Abstract
The aim of this study was to develop an optimal formulation for preparation of edible films from chitosan, pea starch and glycerol using response surface methodology. Three independent variables were assigned comprising chitosan (1–2%), pea starch (0.5–1.5%) and glycerol (0.5–1%) to design an empirical model best fit in physical, mechanical and barrier attributes. Impacts of independent variables on thickness, moisture content, solubility, tensile strength, elastic modulus, elongation at break and water vapor permeability of films were evaluated. All the parameters were found to have significant effects on physical and mechanical properties of film. The optimal formulation for preparation of edible film from chitosan, pea starch and glycerol was 1% chitosan, 1.5% pea starch and 0.5% glycerol. Edible films with good physical and mechanical properties can be prepared with this formulation and thus this formulation can be further applied for testing on coating for fruit and vegetables.
Keywords: Pea starch, Chitosan, Plasticizer, Edible films, Box–Behnken design
Introduction
Many efforts have been made to develop and test edible films for further utilisation to extend shelf life of fresh produce (Arnon et al. 2014; Dhall 2013; Gómez-Estaca et al. 2009; Valencia-Chamorro et al. 2010). Chitosan has been found to have a great potential for wide range of application in formulation of edible films due to its biodegradability, biocompatibility, antimicrobial activity and non-toxicity (Pelissari et al. 2009; Sánchez-González et al. 2010). Pea starch has small granules size, 2–40 µm (Ratnayake et al. 2002) and high amylose content (60–70%) (Hilbert and Macmasters 1945), thus it can provide a good transparent film with good physical, and mechanical properties with composite materials. Glycerol has been widely used as plasticizer for development of starch based films (Santacruz et al. 2015).
Previous studies indicated that physical and mechanical properties of the films could be significantly affected by ingredients concentration of the formulation (van den Broek et al. 2015; Zhang et al. 2015). RSM was applied for optimisation because it has been useful in finding the relationships between different independent and response variables while minimizing the number of experiments and usage of resources (Dailey and Vuong 2016). The findings of this study can be utilised for further application on coating fruit and vegetables. Therefore the aim of this study was to develop an optimal formulation for preparation of edible film from pea starch, chitosan and glycerol using RSM.
Materials and methods
Materials
Chitosan (medium molecular weight Poly (d-glucosamine) deacetylated chitin, ≥75% deacetyleated) and acetic acid were purchased from Sigma-Aldrich USA. Pea starch was supplied by Yantai Shuangta Food Co. Ltd China and was used as a film forming material. Glycerol was purchased from Ajax Finechem Pty Ltd. Australia.
Edible film preparation
Films were prepared by the casting process and dehydrating the suspension solution in petri plates. Suspension solution was prepared by dissolving 1 g chitosan (1–2%) in 100 ml of 0.7% (v/v) of aqueous acetic acid solution (Maciel et al. 2014). Pea starch powder (0.5–1.5%) was mixed to the above solution under control heating conditions (80 °C) with continuous stirring until the gelatinization temperature was reached. The ranges of chitosan (1–2%), pea starch (0.5–1.5%) and glycerol (0.5–1%) were selected based on previous studies (Chillo et al. 2008; Maran et al. 2013b; Santacruz et al. 2015) and our preliminary studies (results not shown). The film forming dispersion solution of starch–chitosan was cooled to room temperature before glycerol (plasticizer; 0.5–1%) was added. The solution was stirred for further 20 min to allow through mixing and removal of air bubbles. Film forming suspension solution (about 20 g) was casted in the petri dishes (10 cm in diameter) and dried at 30 °C for 24 h. Dried films were peeled off and used for further analysis.
Characteristics of pea starch–chitosan film
Physical properties
Thickness
The thickness of film was measured according to previously reported method (Saberi et al. 2015) using a digital micro-meter (Mitutoyo, Co., Model ID-F125, Japan). The sensitivity of the instrument was 0.001 mm. Film sample was placed under the nobe and thickness values in mm was recorded. Random value from at least 10 different points was noted for individual film sample and average was calculated. Results from thickness measurement were also used for further calculation of water vapour permeability (WVP) of the samples.
