Abstract
Introduction
Internet gaming disorder (IGD) and alcohol dependence (AD) have been reported to share clinical characteristics including craving and over-engagement despite negative consequences. However, there are also clinical factors that differ between individuals with IGD and those with AD in terms of chemical intoxication, prevalence age, and visual and auditory stimulation.
Methods
We assessed brain functional connectivity within the prefrontal, striatum, and temporal lobe in 15 patients with IGD and in 16 patients with AD. Symptoms of depression, anxiety, and the attention deficit hyperactivity disorder were assessed in patients with IGD and in patients with AD.
Results
Both AD and IGD subjects have positive functional connectivity between the dorsolateral prefrontal cortex (DLPFC), cingulate, and cerebellum. In addition, both groups have negative functional connectivity between the DLPFC and the orbitofrontal cortex. However, the AD subjects have positive functional connectivity between the DLPFC, temporal lobe and striatal areas while IGD subjects have negative functional connectivity between the DLPFC, temporal lobe and striatal areas.
Conclusions
AD and IGD subjects may share deficits in executive function, including problems with self-control and adaptive responding. However, the negative connectivity between the DLPFC and the striatal areas in IGD subjects, different from the connectivity observed in AD subjects, may be due to the earlier prevalence age, different comorbid diseases as well as visual and auditory stimulation.
Keywords: Alcohol dependence, Internet gaming disorder, Brain connectivity, Immaturity
1. Introduction
1.1. Clinical characteristics of internet gaming disorder and alcohol dependence
Clinical studies of internet addiction have suggested the following diagnostic criteria: time spent longer than initially intended and planned, time distortion, compulsive behaviors, failure of stopping or controlling use, deception about extent of use, utilization of the internet activity to cope with or escape problems, and preoccupation with internet use when offline (Atmaca, 2007; Shapira et al., 2003; Young, 1996). More specifically, DSM-V also suggested internet gaming disorder in the section for future study (American Psychiatric Association, 2013). The clinical characteristics of internet gaming disorder (IGD) include craving and over-engagement despite negative consequences. Many of these behavioral symptoms are shared with those observed in patients with alcohol dependence (AD) (Karim & Chaudhri, 2012). The craving induced by alcohol or other substances has been closely associated with activity of the dorsolateral prefrontal cortex (DLPFC) (George et al., 2001). The DLPFC is also thought to play important roles in mediating the clinical symptoms of alcohol dependence, including impulsivity, aggravation of abuse potential, and executive dysfunction (Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014). Deficits of DLPFC function have been reported in subjects with IGD (Han, Kim, Lee, Min, & Renshaw, 2010; Ko et al., 2009). For example, brain activity within the DLPFC has also been positively correlated with craving in response to online game cues in subjects with IGD (Ko et al., 2009).
However, there are also clinical characteristics that differ between individuals with IGD and those with AD. First, IGD does not involve chemical intoxication or physical withdrawal as is the case with alcohol, nicotine, and drug use disorders (Grant, Potenza, Weinstein, & Gorelick, 2011). Second, the main sensory system inputs in response to internet game play are the result of visual and auditory attention (Dong, Huang, & Du, 2012). Extreme internet game play may cause visual acuity loss or hearing problems (Bovo, Ciorba, & Martini, 2011; DellaCroce & Vitale, 2008). Long term game play in pro-gamers has been correlated with the increased cortical volume within the parietal cortex which might be related to increased visuo-spatial attention (Hyun et al., 2013; Song, Han, & Shim, 2013). For these reasons, we believe that internet game play may change activity within brain regions which are related to visual and auditory stimulation. Third, demographic cohort studies of alcohol dependence report a wide range of ages from childhood to old adults (Peltzer & Phaswana-Mafuya, 2013). In a USA national longitudinal study of adolescent health, life time alcohol dependence reaches a peak age of onset at 23 years (Haberstick et al., 2014). However, few studies report cases of internet gaming disorder in patients over 40 years of age (Choi et al., 2009; Lee, Han, Kim, & Renshaw, 2013; Whang, Lee, & Chang, 2003). In a survey of 908 Dutch adolescents and adults, with ages ranging from 14 to 80, the most vulnerable period for online game addiction was adolescence (Haagsma, Pieterse, & Peters, 2012). For these reasons, some investigators regard IGD as an impulse control disorder (Beard & Wolf, 2001). Other investigators regard it as a behavioral addiction (Grant et al., 2011).
1.2. Brain connectivity in internet gaming disorder vs alcohol dependence
Several cue-induced functional magnetic resonance imaging studies of internet gaming disorder have noted that brain regions which activate in response to online game cues in patients with online game addiction are similar to those observed following alcohol cue presentation in patients with alcohol dependence (Han et al., 2010; Ko et al., 2009; Leeman & Potenza, 2013). Corticostriatal tracts, including the dorsolateral prefrontal cortex, limbic lobe, and striatal areas are thought to be candidate regions that facilitate craving for maintaining alcohol in patients with alcohol dependence (Filbey et al., 2008; Lopez, Akil, & Watson, 1999). Interestingly, the same tract has been reported to activate in patients with online game addiction (Han et al., 2010; Ko et al., 2009).
