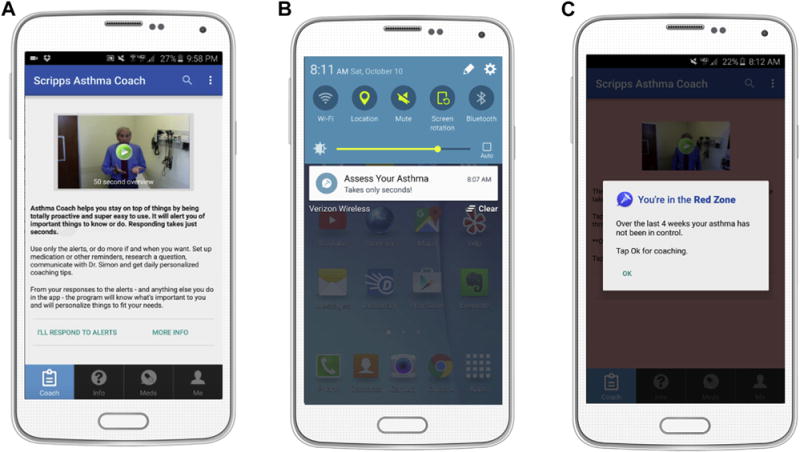
Screenshots demonstrating user engagement and self-assessment functionality. A, Embedded videos and accompanying text provide a user-friendly experience. B, Proactive alerts appear on the home screen and prompt the patient to actively engage in self-care and self-assessment. C, Assessment of asthma control is made using the ACT survey, with results reported as red, yellow, or green. Individualized coaching is provided on the basis of entries (Figure 2).
The app contains an extensive collection of evidence-based educational materials, which are available in both written format and videos. The educational materials are easily modifiable, and can be tailored to fit other treatment centers and disease models. In this study, the videos featured Scripps Clinic Allergists discussing asthma self-care and demonstrating correct usage of medication. A total of 48 unique videos were available to patients, with content including discussion of triggers, importance of medication adherence, the role of different medications, inhaler technique and troubleshooting, use of a peak flow meter, and management of acute symptoms (Figure 2). These materials were provided on demand, as a simple and readily available resource for virtually every aspect of asthma self-care.
FIGURE 2.
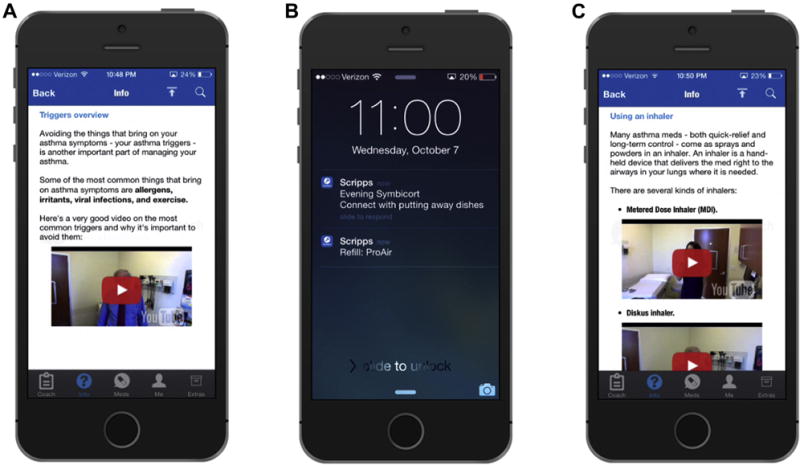
Screenshots demonstrating individualized coaching. “Coaching” is provided on the basis of recorded patient entries and inquiries as well as assessment of knowledge regarding asthma self-care. Patient data are continually collected and analyzed by the app to provide personalized content. Personalized content may include (A) discussions of triggers, (B) strategies to improve medication adherence such as coordinating with daily activities (dishwashing, showering, etc), and (C) overview of inhaler technique among other topics relevant to effective self-care.
