Abstract
The performance of the sunflower oil in deep-fat frying was assessed by evaluating the efficacy of linoleic acid level and composition of tocopherol isomeric on its frying stability. The oil was used as a frying media to fry potato strips for 6 h daily for 7 days. Standard procedures for the measurement of used frying oil degradation such as fatty acid composition, acid value, anisidine value, conjugated diene value, total polar compounds and tocopherol concentration were used. At analogous composition of tocopherol isomers, the high oleic sunflower oil with smaller value of linoleic acid content indicated higher frying stability than the oil with higher linoleic acid level. This, indicating that the high oleic sunflower oil frying efficiency was depended mainly with the oil linoleic acid content and the composition of tocopherol isomers showed no significant effect. Also, the α-tocopherol degradation was lower compared to the corresponding degradation of γ-tocopherol.
Keywords: Sunflower oil, Deep-fat frying, Frying stability, Tocopherol isomeric, Linoleic acid
Introduction
Pleasant flavor of fried food and remarkable economic profit cause the deep-fat frying grow one of the most favorite food cooking procedures applied in restaurant and household kitchen. Moreover, ready-to-eat and pre-cooked food sale which also recourse to the deep-frying process has significantly enhanced in the western world and is quickly extending all over the developing countries.
Frying can induced all of the food compounds to take part in a chain of chemical and physical changes. These alterations not only comprise the dissociation reactions of the components such as triacylglycerols of oil and raw material nutrients, but also comprise the interactions between these components (Chu and Luo 1994; Dobarganes et al. 2000). Furthermore, deep-fat frying is a complex physicochemical proceedings which is concurrently affected with many factors such as temperature, time, frying oil and fried material character, steady or periodic heating, model of fryer, application of filters and oil supplement (Chatzilazarou et al. 2006; Kalogianni et al. 2010; Rojo and Perkins 1987). So, numerous compounds are generated because of these chemical states and complex substances. Moreover, the deep-fat frying chemical reaction pathway is considerable various depend on the frying with or without food (Barrera-Arellano et al. 1997; Houhoula et al. 2002).
Oil is a superb medium for transition of heat, therefore the food is swiftly heated and prepared when it is plunged within the oil (Alvis et al. 2009). During deep-fat frying, mass transfer causes the dissipation of oil, protein, carbohydrate, vitamin and moisture from fried food and product oil uptake (Krokida et al. 2000; Sosa-Morales et al. 2006). Thus, both frying oil and fried product affect on each other and jointly boost the incidence of complex reaction pathways. Oxidation, polymerization, isomerization and hydrolysis are the main chemical reactions happened during deep-fat frying process (Choe and Min 2007; Velasco et al. 2009). These reactions eventuated in the production of hydrocarbons, free fatty acids, aldehydes, ketones, alcohols, acids and lactones (Pokorny 1989), epoxy and cyclic compounds, mono and diglycerides (Rojo and Perkins 1987).
It is clear that autoxidation is the major deterioration reaction which is ascribed to the vegetable oil rancidity. It is also the main reaction happened within deep-fat frying with the rise of temperature. The thermal oxidation mechanism is basically alike with the mechanism of autoxidation, the dissimilar just in the velocity of reaction (Houhoula et al. 2003). Oligomers and polar compounds which generated within thermal oxidation of deep-fat frying period with extent range of 10–16 and 20–27%, respectively, have been suggested to definite the refusal of edible oil after frying process (Bastida and Sánchez-Muniz 2002; Paul and Mittal 1997).
Vitamin E, flavonoids, ascorbic acid and carotins are non-toxic and easily biodegradable natural antioxidants. Vitamin E (tocopherol) is an important natural antioxidant. Tocopherols are very active, however their characteristics of oxidative stability are strongly depended on concentration and temperature (Hamblin 1999; Warner 2005). It was as well found that the tocopherol performance is strongly depended on the nature of antioxidant and structure of fatty acid (Warner 2005).
The objective of this study was to evaluate the influence of deep-fat frying conditions on the oxidative stability, tocopherol isomeric content and alterations of the most dominant fatty acid of oil during French fries preparation.