Moisture content (MC)
Films were cut into 15 × 40 mm strips and placed into the aluminum dishes for drying at 110 °C for 24 h. Films were then cooled for 2 h after removal from the oven and the weight was measured using a four decimal balance (HA-180 M, A&D company Ltd, Japan). MC was calculated based on weight difference (Eq. 1). All the measurements were carried out in triplicate and the values are expressed as means ± standard deviations.
| 1 |
Solubility
Solubility of film was measured according to the method reported in a previous study (Ojagh et al. 2010). Film specimens (40 × 15 mm) were dried to a constant weight at 110 °C for 24 h. Each sample was then placed into the glass-jar containing 50 ml of distilled water and subsequently shaken at 25 rpm at room temperature for 24 h. Undissolved portion of the film was collected and dried in the oven at 110 °C for 24 h to reach a constant weight. Solubility was calculated based on weight difference as shown in Eq. 2.
| 2 |
Barrier properties
Water vapour permeability (WVP)
Gravimetrically method, ASTM E96 procedure (ASTM 1996), with a 75% RH gradient at 25 °C was used to measure the WVP of the film. Permeation cells (0.7065 mm2 film area) containing anhydrous CaCl2 (0% RH) were sealed tightly by the sample film using parafilm. Covered permeation cells were placed in a desiccator having saturated NaCl solution (75% RH). RH inside the permeation cell was always lower than outside, and water vapour transport was determined using the weight gain of the cell at a steady state of transfer. Changes in the weight of the cell were recorded and plotted as a function of time. The slope of each line was evaluated by linear regression (R2 > 0.99), and the water vapour transmission rate was calculated through the slope of the straight line (g/s) divided by the test area (m2). After the permeation tests, the film thickness was measured and WVP (g Pa−1 s−1 m−1) was calculated as:
| 3 |
Δm/Δt, weight of moisture gain per unit time (gs−1) and can be calculated by the slope of the graph. A, area of the exposed film surface (m2), T, thickness of the film (mm), ΔP, represents the water vapour pressure difference inside and outside of the film (Pa) (Saberi et al. 2015).
Mechanical properties
The mechanical properties of the films were determined according to the method described by Saberi et al. (2015) with modification using a Texture Analyzer (LLOYD Instrument LTD, Fareham, UK). Film specimens (15 × 40 mm) were used for all mechanical tests. The maximum load (N) and extension (mm) curves were recorded to calculate tensile strength (TS), elongation at break (E) and Elastic Modulus (EM) of the films using a tensile test at crosshead speed of 1 mm/s and initial grip distance 40 mm.
Experimental design and statistical analysis
Response surface methodology (RSM)
The statistical analysis and regression model study was performed with JMP software (Version 22, SAS, Cary, NC, USA). A Box–Behnken design at three levels for each independent variables at three center points replicates was employed for study. Fifteen different edible coating formulations comprising chitosan (1–2%), pea starch (0.5–1.5%) and glycerol (0.5–1%) were used to get the best optimal combination. Effect of polysaccharide biopolymers blended with plasticizer (independent variables) on properties of casted film (response functions, Y) comprising thickness, WVP, solubility, moisture content, elongation at the break, elastic modulus, and tensile strength, was observed. In the process of optimization of coating formulation, response variables were related to independent variables by a second order polynomial equation (Eq. 4).
| 4 |
Xi, independent variables; β 0, intercept; β i, β ii , β ij, regression coefficients for intercept, linear, quadratic, and interaction terms; k, number of variables.
The independent variables and their code variable levels are shown in Table 1. The JMP software was also employed to develop the model equations, to graph 3D plots, 2D contour plots of the responses, as well as predicting the optimum conditions of the independent variables. The three independent variables were assigned as: X1 (chitosan concentration %), X2 (pea starch, %) and X3 (glycerol, %). Thus, the function containing these three independent variables is expressed as follow
| 5 |
Table 1.