However, recent studies using resting state fMRI have also noted differences in brain connectivity between individuals with AD and those with IGD. Resting state functional MRI has been used to assess the integration and connectivity of neural activities during the resting state (Jiang et al., 2008). Functional connectivity refers to the temporal correlation of a neurophysiological index (signal synchronicity of low frequency fluctuation activity) measured among different brain areas which are consistent with intrinsic brain network organization or network dysfunction (Biswal, Yetkin, Haughton, & Hyde, 1995; Friston, Frith, Liddle, & Frackowiak, 1993). Positive connection refers to positive correlations between functionally related brain regions and negative connections indicate negative correlations between brain regions with opposing functional roles (Greicius, Krasnow, Reiss, & Menon, 2003). Strong (more) connectivity between regions A and B indicates that both regions A and B are activated simultaneously (Biswal et al., 1995; Friston et al., 1993). Seed base analysis requires an a priori a region which is selected to serve as a seed, from which to evaluate connectivity with other brain regions (Biswal et al., 2010). Recently, this technique has been used to detect abnormal functional integration in individuals with a range of psychiatric disorders (Deco, Jirsa, & McIntosh, 2011). Khalili-Mahani et al. (2012) have reported that activity within the medial frontal cortex, dorsolateral prefrontal cortex, parietal lobe, temporal lobe, and cerebellum was more connected as a result of alcohol intake. In patients addicted to different drugs (heroin), increased functional connectivity between nucleus accumbens, ventral/rostal anterior cingulate gyrus, amygdala, and orbitofrontal cortex has been as observed (Ma et al., 2010). In the case of internet gaming disorder, patients with IGD showed enhanced functional connectivity in the brainstem, inferior parietal lobule, left posterior cerebellum, and left middle frontal gyrus, relative to healthy control subjects (Dong et al., 2012). In addition, IGD subjects showed decreased functional connectivity in the temporal, occipital and parietal brain regions (Dong et al., 2012). Because the DLPFC is thought to mediate clinical symptoms of AD and IGD (Han et al., 2010; Jasinska et al., 2014; Ko et al., 2009), we selected the DLPFC as a seed region to assess functional connectivity within corticostriatal–limbic tracts in AD and IGD.
1.3. Hypothesis
Based on previous studies of alcohol dependence and internet gaming disorder, deficits of DLPFC function have been commonly observed in individuals with either disorder. However, no study has directly compared AD subjects and IGD subjects, in part due to differences in the prevalence age. In this direct comparison of AD subjects and IGD subjects, we hypothesized that there would be differential brain functional connectivity from the DLPFC to the striatum and temporal lobe between AD subjects and IGD subjects.
2. Methods
2.1. Subjects
Among 303 patients who visited the online game clinic and research center and agreed to participate in a fMRI research study, 20 internet gaming disorder (IGD) inpatients in their thirties were recruited. Twenty male patients approximately of the same age with alcohol dependence (AD) also agreed to participate in our research. The inclusion criteria for IGD were as follows: (1) The criteria for internet gaming disorder in the current study as suggested by the research criteria in DSM-V (American Psychiatric Association, 2013). In addition, more conditions were added: (2) individuals in their thirties; (3) online game play time ≥ 4 h per day/30 h per week; and (4) Young Internet Addiction Scale scores > 50. The inclusion criteria for alcohol dependence include (1) individuals who met the criteria for alcohol dependence (American Psychiatric Association, 2013); (2) individuals in their thirties; (3) individuals with a Michigan alcohol screening test (MAST) score of > 19 for alcohol problems; and (4) individuals with impaired behaviors or distress due to maladaptive patterns. The exclusion criteria for internet gaming disorder and alcohol dependence include (1) patients with history or current episode of other Axis I psychiatric diseases; (2) patients with other substance abuse history (except for tobacco); (3) patients with medical illness; and (4) patients with claustrophobia. Three AD subjects and three IGD subjects were excluded due to comorbidity with major depressive disorder. One AD subject and one IGD subject were excluded due to comorbidity with ADHD. One IGD subject failed to complete the fMRI scan due to claustrophobia. Finally, 15 IGD inpatients and 16 AD inpatients completed the research protocol. The Chung Ang University Hospital Institutional Review Board approved the research protocol for this study. Written informed consent was provided by all participants.
2.2. Study procedure
During the detox period, both AD subjects and IGD subjects were hospitalized to ensure alcohol or internet game playing abstinence. On hospital days 10–12, fMRI scanning was performed in both AD subjects and IGD subjects. Over a period of 5–10 days, patients with alcohol dependence were detoxified with lorazepam, thiamine, and multivitamins in accordance with published protocols (Asplund, Aaronson, & Aaronson, 2004). After detoxification, craving for alcohol, the severity of internet addiction, symptoms of depression, anxiety, and attention deficit hyperactivity disorder (ADHD) and severity of clinical global status (CGI-s) were assessed with the Young Internet Addiction Scale (YIAS) (Young, 1996), the Korean Alcohol Urge Questionnaire (AUQ-K) (Kim et al., 2008), the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), Beck Anxiety Inventory (BAI) (Beck, Epstein, Brown, & Steer, 1988), and the adult ADHD rating scale (K-AADHD) (Kim, 2003), respectively. A BDI score >21 is “suggestive of clinical depression” and BAI scores >22 reflect an anxiety state of sufficient severity to merit clinical assessment. Both IGD and AD subjects were allowed to use lorazepam for insomnia and anxiety. However, 24 h before the time of scan, lorazepam was not permitted.