Materials and methods
Materials
Fresh potatoes were obtained from a local market (Tonekabon, Iran), and kept in a refrigerator at 5 °C until experiment. Sunflower oils as refined, bleached and deodorized were purchased from Parto Daneh Khazar Co. (Behshahr, Iran). All chemicals and solvents used in this investigation were of analytical grade and provided by Sigma (USA) and Merck (Germany).
Sample preparation
Potatoes were washed, peeled and cut into strips (1 × 1 × 6 cm) with a stainless steel slicer. 2.5 L of sunflower oil was placed in a deep-fryer (Tefal model 1250, France) with 2.5-L capacity and heated at 180 °C for 6 h daily for 7 days. Each sample of the potato slices (100 g) were randomly selected and fried 6 min/frying day. At the end of each frying day, the frying oil (15 g) was filtered into a screw-cap vial and kept in the dark condition at 4 °C until analyzed. In total, two kinds of sunflower oil samples (SOS) were selected; SOS1 and SOS2 with 9.1 and 4.4% of linoleic acid content, respectively.
Analysis
Measurement of fatty acid composition
The most dominant fatty acids of the oil samples were evaluated by gas–liquid chromatography. Methyl esters of fatty acids were prepared by mixing oil and hexane (0.3 g in 7 mL) with methanolic potassium hydroxide (2 mL, 7 N) at 50 °C for 15 min. Esters of fatty acids were identified by HP-5890 chromatograph (Hewlett-Packard, CA, USA) equipped with a flame ionisation detector and a CP-FIL 88 (Supel Co., Inc., Bellefonte, PA, USA) fused silica capillary column (60 m length × 0.22 mm i.d × 0.2 µm film thickness). Helium was as a carrier gas with a flow rate of 0.7 mL/min. The temperature of the oven, the injector and the detector was 198, 280 and 250 °C, respectively. Results were expressed based on the relative percentage of areas (Farhoosh et al. 2008).
Determination of acid value
The acid value (AV) was determined according to the AOCS Official Method Cd 3d-63 (AOCS 1998). For this purpose, initially, the oil sample (5 g) was dissolved in toluene–isopropyl alcohol solution (1:1 v/v, 50 mL). Then, the obtained solution was titrated with standard solution of potassium hydroxide in isopropyl alcohol (0.1 M) to a pink end point in the presence of phenolphthalein as the indicator. The AV was calculated as following formula and expressed in mg of KOH per g of oil.
where V is the KOH volume added to the oil sample, C is the concentration of KOH and W is the mass of the oil sample in grams.
Determination of anisidine value
Anisidine value (AnV) of oil sample was analyzed according to the AOCS Official Method Cd 18-90 (AOCS 1998). The oil sample (4 g) solution in isooctane (25 mL), incorporated with p-anisidine reagent (1 mL) was prepared. Absorbance of a solution at 350 nm was measured by Shimadzu Spectrophotometer Model UV-2101PC UV–VIS (Shimadzu Inc., Columbia, MD) and p-anisidine reagent used as blank in the reference cuvette.
Determination of conjugated diene value
The oil samples conjugated diene value (CDV) were determined according to the AOCS Official Method Ti 1a-64 (AOCS 1998). The oil sample was dissolved in isooctane to attain stock solution of 1 g/L. Then, stock solution was diluted with isooctane to provide test solution (0.4 g/L). The absorbance of test solution at 233 nm was determined and then converted to percentage conjugated dienoic acid.
Determination of total polar compounds
The total polar compounds (TPC) was determined according to the procedure described by Farahmandfar et al. (2015). In detail, 63–100 μm of silica gel 60 (95 parts), dried (12 h, 160 °C), was mixed with water (five parts) and shaked vigorously (1 min) and kept 12 h. Then, silica gel 60 (1 g) was compressed and filled between two cotton wool balls into a pipette tip (5 mL). The oil sample (500 mg) was pipetted into a volumetric flask (5 mL). After that, it was dissolved in toluene (4 mL) and filled with the toluene. The solution (1 mL) was pipetted on top of the pipette tip, under a well ventilated fume hood. The solution was soaked in and then the pipette tip was washed with eluent (1 mL) and after soaking in, were added with eluent (7 mL). After the 15 min of elution operation, the end of the tip was washed with toluene (500 μL). Then the solvent was removed and weighing TPC in percentage (w/w) was performed by the following formula:
where W is the weight of oil sample and W1 is the weight of nonpolar compounds in milligrams.