Box–Behnken design employed for formulation of edible coating composition
| Run | Independent variables | Dependent variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Responses | |||||||||
| X1 (%) | X2 (%) | X3 (%) | T (mm) | WVP × 10−10 (gs−1m−1Pa−1) | S (%) | M (%) | EM (N/m2) | EB (mm) | TS (N/m) | |
| 1 | 1 | 1 | 0.5 | 0.0768 | 9.62 | 44.7 | 22.7 | 401.62 | 6.2 | 54.125 |
| 2 | 1 | 0.5 | 0.75 | 0.0736 | 4.16 | 52.7 | 42.1 | 681.93 | 10.5 | 26.64 |
| 3 | 1 | 1.5 | 0.75 | 0.0911 | 8.5 | 44.4 | 27.9 | 372.17 | 5.7 | 54.545 |
| 4 | 1 | 1 | 1 | 0.0841 | 5.09 | 53.1 | 40.4 | 372.79 | 8.2 | 30.46 |
| 5 | 1.5 | 0.5 | 0.5 | 0.0859 | 9.56 | 40.8 | 24.2 | 670.85 | 6.7 | 70.59 |
| 6 | 1.5 | 1.5 | 0.5 | 0.0677 | 10.56 | 52.6 | 18.0 | 5815.4 | 4.2 | 266.96 |
| 7 | 1.5 | 1 | 0.75 | 0.1246 | 16.25 | 54.0 | 31.0 | 191.50 | 5.6 | 65.64 |
| 8 | 1.5 | 1 | 0.75 | 0.1135 | 8.63 | 48.9 | 29.7 | 407.86 | 5.4 | 57.105 |
| 9 | 1.5 | 1 | 0.75 | 0.0919 | 7.12 | 47.1 | 28.0 | 465.56 | 5.5 | 50.105 |
| 10 | 1.5 | 0.5 | 1 | 0.1055 | 7.15 | 55.2 | 34.8 | 406.23 | 7.7 | 35.47 |
| 11 | 1.5 | 1.5 | 1 | 0.1596 | 16.4 | 50.3 | 30.3 | 249.92 | 6.6 | 45.165 |
| 12 | 2 | 1 | 0.5 | 0.1074 | 23.7 | 46.9 | 13.9 | 1970.9 | 4.7 | 149.015 |
| 13 | 2 | 0.5 | 0.75 | 0.1096 | 12.8 | 42.8 | 24.2 | 548.57 | 5.6 | 78.3 |
| 14 | 2 | 1.5 | 0.75 | 0.13 | 34.4 | 47.3 | 17.8 | 600.39 | 3.4 | 142.935 |
| 15 | 2 | 1 | 1 | 0.1262 | 15 | 48.8 | 26.6 | 414.15 | 5.1 | 93.145 |
Independent variables: X1, Chitosan (1–2%); X2, Starch (0.5–1.5%); X3, Glycerol (0.5–1%)
Responses (Y): T, thickness (mm); WVP, water vapour permeability (gs−1 m−1 pa−1); S, solubility (%); M, moisture (%): EM, elastic modulus (N/m2); EB, elongation at break (mm), TS, tensile strength (N/m)
Statistical analysis
JMP (Version 11, SAS Cary, NC, USA) was used to predict the optimal conditions of independent variables using 3D contour plots. Analysis of variance ANOVA, the coefficient of determination (R2) and adjusted the coefficient of determination (Adj-R2) were used to assess the validity of the model. Analysis of variance (one-way ANOVA) was used to compare the mean differences of the samples. SPSS 16.0.0 statistical software for windows (SPSS IBM, USA) was used for data treatment and statistical analysis. Comparison of the mean was considered to be statistically significant at p < 0.05.
Results and discussion
Fitting of the model
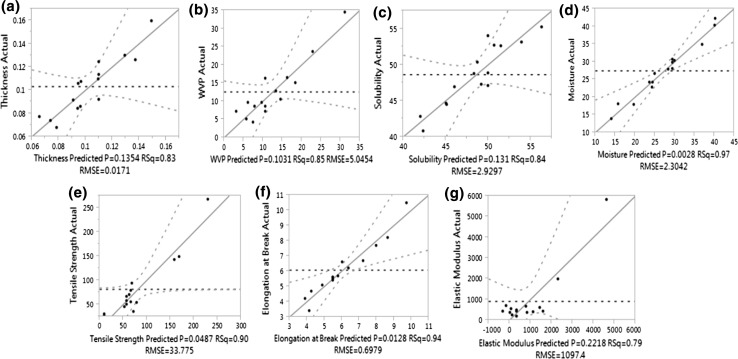
Different analysis sources of variation, such as lack of fit, R2, Predicted Residual Sum of Square (PRESS) for the models, F ratio and Prob > F were analyzed to identify the fitting of the RSM mathematical models. The results (Table 2; Fig. 1) showed that the value of the coefficient of determination (R2) was in the range of 0.79–0.97, reflecting that at least 79% of the predicted values could be matched with the actual values. Values of F ratio for physical parameters (thickness, solubility and moisture content) (2.791, 3.26 and 2.84) and lack of fit (0.5, 0.51 and 0.78) showed that the designed model was efficient in predicting the physical properties of the film.
Table 2.