The true innovation is the ability of the app to quickly provide personalized content in “real time.” Patient data are continually collected by the app, including ACT survey results, patient knowledge queries, usage of educational/instructional content, current medication list, and peak flow values. This information is compiled and analyzed dynamically to recognize worrisome trends in user data, thereby creating individualized interventions and proactive alerts based on National Asthma Education and Prevention Program treatment guidelines. A log of all patient interactions and guidance is recorded within the app, and available on the patient’s phone in a journal format that may be emailed to their provider if desired. Of note, alterations to the treatment plan agreed upon in clinic, such as changes in controller medications, were not made within the app. Instead, coaching encouraged avoidance of relevant triggers, adherence to the current treatment plan, and proper management of acute symptoms. Persistent symptoms that may require changes to the treatment plan would require a clinic visit. For example, patients who scored in the yellow (16–19) or red (<16) zone on the ACT survey received an alert the next day to ensure improvement in symptoms. If control remained poor, an alert provided the patient with the clinic telephone number and their treating physician’s email address. Clinic follow-up, however, was not mandated or recorded in the study.
Measured outcomes
The primary measured outcome was the ACT score after 4 months of app usage. Secondary end points included ACT scores after 5 and 10 weeks of app use. Clinical variables and other assessments of asthma control were obtained by a retrospective chart review. Specifically, we obtained forced expiratory volume in 1 second (FEV1) within 6 months before and after the enrollment date, when available. Prescribed courses of systemic corticosteroids before and after study enrollment were also elicited. An end-of-study survey was used to assess various aspects of the user experience.
Statistical calculations
Paired 2-tailed t tests were used to test for significant differences in continuous variables: ACT scores, number of prescribed courses of systemic steroids, and changes in the percent of predicted FEV1. Chi-square tests for categorical variables were used to test for significant differences in distribution of ACT categories (red, yellow, and green) and patients requiring systemic corticosteroids (yes/no variable). Correlation between change in ACT score and change in FEV1 was assessed by Spearman rank correlation coefficient in all patients with available prestudy and poststudy spirometry data. A multivariable linear regression model was used to evaluate body mass index, age, sex, and month of enrollment as independent predictors of a change in ACT scores.
RESULTS
Study population
A total of 60 patients with asthma were enrolled for the purpose of this study. Patients’ age ranged from 17 to 82 years, with a mean of 50 (standard error of mean, 2.2; Table I). Seventy-nine percent (30 of 38) of the patients with prior testing were noted to be atopic, with the criteria being sensitization to a least 1 common aeroallergen identified by skin prick testing or specific immunoglobulin E (IgE) testing. Sixty-five percent (n = 39) of the patients were treated with a combination inhaled corticosteroid and long-acting beta-agonist at the time of study enrollment. Furthermore, 95% of the patients were treated with either an inhaled corticosteroid and long-acting beta-agonist or inhaled corticosteroid alone for maintenance therapy. Only 3 patients were “off” maintenance inhalers at study enrollment, with 2 of the 3 refusing these medications. Forty-four percent (16 of 34) of the patients with available prestudy spirometry had an FEV1 of 80% predicted or less in the 6 months preceding enrollment. Forty percent (n = 24) of the patients had been prescribed at least 1 course of systemic corticosteroids in the 6 months preceding enrollment. ACT scores obtained at the time of enrollment revealed a mean score of 16.6. Forty percent (n = 24) of the patients recorded a score of less than 16.
TABLE I.
Clinical characteristics of the study population
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Women | 41 (68) |
| Men | 19 (32) |
| Age (y) | |
| Mean (SD; SEM) | 50.1 (17.0; 2.2) |
| Median (min; max) | 51.5 (17; 82) |
| BMI (kg/m2), mean | 30.0 |
| Race/ethnicity, n (%) | |
| White/Non-Hispanic | 50 (83) |
| White/Hispanic | 4 (7) |
| Asian | 4 (7) |
| Other | 2 (3) |
| Atopic by skin testing or specific IgE, n (%)* | |
| Yes | 30 (79) |
| No | 8 (21) |
| Maintenance inhaler at study enrollment, n (%) | |
| ICS | 18 (30) |
| ICS/LABA | 39 (65) |
| None or refused | 3 (5) |
| FEV1 % predicted within 6 mo of study enrollment, n (%)* | |
| <60% predicted | 5 (14) |
| 60%–80% predicted | 11 (31) |
| >80% predicted | 19 (54) |
| Patients receiving systemic corticosteroids in the last | 24 (40) |
| 6 mo before enrollment, n (%) | |
| ACT score at study enrollment | |
| Mean ± SD | 16.6 ± 4.6 |
| Median (min; max) | 17 (5; 24) |
| Red: score <16, n (%) | 24 (40) |
| Yellow: score 16–19, n (%) | 17 (28) |
| Green: score >19, n (%) | 19 (32) |
BMI, Body mass index; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; IgE, immunoglobulin E; LABA, long-acting beta-agonist; SEM, standard error of mean.