Measurement of tocopherols
The high-performance liquid chromatography (Varian Associates, Inc., Walnut Creek, CA) was used to evaluate the concentration of tocopherols according to the AOCS Official Method Ce 8-89 (AOCS 1998). Conditions that were observed in this test: fluorescence detector (TSP brand and FL 2000 model, Varian Associates, Inc.) with excitation 290 nm and emission 330 nm; analytical column with 4.6 mm × 25 cm and 5-µm particle size; silica (Supelcosil LC-Si, Supelco) for normal phase and C18 (Supelco Discovery C18) for reversed phase; flow rate of 1 mL/min. A mixture of 0.5% isopropanol and 99.5% n-hexane was used as the mobile phase. Four tocopherol isomers were considered as external standards for calculations. Results were expressed as µg/g.
Statistical analysis
All results of three independent experiments were expressed as the mean ± standard deviation (SD). The data were analyzed by analysis of variance (ANOVA) and the Duncan’s test for the 5% significance level. Each of which were done with the SPSS software, Version 21 (IBM, New York, USA).
Results and discussion
Oil composition
Tables 1 and 2 show the composition of the main fatty acid and tocopherol isomeric of the sunflower oil. The fatty acid composition of the two sunflower oil samples are partly similar with the exception of the linoleic acid content. The content of linoleic acid in SOS1 and SOS2 were 9.1 and 4.4%, respectively. Also, the distribution of tocopherol isomeric in SOS1 was 269, 492 and 81 µg/g for α, γ, and δ isomers, respectively, while, in SOS2, was 92, 412 and 231 µg/g for α, γ, and δ isomers, respectively. A comparison between SOS1 and SOS2 can present details on the efficacy of the content of linoleic acid as they both have the blend of α, γ, and δ isomers of tocopherol. Warner and Moser (2009) reported that there was no significant difference about frying efficiency of purified sunflower oil with midoleic content incorporated with a blend of α, 30 µg/g; γ, 590 µg/g and δ, 160 µg/g than the blend of α, 360 µg/g; γ, 360 µg/g and δ, 60 µg/g. Therefore, the discrepancy in the comparative quantity of individual isomers of tocopherol between high oleic sunflower oil samples is not anticipated to significantly influence the oil frying stability. As shown in Table 1, the fatty acid composition of the oil samples had a significant difference before and after deep-fat frying process, with the discrepancy existing much more featured for SOS1. At the end of frying process, the reduction in the relative portion of oleic acid was 9.85 and 8.3% for SOS1 and SOS2, respectively. During the identical frying course, nevertheless, the linoleic acid value in SOS1 reduced by 16.48%, however there was not significant difference for this parameter in SOS2, indicating that, in high oleic sunflower oil, the amount of more than 9% linoleic acid causes an increase in sensitivity of oil to thermooxidation within deep-fat frying operation. Dobarganes et al. (1993) as well found a significant reduction in the contents of trilinolein of a high oleic sunflower oil having 9.5% content of linoleic acid within frying at 180 °C for 10 h.
Table 1.