2.3. Acquisition and processing of image data
All imaging data were acquired using a 1.5 Tesla Espree MRI scanner (SIEMENS, Erlangen, Germany). Functional image parameters included a gradient-echo planar sequence sensitive to blood oxygen level-dependent (BOLD) contrast (repetition time (TR) = 2000 ms, echo time (TE) = 50 ms, and flip angle = 90°), whole brain volumes with 5.0 mm-thick transverse slices, a voxel size of 3.5 × 3.5 × 5.0 mm, no interslice gap, and in-plane resolution = 64 × 64 pixels. During neuronal activation, oxygen is consumed. Hemoglobin delivers oxygen to activated neurons. The MRI scanner detects the differences of magnetic susceptibility (magnetic signal variation) between oxyhemoglobin and deoxyhemoglobin (Biswal et al., 1995; Friston et al., 1993). This difference has been termed BOLD for blood-oxygen-level dependent contrast. Patients lay still in the scanner with their eyes closed but remained awake. For anatomical imaging, 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) data were collected with the following parameters: TR = 1500 ms, TE = 3.00 ms, FOV = 256 × 256 mm, 128 slices, and 1.0 × 1.0 × 1.33 mm voxel size. Images were realigned to Talairach space using the anterior and posterior commissures and the sagittal sulcus plane.
Functional images were analyzed using Brain Voyager software (BVQX 1.9; Brain Innovation, Maastricht, Netherlands). During preprocessing, the fMRI data were co-registered to the anatomical 3D data sets for each participant using the provided multi-scale algorithm. Individual 3D structural images were spatially normalized to standard Talairach space (Talairach & Tournoux, 1988). A nonlinear transformation was applied to the T2*-weighted fMRI time series data. Slice scan time and 3D motion correction were applied and the functional data were spatially smoothed using a Gaussian kernel with a full width at half maximum (FWHM) of 6 mm and temporally filtered using linear trend removal and Fourier analysis high pass filtering with a cutoff of 3 cycles of the full time course. Functional connectivity between the DLPFC and all other regions of the brain was assessed using BrainVoyager QX and by drawing a cube of 10 mm on each side, centered on the DLPFC using Talairach coordinates (0, 49, 31) (Fig. 1). This procedure requires subjects to be excluded from fMRI analysis when translations are larger than 3 mm in the x, y or z dimensions or when rotations are larger than 3° around the x, y or z axes (attributed to excessive head movement). No subjects were excluded from analysis based on these criteria.
Fig. 1.
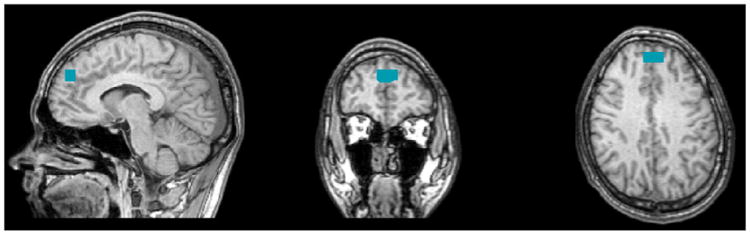
Region of seed. After putting a cursor at Talairach code (0, 49, 31), a cube of 10 mm has moved on each side of X axis from 0 (center) to +5 (right side) and from 0 (center) to −5 (left side).
2.4. Statistical analysis
Differences in demographic data including age, education, CGI-s BDI scores, BAI scores, K-AADHD scores, AUQ-K scores, and YIAS scores between AD subjects and IGD subjects were compared using the Mann–Whitney U test. The average time course of the blood oxygen level-dependent (BOLD) signal was extracted from the DLPFC seed region and used as the model predictor in a general linear model analysis (GLM) to determine brain regions temporally correlated with it in the form of individual subject statistical parametric maps (SPM) of temporal correlation coefficients (β-values). No other predictors were used in the subject GLM analysis. A higher-level multi-subject random effects analysis (RFX) GLM was applied to generate and compare group SPMs of functional connectivity with the DLPFC. A post-hoc region of interest analysis calculated average beta-values using a mask of the significantly higher functional connectivity in IGD vs. AD in the superior temporal gyrus. As a second-level analysis in subjects with AD and IGD subjects, the correlations between mean β values in the frontal, cingulate gyrus, temporal, insular, and nucleus accumbens and mean clinical rating scale scores were analyzed using partial correlations controlling for age. To correct for multiple comparisons, statistical significance was set at p < 0.005 (0.05/10, 5 clinical scales + 5 regions).
3. Results
3.1. Demographic data
For age and sex matching between IGD and AD, male patients in their 30s were recruited. However, we failed to completely match the age within the two groups. The mean age in the AD (35.1 ± 2.4) cohort was higher than that in the IGD cohort (31.6 ± 1.5) (z = 3.86, p < 0.01). The Korean Alcohol Urge Questionnaire (AUQ-K) score in AD subjects (22.9 ± 6.6) was higher than that in IGD subjects (10.6 ± 4.2) (z = 4.39, p < 0.01). The Young Internet Addiction Scale (YIAS) score in IGD individuals (63.7 ± 26.6) was higher than that in AD individuals (35.4 ± 6.8) (z = −2.28, p = 0.02). However, there were no significant differences in sex, education years, Clinical Global Index-severity (CGI-s), BDI scores, BAI scores, adult ADHD rating scale scores and smoking between the two groups (Table 1).