Atopy status and spirometry results were not available by retrospective chart review for the entire study population.
Primary end point—ACT scores
Only 32% (n = 19) of the patients were noted to have an ACT score in the green zone (>19) at the time of study enrollment. This percentage of patients with asthma “well-controlled” increased consistently throughout the study period to 59% at 5 weeks, 69% at 10 weeks, and 78% (n = 45, all P < .0001) at the 16-week final survey (Figure 3, A). Of the patients with ACT scores in the red or yellow zone (≤19) at the time of study enrollment, 72% (P < .0001) achieved good control (>19) at study completion (Figure 3, B).
FIGURE 3.
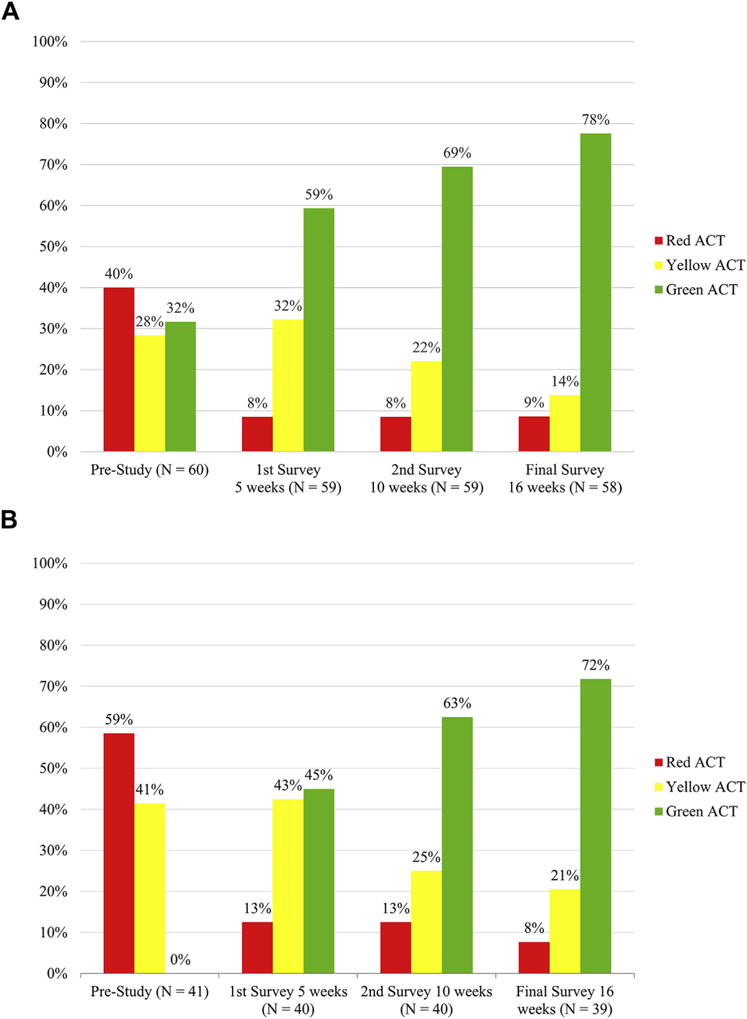
Distribution of ACTscores throughout the study period. A, ACTscore distribution for all patients is depicted, with notable trend toward well-controlled asthma. B, ACTscore distribution of patients beginning in the red or yellow zone is depicted; 72% of these patients achieved a final ACT score of 20 or more despite “poorly controlled” or “not well controlled” asthma at the time of study enrollment. *Key: Green, >19; yellow, =16–19; red, <16.
The mean ACT score increased by 3 points (P < .0001) after only 5 weeks of app use, with an overall increase of 3.9 points (P < .0001) noted at study conclusion (Figure 4). The magnitude of increase was greater in those patients who were “not well controlled” (ACT score ≤19) at the time of enrollment. In this population, ACT scores increased by 5.7 (P < .0001) from 14.3 (red zone) to 20.1 (green zone).