Fatty acid composition (%) of sunflower oil before and after deep fat frying process
| Fatty acids | SOS1 | SOS2 | ||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | |
| C16:0 | 4.5 ± 0.1a | 4.6 ± 0.3a | 3.7 ± 0.3a | 3.8 ± 0.1a |
| C18:0 | 3.4 ± 0.4a | 3.3 ± 0.2a | 3.9 ± 0.1a | 3.7 ± 0.3a |
| C18:1 | 80.2 ± 1.4a | 72.3 ± 1.6b | 85.4 ± 1.3a | 78.3 ± 1.8b |
| C18:2 | 9.1 ± 0.4a | 7.6 ± 0.2b | 4.4 ± 0.1a | 4.0 ± 0.3a |
| C18:3 | 0.5 ± 0.2a | 0.5 ± 0.1a | 0.4 ± 0.3a | 0.3 ± 0.2a |
| SFA | 8.1 ± 0.4a | 8.2 ± 0.3a | 7.8 ± 0.2a | 8.1 ± 0.3a |
| MUFA | 81.2 ± 1.3a | 70.6 ± 1.9b | 87.2 ± 1.3a | 79.6 ± 2.4b |
| PUFA | 9.5 ± 0.6a | 6.7 ± 0.4b | 5.4 ± 0.4a | 4.7 ± 0.4a |
Mean ± SD within a row with the same superscript are not significantly different (p < 0.05)
SOS sunflower oil sample
Table 2.
Tocopherol composition (µg/g) of sunflower oil before and after deep fat frying process
| Tocopherols | SOS1 | SOS2 | ||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | |
| α-tocopherol | 269 ± 9 | 43 ± 2 | 92 ± 1 | Tr |
| β-tocopherol | Tr | Tr | Tr | Tr |
| γ-tocopherol | 492 ± 5 | 28 ± 3 | 412 ± 7 | Tr |
| δ-tocopherol | 81 ± 3 | Tr | 231 ± 3 | Tr |
| Total | 842 ± 11 | 71 ± 4 | 735 ± 10 | Tr |
SOS sunflower oil sample, Tr traces
Acid value
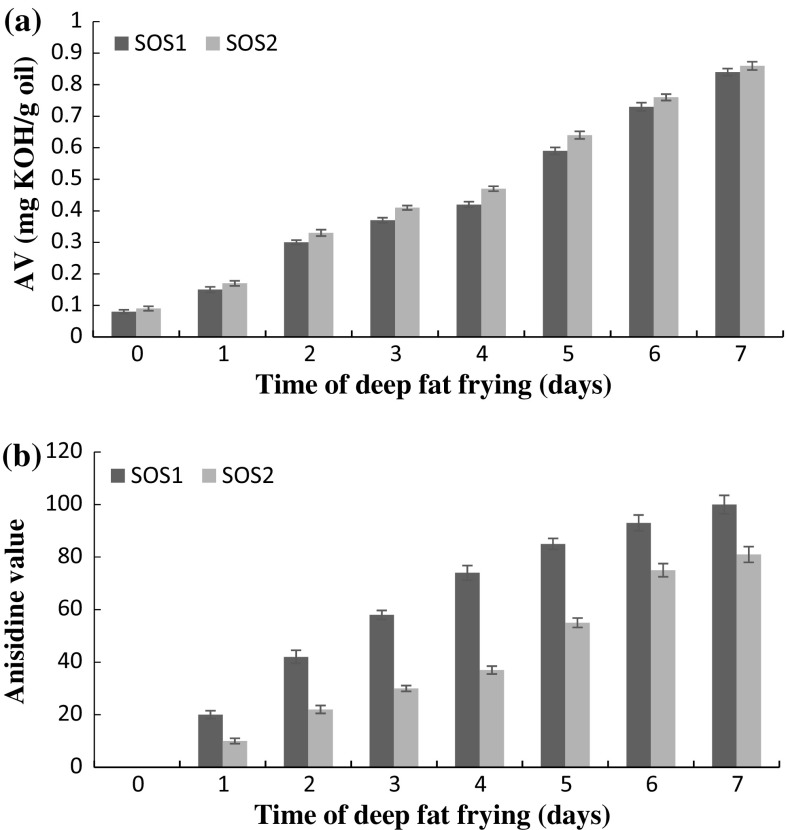
AV is momentous oil quality factor related to the free fatty acids presence. Free fatty acids are produced during triacylglycerol hydrolysis and as deterioration resultants from oxidised triacylglycerols. AV amount enhanced slowly within the frying process and did not surpass from 1 mg/g limit. This result is equivalent with <2% limit for free fatty acids appointed by European regulations (Firestone 2006). The oils AV before frying operation was lower than 0.1 mg/g, representing oils high quality processing. As shown in Fig. 1a, there was not significant difference in the amount of AV between the two oil samples, suggesting that, in the assessed high oleic sunflower oils, the linoleic acid content and the composition of tocopherol have no significant effect on the triacylglycerols hydrolytic reaction happening within French fries frying.