ANOVA study for the model fitting
| Parameters | T (mm) | WVP × 10−10 (gs−1 m−1 Pa−1) | S (%) | MC (%) | EM (N/m2) | EB (mm) | TS (N/m) |
|---|---|---|---|---|---|---|---|
| Lack of fit | 0.51 | 0.51 | 0.78 | 0.19 | 0.01 | 0.01 | 0.03 |
| R2 | 0.83 | 0.85 | 0.84 | 0.97 | 0.79 | 0.94 | 0.90 |
| Adjusted R2 | 0.54 | 0.59 | 0.54 | 0.91 | 0.40 | 0.84 | 0.71 |
| F ratio of model | 2.791 | 3.26 | 2.84 | 17.67 | 2.05 | 9.09 | 4.83 |
| Prob > F | 0.50 | 0.51 | 0.74 | 0.003 | 0.22 | 0.01 | 0.05 |
| Press | 0.0158 | 1377.8 | 338.5 | 376.0 | 957,664.1 | 38.65 | 89,595.6 |
WVP, water vapour permeability (gs−1 m−1 pa−1); S, solubility (%); MC, moisture content (%); EM, elastic modulus (N/m2); EB, elongation at break (mm); TS, tensile strength (N/m)
Fig. 1.
Correlation between predicted and experimental values for thickness (a), WVP (b), solubility (c), moisture content (d), tensile strength (e), Elongation at break (f) and elastic modulus (g)
For barrier properties of the film, statistics showed that values of PRESS, F value and lack of fit were 1377.8, 3.26 and 0.51, indicating that the mathematical model is a good predictor of WVP of film (Table 2). The results also indicated that predicted model for mechanical properties of films had high PRESS values for EM, EB and TS (957,664.1, 38.65 and 89595) and the coefficient of determination (R2) ranged from 0.79 to 0.94, indicating that the model is also reliable for predicting the mechanical properties of the chitosan–pea starch edible film.
Empirical model for prediction of film properties
Through applying multiple regression analysis on the experimentally attained data, the empirical model was developed by fitting the experimental data obtained from Box–Behnken design into second order polynomial mathematical equation (Eq. 4). The model could be fitted to the following second order polynomial Eqs. (6)–(12). In order to investigate the relationship between process variables and response variables from the developed mathematical model equations 3D contour plots were constructed between two independent variables while keeping the 3rd variable constant.
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
Effect of operating parameters on properties of film
Thickness
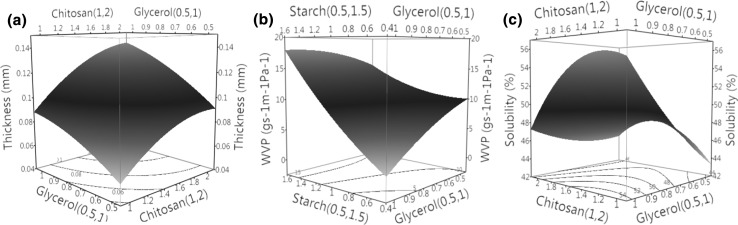
Thickness affects the structure of film in relations of drying kinetics, WVP and film opacity (Maran et al. 2013a). Results showed that thickness varies from 0.06 to 0.16 mm with the change in the amount of dry matter in film suspension solution (Table 1). Analysis results showed that chitosan and glycerol had a significant impact on the thickness of the film (Table 2) whereas, starch did not show any significant impact on thickness of the film (p > 0.05). In addition, the results in Fig. 2a showed that higher the content of chitosan resulted in the formation of film with greater thickness. Chitosan is a positively charged molecule and being a positively charged moiety it have wide hydration layers with highly retained water molecules which participates in the film structure thus inhibiting the chain approximation and giving rise to thicker films. Similar explantation related to the chitosan concentration and thickness of the films has been reported previously in the literature (Bonilla et al. 2013). The other possible explanation could be the over loading of the suspension solution. Unoptimized formulations in most of the previous studies is subject to increase in the unwanted film thickness as a result of overloading of suspension solution which hinders the permeability control of film eventually (Bof et al. 2015). Correlation between permeability properties and thickness can be explained by Fick’s law of diffusion, which says that permeability is inversely proportional to the thickness of the film. Hence higher the thickness lesser will be the mass transfer through the film due to more resistance. Similar explantation has been provided in the literature on the water vapour permeability and thickness effect of hydrophilic films by McHugh et al. (1993).
Fig. 2.