FIGURE 4.
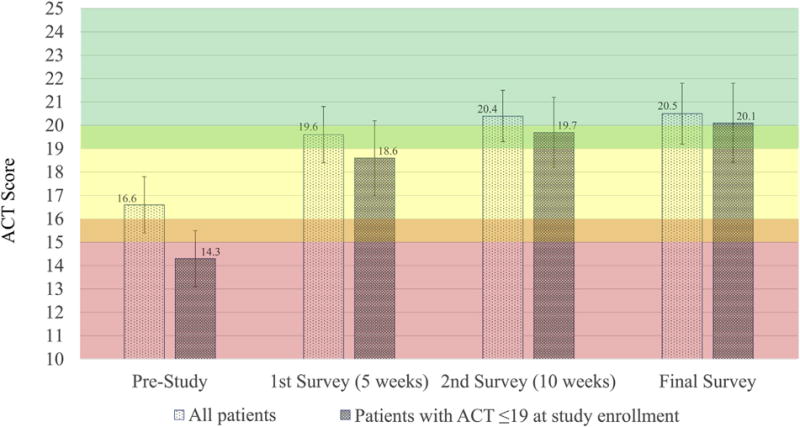
Mean ACTscores throughout the study period. ACTscores with 95% CIs are represented, with a consistent positive trend. A mean increase of 3.9 and 5.7 (both P <.05) points was noted across all patients and those beginning the study with an ACTscore of 19 or less, respectively.
Multivariable analysis showed no association between ACT outcomes and age, sex, and month of enrollment. Higher body mass index, however, was associated with greater improvement in ACT scores over the study period (see Table E1 in this article’s Online Repository at www.jaci-inpractice.org).
Secondary end points—Spirometry and courses of systemic steroids
There was a nonsignificant decrease in the number of patients requiring systemic corticosteroids when comparing the 6 months before versus 6 months after study enrollment: 24 patients (40%) versus 17 patients (28%) (P = .065). There was, however, a statistically significant decrease in the number of courses of systemic steroids per patient from 0.5 to 0.3 (P = .046).
Seventeen of the 60 patients had recorded results of spirometry within 6 months both before and after study enrollment, which was available for review in our electronic medical record. Within this population, a mean increase of 7.9 was noted in the FEV1 percent predicted (P = .03). Positive correlation (rs = 0.44) was noted between changes in ACT scores and changes in FEV1.
User experience, engagement, and feedback
All 60 patients completed the prestudy ACT, 59 patients (98%) completed the second and third surveys, and 58 (97%) completed the final survey. Of the 2 patients who did not complete the final survey, both had reported improvement in their ACT scores before the final survey. Patients received on average 2 to 3 proactive alerts per week. Six months after the study began, 43 (72%) patients continued to use the mobile app. Because of staggered enrollment of patients in the study, 9 of these patients had simply not completed the 4-month study period. Nonetheless, 34 (67%) of the remaining 51 patients had completed the study and continued to use the app without encouragement, further compensation, or enhancements to the app.
The end-of-study survey was used to assess overall user experience and satisfaction with the app. Patients were asked to rate their degree of agreement or disagreement with various statements. A score of 10 indicated strong agreement, whereas a score of 0 indicated strong disagreement. In response to the statement “Using the app was easy,” patients scored the app a mean of 9.3 (Figure 5). In response to the statements “The overall app experience felt relevant/personalized to my asthma” and “The app helped me manage my asthma more effectively,” mean scores were 7.9 and 7.4, respectively.
FIGURE 5.