Fig. 1.
a Changes in acid value during deep-fat frying process in sunflower oils. b Changes in anisidine value during deep-fat frying process in sunflower oils
Anisidine value
The AnV evaluates the secondary products of oxidation, including non-volatile carbonyl compounds generated from hydroperoxides. The AnV alteration of oil samples are shown in Fig. 1b. Irrespective of composition of oil, this index rised consistently, but its severity was reduced in the late of frying period. Aladedunye and Przybylski (2009) reported that the degradation of carbonyl compounds and the absorption of these compounds by fried product causes reduce the severity of AnV growth of oil during deep-fat frying operation. The highest AnV was observed for SOS1, suggesting greater deterioration of unsaturated fatty acids by thermooxidation reaction. The amount of AnV was 19% higher in SOS1 than SOS2 at the end of the frying course.
Conjugated diene value
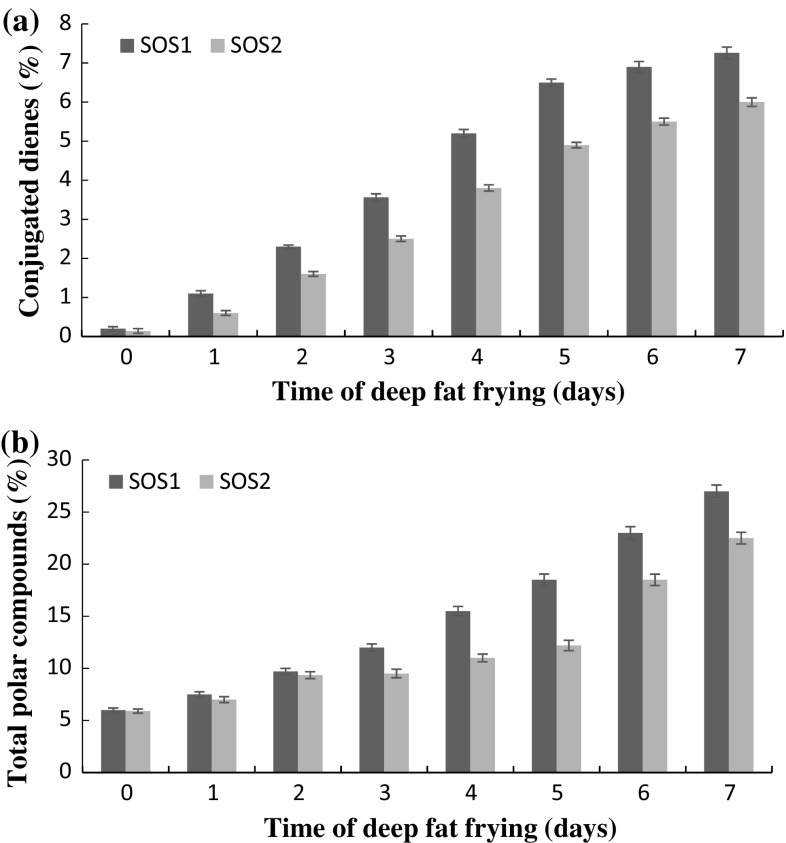
Conjugated dienes are generated during the unsaturated fatty acid oxidation to attain more stable radical. For linoleic acid, the primary radical electron at C11 is delocalized by five systems of carbon from C9 to C13 and next a radical at C9 or C13 generates a stable conjugated diene with radical at C11 (Choe and Min 2005). As shown in Fig. 2a, the amounts of CDV enhanced with frying time and its higher were observed for SOS1. The higher the amount of polyunsaturated fatty acids in the oil, higher the percentage of conjugated diene produced within frying period. This was the cause why SOS1, that had high levels of linoleic acid, contain more conjugated diene compared to SOS2. Moreover, the increment rate in the CDV was sharped within frying until the fifth day, after which it reduced, because conjugated diene compounds will be converted into polymer components with rise in frying course.