Table 1.
Demographic characteristics in AD subjects and IGD subjects.
| Alcohol patients (15) | IGD patients (16) | Statistics | |
|---|---|---|---|
| Sex (male/female) | 15/0 | 16/0 | – |
| Age | 35.1 ± 2.4 | 31.6 ± 1.5 | z = 3.86, p < 0.01* |
| Education years | 12.1 ± 2.5 | 12.3 ±1.6 | z = −0.53, p = 0.61 |
| CGI-s | 4.4 ± 0.8 | 4.2 ± 0.9 | z = 0.82, p = 0.41 |
| BDI score | 14.1 ± 7.8 | 10.6 ± 5.4 | z = 1.21, p = 0.22 |
| BAI | 6.8 ± 3.3 | 6.4 ±3.4 | z = 0.39, p = 0.69 |
| K-AADHDS | 10.4 ± 5.8 | 10.6 ± 4.6 | z = −0.21, p = 0.82 |
| AUQ-K score | 22.9 ± 6.6 | 10.6 ± 4.2 | z = 4.39, p < 0.01* |
| YIAS score | 35.4 ± 6.8 | 65.9 ± 15.4 | z = −4.45, p < 0.01* |
| Smoking (smk/n-smk) | 12/3 | 9/7 | χ2 = 2.00, p = 0.16 |
AD: subjects with alcohol dependence, IGD: subjects with internet gaming disorder, CGI-s: Clinical Global Index-severity, BDI: Beck Depressive Inventory, BAI: Beck Anxiety Inventory, K-AADHDS: Korean adult attention deficit hyperactivity disorder, AUQ-K: Korean Alcohol Urge Questionnaire, YIAS: Young Internet Addiction Scale, YIAS: Young Internet Addiction Scale, smk/n-smk: smoker/non-smoker.
statistically significant.
3.2. Different DLPFC-temporal functional connectivity between PAD and IGD
Controlling for age, we found that the AD group showed positive DLPFC seed connectivity with the anterior cingulate gyrus, right posterior cingulate gyrus, right superior frontal gyrus, right superior temporal gyrus, right nucleus accumbens, both occipital lingual gyri, right cerebellar tonsil, and left insular (p FDR < 0.01 = 0.000248). In addition, the AD group showed negative DLPFC seed connectivity with the medial frontal gyrus, controlling for age (p FDR < 0.01 = 0.000248) (Table 2) (Fig. 2). Controlling for age, we found that the IGD group showed positive DLPFC seed connectivity with the anterior cingulate gyrus, right frontal rectal gyrus, and cerebellum (p FDR < 0.01 = 0.000280). In addition, the IGD group showed negative DLPFC seed connectivity with the middle frontal gyrus, bilateral uncus, and both caudate heads, controlling for age (p FDR < 0.01 = 0.000280) (Table 2) (Fig. 3).
Table 2.
Brain connectivity in subjects with alcohol dependence and subjects with internet game disorder.
| Talairach code | Statistics | Voxels | Regions | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Brain connectivity in patients with alcohol dependence | |||||
| 16 | 0 | 7 | p FDR < 0.01 = 0.000248 | 200 | Right nucleus accumbens |
| 10 | 41 | 18 | p FDR < 0.01 = 0.000248 | 1500 | Right superior frontal gyrus, BA 9 |
| 54 | −24 | 0 | p FDR < 0.01 = 0.000248 | 1500 | Right superior temporal gyrus, BA22 |
| 37 | 0 | 39 | p FDR < 0.01 = 0.000248 | 1500 | Right medial frontal gyrus, BA 6 |
| 10 | 41 | 0 | p FDR < 0.01 = 0.000248 | 1500 | Right anterior cingulate gyrus, BA 32 |
| 10 | −64 | 16 | p FDR < 0.01 = 0.000248 | 700 | Right posterior cingulate, BA 30 |
| 1 | −72 | 0 | p FDR < 0.01 = 0.000248 | 400 | Right occipital lingual gyrus |
| 40 | −60 | −35 | p FDR < 0.01 = 0.000248 | 300 | Right cerebellar tonsil |
| −13 | 42 | 0 | p FDR < 0.01 = 0.000248 | 1500 | Left anterior cingulate gyrus, BA 32 |
| −37 | 0 | 42 | p FDR < 0.01 = 0.000248 | 500 | Left medial frontal gyrus, BA 6 |
| −36 | 0 | 15 | p FDR < 0.01 = 0.000248 | 400 | Left insular, BA13 |
| −18 | −50 | 0 | p FDR < 0.01 = 0.000248 | 500 | Left occipital lingual gyrus |
| Brain connectivity in patients with internet gaming disorder | |||||
| 30 | 45 | 24 | p FDR < 0.01 = 0.000280 | 1500 | Right middle frontal gyrus, BA 10 |
| 10 | 49 | 9 | p FDR < 0.01 = 0.000280 | 1500 | Right anterior cingulate, BA 42 |
| 10 | 34 | −19 | p FDR < 0.01 = 0.000280 | 1000 | Right frontal rectal gyrus, BA 11 |
| 22 | 1 | −35 | p FDR < 0.01 = 0.000280 | 700 | Right uncus, BA36 |
| 7 | 21 | 1 | p FDR < 0.01 = 0.000280 | 200 | Right caudate head |
| 30 | −69 | −35 | p FDR < 0.01 = 0.000280 | 400 | Right cerebellum posterior lobe |
| −24 | 0 | −33 | p FDR < 0.01 = 0.000280 | 700 | Left uncus, BA36 |
| −17 | 0 | −14 | p FDR < 0.01 = 0.000280 | 300 | Left parahippocampal gyrus, BA34 |
| −7 | 22 | 0 | p FDR < 0.01 = 0.000280 | 200 | Left caudate head |
| −30 | −67 | −35 | p FDR < 0.01 = 0.000280 | 400 | Left cerebellum posterior lobe |
Fig. 2.