Response surface plots showing the interaction impact of independent variables on the thickness (a), WVP (b) and solubility (c) of the edible film
Water vapour permeability
WVP is the main parameter used to explain the possible mass transfer mechanisms through the film surface. For edible coatings it should be low to prevent moisture loss from the fresh produce (Ma et al. 2008). The value of WVP varied significantly (p < 0.05) with the concentration of polymer and plasticizer. Coating formulation comprising 1% chitosan; 0.5% starch and 0.75% glycerol showed the minimum permeability values (4.1 × 10−10 gs−1 m−1 Pa−1) whereas increased concentration of starch and glycerol (1.5% pea starch and 0.75% glycerol) resulted into higher WVP response (34.4 × 10−10 gs−1 m−1 Pa−1 (Table 1). Biopolymer-plasticizer chemistry proposed a significant impact on WVP of the casted film. Binding between the NH2 and OH functional groups forms a crosslinking network in the film structure and slow down the rate of permeability. Figure 2b shows the behaviour of increasing starch concentration on the film permeability attributes which could be due to the hydrophilic nature of starch that fails to resist the migration of water through the film surface. These results are in line with the previous study (Pelissari et al. 2009) where increased concentration of starch enhanced the WVP rate. Another possible reason for these observations behaviour could be explained on the basis of the oval granular structure of the pea starch, where the arrangement may leave some inter-granular spaces which at lower concentration are filled by other partaking ingredients but at higher concentration are available for free mass transfer. The WVP of the coated film gradually decreased as the concentration of chitosan increased from 1 to 2% (Table 1). The decreasing WVP value may be due to hydrophobic acetyl group of incompletely deacetylated chitosan or due to intense hydrogen bond interactions between NH2 and OH functional groups. These interaction may be dominated over hydrophilic interactions thus reducing the availability of free hydrophilic groups at lower starch concentration. Glycerol also increases the mobility by reducing the rigidity and destabilisation of chain arrangements (by easily interacting with the starch chain) by minimizing the starch intermolecular and intramolecular hydrogen bonds with starch–glycerol hydrogen bonds which disrupted the crystalline pattern of starch and facilitates the movement of water (Singh et al. 2009; Xu et al. 2005).
Solubility
Water solubility describes the water resistance and integrity of the edible film. Solubility of the chitosan–starch film was significantly affected by glycerol and chitosan concentrations (p < 0.05). Results showed that the solubility of casted film was in the range of 40.8–55.2% and increases with the increase in glycerol concentration (Table 1; Fig. 2c) and decreases with higher chitosan concentration. The possible reason may be due to glycerol which can disrupt the crystalline structure of the starch and causes breakage of hydrogen bonds and formation of new hydrogen bonds between exposed OH group of amylose and amylopectin and glycerol (Ratnayake et al. 2002). These findings are also in agreement with the previous reported work (Maran et al. 2013a; Mehyar and Han 2004). These results could be explained from the fact that higher chitosan concentration induces the strong interactions between the two polymers and lowers the resulting solubility. These observations supports the previous studies where the solubility proportionally decreased as starch was blended with chitosan at higher concentration (Bourtoom and Chinnan 2008; Kanmani and Lim 2013).
Moisture content (MC)
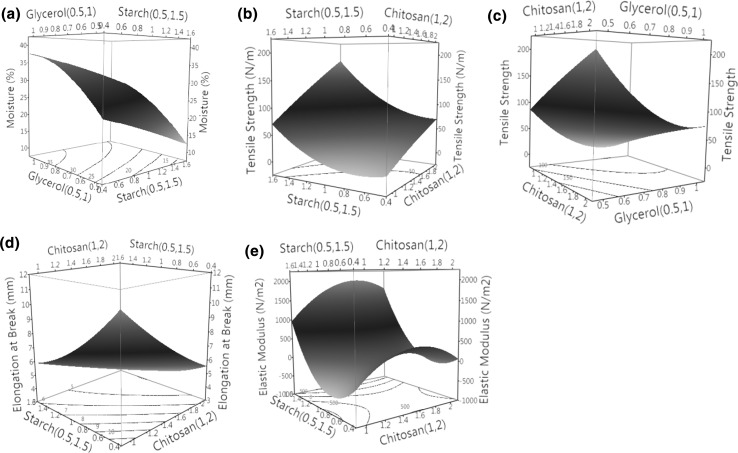
MC describes the available moisture present in the film. Variations in the moisture content are shown in the Fig. 3a. Glycerol and starch were found to affect the moisture level in the film significantly (p < 0.05) and MC was higher at higher concentration of glycerol, 1% (Table 1). This may be due to the hydrophilic nature of the glycerol which assist in the formation of hydrogen bonding with free OH groups (Cerqueira et al. 2012). Similar results were reported in previous studies on the increasing effect of glycerol on MC (Saberi et al. 2016; Sanyang et al. 2015b). Starch also facilitates the retention of MC (due to its hydrophilic nature) in films as compared to chitosan (Fig. 3a). Lower MC at higher chitosan concentration may be due to higher interactions among the molecules leaving behind no free hydrophilic groups for interaction with the water molecules.