Fig. 2.
a Changes in conjugated diene value during deep-fat frying process in sunflower oils. b Changes in total polar compounds during deep-fat frying process in sunflower oils
Total polar compounds
Determination of polar compounds presents the most precise evaluation of the thermooxidative deterioration of frying oils because it evaluates directly the entire degraded components existing in the oil. Figure 2b shows alterations in polar compounds within expanded frying operation. The generation rate of polar compounds was analogous in the two oil samples for the first 2 frying days, afterward SOS2 commenced to represent better frying stability compared to SOS1. Despite a similar distribution of tocopherol isomeric, significant difference (p < 0.05) in TPC was observed between SOS1 and SOS2 at the end of the frying course. This result was in agreement with the conclusion reported for frying efficiency of purified sunflower oil with midoleic content incorporated with various tocopherol isomeric composition of α, γ and δ (Warner and Moser 2009). Therefore, the discrepancies in the generation of TPC between SOS1 and SOS2 can only be illustrated by their contents of linoleic acid. Warner and Mounts (1993) reported that unsaturated fatty acid is one of the substantial factor influencing the generation of TPC.
Tocopherol concentration
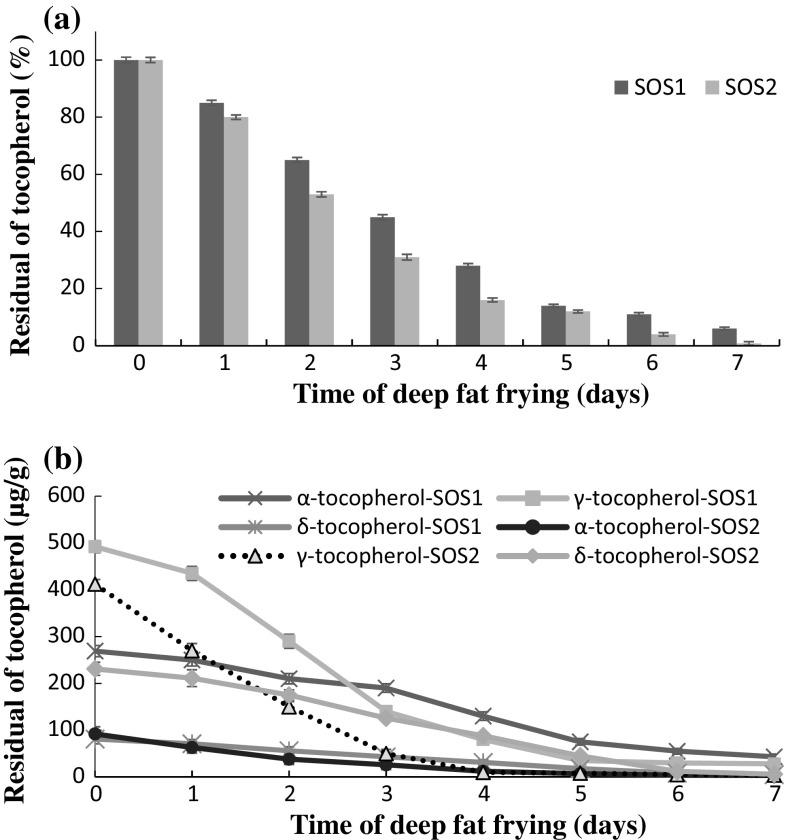
Tocopherols are naturally existing in most vegetable oils and their rates of deterioration can present a sign of the frying oils stability (Normand et al. 2001). As shown in Fig. 3a, the tocopherol loss was quicker in SOS2 than SOS1. At the end of the frying period, tocopherol was entirely degraded in SOS2, whilst up to 6% was still discovered in SOS1. The lower deterioration of tocopherol in SOS1 may be linked to its further value of unsaturation because tocopherols are recognized to deteriorate quicker as the value of saturation in the oil raises (Normand et al. 2001). According to Fig. 3b, the comparative tocopherol isomeric degradation was γ > α > δ and γ > δ > α in SOS1 and SOS2, respectively. The isomer of tocopherol existing in higher amount deteriorates at much quicker rate compared to the one existing in lesser value (Warner and Moser 2009). So, higher primary values of γ-isomer than α-isomer in SOS1 and SOS2 may illustrate the quicker γ-tocopherol deterioration seen in this investigation.