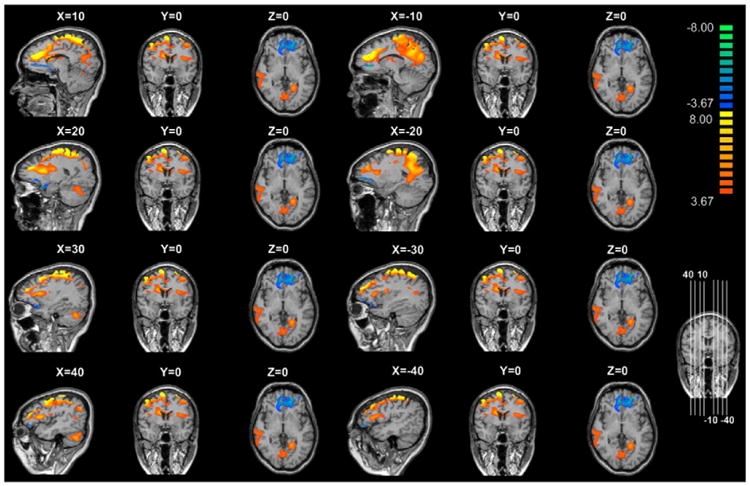
Brain connectivity in subjects with alcohol dependence. Color bar, yellow (orange): positive functional connectivity between the dorsolateral prefrontal cortex (DLPFC) seed and the anterior cingulate gyrus, right posterior cingulate gyrus, right superior frontal gyrus, right superior temporal gyrus, right nucleus accumbens, both occipital lingual gyri, right cerebellar tonsil, and left insular (p FDR < 0.01 = 0.000248), blue: negative functional connectivity between the DLPFC seed and the medial frontal gyrus (p FDR < 0.01 = 0.000248).
Fig. 3.
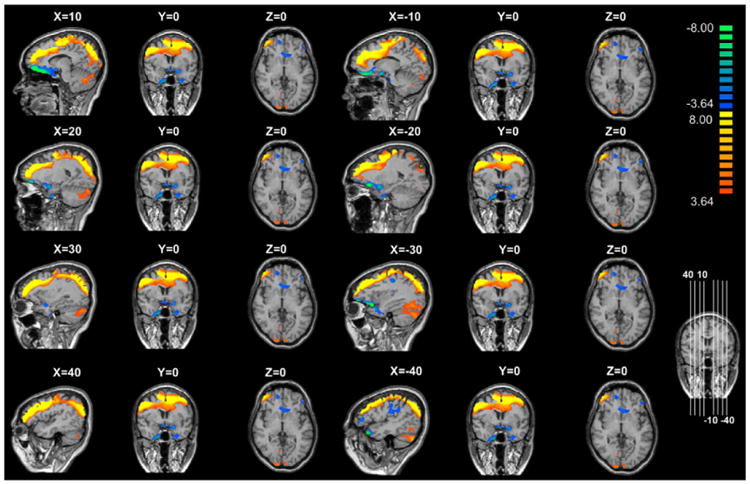
Brain connectivity in subjects with internet gaming disorder. Color bar, yellow (orange): positive functional connectivity between the dorsolateral prefrontal cortex (DLPFC) seed and the anterior cingulate gyrus, right frontal rectal gyrus, and cerebellum (p FDR < 0.01 = 0.000280), blue: negative functional connectivity between the DLPFC seed and the middle frontal gyrus, bilateral uncus, and both caudate heads (p FDR < 0.01 = 0.000280).
In a group comparison between AD and IGD, controlling for age, the higher functional connectivity between the DLPFC seed and the left brain stem, insular, amygdalar and right parahippocampal gyrus was apparent in AD, relative to IGD (p FDR < 0.05 = 0.00299). However, controlling for age, higher functional connectivity between the DLPFC seed and the left superior frontal gyrus and left middle frontal gyrus was apparent in IGD, relative to PAD (p FDR < 0.05 = 0.00299) (Table 3) (Fig. 4).
Table 3.