Fig. 3.
3D Contour plots for moisture % (a), tensile strength (b, c), elongation at break (d) and Elastic modulus (e) showing the interaction impact of independent variables on the pea starch: chitosan film
Mechanical properties: tensile strength (TS), elongation at break (EB) and elastic modulus (EM)
TS is an important property of the films as it greatly affects the utility of the film for its application in shelf life extension of fresh produce during storage. The stability of film was measured on the basis of TS, EB, and EM. Figure 3b–e shows the effect of additives on the mechanical properties of edible film. Pea starch and chitosan blend provided a film with good mechanical properties which illustrate the compatibility of hydrocolloids. TS, EB and EM values of chitosan–pea starch film are presented in Table 1. It was found that strength of the film was significantly affected by varying polymeric concentration (p < 0.05). TS was maximum with 1.5% chitosan and 1.5% of starch blended with lowest concentration of glycerol (0.5%) and depicts the greatest integrity of film forming components. TS varied between 26.6 to 266.9 N/m (Table 1). This is due to the formation of dominating inter-molecular hydrogen bonding between NH2 and OH groups of chitosan–starch which increases with the increase in polymer concentration. Interaction of polymers increases the stability because of the participating functional groups in the bonding. Chitosan–starch hydrogen bonding is the intrinsic factor which supports the mechanical and physical properties of the film. These results are in agreement with previous research where tensile properties of starch films were improved significantly when chitosan was incorporated into the starch solution (Xu et al. 2005; Zhai et al. 2004).
It is important to note that plasticizer also has a significant (p < 0.05) effect on the TS of the film (Fig. 3c). TS increases with the decrease in the plasticizer concentration and was maximum (266.9 N/m) at 0.5% glycerol. This phenomenon can be explained by the role of glycerol on diminishing the strong intra–molecular hydrogen bonding between starch and chitosan molecules as previously reported by Sanyang et al. (2015a). The effectiveness of glycerol for TS reduction is due to hydrophilic nature of the compound which hold more H2O molecules and resulted into more intense plasticizing effect. This arrangement increases the spatial difference between the polymer chains and decrease the TS. Moreover, a more vigorous relationship between tensile properties and moisture content was observed portraying the negative effect of moisture which might have caused extra plasticizing effect hence lower the tensile strength of starch film. The results are in line with the precious study reported by Chinma et al. (2015) which showed that tensile properties of starch films decreased with increase in film humidity.
The % E and EM varies between 3.4–10.5 mm and 191.4–5815.4 N/m2 respectively (Table 1). % E was greater when higher chitosan concentration was applied and lower with higher starch concentrations. Both chitosan and starch were found to affect the elongation property of films at a significant (p < 0.05) rate. Similarly EM value was higher for 1% chitosan, 1.5% starch and 0.5% glycerol (5815.4 N/m2) and minimum with higher concentration of glycerol. High starch concentration results in a lower ability of edible film for stretching where plasticizer influences the flexibility of film by occupying the free space between the polymers.
Optimization and validation of coating formulation
Optimal edible film formulation was achieved by optimizing chitosan, starch and glycerol for physical, mechanical and barrier properties. RSM was used for the optimization of the coating formulation. Based on the effect of independent variables (chitosan, starch and glycerol) on the response values for physical and mechanical properties of film, the optimal conditions for formulation of this film were determined to be chitosan 1%, starch 1.5% and glycerol 0.5%. To validate these predicted conditions, this formulation was tested in triplicate experiments and the results showed that the actual values for physical and mechanical properties were found to be similar to the predicted values (Table 3). These results shows that this formulation can be applied to prepare the pea starch film with good physical and mechanical properties for further utilisation.
Table 3.