Fig. 3.
a Changes in total tocopherol composition during deep-fat frying process in sunflower oils. b Changes in individual tocopherol isomeric deterioration during deep-fat frying process in sunflower oils
Conclusion
The results of this investigation indicated that the frying performance of sunflower oil is affected with its content of linoleic acid compared to the composition of tocopherol isomers. Whilst sunflower oil with high oleic content may present higher frying stability than the common oil. This study showed that more than 9% of linoleic acid in sunflower oil improved its stability during deep-fat frying process.
References
- Aladedunye FA, Przybylski R. Degradation and nutritional quality changes in oil during frying. J Am Oil Chem Soc. 2009;86:149–156. doi: 10.1007/s11746-008-1328-5. [DOI] [Google Scholar]
- Alvis A, Velez C, Rada-Mendoza M, Villamiel M, Villada HS. Heat transfer coefficient during deep-fat frying. Food Control. 2009;20:321–325. doi: 10.1016/j.foodcont.2008.05.016. [DOI] [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists Society. Champaign: AOCS Press; 1998. [Google Scholar]
- Barrera-Arellano D, Márquez-Ruiz G, Dobarganes MC. A simple procedure to evaluate the performance of fats and oils at frying temperatures. Grasas Aceites. 1997;48:231–235. doi: 10.3989/gya.1997.v48.i4.794. [DOI] [Google Scholar]
- Bastida S, Sánchez-Muniz FJ. Polar content vs. TAG oligomer content in the frying-life assessment of monounsaturated and polyunsaturated oils used in deep-frying. J Am Oil Chem Soc. 2002;79:447–451. doi: 10.1007/s11746-002-0504-8. [DOI] [Google Scholar]
- Chatzilazarou A, Gortzi O, Lalas S, Zoidis E, Tsaknis J. Physicochemical changes of olive oil and selected vegetable oils during frying. J Food Lipids. 2006;13:27–35. doi: 10.1111/j.1745-4522.2006.00032.x. [DOI] [Google Scholar]
- Choe E, Min DB. Chemistry of oxidative stabilities of edible oils. In: Akoh C, editor. Healthful lipids. Champaign: AOCS Press; 2005. pp. 558–590. [Google Scholar]
- Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72:77–86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Chu YH, Luo S. Effects of sugar, salt and water on soybean oil quality during deep frying. J Am Oil Chem Soc. 1994;71:897–900. doi: 10.1007/BF02540470. [DOI] [Google Scholar]
- Dobarganes MC, Márquez-Ruiz G, Pérez-Camino MC. Thermal stability and frying performance of genetically modified sunflower seed (Helianthus annuus L.) oils. J Agric Food Chem. 1993;41:678–681. doi: 10.1021/jf00028a033. [DOI] [Google Scholar]
- Dobarganes MC, Márquez-Ruiz G, Velasco J. Interactions between fat and food during deep-frying. Eur J Lipid Sci Technol. 2000;102:521–528. doi: 10.1002/1438-9312(200009)102:8/9<521::AID-EJLT521>3.0.CO;2-A. [DOI] [Google Scholar]
- Farahmandfar R, Asnaashari M, Sayyad R. Comparison antioxidant activity of Tarom Mahali rice bran extracted from different extraction methods and its effect on canola oil stabilization. J Food Sci Technol. 2015;52:6385–6394. doi: 10.1007/s13197-014-1702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhoosh R, Niazmand R, Rezaei M, Sarabi M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Technol. 2008;110:587–592. doi: 10.1002/ejlt.200800004. [DOI] [Google Scholar]