Comparison of brain connectivity between subjects with alcohol dependence and internet gaming disorder.
| Talairach code | Statistics | Voxels | Regions | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Alcohol dependence > internet gaming disorder | |||||
| 24 | 0 | −18 | p FDR < 0.05 = 0.00299 | 80 | Right parahippocampal gyrus |
| 26 | 0 | −18 | p FDR < 0.05 = 0.00299 | 60 | Right amygdala |
| −10 | −23 | −21 | p FDR < 0.05 = 0.00299 | 70 | Left brain stem pons |
| −30 | −21 | 16 | p FDR < 0.05 = 0.00299 | 70 | Left insula, BA13 |
| Alcohol dependence < internet gaming disorder | |||||
| −20 | 55 | 20 | p FDR < 0.05 = 0.00299 | 100 | Left superior frontal gyrus, BA10 |
| −34 | 46 | 22 | p FDR < 0.05 = 0.00299 | 80 | Left middle frontal gyrus, BA10 |
Fig. 4.

Comparison of functional connectivity from DLPFC seed to other regions between AD and IGD. Color bar, yellow (orange): higher functional connectivity between the dorsolateral prefrontal cortex (DLPFC) seed and the left brain stem, insular, amygdalar and right parahippocampal gyrus was apparentin alcohol dependence (AD) subjects, relative to internet gaming disorder (IGD) subjects (p FDR < 0.05 = 0.00299), blue: higher functional connectivity between the DLPFC seed and the left superior frontal gyrus and left middle frontal gyrus was apparent in IGD, relative to AD (p FDR < 0.05 = 0.00299).
3.3. Correlation results
Controlling for age, we found that the mean β values of the superior temporal gyrus region were marginally correlated with AUQ-K scores in AD subjects (r = 0.46, p = 0.08). There were no statistically significant differences in the correlations between K-AADHD, BDI, BAI, and CGI-s scores and mean β values of the superior temporal gyrus in AD. There were no statistically significant differences in the correlations between AUQ-K, K-AADHD, BDI, BAI, and CGI-s scores and mean β values of other clusters in AD. There were no statistically significant differences in the correlations between YIAS, K-AADHD, BDI, BAI, and CGI-s scores and mean β values of other clusters in IGD.
4. Discussion
To the best of our knowledge, the current study is the first to assess DLPFC-seeded functional connectivity in patients with AD and patients with IGD.
4.1. Similarities in functional connectivity between alcohol dependence and internet gaming disorder
As reported in previous studies of substance addiction and behavioral addiction, increased functional connectivity between the DLPFC and the cingulate gyrus and cerebellum has previously been reported in both AD and IGD subjects (Camchong, Stenger, & Fein, 2013; Fryer et al., 2013; Liu et al., 2010). Increased functional connectivity between the DLPFC and the anterior cingulate gyrus has also been reported in long term abstinent alcoholics (Camchong et al., 2013). In addition, self-reported craving for alcohol has been associated with brain activation in the ventral anterior cingulate gyrus, medial frontal cortex, and cerebellum (Fryer et al., 2013). Increased functional connectivity between the cerebellum, cingulate gyrus, and dorsolateral prefrontal cortex was found in a resting state fMRI study of AD (Liu et al., 2010). In a review of substance addiction, Baler and Volkow (2006) suggested that decreased inhibitory control of the DLPFC would increase the saliency of drugs of abuse within the orbitofrontal cortex. The DLPFC, orbitofrontal cortex, and anterior cingulate are thought to play an important role in executive function, including self-control as well as adaptive responding in human addiction processing (Yucel & Lubman, 2007). Although the role of the cerebellum in addiction remains controversial, it may contribute to control of executive function (Stoodley & Schmahmann, 2009). Considering the common findings of increased functional connectivity between the DLPFC, cingulate gyrus, and cerebellum as well as decreased functional connectivity between the DLPFC and the orbitofrontal cortex which are observed in both AD and IGD in the present study, these two addictive disorders may share deficits in executive function, including problems with self-control and adaptive responding.
4.2. Differences in functional connectivity between alcohol dependence and internet gaming disorder
Differences in functional connectivity between AD and IGD subjects may be seen in the different linkage between the DLPFC and the temporal and striatal regions. AD subjects showed positive functional connectivity between the DLPFC seed and the temporal and striatal regions while IGD subjects showed negative functional connectivity between the DLPFC and the temporal cortex and striatum. In a resting state fMRI study, alcohol patients with abstinence showed higher functional connectivity than healthy comparison subjects between a nucleus accumbens seed and the DLPFC and superior frontal gyrus (Camchong et al., 2013). The nucleus accumbens is a critical reward processing region which mediates appetitive drive as well as reward processing (Everitt & Robbins, 2005; Taha & Fields, 2006). The nucleus accumbens in alcohol dependence is known to be linked with the lack of flexible adaptive behavior in response to alcohol cues and the inability to extinguish alcohol-related habits (Chen et al., 2011). The nucleus accumbens, limbic and paralimbic systems including the temporal lobe play a critical role in emotional responsiveness and regulation of behavior in the context of rewarding and punishing outcomes (Drevets et al., 1997; Kelly et al., 2009). Poor regulation of behaviors and emotion are core features of alcohol dependence (Berking et al., 2011; Drevets et al., 1997). In our results, the mean β values of the superior temporal gyrus were marginally correlated with AUQ-K scores in AD subjects. AD subjects show inappropriate expressions of emotion, including extreme emotional bursting, negative affect, and mood swings in response to awkward social situations (Berking et al., 2011; Fox, Hong, & Sinha, 2008). Difficulty in emotional regulation is considered to be one of the important trigger factors for relapse in AD (Berking et al., 2011; Cooper, Frone, Russell, & Mudar, 1995).