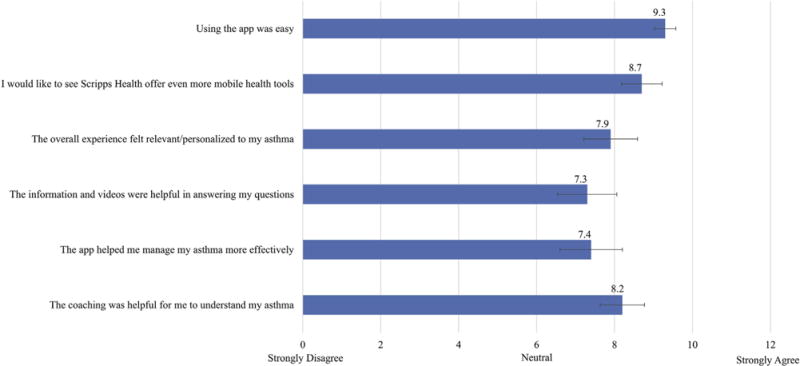
Patient satisfaction and user engagement. After 4 months of using Scripps Asthma Coach, patients were asked to rate their level of agreement or disagreement with various aspects of their experience with the app. Fifty-eight of 60 patients completed the survey. Responses indicated that patients found the app easy-to-use, personalized, and helpful in managing their asthma. 95% CIs are included.
DISCUSSION
Although cognizant of the limitations in this proof-of-concept, quasi-experimental study, the use of a minimally burdensome and proactive mobile app was associated with improvement in asthma control in 60 adults with previously uncontrolled asthma over a 4-month study period. Use of the app was associated with a statistically and clinically significant improvement in ACT scores. On retrospective chart review, we found a statistically significant improvement in FEV1, although the number of patients with before and after spirometry data was limited (~30%). There was also an association with a significant decrease in the total number of systemic corticosteroid prescribed, though the decrease in the number of patients receiving systemic corticosteroids did not reach statistical significance. This may reflect that the most poorly controlled patients required most courses. Our app is novel in its design aimed at limiting user burden while extending relevant physician education material through a range of instructional videos. In the end-of-study survey, patients reported that the app was easy to use and helped them manage their asthma more effectively.
There is growing interest among patients, caregivers, and stakeholders in the adaption of mobile technology to assist in the management of chronic medical diseases. Patient interest is evidenced by the rising number of available and downloaded asthma-related smartphone apps. Younger patients have been noted to voice preference for mobile asthma resources over similar offerings on paper.17 Our study demonstrates that interest in engaging in self-care through mobile technology is not unique to younger patients, but also applies to older adults. The mean age of our subject cohort was 50 years, with age ranging from 17 to 82 years.
The efficacy of using mobile technology to manage asthma has already been demonstrated, though methods and outcomes have varied significantly. Early efforts focused on improving medication adherence by using short messaging service (SMS) text messages to remind patients to take medications as scheduled. A nearly 20% absolute increase in medication adherence has been observed with the use of SMS medication reminders.14,18 The impact on objectively measured asthma outcomes such as spirometry and urgent health care utilization has been less clear.19 One study showed that SMS medication reminders can improve patient-perceived control of asthma, although no significant changes in FEV1 or emergency department visits were concomitantly observed.14
More recently, medication reminders have evolved from these scheduled SMS text messages to smartphone apps. App-based medication reminders have been used to encourage medication adherence in an inner-city, adolescent African American population with poorly controlled asthma. This app used monetary awards to encourage medication adherence among inner-city youth. Results again showed improved medication adherence as well as an increase in ACT scores.15 Certainly, this is a meaningful result and demonstrates proof-of-principle in a challenging patient population.
Finally, several studies have attempted to address patient nonadherence with the use of mobile patient treatment plans.7,17,20,21 These treatment plan apps typically require some form of regular patient input, such as reported respiratory symptoms, medication use, and peak expiratory flow measurements. Basic treatment plan recommendations are then based on these entries. The largest of these studies unfortunately did not show statistical difference when comparing their mobile treatment plan to the same algorithm on paper.20 It should be noted that this particular app did not provide comprehensive, proactive, and individualized recommendations. Importantly, recent mobile apps using a more adaptive and individualized treatment model have successfully demonstrated a significant improvement in asthma outcomes.7,17,21 Scripps Asthma Coach builds on the success of these apps by providing personalized self-care support without the need for daily patient inputs.
Evidence indicates that user engagement and use of smartphone apps for self-care tends to decrease with time.22,23 Many users report achieving mastery of self-management content described within prior apps.22 Others report feeling burdened by intrusive alerts, repetitive content, or faulty technical features.22 Decreasing user engagement due to mastery of the app content is less concerning, and likely translates into an enduring improvement in asthma knowledge. The problem of perceived burden may be addressed through design alterations. Before building the Scripps Asthma Coach app, we hypothesized that user engagement could be better achieved through a reduction in repetitive content and elimination of burdensome inputs. The app was designed to provide timely and individualized content without the need for daily user inputs. As a result, adherence and user engagement with our app remained strong throughout the study period. Fifty-eight of 60 patients completed the final ACT survey. Although the compensation provided certainly impacted adherence to our surveys, user engagement continued beyond the study period, with two-third of the patients recording ongoing use despite no further monetary incentives.