- Firestone D. Regulation of frying fat and oil. In: Erickson MD, editor. Deep frying: chemistry, nutrition, and practical applications. Champaign: AOCS press; 2006. pp. 373–385. [Google Scholar]
- Hamblin P. Oxidative stabilization of synthetic fluids and vegetable oils. J Synth Lubr. 1999;16:157–181. doi: 10.1002/jsl.3000160206. [DOI] [Google Scholar]
- Houhoula DP, Oreopoulou V, Tzia C. A kinetic study of oil deterioration during frying and a comparison with heating. J Am Oil Chem Soc. 2002;79:133–137. doi: 10.1007/s11746-002-0447-0. [DOI] [Google Scholar]
- Houhoula DP, Oreopoulou V, Tzia C. The effect of process time and temperature on the accumulation of polar compounds in cottonseed oil during deep-fat frying. J Sci Food Agric. 2003;83:314–319. doi: 10.1002/jsfa.1314. [DOI] [Google Scholar]
- Kalogianni EP, Karastogiannidou C, Karapantsios TD. Effect of potato presence on the degradation of extra virgin olive oil during frying. Int J Food Sci Technol. 2010;45:765–775. doi: 10.1111/j.1365-2621.2010.02188.x. [DOI] [Google Scholar]
- Krokida MK, Oreopoulou V, Maroulis ZB. Water loss and oil uptake as a function of frying time. J Food Eng. 2000;44:39–46. doi: 10.1016/S0260-8774(99)00163-6. [DOI] [Google Scholar]
- Normand L, Eskin NMA, Przybylski R. Effect of tocopherols on the frying stability of regular and modified canola oils. J Am Oil Chem Soc. 2001;78:369–373. doi: 10.1007/s11746-001-0270-7. [DOI] [Google Scholar]
- Paul S, Mittal GS. Regulating the use of degraded oil/fat in deep-fat/oil food frying. Crit Rev Food Sci Nutr. 1997;37:635–662. doi: 10.1080/10408399709527793. [DOI] [PubMed] [Google Scholar]
- Pokorny J. Flavor chemistry of deep fat frying in oil. In: Min DB, Smouse TH, Zhang SS, editors. Flavor chemistry of lipid foods. Champaign: AOCS Press; 1989. pp. 113–154. [Google Scholar]
- Rojo JA, Perkins EG. Cyclic fatty acid monomer formation in frying fats. I. Determination and structural study. J Am Oil Chem Soc. 1987;64:414–421. doi: 10.1007/BF02549306. [DOI] [Google Scholar]
- Sosa-Morales ME, Orzuna-Espiritu R, Velez-Ruiz JF. Mass, thermal and quality aspects of deep-fat frying of pork meat. J Food Eng. 2006;77:731–738. doi: 10.1016/j.jfoodeng.2005.07.033. [DOI] [Google Scholar]
- Velasco J, Marmesat S, Dobarganes MC. Chemistry of frying. In: Sahin S, Sumnu SG, editors. Advances in deep-fat frying of foods. Boca Raton: CRC Press; 2009. pp. 33–56. [Google Scholar]
- Warner K. Effects on the flavor and oxidative stability of stripped soybean and sunflower oils with added pure tocopherols. J Agric Food Chem. 2005;53:9906–9910. doi: 10.1021/jf0517593. [DOI] [PubMed] [Google Scholar]
- Warner K, Moser J. Frying stability of purified mid-oleic sunflower oil triacylglycerols with added pure tocopherols and tocopherol mixtures. J Am Oil Chem Soc. 2009;86:1199–1207. doi: 10.1007/s11746-009-1461-9. [DOI] [Google Scholar]
- Warner K, Mounts TL. Frying stability of soybean and canola oils with modified fatty acid composition. J Am Oil Chem Soc. 1993;70:983–988. doi: 10.1007/BF02543024. [DOI] [Google Scholar]