The IGD subjects showed decreased functional connectivity between the DLPFC seed and the temporal and caudate regions. Brain activation mismatch (increased activity of the DLPFC and decreased activation of the caudate) was reported in a comparison study between subjects with pediatric bipolar disorder and ADHD (Passarotti, Sweeney, & Pavuluri, 2010). The mismatch between the DLPFC and the striatal areas in these young subjects is thought to be due to immaturity of the frontostriatal circuitry including the DLPFC and the caudate (Durston et al., 2003). The temporal cortex is associated with visual and auditory function (Lewald, Staedtgen, Sparing, & Meister, 2011; Robins, Hunyadi, & Schultz, 2009). The superior temporal gyrus, as a primary auditory cortex, is responsible for the perception of sound (Robins et al., 2009). The inferior temporal gyrus is associated with the representation and detection of complex object features (Lewald et al., 2011). Fronto-temporal circuits including the DLPFC, fusiform gyrus, and superior temporal cortex have been associated with suppression of irrelevant information as well as suppression of distractors during attentional regulation (Proverbio, Del Zotto, Crotti, & Zani, 2009; Zikopoulos & Barbas, 2006). Long duration game play requiring sustained attention despite distracting background sounds may stimulate visual and auditory brain regions (Hyun et al., 2013; Song et al., 2013). However, brain regions which are activated by visual and auditory stimulation for an extended period may not easily be excited or de-activated (Dong et al., 2012). Taken together, the negative connectivity between the DLPFC and the temporal regions in IGD subjects suggests that extended exposure to internet game play may decrease fronto-temporal connectivity in response to the presentation of complex visual and auditory stimulation.
There are several limitations to the current research. First, although we statistically controlled for age while assessing brain activity, the mean age of AD subjects was higher than that of the IGD subjects. This may affect the results and interpretation. Second, the current study did not include vision and hearing assessments. These issues should be considered in future studies. Finally, there were no healthy control subjects in the current study similar to other published 2 group comparison fMRI studies (Meda et al., 2012). However, the current study is the first to assess directly the differences of functional connectivity in AD subjects and IGD subjects within similar age ranges.
In summary, both AD and IGD subjects have positive functional connectivity between the DLPFC, cingulate gyrus, and cerebellum. In addition, both groups have negative functional connectivity between the DLPFC and the OFC. However, the AD subjects have positive functional connectivity between the DLPFC, temporal lobe and striatal areas while IGD subjects have negative functional connectivity between the DLPFC, temporal lobe and striatal areas. The recognition of differences in clinical symptoms and biological characteristics may be important for establishing policies that prevent internet gaming disorder and developing treatment plans for patients with IGD.
Acknowledgments
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120013) and Korea Creative Content Agency (R2014040055).
Role of funding sources: Funding for this study was provided by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120013) and Korea Creative Content Agency (R2014040055).
Footnotes
Contributors: Ministry of Health & Welfare, Republic of Korea had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. Doug Hyun Han and Perry Renshaw designed the study and wrote the protocol. Ji Won Han and Boong Nyun Kim conducted literature searches and provided summaries of previous research studies. Doug Hyun Han conducted the statistical analysis. Ji won Han and Nicolas Bolo wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Conflict of interest: All authors declare that they have no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatry Association; 2013. [Google Scholar]
- Asplund CA, Aaronson JW, Aaronson HE. 3 regimens for alcohol withdrawal and detoxification. Journal of Family Practice. 2004;53:545–554. [PubMed] [Google Scholar]
- Atmaca M. A case of problematic internet use successfully treated with an SSRI-antipsychotic combination. Progress in Neuropsychopharmacology & Biological Psychiatry. 2007;31:961–962. doi: 10.1016/j.pnpbp.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Beard KW, Wolf EM. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychology, Behavior and Social Networking. 2001;4:377–383. doi: 10.1089/109493101300210286. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive–behavioral therapy for alcohol dependence. Journal of Consulting and Clinical Psychology. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bovo R, Ciorba A, Martini A. Environmental and genetic factors in age-related hearing impairment. Aging Clinical and Experimental Research. 2011;23:3–10. doi: 10.1007/BF03324947. [DOI] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 2013;37:75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, et al. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcoholism: Clinical and Experimental Research. 2011;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Son H, Park M, Han J, Kim K, Lee B, et al. Internet overuse and excessive daytime sleepiness in adolescents. Psychiatry and Clinical Neurosciences. 2009;63:455–462. doi: 10.1111/j.1440-1819.2009.01925.x. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature Review Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- DellaCroce JT, Vitale AT. Hypertension and the eye. Current Opinion in Ophthalmology. 2008;19:493–498. doi: 10.1097/ICU.0b013e3283129779. [DOI] [PubMed] [Google Scholar]