Validation of predicted values for physical, mechanical and barrier properties of pea starch: chitosan blended film
| Variables response | Predicted value | Experimental value (n = 3) |
|---|---|---|
| Thickness (mm) | 0.055 ± 0.01 | 0.058 ± 0.03 |
| WVP (gs−1 m−1 Pa−1) | 5.29 ± 0.07 | 5.27 ± 0.03 |
| Solubility (%) | 45.53 ± 0.1 | 48.12 ± 0.06 |
| Moisture content (%) | 17.73 ± 0.03 | 19.14 ± 0.08 |
| Elastic modulus (N/m2) | 3543.53 ± 2.56 | 3559.25 ± 5.69 |
| Elongation at break (mm) | 4.6 ± 0.92 | 5.0 ± 1.34 |
| Tensile strength (Nm−2) | 173.7 ± 2.13 | 181.8 ± 1.78 |
Conclusion
RSM has been successfully applied to optimize the best formulation for preparation of pea starch film for further utilisation in coating vegetable and fruits. All three tested ingredients (pea starch, chitosan and glycerol) were found to have different effects on physical, and mechanical properties of film. The results showed that optimal formulation for preparation of pea starch film were chitosan 1% pea starch 1.5% and glycerol 0.5% which had satisfactory thickness, good WVP, solubility, moisture content and mechanical properties. These findings can be further applied for coating vegetables and fruit.
Acknowledgements
This research was supported by the University of Newcastle, Australian Research Council (ARC) Training Centre for Food and Beverage Supply Chain and Optimisation (IC140100032). NSW Department of Primary Industries is a partner organisation in the Training Centre.
Contributor Information
Rahul Thakur, Email: Rahul.thakur@uon.edu.au.
Quan V. Vuong, Email: vanquan.vuong@newcastle.edu.au
References
- Arnon H, Zaitsev Y, Porat R, Poverenov E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol Technol. 2014;87:21–26. doi: 10.1016/j.postharvbio.2013.08.007. [DOI] [Google Scholar]
- ASTM . Standard test method for water vapor transmission of materials, method E96/E96M e 13. Phialdelphia: American Society for Testing and Materials; 1996. [Google Scholar]
- Bof MJ, Bordagaray VC, Locaso DE, García MA. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocolloids. 2015;51:281–294. doi: 10.1016/j.foodhyd.2015.05.018. [DOI] [Google Scholar]
- Bonilla J, Atarés L, Vargas M, Chiralt A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J Food Eng. 2013;114:303–312. doi: 10.1016/j.jfoodeng.2012.08.005. [DOI] [Google Scholar]
- Bourtoom T, Chinnan MS. Preparation and properties of rice starch–chitosan blend biodegradable film LWT—Food. Sci Technol. 2008;41:1633–1641. [Google Scholar]
- Cerqueira MA, Souza BWS, Teixeira JA, Vicente AA. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—a comparative study. Food Hydrocolloids. 2012;27:175–184. doi: 10.1016/j.foodhyd.2011.07.007. [DOI] [Google Scholar]
- Chillo S, Flores S, Mastromatteo M, Conte A, Gerschenson L, Del Nobile M. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J Food Eng. 2008;88:159–168. doi: 10.1016/j.jfoodeng.2008.02.002. [DOI] [Google Scholar]
- Chinma CE, Ariahu CC, Alakali JS. Effect of temperature and relative humidity on the water vapour permeability and mechanical properties of cassava starch and soy protein concentrate based edible films. J Food Sci Technol. 2015;52:2380–2386. doi: 10.1007/s13197-013-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey A, Vuong Q. Optimum conditions for microwave assisted extraction for recovery of phenolic compounds and antioxidant capacity from macadamia (Macadamia tetraphylla) skin waste using water. Processes. 2016;4:2. doi: 10.3390/pr4010002. [DOI] [Google Scholar]
- Dhall RK. Advances in edible coatings for fresh fruits and vegetables: a review. Crit Rev Food Sci Nutr. 2013;53:435–450. doi: 10.1080/10408398.2010.541568. [DOI] [PubMed] [Google Scholar]
- Gómez-Estaca JG, Giménez B, Montero P, Gómez-Guillén MC. Incorporation of antioxidant borage extract into edible films based on sole skin gelatin or a commercial fish gelatin. J Food Eng. 2009;92:78–85. doi: 10.1016/j.jfoodeng.2008.10.024. [DOI] [Google Scholar]
- Hilbert G, Macmasters M. Pea starch: a starch with high amylose content. J Biol Chem. 1945;38:229. [PubMed] [Google Scholar]
- Kanmani P, Lim ST. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013;141:1041–1049. doi: 10.1016/j.foodchem.2013.03.103. [DOI] [PubMed] [Google Scholar]
- Ma X, Chang PR, Yu J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr Polym. 2008;72:369–375. doi: 10.1016/j.carbpol.2007.09.002. [DOI] [Google Scholar]