Scripps Asthma Coach resulted in an increase of 3 points in the mean ACT score after only 5 weeks of app use, with continued increase of 3.9 points at study completion. Those with uncontrolled asthma (ACT score <20) benefited the most, with a mean increase of 5.7 points. In comparison, Burbank et al21 observed an increase of 2 points (from 16 to 18) in the median ACT score with their mobile asthma action plan targeted at adolescents.21 Mosnaim et al15 observed an increase of 3 points in the ACT score in 58% of their inner-city adolescent patients. It is certainly worth noting that demographic characteristics of patients in our study differed substantially in terms of socioeconomic status, age, and asthma phenotypes. This makes a direct comparison in outcomes difficult to interpret. Instead, our study emphasizes the extension of a mobile asthma app to manage a wide age range of patients including older adults, a population often thought to be less responsive to the adoption of new technologies.
Limitations of the study include underrepresentation of minorities, the uninsured, and those who might be less motivated to use a smartphone app, which may hinder generalizability to other populations. The low patient enrollment rate may reflect poor response to paper mailings, disinterest in experimental studies, or varying interest in mobile health technology. Spirometry results were not available by retrospective chart review for all patients (~30%). Similarly, courses of systemic corticosteroids were counted only if prescribed within the Scripps Clinic electronic medical record. The relatively short study period limits analysis of long-term user engagement and durability of improved outcomes. Assessment of seasonal variability is also limited by the 4-month study period though much of the calendar year was represented because of staggered enrollment. Most patients began the study during the late summer and ended in the winter months. Multivariate analysis did not reveal outcome differences based on month of enrollment. Although inclusion criteria required recent asthma-related urgent health care utilization (2 visits over the last year), it is possible but unlikely that patients were in acute exacerbation at the time of their first ACT survey. Urgent health care visits could have occurred at any time over the last year, and several time barriers separated candidate identification from their first ACT survey (ie, paper mailing, candidate response, and enrollment). Last, our study used a single-arm, treatment-only design. The confounding effects of repeated completion of the ACT surveys, compensation, and any observer-expectancy bias can therefore not be determined. A new trial featuring a larger, more diverse population and prospective collection of additional outcome data is currently planned to further evaluate and prove the clinical utility of this new model and technology.
Overall, our study adds to the growing body of evidence that indicates that smartphone apps are an effective means to improve asthma control when used continually. Adding to the appeal, mobile apps such as the Scripps Asthma Coach are highly scalable, and therefore a cost-effective means to reach a large number of patients. Our approach is novel in that it purposefully limits burdensome patient inputs and avoids repetitive content. Our survey results indicate that this minimally intrusive model is associated with high patient satisfaction. We believe that this model will help sustain user engagement, and is adaptable to treat any number of chronic diseases.
What is already known about this topic?
Smartphone applications (apps) have been shown to improve asthma control, particularly among urban youth. These apps have typically included medication reminders and required regular input of information, which may lead to a decrease in sustained use.
What does this article add to our knowledge?
In a population of patients with poorly controlled asthma with broad age range from 17 to 82 years, individualized self-care and treatment support delivered proactively via a smartphone app improved asthma outcomes without the need for regular inputs.
How does this study impact current management guidelines?
Smartphone apps provide a cost-effective, easily scalable, and efficacious way to improve asthma outcomes in varied patient populations with minimal burden on the user.
Acknowledgments
Funding was provided by a research grant from Scripps Clinic Medical Group. The smartphone application was designed by Scripps Clinic, Division of Allergy, Asthma and Immunology in conjunction with URXmobile System, a company that has a commercial interest in the design and implementation of mobile self-care apps.
B. D. Modena has received research support from the National Institutes of Health (CTSA KL2 TR001112 grant). R. A. Simon has received lecture fees from Merck and Novartis and has a small (<5%) equity position in URXmobile, the company that developed the technology behind the application described in the article.