- Dong G, Huang J, Du X. Alterations in regional homogeneity of resting-state brain activity in internet gaming addicts. Behavioral and Brain Functions. 2012;8:41. doi: 10.1186/1744-9081-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behaviors. 2008;33:388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: The principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow & Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, Watson TD, Shanbhag H, et al. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biological Psychology. 2013;92:282–291. doi: 10.1016/j.biopsycho.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to behavioral addictions. American Journal of Drug and Alcohol Abuse. 2011;36:233–241. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma MC, Pieterse ME, Peters O. The prevalence of problematic video gamers in the Netherlands. Cyberpsychology, Behavior and Social Networking. 2012;15:162–168. doi: 10.1089/cyber.2011.0248. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Young SE, Zeiger JS, Lessem JM, Hewitt JK, Hopfer CJ. Prevalence and correlates of alcohol and cannabis use disorders in the United States: Results from the national longitudinal study of adolescent health. Drug and Alcohol Dependence. 2014;136:158–161. doi: 10.1016/j.drugalcdep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Kim YS, Lee YS, Min KJ, Renshaw PF. Changes in cue-induced, prefrontal cortex activity with video-game play. Cyberpsychology, Behavior and Social Networking. 2010;13:655–661. doi: 10.1089/cyber.2009.0327. [DOI] [PubMed] [Google Scholar]
- Hyun GJ, Shin YW, Kim BN, Cheong JH, Jin SN, Han DH. Increased cortical thickness in professional on-line gamers. Psychiatry Investigation. 2013;10:388–392. doi: 10.4306/pi.2013.10.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:1648–1652. doi: 10.1158/1055-9965.EPI-07-2791. [DOI] [PubMed] [Google Scholar]
- Karim R, Chaudhri P. Behavioral addictions: An overview. Journal of Psychoactive Drugs. 2012;44:5–17. doi: 10.1080/02791072.2012.662859. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP, et al. Effects of morphine and alcohol on functional brain connectivity during “resting state”: A placebo-controlled crossover study in healthy young men. Human Brain Mapping. 2012;33:1003–1018. doi: 10.1002/hbm.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ. Validation of Korean Adult ADHD Scale (K-AADHDS) The Korean Journal of Clinical Psychology. 2003;22:897–911. [Google Scholar]
- Kim CM, Kim SG, Kim MJ, Kim HC, Oh KW, Kim HJ, et al. The study on reliability and validity of Korean Alcohol Urge Questionnaire (AUQ-K) for alcohol dependence. Korean Society of Biological Psychiatry. 2008;15:204–210. [Google Scholar]
- Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, et al. Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatric Research. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Lee YS, Han DH, Kim SM, Renshaw PF. Substance abuse precedes Internet addiction. Addictive Behaviors. 2013;38:2022–2025. doi: 10.1016/j.addbeh.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. A targeted review of the neurobiology and genetics of behavioural addictions: An emerging area of research. Canadian Journal of Psychiatry. 2013;58:260–273. doi: 10.1177/070674371305800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewald J, Staedtgen M, Sparing R, Meister IG. Processing of auditory motion in inferior parietal lobule: Evidence from transcranial magnetic stimulation. Neuropsychologia. 2011;49:209–215. doi: 10.1016/j.neuropsychologia.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao XP, Osunde I, Li X, Zhou SK, Zheng HR, et al. Increased regional homogeneity in internet addiction disorder: A resting state functional magnetic resonance imaging study. Chinese Medical Journal. 2010;123:1904–1908. [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biological Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, et al. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biological Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Phaswana-Mafuya N. Problem drinking and associated factors in older adults in South Africa. African Journal of Psychiatry. 2013;16:104–109. doi: 10.4314/ajpsy.v16i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Del Zotto M, Crotti N, Zani A. A no-go related prefrontal negativity larger to irrelevant stimuli that are difficult to suppress. Behavioral and Brain Functions. 2009;5:25. doi: 10.1186/1744-9081-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Hunyadi E, Schultz RT. Superior temporal activation in response to dynamic audio-visual emotional cues. Brain and Cognition. 2009;69:269–278. doi: 10.1016/j.bandc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira NA, Lessig MC, Goldsmith TD, Szabo ST, Lazoritz M, Gold MS, et al. Problematic internet use: Proposed classification and diagnostic criteria. Depression and Anxiety. 2003;17:207–216. doi: 10.1002/da.10094. [DOI] [PubMed] [Google Scholar]
- Song WH, Han DH, Shim HJ. Comparison of brain activation in response to two dimensional and three dimensional on-line games. Psychiatry Investigation. 2013;10:115–120. doi: 10.4306/pi.2013.10.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. Journal of Neuroscience. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Whang LS, Lee S, Chang G. Internet over-users' psychological profiles: A behavior sampling analysis on internet addiction. Cyberpsychology, Behavior and Social Networking. 2003;6:143–150. doi: 10.1089/109493103321640338. [DOI] [PubMed] [Google Scholar]
- Young KS. Psychology of computer use: XL. Addictive use of the Internet: A case that breaks the stereotype. Psychological Reports. 1996;79(3 Pt 1):899–902. doi: 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: Implications for diagnosis, treatment and prevention. Drug and Alcohol Review. 2007;26:33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. Journal of Neuroscience. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