- Maciel VBV, Yoshida CMP, Franco TT. Development of temperature indicator prototype: cardpaper coated with chitosan intelligent films. J Agric Chem Environ. 2014;03:5–10. [Google Scholar]
- Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K. Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr Polym. 2013;92:1335–1347. doi: 10.1016/j.carbpol.2012.09.069. [DOI] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K. Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr Polym. 2013;92:1335–1347. doi: 10.1016/j.carbpol.2012.09.069. [DOI] [PubMed] [Google Scholar]
- McHugh TH, Avena-Bustillos R, Krochta J. Hydrophilic edible films: modified procedure for water vapor permeability and explanation of thickness effects. J Food Sci. 1993;58:899–903. doi: 10.1111/j.1365-2621.1993.tb09387.x. [DOI] [Google Scholar]
- Mehyar G, Han J. Physical and mechanical properties of high-amylose rice and pea starch films as affected by relative humidity and plasticizer. J Food Sci. 2004;69:E449–E454. doi: 10.1111/j.1365-2621.2004.tb09929.x. [DOI] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010;122:161–166. doi: 10.1016/j.foodchem.2010.02.033. [DOI] [Google Scholar]
- Pelissari FM, Grossmann MVE, Yamashita F, Pineda EAG. Antimicrobial, mechanical, and barrier properties of cassava starch–chitosan films incorporated with oregano essential oil. J Agric Food Chem. 2009;57:7499–7504. doi: 10.1021/jf9002363. [DOI] [PubMed] [Google Scholar]
- Ratnayake WS, Hoover R, Warkentin T. Pea starch: composition, structure and properties—a review. Starch-Stärke. 2002;54:217–234. doi: 10.1002/1521-379X(200206)54:6<217::AID-STAR217>3.0.CO;2-R. [DOI] [Google Scholar]
- Saberi B, Vuong Q, Chockchaisawasdee S, Golding J, Scarlett C, Stathopoulos C. Mechanical and physical properties of pea starch edible films in the presence of glycerol. J Food Process Preserv. 2015 doi: 10.3390/foods5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi B, Vuong Q, Chockchaisawasdee S, Golding J, Scarlett C, Stathopoulos C. Water sorption isotherm of pea starch edible films and prediction models. Foods. 2016;5:1. doi: 10.3390/foods5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-González L, González-Martínez C, Chiralt A, Cháfer M. Physical and antimicrobial properties of chitosan–tea tree essential oil composite films. J Food Eng. 2010;98:443–452. doi: 10.1016/j.jfoodeng.2010.01.026. [DOI] [Google Scholar]
- Santacruz S, Rivadeneira C, Castro M. Edible films based on starch and chitosan: effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical treatment. Food Hydrocolloids. 2015;49:89–94. doi: 10.1016/j.foodhyd.2015.03.019. [DOI] [Google Scholar]
- Sanyang M, Sapuan S, Jawaid M, Ishak M, Sahari J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers. 2015;7:1106. doi: 10.3390/polym7061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J Food Sci Technol. 2015;53:326–336. doi: 10.1007/s13197-015-2009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Belton PS, Georget DMR. The effects of iodine on kidney bean starch: films and pasting properties. Int J Biol Macromol. 2009;45:116–119. doi: 10.1016/j.ijbiomac.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Valencia-Chamorro SA, Perez-Gago MB, Del Rio MA, Palou L. Effect of antifungal hydroxypropyl methylcellulose-lipid edible composite coatings on Penicillium decay development and postharvest quality of cold-stored “Ortanique” mandarins. J Food Sci. 2010;75:S418–S426. doi: 10.1111/j.1750-3841.2010.01801.x. [DOI] [PubMed] [Google Scholar]
- van den Broek LA, Knoop RJ, Kappen FH, Boeriu CG. Chitosan films and blends for packaging material. Carbohydr Polym. 2015;116:237–242. doi: 10.1016/j.carbpol.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Xu YX, Kim KM, Hanna MA, Nag D. Chitosan–starch composite film: preparation and characterization. Ind Crops Prod. 2005;21:185–192. doi: 10.1016/j.indcrop.2004.03.002. [DOI] [Google Scholar]
- Zhai M, Zhao L, Yoshii F, Kume T. Study on antibacterial starch/chitosan blend film formed under the action of irradiation. Carbohydr Polym. 2004;57:83–88. doi: 10.1016/j.carbpol.2004.04.003. [DOI] [Google Scholar]
- Zhang L, Li R, Dong F, Tian A, Li Z, Dai Y. Physical, mechanical and antimicrobial properties of starch films incorporated with epsilon-poly-l-lysine. Food Chem. 2015;166:107–114. doi: 10.1016/j.foodchem.2014.06.008. [DOI] [PubMed] [Google Scholar]