Abbreviations
- ACT
Asthma Control Test
- App
application
- FEV1
forced expiratory volume in 1 second
- IgE
immunoglobulin E
- SMS
short messaging service
Footnotes
Conflicts of interest: K. A. Cook declares no relevant conflicts of interest.
References
- 1.Centers for Disease Control and prevention. Asthma. Available from: www.cdc.gov/nchs.fastats/asthma.htm. Accessed March 6, 2015.
- 2.Eakin MN, Rand CS. Improving patient adherence with asthma self-management practices: what works? Ann Allergy Asthma Immunol. 2012;109:90–2. doi: 10.1016/j.anai.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25:107–30. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Apter AJ, Wan F, Reisine S, Bender B, Rand C, Bogen DK, et al. The association of health literacy with adherence and outcomes in moderate-severe asthma. J Allergy Clin Immunol. 2013;132:321–7. doi: 10.1016/j.jaci.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Available from: http://www.ncbi.nlm.nih.gov/books/NBK7232. Accessed September 30, 2015. [PubMed]
- 6.Chen SY, Sheu S, Chang CS, Wang TH, Huang MS. The effects of the self-efficacy method on adult asthmatic patient self-care behavior. J Nurs Res. 2010;18:266–74. doi: 10.1097/NRJ.0b013e3181fbe33f. [DOI] [PubMed] [Google Scholar]
- 7.Licskai C, Sands TW, Ferrone M. Development and pilot testing of a mobile health solution for asthma self-management: asthma action plan smartphone application pilot study. Can Respir J. 2013;20:301–6. doi: 10.1155/2013/906710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janson SL, McGrath KW, Covington JK, Cheng SC, Boushey HA. Individualized asthma self-management improves medication adherence and markers of asthma control. J Allergy Clin Immunol. 2009;123:840–6. doi: 10.1016/j.jaci.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Better Outcomes of Asthma Treatment (BOAT) Study Group Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–77. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessels RP. Patients’ memory for medical information. J R Soc Med. 2003;96:219–22. doi: 10.1258/jrsm.96.5.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huckvale K, Morrison C, Ouyang J, Ghaghda A, Car J. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med. 2015;13:58. doi: 10.1186/s12916-015-0303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu AC, Carpenter JF, Himes BE. Mobile health applications for asthma. J Allergy Clin Immunol Pract. 2015;3:446–8. e441–16. doi: 10.1016/j.jaip.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chronic physical illness. self-management and behavioural interventions. Int J Integrated Care (IJIC) 2009;9:3–4. [Google Scholar]
- 14.Lv Y, Zhao H, Liang Z, Dong H, Liu L, Zhang D, et al. A mobile phone short message service improves perceived control of asthma: a randomized controlled trial. Telemed J E Health. 2012;18:420–6. doi: 10.1089/tmj.2011.0218. [DOI] [PubMed] [Google Scholar]
- 15.Mosnaim G, Li H, Martin M, Richardson D, Belice PJ, Avery E, et al. A tailored mobile health intervention to improve adherence and asthma control in minority adolescents. J Allergy Clin Immunol Pract. 2015;3:288–290. e281. doi: 10.1016/j.jaip.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Farooqui N, Phillips G, Barrett C, Stukus D. Acceptability of an interactive asthma management mobile health application for children and adolescents. Ann Allergy Asthma Immunol. 2015;114:527–9. doi: 10.1016/j.anai.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. Respir Med. 2010;104:166–71. doi: 10.1016/j.rmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Tran N, Coffman JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma. 2014;51:536–43. doi: 10.3109/02770903.2014.888572. [DOI] [PubMed] [Google Scholar]
- 20.Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ. 2012;344:e1756. doi: 10.1136/bmj.e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burbank AJ, Lewis SD, Hewes M, Schellhase DE, Rettiganti M, Hall-Barrow J, et al. Mobile-based asthma action plans for adolescents. J Asthma. 2015;52:583–6. doi: 10.3109/02770903.2014.995307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatara N, Arsand E, Skrovseth SO, Hartvigsen G. Long-term engagement with a mobile self-management system for people with type 2 diabetes. JMIR Mhealth Uhealth. 2013;1:e1. doi: 10.2196/mhealth.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. 2013;15:e235. doi: 10.2196/jmir.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]


